Summary
Despite significant reductions in morbidity and mortality secondary to availability of effective combination antiretroviral therapy (cART), human immunodeficiency virus (HIV) infection still accounts for 1.5 million deaths annually. The majority of deaths occur in sub-Saharan Africa where rates of opportunistic co-infections are disproportionately high. In this review, we discuss the immunopathogenesis of five common infections that cause significant morbidity in HIV-infected patients globally. These include co-infection with Mycobacterium tuberculosis, Cryptococcus neoformans, hepatitis B virus (HBV), hepatitis C virus (HCV), and Plasmodium falciparum. Specifically, we review the natural history of each co-infection in the setting of HIV, the specific immune defects induced by HIV, the effects of cART on the immune response to the co-infection, the pathogenesis of immune restoration disease (IRD) associated with each infection, and advances in the areas of prevention of each co-infection via vaccination. Finally, we discuss the opportunities and gaps for future research.
Keywords: HIV, immunology, tuberculosis, cryptococcosis, hepatitis B, hepatitis C, malaria, immune restoration disease
Introduction
Despite the increasing availability of combination antiretroviral therapy (cART), nearly 1.5 million deaths were attributed to human immunodeficiency virus (HIV) in 2010 (1). The immunodeficiency caused by chronic HIV infection increases the risk of co-infection with pathogens that are controlled by innate and adaptive cellular immune responses and some that are controlled by phagocytic antibody responses. Furthermore, administration of cART in the setting of HIV co-infection does not always restore the pathogen-specific immune response to normal levels.
Here we review the immunopathogenesis of the five leading infectious diseases that continue to cause significant morbidity and mortality in HIV-infected individuals globally: tuberculosis (TB), cryptococcosis, hepatitis B virus (HBV), hepatitis C virus (HCV), and malaria. Understanding the complex interaction between HIV, these co-infections, and the host immune response is central to developing new strategies for optimal treatment and prevention. Further, we discuss the beneficial impact and potential risks of cART on the natural history of these co-infections, with a particular focus on immune restoration disease (IRD) (Table 1).
Table 1.
| Characteristics of immune reconstitution in HIV infection | Available evidence for specific pathogen-related IRD | ||||
|---|---|---|---|---|---|
| TB-IRIS | C-IRIS | HBV-IRD | HCV-IRD | ||
| High pathogen load | ✓ | ✓ | ✓ | NAc | |
| Severe CD4+ T cell depletion before ART | ✓ | ✓ | ✓ | NAc | |
| Replenishment of CD4+ T- cells | a) Blood | ✓ | ✓ | ✓ | ✓ |
| b) Other compartments | ✓a | ✓a | NA | NA | |
| Restoration of pathogen-specific T cell responses | a) Memory response | ✓ | ✘b | ✘b | ✓ |
| b) Site-specific action | ✓ | NA | NA | ||
| Increased activity of innate immune system | ✓ | ✓ | ✓ | ✓ | |
TB – tuberculosis; C –cryptococcus; HBV – hepatitis B virus; HCV – hepatitis C virus; IRIS – immune reconstitution inflammatory syndrome; IRD – immune restoration disease. NA – information not available
in CSF
not seen longitudinally in blood;
not demonstrated in two small studies.
Mycobacterium tuberculosis co-infection
Epidemiology and global burden of disease
The World Health Organization (WHO) has estimated that approximately 14 million people worldwide have HIV and Mycobacterium tuberculosis co-infection and that TB is the most common opportunistic infection in individuals with HIV infection, accounting for about 26% of acquired immunodeficiency syndrome (AIDS)-related deaths. In 2010, the WHO estimated that 39% of new TB cases occurred in people with HIV co-infection (2).
Natural history of tuberculosis infection
M. tuberculosis is an obligate intracellular pathogen that infects approximately one third of the world’s population (about 2 billion people) and is primarily controlled by cell-mediated immune responses. Primary infection with M. tuberculosis affects the lungs, where alveolar macrophages and dendritic cells (DCs) are infected and generate an innate immune response that may resolve the infection or be circumvented leading to primary pulmonary TB. The majority of infected individuals achieve long-term control of M. tuberculosis infection by innate and adaptive immune responses resulting in latent tuberculosis infection (LTBI). In the absence of HIV infection, 5–10% of individuals with LTBI experience reactivation of the infection at some time during their lifetime when immune control is lost, usually associated with aging or medications and illness that impair immunocompetence. It also appears that many individuals experience phases of subclinical TB before presenting with active TB (3). In contrast, 5–15% of individuals with HIV infection and LTBI experience reactivation of M. tuberculosis infection every year, and HIV infection increases the risk of reactivation by about 20-fold (4). HIV infection also increases the risk of acquiring primary M. tuberculosis infection by 2.2 – 5.5-fold (5, 6).
Immune response to M. tuberculosis and pathogenesis
Immune control of M. tuberculosis infection is mediated by the concerted effects of multiple cell types, including CD4+ and CD8+ T cells, CD1-restricted T cells, B cells, macrophages, neutrophils, fibroblasts, and multinucleated giant cells that all contribute to granuloma formation to contain the infection (3, 7). The inflammatory process that kills or ‘walls-off’ the mycobacterial infection involves chemokines and cytokines that promote T-helper 1 (Th1) cell chemotaxis and/or function such as CXCL9, CXCL10, CXCL11 (ligands for CXCR3), and IL-18, chemokines that promote monocyte chemotaxis and function such as CCL2, Th1 cytokines such as IL-12, IL-23, and IFN-γ, granulysin and other cytotoxic molecules produced by CD8+ T cells, and macrophage products such as nitric oxide synthetase-2 and tumor necrosis factor-α (TNF-α).
The impact of HIV on M. tuberculosis infection
Given the complexity of this immune response, there are multiple ways that HIV can alter the immune response to M. tuberculosis (8). CD4+ T-cell depletion appears to be particularly important in the failure to generate or the loss of a cellular immune response to M. tuberculosis in patients with HIV infection. The rate of TB reactivation in HIV-infected patients increased with declining CD4+ T-cell counts, and patients with CD4+ T-cell counts <200/microliter were particularly susceptible to disseminated TB, presumably reflecting the poor granuloma formation in patients with this degree of immunodeficiency (9). Studies in simian immunodeficiency virus (SIV)-infected macaques demonstrated that reactivation of LTBI was associated with CD4+ T-cell depletion rather than the level of SIV replication (10). CD4+ T-cell depletion was associated with a decline in memory (CD27+CD45RO+) CD4+ T cells that recognize M. tuberculosis antigens (11, 12), a decline in polyfunctional antigen-specific CD4+ T cells, and a relative increase in IFN-γ+ CD8+ T cells (13). Other HIV-induced immune defects may also facilitate M. tuberculosis infection and disease, including suppression of cellular immune responses by regulatory T (Treg) cells (14) and impairment of TNF-α-mediated apoptotic responses to M. tuberculosis (15, 16). HIV infection is also associated with depletion of CD4+ T cells in granulomas (8), and both lymph nodes and other tissues infected by M. tuberculosis demonstrate large numbers of neutrophils and necrosis (17). Increased TNF-α activity within granulomas may increase necrosis, though studies of cytokine production in granulomas from patients with HIV and M. tuberculosis co-infection have been inconclusive (8).
Impact of cART
Suppression of HIV replication by cART leads to an increase in naive (CD27+CD45RA+) and central memory (CD27+CD45RA−) CD4+ T cells and increased IFN-γ+ or polyfunctional T-cell responses to the region of difference-1 (RD-1) antigens 6 kDa early secretory antigenic target (ESAT-6) and 10 kDa culture filtrate antigen (CFP10) (13, 18). Even though T-cell function in HIV-TB co-infected patients is not the same as in patients with M. tuberculosis mono-infection, the improvement of cell-mediated immune responses is associated with a reduction in the rate of both primary TB and reactivation of LTBI by at least 65%, irrespective of the CD4+ T-cell count at which cART is commenced (19). However, the incidence of TB disease increases during the first three months of cART before subsequently declining, predominantly in patients with CD4+ T-cell counts of <50/microliter (20). This has been referred to as ART-associated TB (21). A study conducted in Haiti demonstrated that rates of death were about 3.25 times higher in patients who developed TB during the first 3 months of cART (22). While some of these cases may be undiagnosed incident TB, most cases appeared to be IRD caused by restoration of an immune response against subclinical M. tuberculosis infection. ART-associated TB is characterized by increased Th1 responses to the RD1 antigens of M. tuberculosis with increased production of IFN-γ, the IFN-γ-inducible chemokines CXCL10 and CXCL9, and IL-18 (23–25).
Tuberculosis-associated immune reconstitution inflammatory syndrome
Commencement of cART in HIV-infected patients who have recently received treatment for TB may also result in IRD that presents as what appears to be a ‘paradoxical’ worsening of TB clinical disease characterized by a pronounced and/or atypical inflammatory response (Fig. 1). This affects approximately 20–25% of HIV patients with treated TB during the first 3 months of cART and is usually referred to as paradoxical TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) (21, 26–28). Some cases of subclinical TB that are ‘unmasked’ after cART is commenced (ART-associated TB) also exhibit atypical inflammation and may be referred to as unmasking TB-IRIS (21).
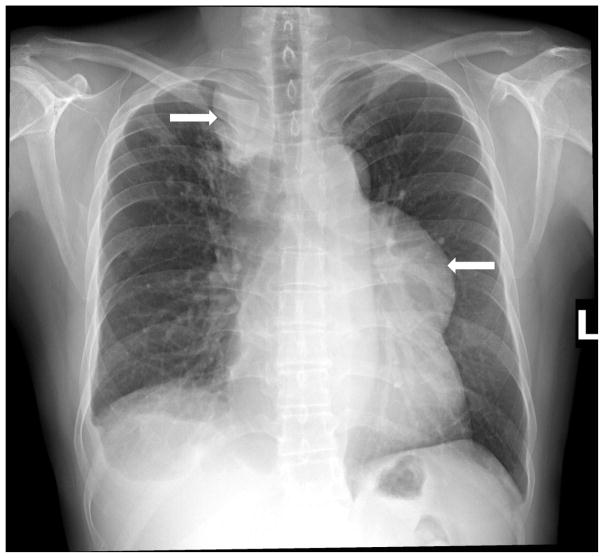
Fig. 1. Chest x-ray of a patient with TB-IRIS showing massive right paratracheal and left anterior mediastinal lymphadenopathy.
The patient exhibited evidence of systemic inflammation, including pronounced fever, for many weeks that eventually resolved on corticosteroid therapy.
High pathogen load and TB-IRIS
The immunopathogenesis of TB-IRIS has been investigated more than any other type of IRD, and information gained has provided fundamental knowledge about the immunopathogenesis of all types of IRD in HIV patients. However, immunological mechanisms may differ for different pathogens (29). Several lines of evidence suggest that a high pathogen load is a major determinant of an immune response that results in immunopathology. Thus, TB-IRIS occurs most commonly in patients with disseminated (extra-pulmonary) TB (21), a shorter period of TB therapy before cART is commenced (26–28), or drug-resistant TB (30), all of which are likely to result in a higher pathogen load. Furthermore, HIV-infected patients with treated TB meningitis who develop TB-IRIS have higher rates of positive cerebrospinal fluid (CSF) culture for M. tuberculosis infection before and after cART than patients who do not develop menigitis TB-IRIS (31). Finally, higher levels of mycobacterial lipoarabinomannan in urine are associated with an increased the risk of developing TB-IRIS (32).
Immunological predictors of TB-IRIS
Severe CD4+ T-cell deficiency (usually CD4+ T cells <50/microliter) is a strong predictor of TB-IRIS (21) as it is for other types of IRD (33). Very low serum levels of antibodies to phenolglycolipid antigen-Tb 1 (PGL-Tb1) (34) and a higher proportion of the V-delta2+ subset of TCRγδ+ T cells that do not express the inhibitory receptors CD94/NKG2 and CD158ah,b (35) have been identified as predictors. These might be determinants of a high pathogen load or be associated with immune dysregulation that predisposes to the restoration of immune responses against M. tuberculosis that cause immunopathology. Those immune responses have yet to be fully characterized.
Th1 responses and TB-IRIS
Bourgarit et al. (35, 36) demonstrated that patients with TB-IRIS exhibit higher proportions of circulating tuberculin-specific IFN-γ+ T cells than controls using enzyme-linked immunospot (ELISpot) assays and argued that Th1 responses were central to the pathogenesis of TB-IRIS. Similar findings were subsequently reported from studies on small numbers of patients using ELISpot assays (37) or polychromatic intracellular flow cytometry (ICS) (38). A role for Th1 cells has been clearly shown in a mouse model of IRD associated with Mycobacterium avium infection, but it was also apparent that activation of myeloid cells contributed to the immunopathology (39). Meintjes et al. (21) confirmed an association of TB-IRIS with increased proportions of IFN-γ+ T cells reacting with several M. tuberculosis antigens using ELISpot assays but also observed that patients who did not develop TB-IRIS also exhibited increased proportions M. tuberculosis-reactive IFN-γ+ T cells and questioned whether they were the cause of the immunopathology. In support of this view, two studies conducted in large numbers of HIV-infected patients with treated TB from South East Asia using whole blood IFN-γ release assays (IGRAs) demonstrated that tuberculin-reactive IFN-γ+ T cells increased after commencing cART in all patients, and although responses were higher in TB-IRIS patients, the difference between patient groups was not statistically significant at the time of the TB-IRIS (23;40). The findings of the latter two studies might be explained by a relative insensitivity of whole blood IGRAs because a study conducted in India using intracellular flow cytometry demonstrated significantly higher IFN-γ+ T-cell responses to tuberculin and ESAT-6 both before and during the first 6 weeks of cART in patients who developed TB-IRIS, though responses also increased in patients who did not develop TB-IRIS (Vignesh R et al., manuscript submitted).
It is unclear why Th1 responses are higher in patients with TB-IRIS and what role they play in the immunopathology. The study of Meintjes et al. (41) did not demonstrate a deficiency of Treg cells (defined as FoxP3+CD4+) in patients with TB-IRIS. Indeed, there was a trend towards higher levels of Treg cells, confirming findings in HIV-infected patients with IRD associated with non-tuberculous mycobacterial infection (42). Taken together, currently available data suggest that Th1 responses against tuberculin and some other M. tuberculosis antigens increase in all HIV-infected patients with treated TB after commencement of cART and that those responses are higher in patients who develop TB-IRIS, though this is less readily demonstrated with whole blood IGRAs.
Innate immune responses and TB-IRIS
There is evidence that perturbations of innate immune responses might be contributing to dysregulation of immune responses against mycobacteria. In HIV-infected patients with TB-IRIS, we demonstrated that production of CCL2 in unstimulated cultures of whole blood was lower both before and after 4 weeks of cART than in matched controls whereas production of IL-18 and CXCL10 were higher during cART (24). Low serum CCL2 levels during TB-IRIS have been reported by others (43) and reduced production of CCL2 might contribute to impaired monocyte function and/or chemotaxis (44). Increased IL-18 and CXCL10 production during cART might promote Th1 responses (44;45).
Further evidence that innate immune responses are perturbed in patients who develop TB-IRIS is provided by the findings of a study conducted in Cambodia which demonstrated that NK cell degranulation capacity (defined as CD107a expression after incubation with K562 cells) was higher before cART in patients who developed TB-IRIS compared to patients who did not develop TB-IRIS (46). In addition, Marais et al. have reported that TB-IRIS presenting as meningitis is associated with higher CSF neutrophil counts at presentation with TB meningitis and after cART (31).
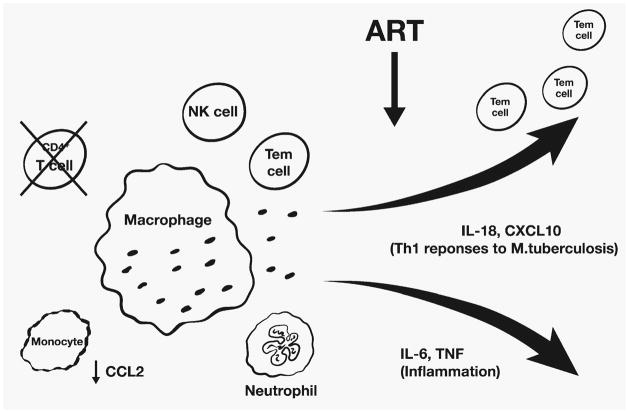
Patients with TB-IRIS also exhibit greater production of pro-inflammatory cytokines after commencing cART, particularly IL-6 and TNF-α (47), which appears to be a feature of IRD in general (48). It is unclear if this is the result of recovering Th1 responses, aberrant innate immune responses, or both. Corticosteroid therapy is effective in patients with TB-IRIS, and this response is associated with suppression of IL-6 and TNF-α production rather than a decline in pathogen-specific T cells (47, 49). Barber et al. (50) have proposed a model of TB-IRIS in which CD4+ T-cell depletion results in the failure of macrophage activation and accumulation of mycobacteria-laden macrophages, which become activated after cART is commenced due to an increase in IFN-γ-producing CD4+ T cells, leading to the excessive production of IL-6 and TNF-α. We suggest that in the context of declining T-cell responses against M. tuberculosis associated with CD4+ T-cell deficiency, the immune system ‘defaults’ to predominantly innate responses, which may be dysfunctional. When HIV infection is suppressed by cART, the recovery of adaptive and innate immune responses occurs in an uncoordinated manner with increased production of IL-18 and CXCL10 driving Th1 responses and increased production of IL-6 and TNF-α driving inflammation (Fig. 2).
Fig. 2. Proposed model of TB-IRIS.
In the context of severe CD4+ T-cell depletion and probable monocyte dysfunction associated with CCL2 deficiency, the immune system defaults to primarily innate immune responses mediated by macrophages, neutrophils, and NK cells. When HIV infection is suppressed by cART, innate and adaptive immune responses against M. tuberculosis recover with expansion of effector memory T cells (Tem). These processes occur in an uncoordinated manner with production of pro-inflammatory cytokines, such IL-6 and TNF-α, that cause inflammation and production of chemokines and cytokines that enhance Th1 responses, such as IL-18 and CXCL10, leading to increased IFN-γ responses against M. tuberculosis antigens.
Vaccine and prevention
Prevention of tuberculosis in HIV-infected patients relies on intensified case finding, infection control, isoniazid prevention, initiation of cART, and potentially immunization against M. tuberculosis infection (51). Vaccination of HIV-infected infants with the currently available vaccine, the M. bovis bacilli live Calmette-Guerin (BCG) vaccine, is not recommended in view of reported vaccine strain disease and death and the impaired BCG-specific immune response (52). Research on TB vaccines for HIV-infected patients has focused on a prime-boost strategy using polyantigenic whole-cell inactivated vaccines. The DarDar study, a phase 3 trial, randomized more than 2000 HIV-infected patients with CD4+ T-cell counts of at least 200 cells/microliter and a BCG scar, with five intradermal doses of a live inactivated M. vaccae vaccine compared to placebo (53). This was stopped early as the vaccine was shown to be effective with a statistically significant protection against definite tuberculosis (53). MOD-901 (a broth-grown inactivated M. vaccae vaccine), three viral vector or subunit vaccines targeting one or a few immunodominant mycobacterial antigens and two polyantigenic vaccines are in development (reviewed in 51); only one of these, VPM 1002, is being trialed as a primary immunogen.
Current gaps and suggested future focus for research in HIV-TB co-infection
Given that worldwide approximately 14 million people are co-infected with HIV and M. tuberculosis and the use of cART is increasing, ART-associated TB, unmasking TB-IRIS, and paradoxical TB-IRIS have become common immunological disorders. They may be avoided by treating HIV infection before severe CD4+ T-cell depletion occurs and by identifying and treating subclinical TB. This should be confirmed in clinical studies. Immune-based diagnostic tests, such as IGRAs, may have a role in the diagnosis of subclinical TB (23, 25), and their use should be evaluated further. Understanding the mechanism of LTBI is essential. Optimal treatment of active TB before cART is commenced reduces the incidence of paradoxical TB-IRIS, but this is associated with higher overall mortality in patients with CD4+ T-cell counts <50/microliter (26–28, 54). However, TB-IRIS is often unavoidable in patients who present with severe CD4+ T-cell deficiency, particularly in resource-limited settings. Therefore, understanding the immunopathogenesis of this condition better may lead to the development of targeted immunomodulatory therapy, and particular attention should be paid to cells of the innate immune system and their mediators.
Cryptococcus co-infection
Epidemiology and global burden of disease
Cryptococcal disease is caused by Cryptococcus spp., a ubiquitous environmental yeast, endemic in many developing countries and in some regions of developed countries. Cryptococcus neoformans (both varieties neoformans and grubii) and C. gattii are the most important in causing human infections (55). The arrival of the HIV pandemic has resulted in a resurgence of cryptococcal disease in the last three decades (56). Infection with cryptococci occurs via the lungs, from where there is dissemination to other body sites, particularly the central nervous system (CNS). The most common clinical manifestation of cryptoccocal infection in HIV-infected patients is cryptococcal meningitis (CM).
Without cART, mortality rates are extremely high in HIV patients with CM. In a cohort of 230 HIV-infected patients with CM in Zambia, the mortality rate was 100% by 7 weeks in patients not given anti-fungal therapy and 100% at 6 months in patients who did receive anti-fungal therapy (57). Globally, nearly a million cases of CM are estimated to occur each year, resulting in an estimated 624,700 deaths within 3 months of infection. Not surprisingly, with high rates of HIV infection and limited resources, Sub-Saharan Africa bears more than 80% of these deaths (58). In this region, estimated deaths due to CM exceed deaths to tuberculosis and are behind only malaria, diarrheal illnesses, and childhood-cluster diseases (58).
Natural history of Cryptococcus infection
Like TB, cryptococcosis may be an acute infection but it is most often due to reactivation of latent infection. Reactivation most commonly occurs in the CNS presenting as either meningoencephalitis or cerebral cryptococcoma (56). Factors leading to reactivation – be it a distinct qualitative or quantitative change in immunity - are uncertain (59), but immunosuppression and particularly HIV infection is a major risk factor for reactivation and disease.
Immune response to Cryptococcus and pathogenesis
Host defense against cryptococcosis has largely been thought to be mediated by cell-mediated immunity (CMI), based on the preponderance of cryptococcosis in HIV/AIDS patients with impaired T-cell immunity, granuloma formation as characteristic of CMI, and studies of adoptive T-cell transfer in CMI-deficient Cryptococcus-susceptible animal models (60). Evidence also suggests a role for innate immunity and antibody-mediated humoral immunity. Protective CMI is based primarily on cryptococcal specific Th1-type CD4+ T cells, which produce IL-12, IL-2, IFN-γ, and TNF-α (61).
IFN-γ has been shown to play a critical role in the control of cryptococcosis (including both CM and pulmonary disease) in murine models and in human cryptococcosis. An increase in early IFN-γ secretion from lung-associated lymph nodes was observed in Cryptococcus-resistant mice compared to Cryptococcus-sensitive mice, and the administration of both an anti-IFN-γ and anti-IL-12 antibody resulted in reduced Cryptococcus clearance (62, 63). In patients with CM, higher IFN-γ levels in CSF were associated with improved rates of CSF Cryptococcus clearance and overall survival (64). In HIV-CM co-infected patients, a higher proportion of IFN-γ+ CD4+ T cells was associated with a lower fungal burden and improved survival at two weeks after cART (65).
Murine models of cryptococcosis demonstrate that CXCR3 and its ligands (CXCL9, CXCL10, and CXCL11) regulate the migration of Th1 cells into sites of inflammation, including the brain (45). CCR5-deficient mice with CM demonstrate increased severe brain damage, swelling, cranial distension, and loss of neural tissue integrity (66). Therefore in CM, the chemokine receptors CXCR3 and CCR5 might be important in T-cell recruitment including CD8+ CTL, facilitating pathogen control and limiting cerebral edema and intracranial hypertension.
Cryptococcus invasion of the CNS is the key to its pathogenesis. The potential paths cryptococcus takes to invade the CNS include a transcellular route via a specific ligand-receptor interaction, a paracellular route after a mechanical or biochemical breakdown of the blood brain barrier (BBB), or via the ‘Trojan horse’ model in which Cryptococcus crosses inside a host monocyte (67). Cryptoccoccus is an intracellular pathogen, capable of being phagocytosed and expulsed from a macrophage without harming the macrophage, i.e. vomocytosis (67). Cryptococcus spp. produces degradative enzymes such as phospholipase, proteinase, and urease, which are able to degrade membranes and compromise intra- and inter-cellular integrity, leading to dissemination into the brain (68).
The Cryptococcus capsule is composed predominantly of polysaccharide, 88% glucoronoxylomannan and 10% galactoxylomannan, is continually shed and interferes with macrophage phagocytosis, depletes complement, and impairs leucocytosis (69). Capsule composition varies greatly between strains, and dynamic changes in size and antigenic properties can lead to variation in the host response (70). Most laboratory studies on cryptococcosis have used C. neoformans as the model organism, and basic science research efforts pivot around a laboratory strain, H99. There is increasing recognition that host immune responses vary for C. gatti (61), and indeed different genotypes of the C. neoformans-C.gattii complex may elicit different immunological response (71).
The impact of HIV on CM
The prominent CD4+ T-cell depletion seen in HIV infection significantly impairs the adaptive immune response to Cryptococcus spp. (72). Effective killing of cryptococcus by alveolar macrophages is also impeded by HIV infection (73). HIV proteins can alter BBB integrity. Tat can disrupt tight junctions and cross the BBB, contributing to BBB degradation, and may act as a chemoattractant for monocytes (74). Gp120 binds to CXCR4 or CCR5, and the changes in brain microvascular endothelial cell causes tight junction dysfunction and an increase in BBB permeability (74). Nef has been associated with increased secretion of CCL2 (74). Further, expression of CCR5 (the predominant HIV-co-receptor) found in the brain increases BBB permeability (75).
HIV-infected monocytes cross the BBB more readily and can repopulate CSF-resident macrophages (74, 76). Human brains infected with HIV demonstrate a generalized diffuse increase in the number of CXCR3+ astrocytes, particularly in areas of gliosis (77). Thus, presence of HIV in the CNS likely facilitates entry of wide range of leucocytes into the perivascular space and Cryptococcus spp. into the brain compartment, together leading to ongoing inflammation and BBB breakdown. Whether HIV-infected monocytes and macrophages are more permissive to intracellular Cryptococcus and vomocytosis is currently unknown.
Impact of cART on CM, including IRD
The preventative effect of cART on reactivation of Cryptococcus infection in HIV-infected patients has not been investigated to the same extent that it has for TB. However, initiation of cART in HIV-infected patients with cryptococcosis may lead to IRD. Cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) may present as a clinical worsening or new presentation of cryptococcal disease after initiation of cART and is thought to be caused by recovery of Cryptococcus-specific immune responses (78).
Like other opportunistic infections, timing of cART initiation in the setting of known CM is contentious. Although survival is improved by early cART initiation in most opportunistic infections (79), delayed ART appears to have a survival advantage in CM. In HIV-CM, delaying cART to more than 5–6 weeks after CM-diagnosis is recommended (80) based on three randomized studies conducted in resource-limited settings showing excess mortality when ART is initiated early (81–83).
Predictors of C-IRIS
Predictors of C-IRIS include a lower baseline CD4+ T-cell count (33, 84) and reduced CSF inflammation (85). We recently completed a longitudinal prospective study HIV-CM co-infection in South Africa, where we enrolled 130 HIV-infected, cART-naive patients presenting with their first episode of CM. We treated these patients with 1mg/kg amphotericin followed by fluconazole and commenced them on cART at a median of 18 days post CM diagnosis and follow up continued for 24 weeks. We showed that persistently positive cultures of Cryptococcus from CSF at the time of initiation of cART and low CD4+ T-cell counts were independent predictors of C-IRIS. We also showed that a persistently positive culture of Cryptococci from CSF at time of cART initiation was associated with significantly poorer clinical outcomes of C-IRIS, cryptococcal relapse, and neurological deterioration (86). Similar to observations with TB-IRIS, a higher pathogen load at the time of cART commencement appears to be a risk factor for C-IRIS.
In contrast to previous studies suggesting that the magnitude of CD4+ T-cell gain was predictive for C-IRIS (87), we showed that a higher increase in CD4+ T-cell count was protective for C-IRIS highlighting immune competence as beneficial. This observation suggests the immunopathology underlying C-IRIS may not reflect the gain of CD4+ T cells but rather the repair of a dysfunctional immune response.
Th1 responses and C-IRIS
In this same study, we also explored T-cell responses to cryptococcal mannoprotein (CMP) before and during 6 months of cART in these patients using whole blood IGRAs and a T-cell activation (CD25+, CD134+) assay developed by Zaunders et al. (89). We found that patients with poor IFN-γ responses pre-cART were at a higher risk of C-IRIS and that C-IRIS was not associated with higher T-cell responses to CMP during cART (348). We interpret these findings as indicating that lower IFN-γ responses contributed to an increased Cryptococcus load and that C-IRIS was not associated with increased blood T-cell responses to CMP. Possible explanations for the latter finding include a predominance of innate immune responses in the immunopathology of C-IRIS, compartmentalization of the immune response to the CNS, or failure to detect T-cell responses against CMP with whole blood assays.
Compartmentalization of the immune response and C-IRIS
Inoculation of Cryptococcus spp. into mice showed a rise in plasma TNF-α and IL-10, which was correlated to fungal burden in the blood and spleen but not in brain (90). The degree and kinetics of anti-inflammatory and pro-inflammatory responses are likely also to differ at different sites, in human cryptococcosis. Most clinical and research studies have focused on peripheral blood. Should C-IRIS be specific to compartmentalized changes in the CNS, direct interrogation of the CSF or other CNS tissue may be necessary to truly understand its immunopathogenesis.
We performed limited studies of chemokine receptor expression on T cells by flow cytometry and chemokine levels in paired plasma and CSF in patients with HIV-CM co-infection and clearly demonstrated a distinctly different immunological profile in the CSF. The T-cell CD4+/CD8+ ratio was significantly lower in CSF than in blood, and CD4+ and CD8+ T cells that expressed both CXCR3 and CCR5 and the concentration of CXCL10, CCL2 and CCL3, were all increased in CSF compared to blood prior to cART initiation (348). We interpret these findings as indicating that enhanced recruitment and/or retention of activated CD8+ T cells and potentially activated monocytes into the CSF is necessary for host response to Cryptococcus.
Vaccine and prevention
Earlier diagnosis of cryptoccocosis is encouraged by measurement of serum cryptococcal antigen by latex agglutination or the newer lateral flow assay (91, 92). The uptake of cryptococcal antigen screening as part of HIV management, particularly in developing countries, remains patchy. The lowered cost of the new lateral flow assay and the ease of use are particularly attractive in resource-limited settings, though the best management algorithm for a positive result remains debated.
There are currently no cryptococcal vaccines in in clinical use or clinical trials. Efforts to develop cryptococcal vaccines have focused on therapeutic vaccines rather than prophylactic vaccines. Live attenuated vaccines and killed vaccines have been unsuccessful. Current research in cryptococcal vaccines has concentrated predominantly on polysaccharide antigens. Vaccine candidates include GXM-protein conjugate vaccines, oligosaccharide vaccines, and peptide mimiotopes of GXM (such as P13) and vaccines against β-1,3-glucans (such as Mab2G8) (60, 61, 93). The ability of cryptococcal mannoproteins to elicit strong T-cell responses has also garnered interest. Antibodies to heat shock protein, glucosylceramide, and melanin have been shown to contribute to antifungal activity (60, 61).
Immunotherapy
Cytokine immunotherapies in murine models have included IL-12 (94) and IL-23 (95), which are not yet available in humans, though exogenous IFN-γ therapy is most promising (61). Two human studies have explored the role of adjunctive, short course exogenous IFN-γ therapy in HIV-infected patients with CM, with the more recent study showing significantly improved rates of CSF cryptococcal clearance (96, 97). Other novel treatment approaches include passive antibody therapy such as M18B7 (monoclonal antibody generated from a mouse immunized with GXM-Tetanus vaccine) and radioimmunotherapy, linking the monoclonal antibody to a radionuclide which emits cytotoxic radiation as site-targeted therapy (60). It is possible that agents such as chemokine receptor antagonists that block trafficking of cells to the CSF, particularly in the setting of C-IRIS, may potentially play a role.
Current gaps and suggested future focus for research in HIV-CM co-infection
Immunology research in CM has been largely extrapolated from laboratory studies in mice, while human studies have been limited to cytokine measurements in stored plasma and occasionally CSF and some studies on polyfunctional T cells. Real-time immunophenotyping of T cells from whole blood and CSF in HIV-CM co-infection would lend further insights. The role of innate immunity and particularly DCs, which not only have direct antifungal activity but are also critical for initiating an effective T-cell response, require further exploration. Understanding cryptococcal latency in HIV-infected patients, specifically, understanding how Cryptococcus spp. interacts with alveolar macrophages, and how cryptococcus survive intra- and extracellular killing may lead to development of novel treatment or prevention strategies. Increased use of clinically derived isolates, specifically from regions of high endemicity such as sub-Saharan Africa, is needed to understand the full complexity of the fungus and its microevolution. Finally, identifying patients who are most suitable for and would most benefit from exogenous IFN-γ therapy or other immunotherapies will be an important area for future research.
Hepatitis B virus co-infection
Epidemiology and global burden of disease
Despite the widespread availability of an effective vaccine, there are currently 300 million people with chronic HBV infection (CHB). Approximately 10% of HIV-infected patients are co-infected with HBV. The prevalence is higher in countries endemic for CHB, including in Asia and Africa, where the prevalence of co-infection can be as high as 25% (98). With the introduction of cART, which also includes agents active against both HIV and HBV (HBV-active cART), liver-related mortality rates have significantly reduced (99–101); however, total and liver-related mortality still remains significantly elevated in some studies of HIV-HBV co-infected patients compared with patients infected with HIV or HBV alone (102–105). Liver-related mortality is now the commonest cause of non-AIDS related death in HIV-infected individuals on cART (101, 105, 106).
Natural history of HBV
Following HBV infection, there is an initial hepatitis that may or may not be symptomatic. Successful clearance and resolution of infection depends on the age and immune status of the individual, with most infections of immunocompetent adults being self-limiting or acute. CHB is characterized by fluctuations in both liver enzymes [primarily alanine aminotransferase (ALT)] and HBV viral load (reviewed in 107). Often, following many decades of a normal ALT (immune tolerant phase), an infected individual may enter the immune clearance phase characterized by repeated inflammatory events, measured by fluctuating ALT, which can result in liver fibrosis and liver disease progression.
During the immune clearance phase, viral suppression is associated with loss of hepatitis B ‘e’ antigen (HBeAg) and development of anti-HBeAg antibodies (HBeAb) and occasionally loss of hepatitis B surface antigen (HBsAg) and development of anti-HBsAg antibodies (HBsAb) (reviewed in 108). A hepatic flare (defined as an increase in ALT > 2x upper limit of normal) often precedes HBeAg or HBsAg seroconversion; however, seroconversion can also occur in the absence of liver damage (109–111). Repeated HF ultimately can lead to development of cirrhosis and ultimately hepatocellular carcinoma.
Immune response to HBV and pathogenesis
T-cell and B-cell dysfunction and CHB
The immune determinants of successful clearance of HBV are not fully understood, but both innate and adaptive immune responses may be important. In CHB, HBV-specific CD4+ and CD8+ T-cell responses are significantly diminished (112, 113). Despite the low frequency in blood, HBV-specific CD8+ T cells are found in the liver, where they may cause an inflammatory response but which are, in isolation, ineffective in clearing HBV infection (112). A generalized CD4+ T-cell hyporesponsiveness and dysregulation has also been demonstrated as measured by an increase in the expression of the T-cell exhaustion marker programmed death-1 (PD-1) expression (114) and altered ratios of total T cells to Treg cells (115, 116), suggesting a role for T-cell dysfunction in the development of CHB. An IL-10-producing subset of regulatory B cells was recently shown to correlate with spontaneous hepatic flare in chronic HBV infection (117). In this study, inhibition of IL-10 rescued HBV-specific CD8+ T-cell function, suggesting a role, also, for B-cell dysfunction in the development of CHB (117).
Innate immune function and CHB
A strong positive correlation was recently demonstrated between activated T cells, monocytes, and NK cells and the IFN-stimulated genes (ISG), CXCL10, and liver flare in chronic HBV (118). CXCL10 is produced by hepatocytes within the HBV-infected liver (119) and is known to correlate with liver fibrosis in HIV-HCV co-infection (120, 121). In mouse models of liver disease, CXCL10 has also recently been shown to induce hepatocyte apoptosis (122). CXCL10 is therefore currently considered a promising candidate marker of intrahepatic immune activity and liver damage, although further work is needed to determine its precise role in inflammation.
The effect of HBV infection on DC function is not well understood, but evidence suggests that DCs in CHB are hyporesponsive with a reduced ability in vitro to prime T cells and produce IL-12 (123–125). Significant functional defects have been demonstrated in both myeloid (m) and plasmacytoid (p) subsets of DCs (126). HBV can impair Toll-like receptor 9 (TLR9) signaling in pDCs leading to reduced IFN-α production (127). However, TLR9 expression was increased in patients with CHB compared to uninfected controls, and TLR9 expression significantly correlated with HBV DNA levels in plasma (128), suggesting a link between TLR9 expression and viral replication.
CHB is also associated with modulation of other TLR pathways. TLR2 expression is reduced in circulating monocytes in CHB compared to uninfected controls (129). This is likely driven by HBeAg binding to and preventing signaling through the TLR2 and TLR4 pathways (130). Others have also shown that HBsAg is associated with a hyporesponsiveness to TLR2 and TLR4 ligation by impaired production of IL-6, IL-8, IL-12, and TNF-α (131). In a mouse model of HBV infection, activation of TLR3, TLR4, TLR5, TLR7, and TLR9 suppress HBV replication (132). TLR7 and TLR9 agonists are effective adjuvants in HBV vaccination (133, 134) and polymorphisms in TLR3 are associated with a risk of developing CHB (135). These studies suggest stimulation of TLR pathways as potential novel therapeutic strategies and worth further investigation into the mechanism by which HBV acts on these pathways.
Circulating NK cell numbers are elevated in individuals who are in the immune-tolerant phase of chronic infection (high plasma HBV DNA but normal ALT) as opposed to those who are in the immune-clearance phase (abnormal ALT), suggesting that NK cells present are not functionally active against HBV in CHB (136). A recent study in individuals with untreated CHB (high ALT and DNA) demonstrated increased expression of the NK cell inhibitory receptor (NKG2A) compared to individuals with inactive HBV (HBeAg and ab negative, low ALT and DNA) or uninfected controls (137). Reversal of the NKG2A blockade resulted in viral clearance in a mouse model of chronic infection (137). NK cells from chronically infected individuals were also recently shown to have an impaired production of IFN-γ in response to TLR9 stimulation (138). These studies suggest that HBV infection inhibits or dampens the NK cell response during CHB and that reversing this response could be an effective novel therapeutic strategy.
Intrahepatic NK cells expressing TNF-related apoptosis-inducing ligand (TRAIL) are enriched in the liver and correlate to liver damage in CHB (139). In addition, intrahepatic TRAIL expression was demonstrated to be highest in livers of patients with chronic hepatitis compared to severe hepatitis, liver cirrhosis, or normal controls (140), suggesting NK cells are active in chronic HBV but perhaps only in the intrahepatic compartment.
The impact of HIV on HBV
There is a significant impact of HIV on the natural history of HBV with accelerated HBV-related liver disease progression and significantly higher liver-related mortality compared to infection with either HIV or HBV alone (104, 141, 142). In patients with HIV-HBV co-infection compared to HBV mono-infection, HBV DNA is significantly higher; there are lower rates of spontaneous HBeAg and HBsAg seroconversion (142, 143), significantly lower ALT levels, and, paradoxically, faster progression to liver disease (142–144). A recent study reported that pre-cART, HIV-HBV co-infected individuals (n=115) compared to HIV mono-infected individuals (n=2105) had lower CD4+ T-cell counts than HIV mono-infected individuals (145), suggesting further immune depletion in co-infection than in HIV infection alone.
Compared to HBV mono-infection, we have previously shown that in HIV-HBV co-infected patients naive to cART, the HBV specific T-cell response was impaired (146–148), and there was significantly increased intrahepatic apoptosis with an increase in liver-infiltrating IL-10-producing CD8+ T cells (149, 150). However, the main driver for accelerated liver disease progression may potentially be related to changes in innate immunity and immune activation.
Lipopolysaccharide, immune activation, and liver disease
HIV significantly depletes CD4+ T cells in the gastrointestinal tract, leading to increased microbial translocation as measured by circulating lipopolysaccharide (LPS) and increased total levels of 16S ribosomal bacterial DNA (16rDNA) (151–153). The resultant systemic low level endotoxemia and chronic immune activation [most commonly measured by LPS and circulating soluble (s)CD14 levels] are believed to be major drivers of HIV disease progression (153–155). Interestingly, LPS can also directly affect multiple cells in the liver. In alcohol-related liver disease, LPS has been shown to directly activate intrahepatic Kupffer cells and human stellate cells, the major cell responsible for liver fibrogenesis (156, 157). LPS is elevated in hepatitis C virus (HCV) infection (155, 158) and HBV mono-infection (155) compared to uninfected controls, and in a cross-sectional study of HIV-HCV co-infected patients, severe liver disease was 19 times more likely in subjects who had LPS levels in the upper quartile (159) or raised 16S ribosomal DNA (160). However, other studies have not shown an association between circulating LPS and liver disease in HBV, HCV, or HIV-HCV co-infection, but they have showed an association with sCD14 (154, 155).
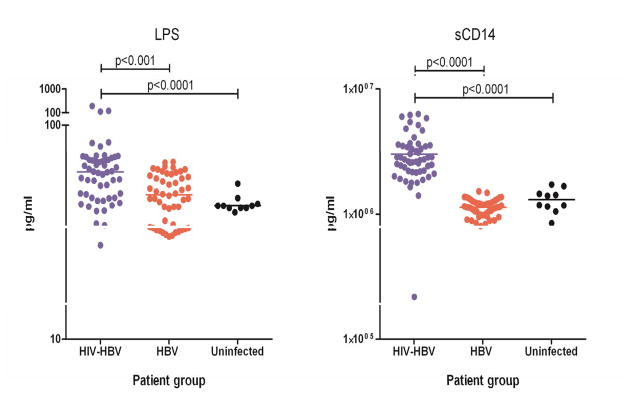
The role of microbial translocation and chronic immune activation in HIV-HBV-associated liver disease is not known. However, we recently demonstrated that LPS and sCD14 are significantly elevated in patients with HIV-HBV co-infection compared to both HBV mono-infection and uninfected individuals (Fig. 3). Following effective HBV-active cART, when both HIV and HBV replication are fully suppressed, LPS and other inflammatory chemokines such as CXCL10 remained persistently elevated compared with uninfected controls. We did not however find an association between LPS and liver diseases (measured by either liver biopsy or elevated liver enzymes). Using a multivariate analysis, we found that significant predictors of elevated liver enzymes on cART included the pro-inflammatory cytokine IFN-γ, IL-6, TNF-α, and CXCL10.
Fig. 3. LPS and immune activation in HIV-HBV co-infection.
Plasma levels of LPS and soluble (s)CD14 (pg/ml) in untreated HIV-HBV co-infection (blue, n=54), HBV mono-infection (red, n=52), and uninfected controls (black, n=10). Individual dots represent a single patient.
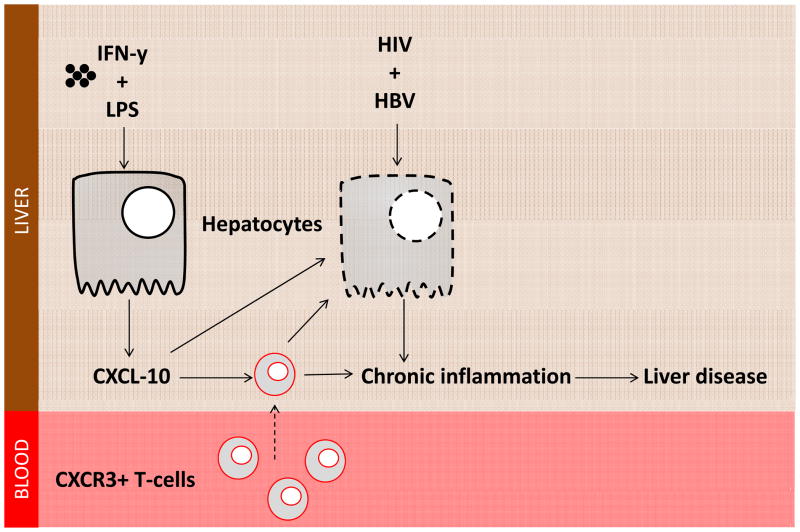
We therefore propose a model of liver disease progression in HIV-HBV co-infection, whereby an HIV-driven increase in circulating LPS contributes to the establishment of a pro-inflammatory intrahepatic environment by inducing hepatocytes, in conjunction with IFN-γ, to produce the chemokine CXCL10. Production of CXCL10 in turn, potentially, promotes chronic immune activation by recruitment of activated T cells, monocytes, and NK cells into the liver and possibly by directly inducing hepatocyte apoptosis (161), promoting chronic liver damage and driving liver disease progression (Fig. 4).
Fig. 4. Proposed model of the role of CXCL10 in liver disease in HIV-HBV co-infection.
Interferon-γ and lipolysaccharide promote CXCL10 production from hepatocytes. HIV and HBV infection is associated with increased apopotosis of hepatocytes. CXCL10 can induce hepatocyte apoptosis and recruits pro-inflammatory CXCR3-expressing T cells to the liver in turn promoting chronic liver inflammation, ultimately contributing to liver disease progression.
Impact of cART on CHB
Management of HIV-HBV co-infection includes the use of nucleoside reverse transcriptase inhibitors (NRTI) that inhibit both the HIV and HBV reverse transcriptase. The use of tenofovir together with lamivudine or emtricitabine, active against both HIV and HBV, is referred to as HBV-active cART (162). HBV-active cART has substantially reduced liver-related mortality (99–101) and in some cases improvements in the severity of liver disease (163), although there are recent reports of liver disease progression in HIV-HBV despite HBV-active therapy (164).
Lack of recovery of HBV-specific T cells following cART
Following treatment of CHB with lamivudine and adefovir, there have been some reports of enhanced, although transient, recovery of HBV-specific and mitogen-responsive CD8+ T cells within the first 12 weeks of treatment (112, 165, 166). Studies of long term tenofovir exposure (up to 48 weeks), however, have shown no long term change in the frequency of HBV-specific CD8+ T cells (167). Factors such as persisting altered Treg levels and elevated PD-1 expression are likely to be contributing to the persistent T-cell exhaustion, even following control of HBV DNA replication (114, 115, 168).
We previously investigated the change in HBV-specific T cells after 48 weeks of HBV-active cART in HIV-HBV co-infected patients with advanced immunosuppression in Thailand (148). Similar to studies in HBV mono-infection, we found no change in the frequency of HBV-specific CD8+ T cells, despite a reduction in HBV DNA and HIV RNA. This finding is in agreement with a recent study demonstrating a diminished HBV-specific CD8+ T-cell response in HIV-HBV co-infected patients on long term adefovir therapy as compared to HBV mono-infected controls (169).
The significant CD4+ T-cell depletion in HIV-HBV co-infected patients, compared to mono-infected patients, almost certainly contributes to the decreased CD8+ T-cell response. In addition, increased frequency of Tregs and increased PD-1 expression also contributed to the hypo-responsiveness of HBV-specific CD8+ T cells in HIV-HBV co-infected patients on long term adefovir treatment (169). Blockade of PD-1 in vitro reversed CD8+ T-cell exhaustion (169), suggesting that novel PD-1 or PD1-ligand-1 (PDL-1) antibodies currently being trialed in the clinic for cancer (170, 171) may potentially be a useful adjunct therapy in improving the HBV-specific T-cell response in HIV-HBV co-infected patients on HBV active cART.
HBeAg and HBsAg seroconversion following cART
HBeAg and HBsAg seroconversion are regarded as the endpoints of treatment for HBV mono-infection, and many clinicians will cease NRTIs when HBsAg (and in some cases HBeAg) seroconversion is sustained for 6 months. In HIV-HBV co-infected patients, HBV-active cART has been associated with high rates of HBeAg seroconversion, up to 57% (172), and high rates of HBsAg seroconversion up to 12% in HIV-HBV co-infected patients over 5 years of therapy (173–175). In patients with CHB who receive NRTI, HBsAg seroconversion was associated with a reduction in quantitative (q)HBsAg (114, 176, 177). In HIV-HBV co-infection, a greater increase in CD4+ T cells following HBV-active cART was associated with greater declines in (q)HBsAg, (178, 179), but this has not yet been associated with HBsAg seroconversion.
In HBV mono-infection, CXCL10 has been associated with hepatic flare and HBsAg loss following NRTIs (180, 181), and IL-21 and the IFN-γ-inducing IL-12 have been associated with HBeAg seroconversion (182–184). In addition, CCL-2 and the inflammatory cytokine IL-4 were elevated in virological responders following IFN-α therapy (185). In a recent case control study of HIV-HBV co-infected patients treated with HBV-active ART, we compared patients who seroconverted to HBeAg (n=12, cases) to those who did not (n=13, controls) and found no significant differences in serum levels of CXCL10, IL-21 and IL-12 in cases and controls prior to or following seroconversion. However, we did find a significantly higher frequency of effector memory CD4+ T cells at the time of seroconversion in cases compared with controls, suggesting that reconstitution of adaptive immunity may potentially drive HBeAg seroconversion in this setting. Further work is required to determine whether a reduction in the frequency of Tregs, as described in HBV mono-infection (115, 116), and/or blockade of PD-1 and reversal of T-cell exhaustion are also associated with seroconversion in HIV-HBV co-infected patients following HBV-active cART.
Hepatic flares and HBV-IRD following cART
There are multiple causes of HF in patients with HIV-HBV co-infection who initiate HBV-active cART, including direct hepatotoxicity of antiretroviral therapy and other medications, co-infection with other hepatotropic viruses, and IRD. In a recent retrospective cohort study of 287 HIV-infected patients co-infected with HBV or HCV (HBV n=70; HCV n=207; HBV+HCV n=10), we found that detectable hyaluronic acid and higher D-dimer, IL-6, IL-8, and sCD14 levels in plasma pre-cART were associated with death in univariate models. Risk of HF was higher with HBV co-infection (24.3%) or both HBV and HCV (50%) than with HCV co-infection only (13.5%). In addition, higher levels of ALT and IL-10 were also associated with HF (186).
In a prospective randomized controlled trial of HIV-HBV co-infected patients who received different HBV-active cART regimens, we found that HF occurred in approximately 25% of participants and was fatal in one participant (187). HF in this setting was more common in HIV-HBV co-infected individuals with elevated ALT and elevated HBV DNA and resulted in HBeAg loss in 75% of cases and HBsAg loss in 25% of cases (188). We demonstrated a significant correlation between HF and the inflammatory chemokines and cytokines CXCL10, sCD30, IL-18, and CCL-2. A significantly elevated CXCL10 in patients with HF compared to those without HF suggested that HF in this setting was consistent with IRD [92], similar to that described for TB-IRD (24). Further studies are needed to better understand the pathogenesis of HBV-IRD to develop better treatment strategies to prevent HBV-IRD.
Vaccine and prevention
Immunological responses to the HBV vaccine are poorer in HIV-infected patients, although patients who received more than 3 doses, those with higher CD4+T-cell counts at vaccination, and the use of cART were all associated with an increased likelihood of response (189, 190). Compared to unvaccinated individuals, patients who were vaccinated prior to HIV acquisition had significantly reduced risks of hepatitis B infection after HIV diagnosis (191), giving further support for childhood vaccination in endemic countries and targeted vaccination in groups at high-risk for HIV-HBV co-infection.
Current gaps and suggested future focus of research in HIV-HBV co-infection
HBV-active cART has had a profound impact on improving the prognosis of patients with HIV-HBV co-infection with substantial reductions in overall and liver related mortality. However, mortality still remains elevated in HIV-HBV co-infection in the era of HBV-active cART. Immune activation and the role of microbial translocation and/or activation of IFN-stimulated genes such as CXCL10 may play an important role in ongoing intrahepatic inflammation. Further work is needed to dissect the immune abnormalities that persist in HIV-HBV co-infected patients on HBV-active cART, specifically in low income countries and in patients who initiate HBV-active cART at low CD4+ T-cell counts. To achieve this, large prospective observational studies will be needed. Systems biology using both blood and liver collected from cohort participants is likely to be very informative. Finally, a better understanding of the immune determinants of HBeAg and HBsAg seroconversion in HIV-HBV co-infection will be important to develop new immunotherapies that could potentially allow for cessation of NRTI.
Hepatitis C virus co-infection
Epidemiology and global burden of disease
Approximately 30% of HIV-infected individuals in the United States and Europe are co-infected with HCV as a result of shared parenteral transmission routes (192, 193). HCV is the leading indication for liver transplantation (194), with increasing numbers also being performed in co-infected individuals (195). Even in the era of cART, end-stage liver disease and its complications remain an important cause of death among HIV-HCV co-infected individuals (196).
Natural history of HCV
The HCV virion is an enveloped particle with a single positive sense strand of RNA. The genome consists of an uninterrupted open-reading frame, prone to mutation resulting in formation of multiple genetically distinct viral variants termed quasispecies (197;198). Acute HCV infection is usually asymptomatic, but 60–80% of cases progress to a chronic phase with the risk of liver cirrhosis over 20–30 years.
Unlike HIV, HCV infection can be cured with antiviral therapy, including in the setting of co-infection. Antiviral treatment for chronic HCV has improved slowly over the last decade with the availability of immune modulators, pegylated INF-α (PEG-IFN) and ribavirin (RBV), which offer approximately a 40–50% cure rate (199). The imminent advent of multiple new direct acting antivirals (DAAs), including HCV protease inhibitors, polymerase inhibitors (nucleoside and non-nucleoside analogues), and NS5A inhibitors, is likely to markedly improve cure rates in both HCV mono-infection and HIV-HCV co-infection (reviewed in 200, 201).
Immune response to HCV and pathogenesis
Acute HCV mono-infection
Early after HCV infection, an innate immune response is evident in the liver and in the blood, featuring induction of antiviral proteins, notably the type 1 IFNs, IFNα and IFNβ. This leads to expression of many IFN-stimulated genes (ISGs) with antiviral properties (202–204). In addition to the ISG response, NK cells are activated in acute HCV infection and display markers of cytotoxicity (205, 206).
In parallel with the innate response, viral antigens are processed by immature DCs to initiate adaptive immune responses, involving both CD4+ and CD8+ T cells (reviewed in 207). An association between HLA class II genotype and HCV clearance suggests that CD4+ T cells are critical (207–209). Consistent with this notion, early, vigorous, and sustained CD4+ T-cell proliferative responses against multiple HCV proteins have been shown to predict disease resolution (210–213). In addition, depletion of CD4+ T cells before re-infection of chimpanzees resulted in persistent, low-level viremia, despite functional intra-hepatic CD8+ T-cell responses (214). Similarly, in both human and chimpanzee studies, strong and broadly targeted HCV-specific CD8+ T-cell responses have been consistently associated with clearance of primary infection (207, 215, 216). These findings argue for a cooperative role for both CD4+ and CD8+ T cells in facilitating clearance (207).
There is increasing evidence that neutralizing antibody responses are important in clearance (207). Viral evasion from the immune selection pressure of neutralizing antibodies has been revealed early in acute HCV infection of liver grafts following transplantation (217), and also in early acute infection, where envelope-targeted neutralizing antibody responses have been associated with an increased likelihood of clearance (218, 219).
Chronic HCV mono-infection
HCV is non-cytolytic, hence hepatocellular injury and death occur via the host immune response, notably CD4+ and CD8+ T cells and the associated cytokine-driven inflammation (reviewed in 220). The severity of hepatic necro-inflammation is a key predictor of the rate of fibrosis, and hence progression to cirrhosis and late stage complications.
Up to 75% of individuals with chronic HCV infection have CD4+ T-cell responses against Core, NS3, NS4, or NS5 proteins (212, 221). The absence of detectable lymphoproliferative responses to HCV antigens in a significant minority of individuals may either relate to antigen-specific CD4+ T cells present in the circulation at a frequency below the sensitivity of traditional assays, lack of recognition of epitopes in variable regions of the HCV polyprotein, or sequestration of HCV-specific T cells within the liver. With regard to the latter, a greater parenchymal concentration of activated CD4+ T cells has been found to correspond not only with more severe hepatitis but also with lower levels of viremia (222–224). Similarly, correlations have been found between the number of lobular CD8+ T cells, serum transaminase levels, and the degree of histological inflammation (225), as well as between higher HCV-specific CD8+ T-cell activity in liver-derived T cells and worsened hepatic necro-inflammatory activity (226). Recent data reveal that many of these cells are Th17 cells expressing CD161 (227). It should be noted, however, that tetramer studies indicate that only a minority of the liver-infiltrating CD8+ T cells are HCV specific (228, 229). Although the HCV-infected liver features T-cell infiltration, the virus persists via a combination of ongoing evolution driving immune escape (230), immune dysfunction with exhaustion of CD8+ T cells (231), and accumulation of CD4+ and CD8+ regulatory T cells (232, 233).
The histological features of the liver in chronic HCV include mononuclear infiltration into both portal tracts and lobules, together with patchy peri-portal and lobular piecemeal necrosis of hepatocytes (234). The leukocyte subsets that infiltrate the portal tracts and lobules exhibit relatively distinct compartmentalization, with a mixture of CD4+ and CD8+ T cells, as well as B cells, accumulating in the portal tracts, whereas CD8+ T cells predominate in the lobules (224). In the peri-portal regions, lymphocytes may form focal aggregates that occasionally take the form of follicles with germinal centers, comprised largely of B cells. Microvesicular fat accumulation (steatosis) is common – either driven by the virus in those infected with genotype 3, or by the host in those infected with other genotypes, but with co-morbid metabolic syndrome, which features steato-hepatitis in the absence of HCV infection (235).
Recruitment of inflammatory cells into the liver is facilitated by expression of the IFN-inducible chemokines that are ligands for CXCR3 (CXCL10, CXCL9, and CXCL11), which are increased in the HCV-infected liver, and correlate with the degree of lobular inflammation (reviewed in 236). In addition, CCR5 and its ligands (CCL3, CCL4, and CCL5) have been shown to be overexpressed. The IFN-inducible chemokines are produced by hepatocytes upon HCV infection in vitro (237). Circulating plasma CXCL10 levels have been shown to correlate with intrahepatic mRNA expression in chronic HCV infection (238). Interestingly, we have recently shown that high plasma levels of CXCL10 in acute HCV infection predict a chronic infection outcome (239).
Hepatic fibrosis
Although the mechanisms of fibrogenesis in chronic HCV remain incompletely defined, it is recognized that activated Kupffer cells produce pro-inflammatory and pro-fibrogenic cytokines, such as TNF-α and transforming growth factor-β (TGF-β), that in turn activate hepatic stellate cells which mediate liver fibrosis (reviewed in 240). Kupffer cells are responsible for clearing gut-derived microbes and microbial translocation products, such as LPS (241). Hence, portal vein-derived LPS and its sensing apparatus on Kupffer cells (CD14, LPS-binding protein, and TLR4) have been implicated in the development of fibrotic liver disease (242). Another important factor in hepatic fibrosis is insulin resistance, which is common in chronic HCV, and has been consistently linked to fibrosis progression, although the underlying mechanism is unclear (reviewed in 243).
The impact of HIV on HCV
HIV-HCV co-infection leads to a higher propensity to chronic hepatitis as well as accelerated progression of hepatic fibrosis, and hence higher rates of cirrhosis, liver failure, and hepatocellular carcinoma (193, 244). Individuals with pre-existing HIV-infection have lower rates of clearance of HCV viremia in acute infection, potentially due to impaired CD4+ T-cell responses (245, 246). Higher rates of HCV persistence are inversely correlated with CD4+ T-cell counts (247). In those with chronic HCV infection, HIV or HBV co-infection as well as increasing age and heavy alcohol use all contribute to an accelerated progression to cirrhosis (235, 248, 249).
Acute HCV
In a cross-sectional study of 47 individuals who had spontaneously cleared HCV, the 30 individuals who were also co-infected with HIV (250) had significantly less frequent HCV-specific immune responses. By analogy with the chimpanzee studies in which CD4+ T-cell depletion resulted in prolonged HCV viremia and viral escape from CD8+ T-cell responses upon re-challenge (214), these data imply that loss of the HCV-specific T-cell repertoire caused by HIV is key to reduced clearance in acute HCV infection (251).
Chronic HCV
Studies of individuals with existing chronic HCV, before and after HIV seroconversion, have shown that HCV viral loads increase following HIV infection (252, 253). The increase occurs before CD4+ T-cell counts decline significantly, indicating additional early HIV-associated effects on the immune system. However, there are conflicting data regarding differences in HCV viral load in co-infected patients when compared with HCV mono-infected individuals (254). The level of HCV viremia is typically correlated inversely with CD4+ T-cell counts in co-infected individuals, suggesting a loss of immunological control of HCV replication (254). One in vitro study demonstrated that the envelope protein of HIV (gp120) increased HCV replication through engagement of the HIV co-receptors, CCR5 and CXCR4 through a TGF-β-dependent pathway (255). Nevertheless, the potentially increased HCV viral load in co-infected individuals does not appear to be a major contributor to the worsened liver disease, whereas altered host mechanisms are likely to be critical (256).
As CD4+ T-cell counts decline in progressive HIV infection, there is also a loss of HCV-specific antibody responses (257), as well as generally consistent evidence for a decline in functional HCV-specific CD8+ T-cell responses in the peripheral blood (258–260). Similarly, HCV-specific IFN-γ producing CD4+ T-cell responses are reduced (261). By contrast, in the liver, HCV-specific CD8+ T-cell responses are maintained (262). This latter finding is consistent with the observation that the co-infected liver is enriched with CD8+ T cells and mRNA expression of relevant chemokines, i.e. CCL3, CCL5, and CXCL10, is increased (263). These findings were extended in our recent immunohistochemical examination of the intrahepatic cellular infiltrate and the profile of chemokines in immune competent co-infected subjects and mono-infected comparison cases (Nguyen et al., manuscript submitted). We found increased numbers of CD8+ T cells in the co-infected liver correlating with increased hepatocyte expression of CCL5. In addition, there was increased expression of IFN-γ correlating with lobular CD4+ T-cell numbers. Taken together, these data indicate an enhanced hepatic pro-inflammatory environment in HIV-HCV co-infection.
Hepatic fibrosis
In HIV-HCV co-infected individuals with undetectable HIV RNA on cART, fibrosis progression rates approach those of HCV mono-infected individuals, demonstrating a direct role of HIV in liver disease progression (264). In HIV-HCV co-infection, there is depletion of Kupffer cells from the liver in vivo (265). HIV infection of both hepatic stellate cell lines and primary cells has been demonstrated in vitro (266), which therefore has the potential to be a significant driver of extracellular matrix deposition. In addition, HIV can lead to disruption to the structural and immunological barrier of the gut resulting in increased microbial translocation, and hence Kupffer cell activation (see section on HBV co-infection) and confer an increased risk of hepatic steatosis, whereas insulin resistance was specifically linked to HCV (267).
Impact of cART, including IRD
Hepatic flare is common in HIV-HCV co-infected patients initiating cART, occurring in up to 18% of subjects (186, 268). Such hepatic flares are usually mild and self-limited, although they can rarely result in hepatic decompensation in individuals with pre-existing cirrhosis (267, 269).
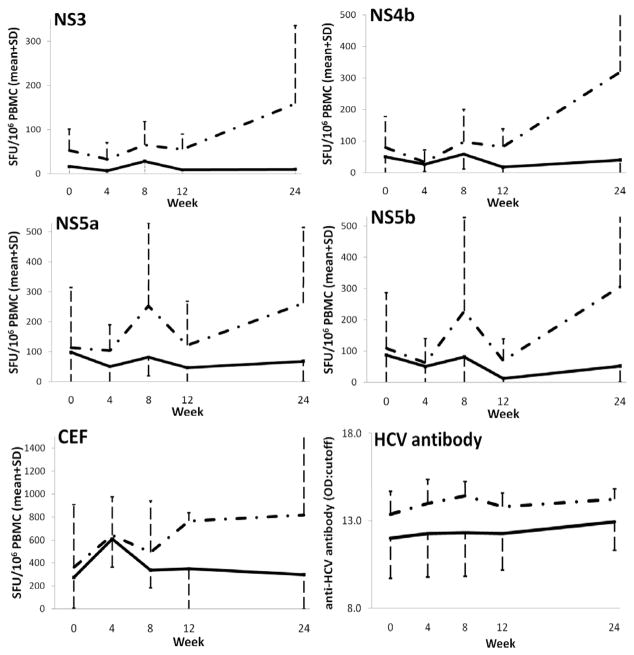
In HIV-HCV co-infected patients who have HF following initiation of cART, serum markers of T-cell activation (sCD26, sCD30), and the chemokine CXCL10 increased in some (270) but not all studies (271). We found that subjects with HF after cART had a higher frequency of IFN-γ-producing T cells targeting non-structural HCV peptides, as well as higher levels of anti-HCV antibodies (271) (Fig. 5). Subjects with elevated HCV-specific T-cell responses also had improved T-cell responses to common viral recall antigens, in keeping with the hypothesis that these findings are associated with generalized immune reconstitution. Others have confirmed this association (272).
Fig. 5. Increasing HCV-specific and non-specific T-cell responses, and higher antibody levels after commencement of cART in subjects with evidence of HCV IRD compared to those without.
Mean IFN-γ spot forming units (SFU) per 106 peripheral blood mononuclear cells in ELISpot assays, and anti-HCV antibody levels, at each time point after commencement of cART for those with HCV IRD (dashed) and without (solid). Error bars indicate standard deviations. HCV NS: non-structural; CEF: Cytomegalovirus/Epstein-Barr virus/Influenza peptides; OD: sample optical density; CO: cut-off value. Reprinted with permission from Cameron et al. (271).
Vaccine and prevention
The limited effectiveness of current prevention strategies against HCV suggest decreases in HCV incidence are unlikely to be achieved in the absence of effective biomedical interventions such as vaccines (273). As described above, the immune responses associated with successful clearance of the virus in acute infection are becoming more clearly understood, and there is growing evidence for partially effective immunological protective mechanisms, including both cross-reactive neutralizing antibody and T-cell responses (274). Only three Phase I trials of candidate prophylactic vaccines have been completed in humans. These included recombinant envelope proteins of HCV genotype 1 administered either with alum (275, 276) or with MF59 (an oil emulsion adjuvant) (277, 278) and a T-cell-based HCV vaccine in humans using adenoviral vectors (279). Two Phase I/II trials of the same adenoviral construct and a modified vaccinia Ankara (MVA) construct administered sequentially or alone, in both healthy volunteers and patients with HCV infection are now underway (280, 281).
Current gaps and suggested future focus of research in HIV-HCV co-infection
The major challenge and highest priority in HIV/HCV immunopathogenesis research is to dissect the immunological, microbial, and metabolic factors contributing to accelerated hepatic fibrosis in those with chronic HCV. In acute HCV infection of HIV-infected subjects, the research priorities include the application of next generation sequencing technologies to longitudinally collected samples from informative cohorts to characterize HCV evolution in the context of varied levels of HIV-associated immune deficiency and in HCV mono-infection to better understand immune escape. As the precursor frequency of HCV-specific CD4+ and CD8+ T cells is very low, a key priority for immunological studies in both contexts includes development and application of assay systems with improved sensitivity for detection and characterization of antigen-specific CD4+ and CD8+ T-cell responses, including effector and regulatory cells (282). Similarly, better systems for investigation of neutralizing antibody responses are required given the high degree of diversity in the HCV quasispecies and the current labor-intensive approach based on incorporating autologous viral envelopes into chimeric viral systems. As the diversity of HCV across populations is even greater than HIV, another priority is to characterize potent and broadly cross-reactive neutralizing antibody and T-cell responses and their target epitopes. In combination, these data will inform treatment strategies for those infected as well as provide a backdrop for application of preventative HCV vaccines in this population.
Malaria co-infection
Epidemiology and global burden of disease
Malaria is caused by five species of parasites of the genus Plasmodium, P. vivax, P. falciparum, P. malariae, P. ovale, and P. knowlesi, which are all transmitted by female Anopheles mosquitoes. P. vivax is more widespread, but P. falciparum, which predominates in Africa, is most deadly, causes the most morbidity and mortality, and thus has been the subject of most research into its interaction with HIV infection. Further, there is considerable overlap in the geography of endemic malaria from P. falciparum and high HIV prevalence. This section therefore concentrates on HIV and P. falciparum co-infection. P. falciparum infection continues to cause more than 1 million deaths each year (283). The threat of emerging artemisinin-resistance (284), the backbone of P. falciparum therapy, mandates for a continued pursuit towards better understanding of malaria immunopathogenesis and vaccine development.
Natural history of malaria
Malaria is an acute illness typified by fevers, chills, and rigors, caused by the blood-stages of the parasite. Severe P. falciparum infection may lead to cerebral involvement and multi-organ failure. Infection in pregnant women may result in miscarriages, anemia, and maternal death.
In malaria endemic areas, humans usually acquire protective natural immunity to severe clinical disease after 1 or 2 episodes of infection (285), whereas immunity to mild disease accumulates very slowly. Adults in malaria endemic areas are therefore at low risk of severe clinical disease, with the exception of pregnant women for whom acquired immunity to normal malaria strains is insufficient to protect them during their first few pregnancies.
Pregnancy-associated malaria results in morbidity to both the mother, mainly presenting as anemia, and to the baby, presenting as low birth weight due to a combination of either intrauterine growth retardation or premature delivery. HIV-malaria co-infection in malaria endemic areas is therefore a significant issue in children infected with HIV via mother-to child transmission from HIV-infected mothers and in HIV-infected women who become pregnant.
Immune response to malaria and pathogenesis
Humoral immunity
Different lines of evidence suggest that immunity to malaria is primarily mediated by humoral immunity. First, passive transfer studies have shown that γ-globulin pooled from hyperimmune individuals can reduce parasitemia and improve outcomes in children with severe clinical malaria (286–288). Second, children younger than 6 months appear to be at lower risk of clinical malaria, which is thought to be due, in part, to the transfer of maternal antibodies (285, 289). Third, titers of antibodies to various malaria antigens increase with age in parallel with the acquisition of protective immunity. In pregnant women, antibodies specific for VAR2CSA increase in a parity-dependent manner (290, 291) and are associated with acquisition of protection against placental malaria (291). A crucial question therefore is how HIV infection affects acquisition and maintenance of antibody-mediated immunity.
Cytokine responses
Increased plasma levels of the pro-inflammatory cytokines TNF-α and IL-6 were first reported in people infected with P. falciparum but not P. vivax (292), which suggested that cytokine secretion was a response to parasitemia, which was generally higher in P. falciparum compared to P. vivax infection. Subsequent studies have shown that elevated pro-inflammatory cytokines were associated with severe disease and cerebral malaria in African children infected with P. falciparum (293–296). Similarly, studies have shown elevation of both pro- and anti-inflammatory cytokines associated with severe malaria in areas of low transmission such as Thailand (297) and India (298), and attempts have been made to define cytokine ‘signatures’ that were associated with severe malaria and which may be of prognostic value (298).
In pregnant women, exposure to malaria without infection was associated with increased pro-inflammatory and Th1 cytokines (299). Placental samples obtained from women with placental malaria at delivery had higher levels of mRNA for TNF-α, IL-8, and IL-1β (300). After adjusting for age and anemia, the levels of IFN-γ, TNF-α, and IL-10 remained elevated in another study of placental samples from women with malaria (301). Placental leukocytes from malaria-infected women are enriched for monocytes, and higher levels of pro-inflammatory cytokine and chemokine secretion by placental leukocytes was associated with the proportion of monocytes, suggesting these cells are an important source of pro-inflammatory cytokines (301). Taken together, these data suggest increased pro-inflammatory cytokine secretion in the placenta in response to malaria infection.
The impact of HIV on malaria
Malaria parasitemia and disease
A meta-analysis of early studies largely comprising children or non-pregnant adults suggested that there was no effect of HIV infection on malaria (302). Several other studies also suggested that there was no association between HIV infection and malaria parasitemia in pregnant women (303–306). However, in two population based cohort studies in rural Uganda (307) and Malawi (308), HIV infection was associated with an increased prevalence of clinical malaria episodes and the degree of parasitemia showed an inverse correlation with CD4+ T-cell counts in HIV-infected individuals. Although there was a relationship between malaria parasitemia and clinical disease and HIV infection, the association was not as strong as with other infections such as tuberculosis (308).
In a large cohort of pregnant women from a malaria endemic region in rural Malawi, HIV infection was associated with increased peripheral parasitemia at enrollment and an increased peripheral and placental parasitemia at delivery (309). Importantly, the increase in parasitemia was more pronounced in multigravidae, suggesting that HIV infection interferes with the development of acquired immunity to P. falciparum. The association between HIV infection and both a higher prevalence of parasitemia and a higher parasite density in pregnant women has been confirmed in subsequent studies and in a comprehensive meta-analysis (310). This analysis confirmed that the effect of HIV infection on parasitemia was more pronounced in multigravidae.
Humoral immunity and pregnancy-associated malaria
Protective immunity to malaria is mediated by acquisition of antibodies to malaria-associated neo antigens expressed on the surface of the infected erythrocyte (311). The most relevant antigen in pregnant women is VAR2CSA (a member of the P. falciparum erythrocyte membrane protein 1 family of proteins that binds specifically to chondroitin sulfate A in placenta). Development of anti-VAR2CSA antibodies mainly occurs in the second trimester of pregnancy and increases with the number of pregnancies (290, 291, 312, 313). Attempts to correlate function of anti-VAR2CSA antibodies with protection from malaria disease have revealed that the ability of serum or purified IgG from pregnant women in malaria endemic areas to block adhesion of infected erythrocytes to chondroitin sulfate A also increases with increasing numbers of pregnancies (299, 312) and correlates with protection to both the mother and the new born baby (314, 315).
We have shown that the ability of antibodies to opsonize infected erythrocytes for phagocytosis by macrophages, which is crucial for clearance of parasites in the placenta, also increases with increasing numbers of pregnancies (316). HIV-infected women had lower levels of antibody to infected erythrocytes expressing VAR2CSA, and this decrease was associated with CD4+ T-cell count (316, 317). When, however, we measured the effect of HIV infection on antibody function in a cohort of pregnant women from Malawi, we found that HIV-infected women had decreased levels of opsonizing activity in their plasma but not a decrease in inhibition of chondroitin sulfate A binding by malaria-infected erythrocytes (316). The decrease in opsonic activity in plasma from HIV-infected women has also been reported in a cohort of pregnant women from Kenya (318). Lower levels of opsonizing activity were also associated with immunodeficiency in HIV-infected women. Opsonizing activity was not decreased in malaria in HIV-uninfected women, consistent with the hypothesis that HIV decreases induction of antibody responses to malaria infection and not immunological memory.
The decrease in opsonic activity in HIV-infected women was clinically significant, since lower levels of these antibodies were associated with anemia in these women (316) and correlated with infant birth weight in other studies (319). Our data suggested that HIV infection blocks some antibody responses to malaria infection but not all, which is consistent with data showing that antibodies specific for VSAPAM and the merozoite antigen AMA-1 are decreased in HIV positive women but not antibodies specific for another merozoite surface antigen MSP-2 (317).
HIV-infected monocyte/macrophages and malaria
We have also shown that HIV infection of primary human macrophages impairs their ability to phagocytose IgG-opsonized targets (320, 321) and, specifically, to phagocytose malaria-infected erythrocytes opsonized with immune serum (322). Coupled with the lower levels of opsonizing antibodies in HIV-infected pregnant women (316), this observation suggests a serious impairment of the ability of macrophages to clear infected red blood cells in the placenta, which is consistent with the increased placental parasitemia evident in HIV-infected pregnant women with malaria.
HIV infection of macrophages induces a transient activation of NFκB, required for the activation of transcription via the HIV promoter or long terminal repeat (LTR) and hence viral replication. Active NFκB is also required for pro-inflammatory cytokine mRNA transcription by macrophages, so it is conceivable that HIV infection, while inhibiting phagocytosis, may stimulate cytokine production and promote immunopathogenesis. Placental blood mononuclear cells purified from HIV-infected mothers constitutively secrete more IL-1β and IL-6 and have more IL-6, IL-1β, and TNF-α mRNA; however, the high basal rates of secretion were associated with a lower response to stimulation with LPS (323). Because of the importance of monocytes in cytokine production in the placenta, we measured the effect of HIV infection on monocyte-derived macrophages exposed to malaria-infected erythrocytes (322), which may recapitulate some responses in HIV-infected pregnant women co-infected with malaria. Our data showed that HIV infection of human monocyte-derived macrophages in vitro decreased NF-κB activation in response to infected red blood cells and consequently decreased pro-inflammatory cytokine secretion.
Rather than support the hypothesis that HIV infection increases immunopathogenesis of placental malaria via cytokine induction, these data suggest that the major effect of HIV infection will be decreased clearance of infected erythrocytes in the placenta by infiltrating monocytes. The inhibition of phagocytosis by HIV infection of human monocyte-derived macrophages is a bystander effect of infection (320); therefore, infection of a relatively small proportion of placental macrophages may cause a more widespread reduction in cytokine response. Careful measurement of cytokine transcription and secretion by infiltrating monocytes in placentas from HIV-infected and uninfected women stratified for gravidity is required to assess the impact of HIV infection on innate immunity to malaria in pregnancy. HIV status also impacts on pro-inflammatory cytokine profiles in children with malaria (324), so the effect of HIV infection on monocyte cytokine production and its relationship to severe malaria disease in children also needs to be addressed.
The effect of malaria on HIV infection
Several studies have shown that malaria infection in both non-pregnant adults (325, 326) and pregnant women (327–329) is associated with an increase in plasma HIV RNA. The magnitude of this increase appears greater in non-pregnant adults [approximately sevenfold greater (325)] than in pregnant women [approximately twofold greater (310)], presumably because the extent of parasitemia is much lower in pregnant women than in semi-immune adults. The increase in plasma HIV RNA is transient and within 28 days of malaria treatment returns to the same level as prior to malaria infection (325). Malaria infection also induces a transient decrease in CD4+ T-cell counts in HIV-infected adults, which also returns to baseline levels following effective antimalarial treatment (330).
The relatively mild and transient increase in plasma HIV RNA suggests that malaria will not have a major impact on HIV disease progression. In one study, there was no effect of malaria on all-cause mortality in HIV-infected people in Uganda (331), while a separate study (332) reported a faster decline in CD4+ T-cell counts following episodes of malaria. It has been suggested that the increase in plasma HIV RNA in malaria in HIV-infected individuals could have an impact on HIV transmission rates and on mother to child transmission (307, 333). The increase in plasma HIV RNA following malaria infection has been suggested to be caused by a malaria-induced increase in the circulating levels of TNF-α (326), which is known to stimulate HIV replication in CD4+ T cells (334, 335).
Impact of cART including IRD
The CD4+ T-cell dependence of anti-malaria antibodies suggests that restoration of CD4+ T-cell counts in HIV-infected individuals with cART will improve acquired immunity to malaria. However, recent research has suggested that cART does not improve innate immune responses to malaria (336). There are few studies on the efficacy of cART in preventing severe malaria in children or adults and non-specifically addressing this question in pregnant women. A prospective study of HIV-infected adults in Uganda receiving either co-trimoxazole prophylaxis alone or in combination with antiretroviral drugs and insecticide treated bed-nets, showed that the frequency of clinical malaria was decreased in individuals receiving cART plus co-trimoxazole compared to those receiving co-trimoxazole alone (332). While treatment was not randomized in this observational study, the analysis adjusted for CD4+ T-cell levels, age, and sex. There are no reported cases of malaria IRD possibly because protective immune responses are humoral rather than cellular.
Vaccine and prevention
Prevention of malaria pivots on vector control and medical prophylaxis for those at risk. The complexity and genome variability of the parasite is challenging, though the observation of acquired natural immunity after repeated exposure in a subset of patients bears optimism. Vaccine research in malaria has focused on pre-erythrocytic vaccines to prevent entry of sporozoites into hepatocytes, erythrocytic phase vaccines aimed to prevent invasion of red cells or prevent parasite sequestration, and sexual stage vaccines, aimed to prevent development of infectious sporozoites in salivary glands of Anopheles mosquitoes, using novel vaccine carriers (337). A phase 3 trial of a candidate malaria vaccine RTS,S/AS01 conducted in seven African countries demonstrated vaccine efficacy of 56% against all clinical malaria infections and 47% against severe malaria during a 12-month follow-up period, in children 5–17 months of age at enrolment and a reduced efficacy of 31% and 26% respectively in children 6–12 weeks of age at enrolment (338, 339). While modest, the subset of responders demonstrated development of antibodies to the circumsporozoite vaccine antigen, and their immune response will be further dissected.
The relatively conserved nature of VAR2CSA compared to other var genes and the fact that antisera from multigravid pregnant women cross-reacts with and cross-inhibits a high proportion of geographically distinct isolates (340, 341) has given hope that a vaccine may be developed for pregnancy-associated malaria based on this antigen (reviewed in 342). As malaria vaccine candidates are developed, a better understanding of correlates of protection is needed. This requires use of assays to measure induction of protective antibody function such as opsonization (316, 319, 343) and inhibition of CSA binding (315) to determine optimum immune responses.
Current gaps and suggested future focus of research in HIV-malaria co-infection
The risk of HIV and malaria co-infection remains high in malaria endemic areas. While malaria may not have a major effect on HIV disease progression, there is the potential for malaria infection of pregnant women to alter mother to child transmission of HIV; this needs to be accurately quantified in adequately powered clinical studies. Conversely, HIV infection adversely affects outcomes for both mother and infant in the context of maternal malaria.
Outstanding questions include what constitutes acquired immunity: the mechanism, its duration, and its immunological correlates (344). Placental immunity, malarial involvement in the CNS, and the immunological cascade leading to P. falciparum-related sepsis needs to be further explored. Vector research to understand impact of its saliva on the host’s cutaneous response may reveal insights into the early phases of P. falciparum infection (344).
Conclusions
Mechanistic studies of HIV-co-infection are particularly complex given the impact of both HIV and the co-infection individually and synergistically on different arms of the immune system and the lack of suitable animal models for the majority of HIV co-infections. Significant investment is required in large multi-center collaborative prospective clinical cohorts with HIV co-infection that incorporate sample collection for basic science research. Carefully matched controls groups, which include patients infected with HIV alone and the co-infection alone, are also needed for these studies. These cohorts should support and foster collaborative efforts between clinical and laboratory medicine. The application of powerful novel technologies such as systems biology (344) or systems immunology (345), mass flow cytometry techniques which enable simultaneous measurement of multiple parameters in a single cell, and novel analysis platforms such as spanning-tree progression analysis of density-normalized events (SPADE) and single cell networking profiling (SCNP) (346, 347) should also be considered.
With the widespread availability of cART and the increasing evidence supporting early initiation of cART, the clinical burden of most HIV-co-infections is likely to reduce in coming years. However, in low income countries where access to cART is still limited, individuals often initiate cART at low CD4+ T-cell counts. The prevalence of certain co-infections such as TB and CM is very high, so HIV-co-infection will remain a significant clinical issue. Improved strategies for management can only be developed with a better understanding of the immunopathogenesis of these diseases and the complexities of cART, including IRD.
Acknowledgments
CCC was supported by the Australian Commonwealth Government for the Australian Postgraduate Award 2009, Australian NHMRC (National Health and Medical Research Council) Postgraduate Scholarship 2010–2012 and Pfizer Neuroscience Research Grant. MC is supported by a Mathilde Krim Biomedical Fellowship from the American Foundation for AIDS Research; MM is supported by an Australian Postgraduate Association scholarship. ARL is an NHMRC Practitioner Fellow (1043607). AJ is supported by an NHMRC project grant 628611. MAF is supported by NHMRC grant 510448 and the Reach Initiative (Research and Education in HIV/AIDS for Resource Poor Countries). SRL is a NHMRC practitioner fellow (1042654) and is supported by the National Institutes of Health (NIH; U19 AI096109).
Footnotes
The authors have no conflicts of interest to declare.
Reference List
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2012. 2013. Available from: URL: http://www.who.int/tb/publications/global_report/en/
- 3.Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G. Tuberculosis and HIV co- infection. PLoS Pathog. 2012;8:e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 6.Crampin AC, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS. 2010;24:417–426. doi: 10.1097/QAD.0b013e32832f51cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 8.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geldmacher C, Koup RA. Pathogen-specific T cell depletion and reactivation of opportunistic pathogens in HIV infection. Trends Immunol. 2012;33:207–214. doi: 10.1016/j.it.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diedrich CR, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geldmacher C, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldmacher C, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland JS, et al. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J Immunol. 2010;184:6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- 14.Sarrazin H, et al. Association between tuberculin skin test reactivity, the memory CD4 cell subset, and circulating FoxP3-expressing cells in HIV-infected persons. J Infect Dis. 2009;199:702–710. doi: 10.1086/596735. [DOI] [PubMed] [Google Scholar]
- 15.Patel NR, et al. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol. 2007;179:6973–6980. doi: 10.4049/jimmunol.179.10.6973. [DOI] [PubMed] [Google Scholar]
- 16.Patel NR, Swan K, Li X, Tachado SD, Koziel H. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J Leukoc Biol. 2009;86:53–60. doi: 10.1189/JLB.0908574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MB, Boyars MC, Veasey S, Woods GL. Generalized tuberculosis in the acquired immune deficiency syndrome. Arch Pathol Lab Med. 2000;124:1267–1274. doi: 10.5858/2000-124-1267-GTITAI. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson KA, et al. Dissection of regenerating T-Cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2009;180:674–683. doi: 10.1164/rccm.200904-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suthar AB, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HIV CAUSAL collaboration. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis. 2012;54:1364–1372. doi: 10.1093/cid/cis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig SP, Riviere C, Leger P, et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48:829–831. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott JH, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 24.Oliver BG, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis. 2010;202:1728–1737. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 25.Oliver BG, Elliott JH, Price P, Phillips M, Cooper DA, French MA. Tuberculosis after commencing antiretroviral therapy for HIV infection is associated with elevated CXCL9 and CXCL10 responses to Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr. 2012;61:287–292. doi: 10.1097/QAI.0b013e31826445ef. [DOI] [PubMed] [Google Scholar]
- 26.Naidoo K, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanc FX, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havlir DV, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin Microbiol Rev. 2009;22:651–663. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meintjes G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marais S, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2013;56:450–460. doi: 10.1093/cid/cis899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conesa-Botella A, et al. Urinary lipoarabinomannan as predictor for the tuberculosis immune reconstitution inflammatory syndrome. J Acquir Immune Defic Syndr. 2011;58:463–468. doi: 10.1097/QAI.0b013e31823801de. [DOI] [PubMed] [Google Scholar]
- 33.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 34.Simonney N, et al. Anti-PGL-Tb1 responses as an indicator of the immune restoration syndrome in HIV-TB patients. Tuberculosis. 2008;88:453–461. doi: 10.1016/j.tube.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Bourgarit A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 36.Bourgarit A, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 37.Tan DB, Yong YK, Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 38.Mahnke YD, et al. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber DL, et al. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tieu HV, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meintjes G, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seddiki N, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 43.Haddow LJ, Dibben O, Moosa MY, Borrow P, Easterbrook PJ. Circulating inflammatory biomarkers can predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2011;25:1163–1174. doi: 10.1097/QAD.0b013e3283477d67. [DOI] [PubMed] [Google Scholar]
- 44.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 c(MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pean P, et al. Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood. 2012;119:3315–3320. doi: 10.1182/blood-2011-09-377523. [DOI] [PubMed] [Google Scholar]
- 47.Tadokera R, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 49.Meintjes G, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2012;186:369–377. doi: 10.1164/rccm.201201-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol. 2012;10:150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Reyn CF, Bakari M, Arbeit RD, Lahey T, Ramadhani A, Egwaga S. New vaccines for the prevention of tuberculosis in human immunodeficiency virus infection. Int J Tuberc Lung Dis. 2012;16:718–723. doi: 10.5588/ijtld.11.0444. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Revised BCG vaccination guidelines for infants at risk for HIV infection. Weekly Epidemiological Record. 2007 May 25; [cited 2013 Jan 25];21Available from: URL: http://www.who.int/immunization/wer8221bcg_May07_position_paper.pdf.
- 53.von Reyn CF, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24:675–685. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdool Karim SS, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon-Chung KJ, Boekhout T, Wickes BL, Fell JW. Systematics of the Genus Cryptococcus and its type species C. neoformans. In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, editors. Cryptococcus - from human pathogen to model yeast. Washington, DC: ASM Press; 2011. pp. 3–15. [Google Scholar]
- 56.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507-vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Mwaba P, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 59.Casadevall A, Pirofski LA. Feasibility and prospects for a vaccine to prevent cryptococcosis. Med Mycol. 2005;43:667–680. doi: 10.1080/13693780500448230. [DOI] [PubMed] [Google Scholar]
- 60.Casadevall A, Dadachova E, Pirofski L. Vaccines and antibody therapies from Cryptococcus neoformans to melanoma. In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, editors. Cryptococcus - from human pathogen to model yeast. Washington, DC: ASM Press; 2011. pp. 537–546. [Google Scholar]
- 61.Hole CR, Wormley FL., Jr Vaccine and immunotherapeutic approaches for the prevention of cryptococcosis: lessons learned from animal models. Front Microbiol. 2012;3:291. doi: 10.3389/fmicb.2012.00291. Available from: URL: PM:22973262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C. B-17 mice Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 63.Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 64.Siddiqui AA, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 65.Jarvis JN, Casazza JP, Stone H, Meintjes G, Harrison TS, Koup R. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal menegitis. J Infect Dis. 2013 doi: 10.1093/infdis/jit099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huffnagle GB, et al. Cutting edge: Role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J Immunol. 1999;163:4642–4646. [PubMed] [Google Scholar]
- 67.Johnston SA, May RC. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell Microbiol. 2013;15:403–411. doi: 10.1111/cmi.12067. [DOI] [PubMed] [Google Scholar]
- 68.Sabiiti W, May RC. Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans. Future Microbiol. 2012;7:1297–1313. doi: 10.2217/fmb.12.102. [DOI] [PubMed] [Google Scholar]
- 69.Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, Heitman J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues ML, Casadevall A, Zaragoza O. The architecture and antigenic composition of the polysaccharide capsule. In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, editors. Cryptococcus: From Human Pathogen to Model Yeast. Washington, D.C: ASM Press; 2011. pp. 43–54. [Google Scholar]
- 71.Wiesner DL, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3:e00196–12. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 73.Ieong MH, Reardon CC, Levitz SM, Kornfeld H. Human immunodeficiency virus type 1 infection of alveolar macrophages impairs their innate fungicidal activity. Am J Respir Crit Care Med. 2000;162:966–970. doi: 10.1164/ajrccm.162.3.9912054. [DOI] [PubMed] [Google Scholar]
- 74.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorce S, Myburgh R, Krause KH. The chemokine receptor CCR5 in the central nervous system. Prog Neurobiol. 2011;93:297–311. doi: 10.1016/j.pneurobio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Ivey NS, MacLean AG, Lackner AA. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol. 2009;15:111–122. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldberg SH, et al. CXCR3 expression in human central nervous system diseases. Neuropathol Appl Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 78.Haddow LJ, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zolopa A, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sax PE. Cryptococcal Meningitis Study Stopped - Early HIV Therapy Clearly Harmful. http://blogsjwatchorg/hiv-id-observations/indexphp/cryptococcal-meningitis-study-stopped-early-hiv-therapy-clearly-harmful/2012/06/02/2012June2 [cited 13 A.D. Jan 6];Available from: URL: http://blogs.jwatch.org/hiv-id-observations/index.php/cryptococcal-meningitis-study-stopped-early-hiv-therapy-clearly-harmful/2012/06/02/
- 81.Makadzange AT, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50:1532–1538. doi: 10.1086/652652. [DOI] [PubMed] [Google Scholar]
- 82.Bisson GP, et al. Early Versus Delayed Antiretroviral Therapy and Cerebrospinal Fluid Fungal Clearance in Adults With HIV and Cryptococcal Meningitis. Clin Infect Dis. 2013 doi: 10.1093/cid/cit019. (published ahead of print) [DOI] [PubMed] [Google Scholar]
- 83.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. ART Initiation within the First 2 Weeks of Cryptococcal Meningitis Is Associated with Higher Mortality: A Multisite Randomized Trial. Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. [Google Scholar]
- 84.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 85.Boulware DR, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang CC, et al. Clinical and mycological predictors of Cryptococcosis-associated Immune reconstitution inflammatory syndrome (C-IRIS) AIDS. 2013 doi: 10.1097/QAD.0b013e3283614a8d. published ahead of print. [DOI] [PubMed] [Google Scholar]
- 87.Bicanic T, et al. Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis: A Prospective Study. J Acquir Immune Defic Syndr. 2009;51:130–134. doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 88.Chang CC, et al. Cryptococcosis-IRIS is associated with lower Cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy. J Infect Dis. 2013 doi: 10.1093/infdis/jit271. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaunders JJ, et al. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40) J Immunol. 2009;183:2827–2836. doi: 10.4049/jimmunol.0803548. [DOI] [PubMed] [Google Scholar]
- 90.Lortholary O, Improvisi L, Rayhane N, et al. Cytokine profiles of AIDS patients are similar to those of mice with disseminated Cryptococcus neoformans infection. Infect Immun. 1999;67:6314–6320. doi: 10.1128/iai.67.12.6314-6320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jarvis JN, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011;53:1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen J, et al. Large Scale Evaluation of the Immuno-Mycologics Inc (IMMY) Lateral Flow and Enzyme-linked Immunoassays for the detection of Cryptococcal Antigen in Serum and Cerebrospinal Fluid. Clin Vaccine Immunol. 2013;20:52–55. doi: 10.1128/CVI.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Datta K, Pirofski LA. Towards a vaccine for Cryptococcus neoformans: principles and caveats. FEMS Yeast Res. 2006;6:525–536. doi: 10.1111/j.1567-1364.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- 94.Clemons KV, Brummer E, Stevens DA. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleinschek MA, et al. Administration of IL-23 engages innate and adaptive immune mechanisms during fungal infection. Int Immunol. 2010;22:81–90. doi: 10.1093/intimm/dxp117. [DOI] [PubMed] [Google Scholar]
- 96.Jarvis JN, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pappas PG, Bustamante B, Ticona E, et al. Recombinant interferon-gamma 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis. 2004;189:2185–2191. doi: 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- 98.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–409. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 99.Rosenthal E, et al. Liver-related mortality in human-immunodeficiency-virus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 Study) J Viral Hepat. 2007;14:183–188. doi: 10.1111/j.1365-2893.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 100.Rosenthal E, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10:282–289. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 101.Smith C, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 102.Hoffmann CJ, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23:1881–1889. doi: 10.1097/QAD.0b013e32832e463a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikolopoulos GK, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis. 2009;48:1763–1771. doi: 10.1086/599110. [DOI] [PubMed] [Google Scholar]
- 104.Thio CL, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 105.Weber R, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2012 doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 106.Weber R, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 107.Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 108.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Benhamou Y. Hepatitis B in the HIV-coinfected patient. J Acquir Immune Defic Syndr. 2007;45 (Suppl):S57–S65. doi: 10.1097/QAI.0b013e318068d1dd. [DOI] [PubMed] [Google Scholar]
- 110.Miailhes P, Trabaud MA, Pradat P, et al. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clin Infect Dis. 2007;45:624–632. doi: 10.1086/520752. [DOI] [PubMed] [Google Scholar]
- 111.Thio CL, Locarnini S. Treatment of HIV/HBV coinfection: clinical and virologic issues. AIDS Rev. 2007;9:40–53. [PubMed] [Google Scholar]
- 112.Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sprengers D, et al. Analysis of intrahepatic HBV-specific cytotoxic T-cells during and after acute HBV infection in humans. J Hepatol. 2006;45:182–189. doi: 10.1016/j.jhep.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 114.Evans A, et al. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759–769. doi: 10.1002/hep.22419. [DOI] [PubMed] [Google Scholar]
- 115.Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 116.Zhang HH, et al. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17 (Suppl):34–43. doi: 10.1111/j.1365-2893.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 117.Das A, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bayard F, Godon O, Nalpas B, et al. T-cell responses to hepatitis B splice-generated protein of hepatitis B virus and inflammatory cytokines/chemokines in chronic hepatitis B patients. ANRS study: HB EP 02 HBSP-FIBRO. J Viral Hepat. 2012;19:872–880. doi: 10.1111/j.1365-2893.2012.01611.x. [DOI] [PubMed] [Google Scholar]
- 119.Zhou Y, et al. Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes. J Biol Chem. 2010;285:12159–12168. doi: 10.1074/jbc.M109.067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harvey CE, Post JJ, Palladinetti P, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- 121.Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220–1229. doi: 10.1002/hep.20167. [DOI] [PubMed] [Google Scholar]
- 122.Sahin H, et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes. Hepatology. 2013;57:797–805. doi: 10.1002/hep.26069. [DOI] [PubMed] [Google Scholar]
- 123.Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138–150. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 124.Beckebaum S, et al. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109:487–495. doi: 10.1046/j.1365-2567.2003.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zheng BJ, et al. Selective functional deficit in dendritic cell--T cell interaction is a crucial mechanism in chronic hepatitis B virus infection. J Viral Hepat. 2004;11:217–224. doi: 10.1111/j.1365-2893.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 126.van der Molen RG, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 127.Xie Q, et al. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect. 2009;11:515–523. doi: 10.1016/j.micinf.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 128.Xu N, Yao HP, Sun Z, Chen Z. Toll-like receptor 7 and 9 expression in peripheral blood mononuclear cells from patients with chronic hepatitis B and related hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29:239–244. doi: 10.1111/j.1745-7254.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- 129.Visvanathan K, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 130.Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55:762–769. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 131.Chen Z, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 132.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma R, Du JL, Huang J, Wu CY. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537–542. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 134.Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005;23:5263–5270. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 135.Rong Y, et al. Association of Toll-like Receptor 3 Polymorphisms with Chronic Hepatitis B and Hepatitis B-Related Acute-on-Chronic Liver Failure. Inflammation. 2012 doi: 10.1007/s10753-012-9560-4. [DOI] [PubMed] [Google Scholar]
- 136.Sprengers D, et al. Different composition of intrahepatic lymphocytes in the immune-tolerance and immune-clearance phase of chronic hepatitis B. J Med Virol. 2006;78:561–568. doi: 10.1002/jmv.20576. [DOI] [PubMed] [Google Scholar]
- 137.Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 138.Ratnam DT, Sievert W, Visvanathan K. Natural killer cells display impaired responses to toll like receptor 9 that support viral persistence in chronic hepatitis B. Cell Immunol. 2012;279:109–115. doi: 10.1016/j.cellimm.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 139.Dunn C, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu FW, et al. Expression of TRAIL in liver tissue from patients with different outcomes of HBV infection. Clin Res Hepatol Gastroenterol. 2012 doi: 10.1016/j.clinre.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 141.Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a study of 150 homosexual men. J Infect Dis. 1989;160:577–582. doi: 10.1093/infdis/160.4.577. [DOI] [PubMed] [Google Scholar]
- 142.Colin JF, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–1310. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 143.Gilson RJ, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 144.Audsley J, et al. HIV replication is associated with increased severity of liver biopsy changes in HIV-HBV and HIV-HCV co-infection. J Med Virol. 2012;84:993–1001. doi: 10.1002/jmv.23236. [DOI] [PubMed] [Google Scholar]
- 145.Thio CL, et al. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS. 2013;27:191–201. doi: 10.1097/QAD.0b013e32835a9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang JJ, et al. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol. 2005;79:3038–3051. doi: 10.1128/JVI.79.5.3038-3051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang JJ, et al. Impaired quality of the HBV-specific T-cell response in HIV-1-HBV co-infection. J Virol. 2009;83:7649–7658. doi: 10.1128/JVI.00183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crane M, et al. No increase in hepatitis B virus (HBV)-specific CD8+ T cells in patients with HIV-1-HBV coinfections following HBV-active highly active antiretroviral therapy. J Virol. 2010;84:2657–2665. doi: 10.1128/JVI.02124-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang JJ, et al. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology. 2007;46:1332–1340. doi: 10.1002/hep.21844. [DOI] [PubMed] [Google Scholar]
- 150.Iser DM, Avihingsanon A, Wisedopas N, et al. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS. 2011;25:197–205. doi: 10.1097/QAD.0b013e3283410ccb. [DOI] [PubMed] [Google Scholar]
- 151.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rajasuriar R, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis. 2010;202:1254–1264. doi: 10.1086/656369. [DOI] [PubMed] [Google Scholar]
- 153.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 154.Marchetti G, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 155.Sandler NG, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 157.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 158.Dolganiuc A, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Balagopal A, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia-Alvarez M, et al. Bacterial DNA translocation and liver disease severity among HIV-infected patients with chronic hepatitis C. J Acquir Immune Defic Syndr. 2012;61:552–556. doi: 10.1097/QAI.0b013e31826ea109. [DOI] [PubMed] [Google Scholar]
- 161.Sahin H, et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes. Hepatology. 2013;57:797–805. doi: 10.1002/hep.26069. [DOI] [PubMed] [Google Scholar]
- 162.Masgala A, Bonovas S, Nikolopoulos GK. Recent advances in the treatment of HIV/HBV and HIV/HCV co-infection. Mini Rev Med Chem. 2012;12:890–904. doi: 10.2174/138955712800959062. [DOI] [PubMed] [Google Scholar]
- 163.Marcellin P, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 164.Martin-Carbonero L, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–79. doi: 10.1097/QAD.0b013e328340fde2. [DOI] [PubMed] [Google Scholar]
- 165.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kondo Y, et al. Recovery of functional cytotoxic T lymphocytes during lamivudine therapy by acquiring multi-specificity. J Med Virol. 2004;74:425–453. doi: 10.1002/jmv.20194. [DOI] [PubMed] [Google Scholar]
- 167.Lau GK, et al. Impact of early viral kinetics on T-cell reactivity during antiviral therapy in chronic hepatitis B. Antivir Ther. 2007;12:705–718. [PubMed] [Google Scholar]
- 168.Fisicaro P, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693. doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 169.Sherman A, et al. Augmentation of HBV specific cellular immunity with PD-1/PD-L1 Blockade in HBV and HIV/HBV coinfected patients treated with Adefovir. AIDS Res Hum Retroviruses. 2013 doi: 10.1089/aid.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kosi L, et al. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J Viral Hepat. 2012;19:801–810. doi: 10.1111/j.1365-2893.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 173.de Vries-Sluijs TE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010;139:1934–1941. doi: 10.1053/j.gastro.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 174.Piroth L, et al. Management and treatment of chronic hepatitis B virus infection in HIV positive and negative patients: the EPIB 2008 study. J Hepatol. 2010;53:1006–1012. doi: 10.1016/j.jhep.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 175.Psevdos G, Jr, Kim JH, Suh JS, Sharp VL. Predictors of loss of hepatitis B surface antigen in HIV-infected patients. World J Gastroenterol. 2010;16:1093–1096. doi: 10.3748/wjg.v16.i9.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fried MW, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47:428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]
- 177.Liaw YF, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 178.Arendt E, et al. Improved immune status corresponds with long-term decline of quantitative serum hepatitis B surface antigen in HBV/HIV co-infected patients. Viral Immunol. 2012;25:442–447. doi: 10.1089/vim.2012.0036. [DOI] [PubMed] [Google Scholar]
- 179.Jaroszewicz J, et al. Hepatitis B surface antigen concentrations in patients with HIV/HBV co-infection. PLoS One. 2012;7:e43143. doi: 10.1371/journal.pone.0043143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tan AT, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330–339. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 181.Jaroszewicz J, et al. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther. 2011;16:915–924. doi: 10.3851/IMP1866. [DOI] [PubMed] [Google Scholar]
- 182.He D, Yan G, Wang Y. Serum levels of interleukin-12 in various clinical states with hepatitis B virus infection. Cell Immunol. 2012;272:162–165. doi: 10.1016/j.cellimm.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 183.Ma SW, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol. 2012;56:775–781. doi: 10.1016/j.jhep.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 184.Wu JF, et al. Serum levels of interleukin-10 and interleukin-12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138:165–172. doi: 10.1053/j.gastro.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 185.Park Y, Park JY, Han KH, Kim HS. Serum cytokine levels in chronic hepatitis B patients receiving peginterferon alpha-2a therapy. Hepatobiliary Pancreat Dis Int. 2012;11:499–506. doi: 10.1016/s1499-3872(12)60214-8. [DOI] [PubMed] [Google Scholar]
- 186.Andrade BB, et al. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons co-infected with HIV and hepatitis viruses. J Infect Dis. 2013 doi: 10.1093/infdis/jit03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Matthews GV, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;48:1062–1069. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 188.Avihingsanon A, et al. Assessment of HBV flare in a randomized clinical trial in HIV/HBV coinfected subjects initiating HBV-active antiretroviral therapy in Thailand. AIDS Res Ther. 2012;9:6. doi: 10.1186/1742-6405-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rey D, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–1165. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 190.Landrum ML, et al. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Landrum ML, et al. The timing of hepatitis B virus (HBV) immunization relative to human immunodeficiency virus (HIV) diagnosis and the risk of HBV infection following HIV diagnosis. Am J Epidemiol. 2011;173:84–93. doi: 10.1093/aje/kwq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 193.McGovern BH. Hepatitis C in the HIV-infected patient. J Acquir Immune Defic Syndr. 2007;45 (Suppl):S47–S56. doi: 10.1097/QAI.0b013e318068d190. [DOI] [PubMed] [Google Scholar]
- 194.2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32(Suppl):2–8. doi: 10.1111/j.1478-3231.2011.02703.x. [DOI] [PubMed] [Google Scholar]
- 195.Terrault NA, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bica I, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 197.Davis GL. Hepatitis C virus genotypes and quasispecies. Am J Med. 1999;107:21S–6S. doi: 10.1016/s0002-9343(99)00376-9. [DOI] [PubMed] [Google Scholar]
- 198.Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelson AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010;84:6096–6102. doi: 10.1128/JVI.00127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McHutchison JG, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 200.Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2011;8:257–264. doi: 10.1038/nrgastro.2011.49. [DOI] [PubMed] [Google Scholar]
- 201.Sulkowski MS. HCV therapy in HIV-infected patients. Liver Int. 2013;33 (Suppl):63–67. doi: 10.1111/liv.12082. [DOI] [PubMed] [Google Scholar]
- 202.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nanda S, et al. Hepatic transcriptome analysis of hepatitis C virus infection in chimpanzees defines unique gene expression patterns associated with viral clearance. PLoS One. 2008;3:e3442. doi: 10.1371/journal.pone.0003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Su AI, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Golden-Mason L, Castelblanco N, O’Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Amadei B, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Post J, Ratnarajah S, Lloyd AR. Immunological determinants of the outcomes from primary hepatitis C infection. Cell Mol Life Sci. 2009;66:733–756. doi: 10.1007/s00018-008-8270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cramp ME, Carucci P, Underhill J, Naoumov NV, Williams R, Donaldson PT. Association between HLA class II genotype and spontaneous clearance of hepatitis C viraemia. J Hepatol. 1998;29:207–213. doi: 10.1016/s0168-8278(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 209.Vejbaesya S, Songsivilai S, Tanwandee T, Rachaibun S, Chantangpol R, Dharakul T. HLA association with hepatitis C virus infection. Hum Immunol. 2000;61:348–353. doi: 10.1016/s0198-8859(99)00131-7. [DOI] [PubMed] [Google Scholar]
- 210.Chang KM, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 211.Diepolder HM, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 212.Ferrari C, et al. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology. 1994;19:286–295. [PubMed] [Google Scholar]
- 213.Lechner F, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Grakoui A, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 215.Gruner NH, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 216.Sobao Y, Tomiyama H, Nakamura S, Sekihara H, Tanaka K, Takiguchi M. Visual demonstration of hepatitis C virus-specific memory CD8(+) T-cell expansion in patients with acute hepatitis C. Hepatology. 2001;33:287–294. doi: 10.1053/jhep.2001.21164. [DOI] [PubMed] [Google Scholar]
- 217.Fafi-Kremer S, et al. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010 Aug 30;207:2019–2031. doi: 10.1084/jem.20090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu L, Fisher BE, Dowd KA, Astemborski J, Cox AL, Ray SC. Acceleration of hepatitis C virus envelope evolution in humans is consistent with progressive humoral immune selection during the transition from acute to chronic infection. J Virol. 2010;84:5067–5077. doi: 10.1128/JVI.02265-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Freeman AJ, Marinos G, Ffrench RA, Lloyd AR. Immunopathogenesis of hepatitis C virus infection. Immunol Cell Biol. 2001;79:515–536. doi: 10.1046/j.1440-1711.2001.01036.x. [DOI] [PubMed] [Google Scholar]
- 221.Botarelli P, et al. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology. 1993;104:580–587. doi: 10.1016/0016-5085(93)90430-k. [DOI] [PubMed] [Google Scholar]
- 222.Abrignani S. Immune responses throughout hepatitis C virus (HCV) infection: HCV from the immune system point of view. Springer Semin Immunopathol. 1997;19:47–55. doi: 10.1007/BF00945024. [DOI] [PubMed] [Google Scholar]
- 223.Freeman AJ, et al. The presence of an intrahepatic cytotoxic T lymphocyte response is associated with low viral load in patients with chronic hepatitis C virus infection. J Hepatol. 2003;38:349–356. doi: 10.1016/s0168-8278(02)00424-5. [DOI] [PubMed] [Google Scholar]
- 224.Khakoo SI, et al. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963–970. [PMC free article] [PubMed] [Google Scholar]
- 225.Fiore G, Angarano I, Caccetta L, et al. In-situ immunophenotyping study of hepatic-infiltrating cytotoxic cells in chronic active hepatitis C. Eur J Gastroenterol Hepatol. 1997;9:491–496. doi: 10.1097/00042737-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 226.Nelson DR, et al. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 227.Billerbeck E, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He XS, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Neumann-Haefelin C, et al. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47:1824–1836. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- 230.Cox AL, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Radziewicz H, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Accapezzato D, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rushbrook SM, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of hepatitis C. Hepatology. 1992;15:567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- 235.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 236.Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009;49:676–688. doi: 10.1002/hep.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Helbig KJ, et al. Differential expression of the CXCR3 ligands in chronic hepatitis C virus (HCV) infection and their modulation by HCV in vitro. J Virol. 2009;83:836–846. doi: 10.1128/JVI.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Askarieh G, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 239.Grebely J, et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology. 2013 doi: 10.1002/hep.26263. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 241.Kirn A, Bingen A, Steffan AM, Wild MT, Keller F, Cinqualbre J. Endocytic capacities of Kupffer cells isolated from the human adult liver. Hepatology. 1982;2:216–222. doi: 10.1002/hep.1840020205. [DOI] [PubMed] [Google Scholar]
- 242.Seki E, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 243.Leandro G, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 244.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 245.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36(Suppl):S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 246.Danta M, et al. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis. 2008;197:1558–1566. doi: 10.1086/587843. [DOI] [PubMed] [Google Scholar]
- 247.Thomas DL, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 248.Martinez-Sierra C, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 249.Piroth L, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–388. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 250.Kim AY, et al. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 2006;3:e492. doi: 10.1371/journal.pmed.0030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim AY, Chung RT. Coinfection with HIV-1 and HCV--a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020–1023. [PubMed] [Google Scholar]
- 253.Thomas DL, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 254.Daar ES, et al. Relation between HIV-1 and hepatitis C viral load in patients with hemophilia. J Acquir Immune Defic Syndr. 2001;26:466–472. doi: 10.1097/00126334-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 255.Lin W, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 256.Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J. 2011;8:346. doi: 10.1186/1743-422X-8-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Netski DM, Mosbruger T, Astemborski J, Mehta SH, Thomas DL, Cox AL. CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis. 2007;195:857–863. doi: 10.1086/511826. [DOI] [PubMed] [Google Scholar]
- 258.Barrett L, et al. Stronger hepatitis C virus-specific CD8+ T-cell responses in HIV coinfection. J Viral Hepat. 2011;18:170–180. doi: 10.1111/j.1365-2893.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 259.Dutoit V, Ciuffreda D, Comte D, Gonvers JJ, Pantaleo G. Differences in HCV-specific T cell responses between chronic HCV infection and HIV/HCV co-infection. Eur J Immunol. 2005;35:3493–3504. doi: 10.1002/eji.200535035. [DOI] [PubMed] [Google Scholar]
- 260.Kim AY, et al. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood. 2005;105:1170–1178. doi: 10.1182/blood-2004-06-2336. [DOI] [PubMed] [Google Scholar]
- 261.Harcourt G, Gomperts E, Donfield S, Klenerman P. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut. 2006;55:1484–1487. doi: 10.1136/gut.2005.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Alatrakchi N, Graham CS, He Q, Sherman KE, Koziel MJ. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J Infect Dis. 2005;191:702–709. doi: 10.1086/427778. [DOI] [PubMed] [Google Scholar]
- 263.Kuntzen T, et al. Intrahepatic mRNA expression in hepatitis C virus and HIV/hepatitis C virus co-infection: infiltrating cells, cytokines, and influence of HAART. AIDS. 2008;22:203–210. doi: 10.1097/QAD.0b013e3282f3553b. [DOI] [PubMed] [Google Scholar]
- 264.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 265.Balagopal A, et al. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS. 2009;23:2397–2404. doi: 10.1097/QAD.0b013e3283324344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tuyama AC, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Guaraldi G, et al. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res. 2011;42:690–697. doi: 10.1016/j.arcmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 268.John M, Flexman J, French MA. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 269.French MA. Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. Med J Aust. 2012;196:318–321. doi: 10.5694/mja12.10089. [DOI] [PubMed] [Google Scholar]
- 270.Yunihastuti E, et al. Antibody and markers of T-cell activation illuminate the pathogenesis of HCV immune restoration disease in HIV/HCV co-infected patients commencing ART. Clin Immunol. 2011;139:32–39. doi: 10.1016/j.clim.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 271.Cameron BA, Emerson CR, Workman C, Kelly MD, Lloyd AR, Post JJ. Alterations in immune function are associated with liver enzyme elevation in HIV and HCV co-infection after commencement of combination antiretroviral therapy. J Clin Immunol. 2011;31:1079–1083. doi: 10.1007/s10875-011-9587-6. [DOI] [PubMed] [Google Scholar]
- 272.Kang F, et al. Transient Liver Injury Associated With the Early Recovery of HCV-Specific T-Cell Responses and HCV Rebound in HIV-1/HCV Coinfected Patients Undergoing Highly Active Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2013;62:135–142. doi: 10.1097/QAI.0b013e3182752d20. [DOI] [PubMed] [Google Scholar]
- 273.Page-Shafer K, Hahn JA, Lum PJ. Preventing hepatitis C virus infection in injection drug users: risk reduction is not enough. AIDS. 2007;21:1967–1969. doi: 10.1097/QAD.0b013e3282ef7701. [DOI] [PubMed] [Google Scholar]
- 274.Osburn WO, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Leroux-Roels G, et al. Immunogenicity and tolerability of intradermal administration of an HCV E1-based vaccine candidate in healthy volunteers and patients with resolved or ongoing chronic HCV infection. Hum Vaccin. 2005;1:61–65. doi: 10.4161/hv.1.2.1554. [DOI] [PubMed] [Google Scholar]
- 276.Leroux-Roels G, et al. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine. 2004;22:3080–3086. doi: 10.1016/j.vaccine.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 277.Frey SE, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Stamataki Z, Coates S, Abrignani S, Houghton M, McKeating JA. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J Infect Dis. 2011;204:811–813. doi: 10.1093/infdis/jir399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Barnes E, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Study of a New MVA Vaccine for Hepatitis C Virus. US National Institutes of Health, ClinicalTrials gov. 2013 [cited 2013 Jan 21];Available from: URL: http://clinicaltrials.gov/ct2/show/NCT01296451?term=HCV+vaccine&rank=14.
- 281.Staged Phase I/II Hepatitis C Prophylactic Vaccine. US National Institutes of Health, ClinicalTrials gov. 2013 [cited 2013 Jan 21];Available from: URL: http://clinicaltrials.gov/ct2/show/NCT01436357?term=NCT01436357&rank=1.
- 282.Keoshkerian E, et al. A novel assay for detection of hepatitis C virus-specific effector CD4(+) T cells via co-expression of CD25 and CD134. J Immunol Methods. 2012;375:148–158. doi: 10.1016/j.jim.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 283.Murray CJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 284.Phyo AP, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 286.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 287.McGregor IA, Williams K, Goodwin LG. Pyrimethamine and Sulphadiazine in Treatment of Malaria. Br Med J. 1963;2:728–729. doi: 10.1136/bmj.2.5359.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sabchareon A, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 289.Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 2001;23:51–59. doi: 10.1046/j.1365-3024.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- 290.Ricke CH, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 291.Salanti A, Dahlback M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kern P, Hemmer CJ, Van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 293.Jakobsen PH, et al. Increased concentrations of interleukin-6 and interleukin-1 receptor antagonist and decreased concentrations of beta-2-glycoprotein I in Gambian children with cerebral malaria. Infect Immun. 1994;62:4374–4379. doi: 10.1128/iai.62.10.4374-4379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Grau GE, Piguet PF, Vassalli P, Lambert PH. Involvement of tumour necrosis factor and other cytokines in immune-mediated vascular pathology. Int Arch Allergy Appl Immunol. 1989;88:34–39. doi: 10.1159/000234744. [DOI] [PubMed] [Google Scholar]
- 295.Kwiatkowski D, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 296.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Day NP, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 298.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006;194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 299.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 300.Moormann AM, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis. 1999;180:1987–1993. doi: 10.1086/315135. [DOI] [PubMed] [Google Scholar]
- 301.Suguitan AL, Jr, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis. 2003;188:1074–1082. doi: 10.1086/378500. [DOI] [PubMed] [Google Scholar]
- 302.Chandramohan D, Greenwood BM. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int J Epidemiol. 1998;27:296–301. doi: 10.1093/ije/27.2.296. [DOI] [PubMed] [Google Scholar]
- 303.Greenberg AE, et al. Plasmodium Falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325:105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 304.Allen S, et al. Human immunodeficiency virus and malaria in a representative sample of childbearing women in Kigali, Rwanda. J Infect Dis. 1991;164:67–71. doi: 10.1093/infdis/164.1.67. [DOI] [PubMed] [Google Scholar]
- 305.Colebunders R, et al. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J Infect. 1990;21:167–173. doi: 10.1016/0163-4453(90)91701-e. [DOI] [PubMed] [Google Scholar]
- 306.Simooya OO, Mwendapole RM, Siziya S, Fleming AF. Relation between falciparum malaria and HIV seropositivity in Ndola, Zambia. BMJ. 1988;297:30–31. doi: 10.1136/bmj.297.6640.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Whitworth JA, Hewitt KA. Effect of malaria on HIV-1 progression and transmission. Lancet. 2005;365:196–197. doi: 10.1016/S0140-6736(05)17752-6. [DOI] [PubMed] [Google Scholar]
- 308.Laufer MK, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 309.Steketee RW, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55(Suppl):42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 310.ter Kuile FO, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71(Suppl):41–54. [PubMed] [Google Scholar]
- 311.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 312.O’Neil-Dunne I, et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun. 2001;69:7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Staalsoe T, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184:618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 314.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 315.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jaworowski A, et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol. 2009;16:312–319. doi: 10.1128/CVI.00356-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mount AM, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 2004;363:1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 318.Keen J, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 2007;4:e181. doi: 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ataide R, et al. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS One. 2010;5:e10807. doi: 10.1371/journal.pone.0010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Leeansyah E, Wines BD, Crowe SM, Jaworowski A. The mechanism underlying defective Fcgamma receptor-mediated phagocytosis by HIV-1-infected human monocyte-derived macrophages. J Immunol. 2007;178:1096–1104. doi: 10.4049/jimmunol.178.2.1096. [DOI] [PubMed] [Google Scholar]
- 321.Kedzierska K, Ellery P, Mak J, Lewin SR, Crowe SM, Jaworowski A. HIV-1 down-modulates gamma signaling chain of Fc gamma R in human macrophages: a possible mechanism for inhibition of phagocytosis. J Immunol. 2002;168:2895–2903. doi: 10.4049/jimmunol.168.6.2895. [DOI] [PubMed] [Google Scholar]
- 322.Ludlow LE, et al. HIV-1 inhibits phagocytosis and inflammatory cytokine responses of human monocyte-derived macrophages to P. falciparum infected erythrocytes. PLoS One. 2012;7:e32102. doi: 10.1371/journal.pone.0032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Reuben JM, et al. Induction of inflammatory cytokines in placental monocytes of gravidae infected with the human immunodeficiency virus type 1. J Interferon Cytokine Res. 1996;16:963–971. doi: 10.1089/jir.1996.16.963. [DOI] [PubMed] [Google Scholar]
- 324.Davenport GC, Hittner JB, Were T, Ong’echa JM, Perkins DJ. Relationship between inflammatory mediator patterns and anemia in HIV-1 positive and exposed children with Plasmodium falciparum malaria. Am J Hematol. 2012;87:652–658. doi: 10.1002/ajh.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hoffman IF, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 326.Kublin JG, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 327.Kapiga SH, Bang H, Spiegelman D, et al. Correlates of plasma HIV-1 RNA viral load among HIV-1-seropositive women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;30:316–323. doi: 10.1097/00126334-200207010-00008. [DOI] [PubMed] [Google Scholar]
- 328.Mwapasa V, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18:1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 329.Inion I, Mwanyumba F, Gaillard P, et al. Placental malaria and perinatal transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;188:1675–1678. doi: 10.1086/379737. [DOI] [PubMed] [Google Scholar]
- 330.Van Geertruyden JP, et al. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 331.Quigley MA, et al. The effect of malaria on mortality in a cohort of HIV-infected Ugandan adults. Trop Med Int Health. 2005;10:894–900. doi: 10.1111/j.1365-3156.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- 332.Mermin J, Lule JR, Ekwaru JP. Association between malaria and CD4 cell count decline among persons with HIV. J Acquir Immune Defic Syndr. 2006;41:129–130. doi: 10.1097/01.qai.0000179427.11789.a7. [DOI] [PubMed] [Google Scholar]
- 333.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 334.Lawn SD, et al. Sustained plasma TNF-alpha and HIV-1 load despite resolution of other parameters of immune activation during treatment of tuberculosis in Africans. AIDS. 1999;13:2231–2237. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 335.Xiao L, Owen SM, Rudolph DL, Lal RB, Lal AA. Plasmodium falciparum antigen-induced human immunodeficiency virus type 1 replication is mediated through induction of tumor necrosis factor-alpha. J Infect Dis. 1998;177:437–445. doi: 10.1086/514212. [DOI] [PubMed] [Google Scholar]
- 336.Finney CA, et al. HIV infection deregulates innate immunity to malaria despite combination antiretroviral therapy. AIDS. 2013;27:325–335. doi: 10.1097/QAD.0b013e32835b3dfa. [DOI] [PubMed] [Google Scholar]
- 337.Tyagi RK, Garg NK, Sahu T. Vaccination Strategies against Malaria: novel carrier(s) more than a tour de force. J Control Release. 2012;162:242–254. doi: 10.1016/j.jconrel.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 338.Agnandji ST, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Agnandji ST, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 340.Hommel M, et al. Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infect Immun. 2010;78:1963–1978. doi: 10.1128/IAI.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Beeson JG, et al. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. J Infect Dis. 2006;193:721–730. doi: 10.1086/500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kane EG, Taylor-Robinson AW. Prospects and Pitfalls of Pregnancy-Associated Malaria Vaccination Based on the Natural Immune Response to Plasmodium falciparum VAR2CSA-Expressing Parasites. Malar Res Treat. 2011;2011:764845. doi: 10.4061/2011/764845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Feng G, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis. 2009;200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Aderem A, et al. A systems biology approach to infectious disease research: innovating the pathogen-host research paradigm. MBio. 2011;2:e00325–10. doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tran TM, Samal B, Kirkness E, Crompton PD. Systems immunology of human malaria. Trends Parasitol. 2012;28:248–257. doi: 10.1016/j.pt.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nolan GP. Flow cytometry in the post fluorescence era. Best Pract Res Clin Haematol. 2011;24:505–508. doi: 10.1016/j.beha.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 347.Qiu P, et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29:886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chang, et al. Chemokine levels and chemokine receptor expression in blood and the CSF of HIV-infected patients with cryptococcal meningitis and C-IRIS. JID. 2013 doi: 10.1093/infdis/jit388. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]