Abstract
Streptococcus pneumoniae is the most common etiological cause of complicated pneumonia, including empyema. In this study, we investigated the serotypes of S. pneumoniae that cause empyema in children. One hundred fifty-six children who were diagnosed with pneumonia complicated with empyema in 13 hospitals in seven geographic regions of Turkey between 2010 and 2012 were included in this study. Pleural fluid samples were collected by thoracentesis and tested for 14 serotypes/serogroups using a Bio-Plex multiplex antigen detection assay. The serotypes of S. pneumoniae were specified in 33 of 156 samples. The mean age ± the standard deviation of the 33 patients was 6.17 ± 3.54 years (range, 0.6 to 15 years). All of the children were unvaccinated according to the vaccination reports. Eighteen of the children were male, and 15 were female. The serotypes of the non-7-valent pneumococcal conjugated vaccine (non-PCV-7), serotype 1, serotype 5, and serotype 3, were detected in eight (14.5%), seven (12.7%), and five (9.1%) of the samples, respectively. Serotypes 1 and 5 were codetected in two samples. The remaining non-PCV-7 serotypes were 8 (n = 3), 18 (n = 1), 19A (n = 1), and 7F/A (n = 1). PCV-7 serotypes 6B, 9V, 14, 19F, and 23F were detected in nine (16.3%) of the samples. The potential serotype coverages of PCV-7, PCV-10, and PCV-13 were 16.3%, 45.4%, and 60%, respectively. Pediatric parapneumonic empyema continues to be an important health problem despite the introduction of conjugated pneumococcal vaccines. Active surveillance studies are needed to monitor the change in S. pneumoniae serotypes that cause empyema in order to have a better selection of pneumococcal vaccines.
INTRODUCTION
Streptococcus pneumoniae is the most commonly identified cause of pneumonia with empyema (1, 2). Although empyema complicates only 1 to 2% of childhood pneumonia cases, these cases are associated with considerable morbidity and mortality (3–5). The emergence and spread of resistant pneumococcal strains have led to an emphasis on the prevention of pneumococcal disease by vaccination (6–8). In February 2000, a pneumococcal conjugate vaccine (PCV) covering serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F was licensed for use in infants and young children and recommended as part of the routine U.S. childhood immunization schedule (9). Before the introduction of the 7-valent PCV (PCV-7), an increase in the incidence of parapneumonic empyema in children was reported (10–18). Despite the introduction of PCV-7, a number of studies have shown that the incidence of empyema in children continues to increase (19, 20).
In 2008, PCV-7 was implemented into the Turkish national immunization schedule at 2, 4, 6, and 12 months of age, and it was replaced with PCV-13 in 2011. In this study, we specified the S. pneumoniae serotypes that cause empyema in children, irrespective of their vaccination status.
MATERIALS AND METHODS
Patients.
Children aged 0 to 18 years in 13 hospitals in seven geographic regions (representing one-third of the total population of Turkey) with a diagnosis of parapneumonic empyema were included in this active-prospective surveillance study between January 2010 and December 2011. All of the children with a clinical diagnosis of pneumonia with empyema were enrolled in this laboratory-based study irrespective of their vaccination status, and pleural fluid was collected at the time of chest drain insertion or via thoracentesis. A total of 156 children with a clinical diagnosis of parapneumonic empyema during the active surveillance period were investigated. The children were evaluated by means of detailed histories, physical examinations, chest radiography, and complete blood counts. Additionally, the vaccination status of the children was determined from the extant vaccination-recording system of the Turkish Ministry of Health. Pleural fluid aspirates were examined to determine the pH, the white blood cell (WBC) count, and the concentrations of glucose, protein, and lactate dehydrogenase (LDH). Appropriate bacterial staining and pleural fluid cultures were also performed in the local hospitals. In addition, many children were subjected to computed tomography and ultrasonographic evaluations of the chest. A pleural fluid sample (minimum, 0.5 ml) from each patient was stored at −20°C and then transported via cold chain to a central laboratory (Pediatric Infectious Diseases, Hacettepe University, Ankara, Turkey) for further analysis. After transport, the samples were stored at −80°C. From this cohort, only 53 cases confirmed by PCR to be caused by S. pneumoniae were included in the present study. The Bio-Plex multiplex antigen detection method was applied to all PCR-proven S. pneumoniae isolates for serotyping.
Empyema, as a complication of pneumonia, was defined according to several major and minor criteria (21). The major criteria were the presence of pus in the pleural space, the need for surgical decortication, and the positivity of a pleural fluid culture. Minor criteria included the following laboratory values or findings: pleural fluid pH ≤ 7.2, LDH ≥ 1,000 U/ml, glucose ≤ 40 mg/dl, WBC count ≥ 10,000/dl, and a positive blood culture.
Laboratory tests.
DNA was prepared from pleural fluid samples using the Qiagen QIAamp DNA blood minikit (Hilden, Germany). The PCR assay was performed using a DNA thermal cycler (GeneAmp PCR system, model 9700; Applied Biosystems, Foster City, CA) to identify S. pneumoniae. The specific gene target was ply for S. pneumoniae (22, 23). PCR-positive empyema fluid samples were treated with proteinase K for 10 min at 56°C, diluted 1:5 in phosphate-buffered saline (PBS), and heat treated at 100°C for 10 min to render them noninfectious. This treatment also liquefied the viscous samples to enable them to be assayed. The processed samples (100 μl per well) were tested in a Bio-Plex multiplex antigen detection assay capable of detecting 14 serotypes/groups (1, 3, 4, 5, 6A, 6B, 7F/A, 8, 9V, 14, 18, 19A, 19F, and 23F) and a pneumococcal C polysaccharide control (23). Each sample was tested in duplicate with a standard curve consisting of purified capsular polysaccharides in PBS and a negative control containing only PBS. To score results, the sample median fluorescence values were divided by the negative control to create a test-to-negative ratio. Samples that provided a ratio of >3 were considered positive.
Informed consent and a case report form were obtained to record the clinical and laboratory findings for evidence of pneumococcal infection, as detailed below. This study was reviewed and approved by the Hacettepe University Institutional Ethics Committee.
RESULTS
A total of 156 children with a diagnosis of pneumonia complicated with empyema were investigated, and the causative agent was found to be S. pneumoniae in 53 (34%) of the 156 patients. Twenty-four patients were included in 2010, and the others were included in 2011. S. pneumoniae serotypes were specified in 33 of them. Twenty of the S. pneumoniae-positive samples could not be serotyped because of the limited number of antigens tested for in the Bio-Plex multiplex antigen detection assay used. The serotype distribution of the 33 subjects is presented in Table 1. All but three children received antibiotics before referral to the study centers. The pleural fluid cultures were negative in all cases.
Table 1.
Serotype distribution of Streptococcus pneumoniae in children with parapneumonic empyema
| Serotype | n (%) |
|---|---|
| PCV-7 serotype | |
| 4 | 0 (0) |
| 6B | 2 (3.6) |
| 9V | 1 (1.8) |
| 14 | 2 (3.6) |
| 18Ca | NA |
| 19F | 3 (5.5) |
| 23F | 1 (1.8) |
| PCV-10 serotypeb | |
| 1 | 8 (14.5) |
| 5 | 7 (12.7) |
| 7Fc | 1 (1.8) |
| PCV-13 serotyped | |
| 3 | 5 (9.1)e |
| 6A | 0 (0) |
| 19A | 1 (1.8) |
| Non-PCV serotype | |
| 8 | 3 (5.5) |
| 18 | 1 (1.8) |
| Nonclassified | 20 (36.4) |
| Total | 55 (100)f |
NA, not applicable (18C was not specifically tested for).
Remaining PCV-10 serotypes except the PCV-7 serotypes.
Serotype 7F/A is considered a PCV-10 and a PCV-13 serotype.
Remaining PCV-13 serotypes except the PCV-10 serotypes.
Two of these were mixed infections of serotypes 1 and 5.
Total number of isolates positive for S. pneumoniae from 53 patients (two were mixed infections). This value was used to calculate serotype coverage.
The mean age ± the standard deviation was 6.17 ± 3.54 years (range, 0.6 to 15 years) in all serotyped S. pneumoniae cases. Two of the empyema isolates were obtained from children <1 year of age. The children had not been vaccinated, according to their vaccination records. Eighteen of the children were male, and 15 were female. No mortality was observed among the cases. Serotype 1 was detected in eight samples (14.5%); the second most common type was serotype 5, which was detected in seven samples (12.7%), and serotype 3 was detected in five (9.1%) samples. Two serotypes were revealed in two samples simultaneously in 2011. While serotypes 1 and 3 were codetected in one sample, serotypes 3 and 5 were codetected in the other. Serotypes 1, 5, and 3 are non-PCV-7 serotypes. The remaining non-PCV7 serotypes were 8 (n = 3), 18 (n = 1), 19A (n = 1), and 7F/A (n = 1). We were able to detect only serotype 18 with the assay used; however, we did not specifically test for serotype 18C. Therefore, serotype 18 is not listed as a PCV serotype in Table 1. The serotypes 7F and 7A were not differentiable by the antigen detection assay used; therefore, the one isolate that was positive for 7F/7A was counted in the PCV-10 and -13 serotype groups.
PCV-7 serotypes were detected in nine of the samples, including serotypes 6B (n = 2), 9V (n = 1), 14 (n = 2), 19F (n = 3), and 23F (n = 1). Serotypes 4 and 18C were not detected in any sample.
Our study was conducted in 13 hospitals in seven geographic regions of Turkey. A total of 55 S. pneumoniae serotype-positive tests from 53 patients' samples, because of coinfections, were used to calculate serotype coverage. Of the S. pneumoniae isolates with an identifiable serotype, 16.3%, 45.4%, and 60% were contained within PCV-7, PCV-10, and PCV-13, respectively. The annual serotype coverages for PCV-7, PCV-10, and PCV-13 were 20.8%, 50%, and 62.5% in 2010 and were 12.9%, 41.9%, and 51.6% in 2011, respectively (Fig. 1).
Fig 1.
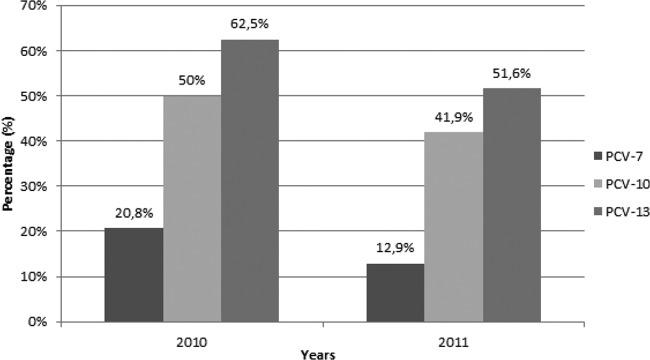
Serotype coverage of pneumococcal conjugated vaccines PCV-7, PCV-10, and PCV-13 in nonvaccinated children with pneumococcal empyema.
DISCUSSION
Although pediatric pneumococcal empyema is a rare complication of pneumonia, it has been increasingly reported in recent years (1, 3, 11, 19, 24–27). The difficulty of culture-confirmed diagnosis remains a problem in pediatric empyema (1, 28). In our study, pleural fluid cultures were negative for all the patients, which may have been related to the use of antibiotics before admission to the study centers. Routine clinical practices, such as the administration of antibiotics prior to thoracentesis, can affect the sensitivity of bacterial cultures. Therefore, nonculture techniques may be necessary to detect the causative organism. Although bacterial culture is considered the standard method, pneumococcal antigen detection in empyema fluid is also a useful diagnostic tool (17, 26). This surveillance method made documentation of the serotype distribution of pneumococci in culture-negative patients possible. Twenty of the isolates could not be serotyped because the Bio-Plex antigen detection assay detected only a limited number of pneumococcal serotypes (14). Although the serotyping method used has a limited ability to detect all pneumococcal serotypes, this non-culture-based rapid assay would assist in the identification of frequency and serotype distribution in the postvaccine era. Antigen detection methods are becoming more important for the serotyping of pneumococcal infections in countries with a high antibiotic usage rate and delayed admission to the hospital, such as Turkey. Information regarding S. pneumoniae serotypes in patients with pneumonia is limited because sampling in children is difficult. Although the spectrum of etiologic bacteria differs from those in empyema and pneumonia, our data provide information about the pneumococcal serotypes that cause pneumonia in Turkey.
Although it is difficult to determine the actual proportion of S. pneumoniae serotypes that cause parapneumonic empyema in children in the absence of knowledge of the other empyema-causing pathogens, S. pneumoniae seems to be one of the leading causes, and serotype 1 is the most common. This finding agrees with the findings of studies by Fletcher et al. (17), Eastham et al. (26), Eltringham et al. (28), and Byington et al. (1). The reason for the high invasive potential of serotype 1 into the pleural space is unknown (17).
Increases in the frequency of serotypes 3, 7F, and 19A in the post-PCV-7 era (24, 27) have been reported. Serotype 3 seemed more likely to be a cause of parapneumonic empyema in our study. One of the non-PCV-7 serotypes, 19A, has been identified in many studies as a cause of invasive pneumococcal disease following the licensure of PCV-7 (29–32), and serotype 7F has been associated with children with severe or fatal pneumococcal infections (33). We detected serotypes 19A and 7F in one patient each.
Some studies have shown a >75% efficacy of the PCV-7 vaccine against invasive disease (34–38). Vaccination reduces radiologically confirmed pneumonia by 20.5 to 37%. In the United States, the effectiveness of PCV-7 against invasive pneumococcal disease (IPD) is controversial. The limited effectiveness of PCV-7 was reported in Utah as a result of the rapid emergence of IPD, particularly empyema, due to non-PCV-7 serotypes (10, 19, 39, 40). These findings encouraged the development of new pneumococcal conjugate vaccines with additional serotypes, namely, PCV-10 and PCV-13 (41, 42). However, clinical trials showing the efficacy of PCV-10 and PCV-13 against consolidated pneumonia and IPD have been limited. The compositions of PCV-10 and PCV-13 might have provided protection against empyema caused by non-PCV-7 serotypes in this study. However, the efficacies of pneumococcal vaccines may differ among different geographic regions due to variations in the serotype spectrum. Surveillance of local serotypes is critical for determining the PCV composition that also provides coverage of IPD-causing serotypes.
Routine vaccination with PCV-7 for children <1 year of age was agreed upon in Turkey at the end of 2008 and was included in the National Immunization Schedule in 2009. PCV-7 was used for 2 years in Turkey before being replaced by PCV-13 in November 2011. In 2010 and 2011, 96% and 97% of the target population, respectively, were vaccinated with PCV-7 (see http://www.sgk.gov.tr). Despite this high coverage rate, the children in our study had not been vaccinated with either PCV-7 or PCV-13 because the majority of the children were older than the requisite age for routine vaccination, according to the Turkish Ministry of Health schedule. Additionally, the baseline data on the prevalence and serotype distribution of S. pneumoniae isolates causing empyema in Turkey before the PCV era are limited. One of the aims of this study was to understand the impact of immunization on invasive pneumococcal infection in Turkey. We plan to monitor the incidence and etiologic agents of pediatric parapneumonic empyema in Turkey after the institution of the new vaccination practices.
After the PCV-7 era, vaccine authorities from various countries are in conflict regarding the selection of new 10- and 13-valent vaccines. The most common pneumococcal serotypes in our study were 1 and 5, which are covered by both the 10- and 13-valent vaccines. Serotype 3 was another important cause of empyema in the present study; this serotype is covered only by PCV-13. The potential serotype coverages of PCV-7, PCV-10, and PCV-13 in our study were 16.3%, 45.4%, and 60%, respectively. According to our data, PCV-13 seems to be the most protective against childhood pneumococcal empyema. Therefore, PCV-13 represents a promising vaccine against empyema.
Although limited, our data provide information regarding the serotype distribution of pneumococcal empyema in children in Turkey. We suggest that regional data are important for determining the most appropriate vaccine for the local S. pneumoniae epidemiological profile. As the incidence of this invasive disease is increasing, vaccines with coverage appropriate for each country are needed.
ACKNOWLEDGMENT
The study was supported by GlaxoSmithKline.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Byington CL, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, Kaplan S, Carroll KC, Daly JA, Christenson JC, Samore MH. 2002. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin. Infect. Dis. 34:434–440 [DOI] [PubMed] [Google Scholar]
- 2. Grisaru-Soen G, Eisenstadt M, Paret G, Schwartz D, Keller N, Nagar H, Reif S. 2013. Pediatric parapneumonic empyema: risk factors, clinical characteristics, microbiology, and management. Pediatr. Emerg. Care 29:425–429 [DOI] [PubMed] [Google Scholar]
- 3. Chonmaitree T, Powell KR. 1983. Parapneumonic pleural effusion and empyema in children: review of a 19-year experience, 1962-1980. Clin. Pediatr. (Phila.) 22:414–419 [DOI] [PubMed] [Google Scholar]
- 4. Varkey B, Rose HD, Kutty CP, Politis J. 1981. Empyema thoracis during a ten year period: analysis of 72 cases and comparison to a previous study (1952-1967). Arch. Intern. Med. 141:1771–1776 [DOI] [PubMed] [Google Scholar]
- 5. Freij BJ, Kusmiesz H, Nelson JD, McCracken GH., Jr 1984. Parapneumonic effusions and empyema in hospitalized children: a retrospective review of 227 cases. Pediatr. Infect. Dis. 3:578–591 [DOI] [PubMed] [Google Scholar]
- 6. Friedland IR, McCracken GH., Jr 1994. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N. Engl. J. Med. 331:377–382 [DOI] [PubMed] [Google Scholar]
- 7. Mastro TD, Ghafoor A, Nomani NK, Ishaq Z, Anwar F, Granoff DM, Spika JS, Thornsberry C, Facklam RR. 1991. Antimicrobial resistance of pneumococci in children with acute lower respiratory tract infection in Pakistan. Lancet 337:156–159 [DOI] [PubMed] [Google Scholar]
- 8. Friedland IR, Klugman KP. 1992. Antibiotic-resistant pneumococcal disease in South African children. Am. J. Dis. Child. 146:920–923 [DOI] [PubMed] [Google Scholar]
- 9. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC) 2010. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 59:1–18 [PubMed] [Google Scholar]
- 10. Byington CL, Samore MH, Stoddard GJ, Barlow S, Daly J, Korgenski K, Firth S, Glover D, Jensen J, Mason EO, Shutt CK, Pavia AT. 2005. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin. Infect. Dis. 41:21–29 [DOI] [PubMed] [Google Scholar]
- 11. Tan TQ, Mason EO, Jr, Wald ER, Barson WJ, Schutze GE, Bradley JS, Givner LB, Yogev R, Kim KS, Kaplan SL. 2002. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 110:1–6 [DOI] [PubMed] [Google Scholar]
- 12. Schultz KD, Fan LL, Pinsky J, Ochoa L, Smith EO, Kaplan SL, Brandt ML. 2004. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics 113:1735–1740 [DOI] [PubMed] [Google Scholar]
- 13. Rees JH, Spencer DA, Parikh D, Weller P. 1997. Increase in incidence of childhood empyema in West Midlands, U. K. Lancet 349:402. 10.1016/S0140-6736(97)80022-0 [DOI] [PubMed] [Google Scholar]
- 14. Finley C, Clifton J, Fitzgerald JM, Yee J. 2008. Empyema: an increasing concern in Canada. Can. Respir. J. 15:85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta R, Crowley S. 2006. Increasing paediatric empyema admissions. Thorax 61:179–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Playfor SD, Smyth AR, Stewart RJ. 1997. Increase in incidence of childhood empyema. Thorax 52:932. [PubMed] [Google Scholar]
- 17. Fletcher M, Leeming J, Cartwright Finn KA. 2006. Childhood empyema: limited potential impact of 7-valent pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 25:559–560 [DOI] [PubMed] [Google Scholar]
- 18. Spencer DA, Iqbal SM, Hasan A, Hamilton L. 2006. Empyema thoracis is still increasing in UK children. BMJ 332:1333. 10.1136/bmj.332.7553.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. 2006. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr. Infect. Dis. J. 25:250–254 [DOI] [PubMed] [Google Scholar]
- 20. Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. 2008. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 27:1030–1032 [DOI] [PubMed] [Google Scholar]
- 21. Hardie W, Bokulic R, Garcia VF, Reising SF, Christie CDC. 1996. Pneumococcal pleural empyemas in children. Clin. Infect. Dis. 22:1057–1063 [DOI] [PubMed] [Google Scholar]
- 22. Ceyhan M, Yildirim I, Balmer P, Borrow R, Dikici B, Turgut M, Kurt N, Aydogan A, Ecevit C, Anlar Y, Gulumser O, Tanir G, Salman N, Gurler N, Hatipoglu N, Hacimustafaoglu M, Celebi S, Coskun Y, Alhan E, Celik U, Camcioglu Y, Secmeer G, Gur D, Gray S. 2008. A prospective study of etiology of childhood acute bacterial meningitis, Turkey. Emerg. Infect. Dis. 14:1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceyhan M, Yildirim I, Sheppard CL, George RC. 2010. Pneumococcal serotypes causing pediatric meningitis in Turkey: application of a new technology in the investigation of cases negative by conventional culture. Eur. J. Clin. Microbiol. Infect. Dis. 29:289–293 [DOI] [PubMed] [Google Scholar]
- 24. Byington CL, Hulten KG, Ampofo K, Sheng X, Pavia AT, Blaschke AJ, Pettigrew M, Korgenski K, Daly J, Mason EO. 2010. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J. Clin. Microbiol. 48:520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buckingham SC, King MD, Miller ML. 2003. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr. Infect. Dis. J. 22:499–504 [DOI] [PubMed] [Google Scholar]
- 26. Eastham KM, Freeman R, Kearns AM, Eltringham G, Clark J, Leeming J, Spencer DA. 2004. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax 59:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obando I, Munoz-Almagro C, Arroyo LA, Tarrago D, Sanchez-Tatay D, Moreno-Perez D, Dhillon SS, Esteva C, Hernandez-Bou S, Garcia-Garcia JJ, Hausdorff WP, Brueggemann AB. 2008. Pediatric parapneumonic empyema, Spain. Emerg. Infect. Dis. 14:1390–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eltringham G, Kearns A, Freeman R, Clark J, Spencer D, Eastham K, Harwood J, Leeming J. 2003. Culture-negative childhood empyema is usually due to penicillin-sensitive Streptococcus pneumoniae capsular serotype 1. J. Clin. Microbiol. 41:521–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore MR, Gertz RE, Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, Hadler JL, Bennett NM, Thomas AR, McGee L, Pilishvili T, Brueggemann AB, Whitney CG, Jorgensen JH, Beall B. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016–1027 [DOI] [PubMed] [Google Scholar]
- 30. Pai R, Moore MR, Pilishuili T, Gertz RE, Whitney CG, Beall B; Active Bacterial Core Surveillance Team 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988–1995 [DOI] [PubMed] [Google Scholar]
- 31. Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstem R, Hanage WP. 2007. Emergence of 19A as a virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26:468–472 [DOI] [PubMed] [Google Scholar]
- 32. Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Butler JC, Rudolph K, Parkinson A. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784–1792 [DOI] [PubMed] [Google Scholar]
- 33. Rückinger S, von Kries R, Siedler A, van der Linden M. 2009. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr. Infect. Dis. J. 28:118–122 [DOI] [PubMed] [Google Scholar]
- 34. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 35. Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, Hackell J. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810–815 [DOI] [PubMed] [Google Scholar]
- 36. O'Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, Kumar G, Parkinson A, Hu D, Hackell J, Chang I, Kohberger R, Siber G, Santosham M. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355–361 [DOI] [PubMed] [Google Scholar]
- 37. Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N, Vaccine Trialists Group 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341–1348 [DOI] [PubMed] [Google Scholar]
- 38. Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, McAdam KP, Biney E, Saaka M, Onwuchekwa U, Yallop F, Pierce NF, Greenwood BM, Adegbola RA; Gambian Pneumococcal Vaccine Trial Group 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-bind, placebo-controlled trial. Lancet 365:1139–1146 [DOI] [PubMed] [Google Scholar]
- 39. Kaplan SL, Mason EO, Jr, Wald ER, Schutze GE, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Yogev R, Barson WJ. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113:443–449 [DOI] [PubMed] [Google Scholar]
- 40. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennet NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A; Active Bacterial Core Surveillance of the Emerging Infections Program Network 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737–1746 [DOI] [PubMed] [Google Scholar]
- 41. Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsene JP, Lommel P, Dieussaert I, Schverman L. 2009. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 28:S66–S76 [DOI] [PubMed] [Google Scholar]
- 42. Vanderkooi OG, Scheifele DW, Girgenti D, Halperin SA, Patterson SD, Gruber WC, Emini EA, Scott DA, Kellner JD, Canadian PCV13 Study Group 2012. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers given with routine pediatric vaccinations in Canada. Pediatr. Infect. Dis. J. 31:72–77 [DOI] [PubMed] [Google Scholar]


