Abstract
It has been proposed that Borrelia burgdorferi infection is present in ∼25% of children with autism spectrum disorders. In this study, antibodies against Borrelia burgdorferi were assessed in autistic (n = 104), developmentally delayed (n = 24), and healthy control (n = 55) children. No seropositivity against Borrelia burgdorferi was detected in the children with and without autism. There was no evidence of an association between Lyme disease and autism.
TEXT
Autism spectrum disorder (ASD) presents before the age of 3 years and is diagnosed when a child displays a constellation of symptoms including impaired verbal and nonverbal communication, deficits in reciprocal social interaction, and repetitive and stereotypic patterns of behavior (1). The potential causes of autism, including genetic and environmental factors, are subjects of intense investigation (2).
Lyme disease, caused by the spirochete Borrelia burgdorferi, is transmitted by ticks and is widespread in many parts of the United States (3). B. burgdorferi infection can cause a systemic infectious disease with a broad spectrum of symptoms affecting the skin, the heart, and the musculoskeletal and nervous systems (4). Laboratory tests for Lyme disease rely predominantly on the detection of serum antibodies against B. burgdorferi proteins using a variety of immunoassay formats (4). Recently, we developed a quantitative luciferase immunoprecipitation system (LIPS) test for detecting B. burgdorferi antibodies using a liquid-phase assay that employed the synthetic VOVO antigen, composed of immunogenic epitopes from the VlsE and OspC proteins fused to the light-emitting protein Renilla luciferase, named VOVO-LIPS. The VOVO-LIPS test showed high diagnostic performance for detecting Lyme disease, with 98% sensitivity and 98% specificity, and had the ability to detect a wide range of antibody titers (5).
Lyme disease has become the target of many controversial concepts. A recent article hypothesized that Borrelia burgdorferi infection might be present in 20 to 30% of patients with ASD (6), and long-term antibiotic therapy has been used as a potential treatment option for presumed Lyme disease in individuals with autism (7). However, a formal study examining the frequency of B. burgdorferi infection in ASD has not been conducted. In this study, a retrospective, cross-sectional cohort was used to assess the frequency of B. burgdorferi seropositivity in young children with and without ASD.
A cohort of children with autism and other controls (n = 183) was evaluated at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD, under the institutional review board approved protocol NCT00298246: Clinical and Immunological Investigations of Subtypes of Autism. The parents of all the children participating in this study provided written informed consent. Inclusion criteria consisted of a confirmed diagnosis of autism and age range between 1 and 6 years old at study entry, and children with and without reported histories of a regressive onset were included. Exclusion criteria included a known neurological disorder (e.g., cerebral palsy) that interfered with ability to complete study procedures. The children with autism and controls were recruited directly through advertisements in the local community. The autistic children included individuals with autism with no regression (n = 59) and autism with regression (n = 45). The children were diagnosed by Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (8), criteria after assessment using the Autism Diagnostic Observation Schedule (ADOS), a clinician-administered structured play interview designed to elicit behaviors relevant to a diagnosis of autism, and the Autism Diagnostic Interview—Revised (ADI-R) (9), a semistructured parent interview concerning all domains of impairment in autism spectrum disorders. The developmentally delayed group (n = 24) was approximately matched to the autism group on developmental level. An additional group of healthy, typically developing children (n = 55) was also studied. All children underwent physical examination. Parents completed medical history forms, which were reviewed by and followed up by a clinician. The medical history form included questions about previous infections in general and asked specifically about a history of Lyme disease. Control serum samples from patients with Lyme disease were tested in parallel. These patients include two with erythema migrans, one with neuroborreliosis, one with post-Lyme disease syndrome, and one with Lyme arthritis. Patients fulfilled the case criteria for confirmed Lyme disease as per the 2011 Centers for Disease Control and Prevention definition (10). Serum samples were collected under institutional review board-approved protocols at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD. All Lyme patients signed forms giving informed consent.
For LIPS serology, serum samples were tested as described previously (7), with the exception that raw light unit (LU) values without subtraction of the buffer blanks are reported in this study. A previously characterized low seropositive erythema migrans serum sample was used to assign the cutoff.
The 104 autism samples and Lyme positive controls were also analyzed in a blinded fashion using an FDA-approved C6 Lyme enzyme-linked immunosorbent assay (ELISA) (Immunetics, Boston, MA). Equivocal samples identified by C6 testing were further evaluated by Western blotting performed with the FDA-approved Borrelia B31 IgG Virastrip kit (Viralab Inc., Oceanside, CA) in accordance with standard CDC recommendations for laboratory diagnosis of Lyme disease (11). GraphPad Prism software (San Diego, CA) was used for statistical analysis.
The average age of the autistic children and controls was 4 years, and the majority of the children were male. All of the children in the study were living on the East Coast and Midwest of the United States. The children with autism (n = 104) were from various U.S. locations, including Maryland (n = 59), Virginia (n = 23), Washington, DC (n = 4), Georgia (n = 4), Pennsylvania (n = 3), New Jersey (n = 3), Ohio (n = 3), North Carolina (n = 2), Missouri (n = 1), Tennessee (n = 1), and Illinois (n = 1). Of note, 82% (85/104) of the autistic children were from Maryland, Virginia, and Pennsylvania, which according to the Centers for Disease Control and Prevention are states with a high incidence of Lyme disease (12). None of the children had a history of previous diagnosis of Lyme disease on the medical history form (with 3 missing data points on this question from 185 subjects).
In the positive-control Lyme patients, the serum antibody levels detected by the VOVO-LIPS ranged from 12,340 LU to 5,909,000 LU, in which all samples were above the cutoff value (Fig. 1). As expected, the two erythema migrans positive-control serum samples had the lowest seropositive antibody levels, whereas the patients with early neuroborreliosis, post-Lyme disease syndrome, and Lyme arthritis had markedly higher antibody levels: 324,900, 1,444,000, and 5,909,000 LU, respectively.
Fig 1.
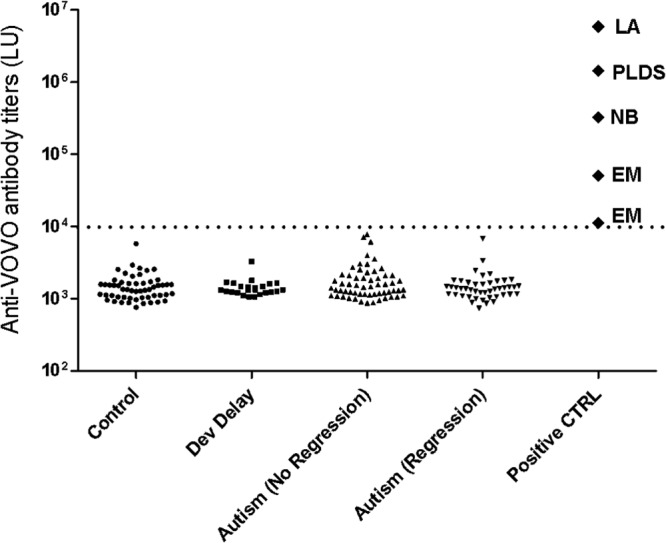
Anti-B. burgdorferi antibody titers in autistic children and controls. Anti-VOVO antibody levels were evaluated in 55 healthy children, 24 children with developmental delay, 59 children with autism without regression, and 45 children with autism with regression. Five additional positive-control (CTRL) B. burgdorferi-infected serum samples included samples from two patients with erythema migrans (EM), one with early neuroborreliosis (NB), one with post-Lyme disease syndrome (PLDS), and one with Lyme arthritis (LA). Each symbol represents a serum sample from an individual subject. The cutoff is shown by the stippled line, and the number of LU for each sample is shown on the y axis.
In comparison, the antibody levels detected in all the autistic children, developmentally delayed children, and healthy control children were seronegative, being below the cutoff value of 10,000 LU (Fig. 1). To independently validate the results, the FDA-approved C6 Lyme ELISA was employed to test the Lyme positive-control and 104 autism samples. Of the autism samples, 101 samples tested negative by C6 Lyme ELISA, and 3 samples had equivocal results. These 3 samples were negative by IgG Western blotting and therefore negative per CDC criteria (11). All the positive-control samples were positive by C6 testing. Based on these results, the upper limit for the 99.5% confidence interval of the proportion of autistic children seropositive for B. burgdorferi (0/104) is 0.0497. In conclusion, our study found no evidence of B. burgdorferi infection in children with autism.
ACKNOWLEDGMENTS
We thank the subjects who volunteered for this study.
This work was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, National Institute of Mental Health, and by the Division of Intramural Research National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Rapin I. 1997. Autism. N. Engl. J. Med. 337:97–104 [DOI] [PubMed] [Google Scholar]
- 2. Miles JH. 2011. Autism spectrum disorders—a genetics review. Genet. Med. 13:278–294 [DOI] [PubMed] [Google Scholar]
- 3. Bacon RM, Kugeler KJ, Mead PS. 2008. Surveillance for Lyme disease—United States, 1992–2006. MMWR Surveill. Summ. 57:1–9 [PubMed] [Google Scholar]
- 4. Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473 [DOI] [PubMed] [Google Scholar]
- 5. Burbelo PD, Issa AT, Ching KH, Cohen JI, Iadarola MJ, Marques A. 2010. Rapid, simple, quantitative, and highly sensitive antibody detection for Lyme disease. Clin. Vaccine Immunol. 17:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bransfield RC, Wulfman JS, Harvey WT, Usman AI. 2008. The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med. Hypotheses 70:967–974 [DOI] [PubMed] [Google Scholar]
- 7. Kuhn M, Grave S, Bransfield R, Harris S. 2012. Long term antibiotic therapy may be an effective treatment for children co-morbid with Lyme disease and autism spectrum disorder. Med. Hypotheses 78:606–615 [DOI] [PubMed] [Google Scholar]
- 8. American Psychiatric Association 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th ed American Psychiatric Association, Arlington, VA [Google Scholar]
- 9. Lord C, Rutter M, Le Couteur A. 1994. Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24:659–685 [DOI] [PubMed] [Google Scholar]
- 10. CDC 2012. Lyme disease (Borrelia burgdorferi). 2011 case definition. CDC, Atlanta, GA: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=752&DatePub=1/1/2011%2012:00:00%20AM [Google Scholar]
- 11. CDC 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 44:590–591 [PubMed] [Google Scholar]
- 12. CDC 2012. Lyme disease data. CDC, Atlanta, GA: http://www.cdc.gov/lyme/stats/index.html [Google Scholar]


