Abstract
The human innate immune response to pathogens is not fully effective and mature until well into childhood, as exemplified by various responses to Toll-like receptor (TLR) agonists in newborns compared to adults. To better understand the mechanistic basis for this age-related difference in innate immunity, we compared tumor necrosis factor alpha (TNF-α) production by monocytes from cord blood (CB) and adult blood (AB) in response to LAM (lipoarabinomannan from Mycobacterium tuberculosis, a TLR2 ligand) and LPS (lipopolysaccharide from Escherichia coli, a TLR4 ligand). LPS or LAM-induced TNF-α production was 5 to 18 times higher in AB than in CB monocytes, whereas interleukin-1α (IL-1α) stimulated similar levels of TNF-α in both groups, suggesting that decreased responses to LPS or LAM in CB are unlikely to be due to differences in the MyD88-dependent signaling pathway. This impaired signaling was attributable, in part, to lower functional TLR4 expression, especially on CD14+ CD16+ monocytes, which are the primary cell subset for LPS-induced TNF-α production. Importantly, the frequency of CD14+ CD16+ monocytes in CB was 2.5-fold lower than in AB (P < 0.01). CB from Kenyan newborns sensitized to parasite antigens in utero had more CD14+ CD16+ monocytes (P = 0.02) and produced higher levels of TNF-α in response to LPS (P = 0.004) than CB from unsensitized Kenyan or North American newborns. Thus, a reduced CD14+ CD16+ activated/differentiated monocyte subset and a correspondingly lower level of functional TLR4 on monocytes contributes to the relatively low TNF-α response to LPS observed in immunologically naive newborns compared to the response in adults.
INTRODUCTION
Human neonates are more susceptible than adults to infections, in part because they have limited antigenic experience. Innate cellular immunity is especially important in newborns since it can provide partial protection against pathogens prior to the development of adaptive immunity. Monocytes and macrophages, along with dendritic cells (DC), are antigen-presenting cells that play a crucial role in innate immunity to microbial infections and link innate with adaptive immunity. However, in some aspects, innate immunity in neonates is distinct from than in adults. Cord blood (CB) monocytes and DC have a reduced ability to process and present antigen to T cells (1) and produce less whole functional interleukin-12 (IL-12) than adult blood (AB) peripheral DC (2). These differences may contribute to functionally different responses to certain microbial molecules in newborns compared to the responses in adults. Lipopolysaccharide (LPS), an important component of Gram-negative bacteria, for example, stimulates less tumor necrosis factor alpha (TNF-α) production by CB whole mononuclear cells (MNCs) than by AB MNCs (3–5). This relatively low response to microbial molecules in newborns is not universal, since crude preparations of heat-killed gut-derived bacteria (Gram-positive or -negative bacteria [6], Streptococcus agalactiae [7], or Staphylococcus aureus [8]) stimulate similar levels of TNF-α, IL-6, and IL-12 in CB and in AB MNCs. Thus, the antigen-presenting cells of newborns have selective differences in response to microbial products.
Recognition of conserved microbial structures (pathogen-associated molecular patterns [PAMPs]) by antigen-presenting cells and other cells is mediated by various pattern recognition receptors (PRRs), including Toll-like receptors (TLR) (9). For example, LPS is recognized by TLR4/Myeloid differentiation molecule 2 (MD2) (10), whereas TLR2, along with coreceptor TLR1 or TLR6, recognizes peptidoglycan and various lipoproteins that are important components in the cell walls of Gram-positive bacteria, Mycobacteria, and protozoan parasites (11, 12).
TLRs and the type I IL-1 receptor (IL-1R, recognizing IL-1α and IL-1β) share a homologous intracellular domain activating a common intracellular signaling pathway that culminates in the translocation of nuclear factor-κB (NF-κB), a nuclear transcription factor that regulates the expression of proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α (reviewed in reference 13). Combinatorial sets of TLR and/or accessory molecules expand and define the microbial molecules recognized. The activation of monocytes by LPS involves several steps and molecular components. Briefly, LPS is first captured by LPS-binding protein (LBP) in the serum and then transported to soluble and membrane CD14 to be transferred and bound to MD2 and TLR4. The formation of this complex activates the intracellular adaptor molecule Myeloid differentiation molecule 88 (MyD88), which triggers a multimolecular cascade of signals that culminates with the translocation of NF-κB to the nucleus for cytokine transcription (14). The heterodimer TLR4/MD2 is critical for the recognition of various lipid A moieties of LPS and its analogues, such as lipid IVa and Rhodobacter sphaeroides diphosphoryl lipid A, by both human and rodent cells (15). Therefore, the regulation of TLR4 expression likely plays an important role in the magnitude of the response to LPS.
Monocytes engage pathogens or their products through PRRs, including TLR, resulting in the production of proinflammatory cytokines. Human monocytes can be classified into 3 subsets, defined by surface expression of the LPS receptor CD14 and the FcγIII receptor CD16—namely, CD14hi CD16− (monocytes expressing CD14 but not CD16), CD14hi CD16+ (monocytes expressing CD14 and CD16), and CD14+ CD16+ (monocytes expressing CD16 and lower CD14) cells, of which the latter two subsets are summarized as CD16+ monocytes and constitute about 10 to 20% of circulating monocytes. CD14+ CD16+ monocytes are poorly phagocytic, express high levels of HLA-DR and PRR, including TLR4, and produce large amounts of TNF-α, IL-1β, and reactive oxygen species in response to microbial insults, while the CD14hi CD16− monocytes are better phagocytic cells and produce limited proinflammatory cytokines (16–19). The proportion of CD16+-expressing monocytes expands in a number of inflammatory conditions (reviewed in reference 20), ranging from arthritis (21), inflammatory bowel disease (22), atherosclerosis (23), and sepsis (24) to infections such as HIV (25–27) and malaria (28, 29). Although TLR expression is known to differ among CD16+ or CD16− monocyte populations in peripheral blood (20), no study to date has documented any alteration of the monocyte subpopulations and the mechanisms underpinning changes in blood monocyte TLR in neonates versus adults.
Although previous studies have indicated reduced expression of TLR4 on cord blood cells compared to its expression in adults (30), other studies have not (31, 32), and the current study addresses these inconsistences. Based on observations that CB polymorphonuclear cells bound less labeled LPS than adult cells (33) and that CB MNCs produced less TNF-α in response to LPS than did adult MNCs (3–5), we hypothesized that CB cells could have reduced expression and/or function of TLR4. In this study, we correlated TLR4 expression on whole monocytes and their subsets (CD16+ or CD16−) with TNF-α production. The present study shows a reduced expression of TLR4 on CB monocytes compared to its expression in adults, according to flow cytometry analysis and inhibition experiments with diphosphoryl lipid A from R. sphaeroides (RsDPLA). This reduced TLR4 expression is a consequence of the reduced frequency of CD14+ CD16+ monocytes in CB compared to their frequency in AB, since this subset is mainly responsible for TLR4 expression and TNF-α production. Importantly, prenatal exposure to transplacentally transferred parasite antigens, as determined by in utero priming to these antigens, was associated with an expanded proportion of CD14+ CD16+ monocytes in cord blood. These CD14+ CD16+ monocytes had increased TLR4 expression and enhanced TNF-α production to LPS.
MATERIALS AND METHODS
Blood samples.
Cord blood (CB) samples were obtained in sterile heparinized tubes from normal-term and healthy deliveries at MacDonald Women's Hospital of University Hospitals of Cleveland and from IMSS Hospital de Ginecobstetricia Luis Castelazo Ayala in Mexico City and were usually processed within 4 h of delivery. CB samples were also obtained from offspring of malaria-infected and uninfected women during pregnancy who delivered at the Msambweni District Hospital, Coast Province, Kenya. Informed consent was obtained from each individual (including healthy adults), and the IRBs of University Hospitals of Cleveland, IMSS in Mexico City, and Ministry of Health in Kenya approved the protocol and procedures.
Cell cultures and activation.
Initial experiments were performed by stimulating whole blood diluted 1:2 with RPMI and supernatants harvested at 6 h for cytokine detection. In preliminary studies, the maximum LPS-induced TNF-α production occurred at approximately 6 h (data not shown). Mononuclear cells (MNCs) were prepared by density-gradient separation (Ficoll-Hypaque; Pharmacia, Uppsala, Sweden). Separations were performed twice with CB samples to remove excess erythrocytes (34), and the viability was >95% as determined by trypan blue exclusion. CD14+ cells were enriched by immunomagnetic negative selection (Miltenyi MACS kit for monocyte enrichment; Miltenyi Biotec, Auburn CA) to >68% CD14+ cells. The major contaminants of CD14+-enriched cells were platelets and erythrocytes, and <5% were T cells (CD3+) and/or B cells (CD19+) as evaluated by flow cytometry. Mononuclear cells or enriched CD14+ monocytes were cultured at 1 × 106 or 0.4 × 106 cells/well, respectively, in 48-well tissue culture plates with complete RPMI (C-RPMI) that included 10% heat-inactivated (56°C for 30 min) autologous plasma, 2 mM l-glutamine, 20 mM HEPES, and 80 μg/ml gentamicin.
Various molecules were added separately to cell cultures at the following concentrations: 25 ng/ml of IL-1α (R&D Systems, Inc., Minneapolis, MN); 1 ng/ml of repurified sonicated Escherichia coli LPS 055:B5 (Sigma, St. Louis MO; donated by D. T. Golenbock, University of Massachusetts, Worcester, MA); 1 μg/ml of sonicated lipoarabinomannan (LAM) (mannose-capped LAM [Man-LAM] derived from Mycobacterium tuberculosis H37Rv, 1.65 ng endotoxin/1 mg of the LAM preparation; kindly proved by J. T. Belisle, Colorado State University). Culture supernatants were collected after 6 h and immediately frozen at −80°C. The LPS preparation at the concentrations used activated TLR4- and not TLR2-transfected CHO-K1 cells (kindly provided by D. T. Golenbock), demonstrating the specificity of the LPS preparation used for TLR4 stimulation (35). The LAM activated only TLR2- and not TLR4-transfected CHO-K1 cells, indicating the specificity of LAM for TLR2 (data not shown). In some experiments, cells were pretreated for 30 min with various concentrations of the TLR4-specific antagonist diphosphoryl lipid A (sonicated) from R. sphaeroides (RsDPLA) (kindly donated by Nilofer Qureshi, University of Kansas City, Kansas City, MO) prior to the addition of LPS. RsDPLA failed to block Pam3Cys (TLR2 ligand)-induced TNF-α production by MNCs from adult and cord blood, demonstrating its specificity for TLR4 compared to TLR2 (data not shown).
Frozen cord blood mononuclear cells were stimulated with recombinant malaria blood stage antigens (recombinant Plasmodium falciparum MSP1-42 or peptide corresponding to T cell epitopes from P. falciparum P0 ribosomal phosphoprotein [PfP0]), crude extract of Schistosoma mansoni, and Brugia malayi adult worm antigens in cultures at 2 × 106/ml, and cytokines were determined in supernatants, as previously described (36).
FACS analysis.
Aliquots of mononuclear cells or enriched monocytes (0.3 × 106 to 2 × 106) were incubated with 100 μg human IgG for 15 min at room temperature for Fc receptor blocking before staining with saturating amounts of isotype control antibodies (γ2a-fluorescein isothiocyanate [FITC] and γ2a-phycoerythrin [PE] antibodies; eBioscience, San Diego, CA) or antibodies directed to the desired cell surface markers (allophycocyanin [APC]-conjugated CD14 [BD-Pharmingen, San Jose, CA], FITC-conjugated CD45 [BD-Pharmingen], PE-conjugated CD14 or FITC-CD14 [BD-Pharmingen], CD16-FITC [BD Pharmingen], FITC-conjugated TLR2 [clone 2.1; e-Bioscience], and PE-conjugated TLR4 [clone HTA125; Santa Cruz Biotechnology, Inc., Santa Cruz, CA] antibodies). The percentages of monocytes were assessed using the antibody pair CD14-APC/CD45-FITC. To determine TLR4 expression on monocyte subsets, 100 μl of whole blood was incubated with unlabeled TLR4 antibody (clone HTA125), and then the red blood cells were lysed with fluorescence-activated cell sorting (FACS) lysing solution (Becton, Dickinson), washed, and then incubated with tricolor-labeled anti-mouse IgG (Caltag, Burlingame, CA). The cells were washed again and incubated with saturating amounts of FITC–anti-CD16 and APC–anti-CD14 (clones 3G8 and Mϕp9, respectively; Becton, Dickinson, San Jose, CA) or CD14-PE. Stained cells were fixed with 1% paraformaldehyde prior to FACS analysis with a Becton, Dickinson FACScalibur flow cytometer, using CellQuest (BD-Bioscience software) for analysis. At least 10,000 cells were analyzed per sample (for purified monocytes) and 30,000 to 100,000 for whole MNCs. Some cytometry graphics were done with WinMDI software, version 2.9 (Joe Trotter, The Scripps Research Institute).
Cytokine analysis.
The TNF-α levels in the supernatants were determined by sandwich enzyme-linked immunosorbent assay (ELISA), using paired antibodies from Endogen (clones 2TNF-H34A and 2TNF-H33) according to the manufacturer's recommended protocols, and the detection limit level was 24 pg/ml. Cytokine levels were calculated by subtracting the spontaneous production from the LPS-induced TNF-α production.
Assessment of intracellular TNF-α by flow cytometry.
MNCs obtained from adult or cord blood were stimulated with 1 ng/ml LPS in RPMI with 10% fetal bovine serum (1 × 106 cells/ml in 24-well tissue culture Costar plates) for 5 h in the presence of 10 μg/ml of brefeldin A (Sigma, St. Louis, MO). Then, cells were harvested in FACS polypropylene tubes (Falcon; Becton, Dickinson) and cell membranes were labeled with saturating amounts of anti-CD14–APC, anti-CD16–FITC, or anti-TLR4–PE antibody for 15 min at room temperature. After washing cells with 1 ml PBA (phosphate-buffered saline [PBS] 0.15 M plus 1% fetal bovine serum plus 0.1% NaN3), they were permeabilized with 1 ml of 1× FACS Perm2 permeabilizing solution (Becton, Dickinson, San Jose CA) for 15 min, followed by centrifugation at 300 × g. The supernatant was decanted, and cells resuspended in the remainder of the permeabilizing solution. Anti-TNF-α–PE antibody was added, and the cells were incubated for 30 min at room temperature in the dark. Then, cells were washed with 1 ml PBA, resuspended in 500 μl of 1% paraformaldehyde in PBS, and kept at 4°C in the dark to be further analyzed in a flow cytometer.
Statistical analysis.
LPS-driven TNF-α production was log-normally distributed, and therefore, the data were log-transformed prior to statistical analysis. The mean fluorescent intensity for CD14+ or TLR4 staining was normally distributed, and therefore, it was not transformed prior to analysis. Comparisons between CB and AB cell TNF-α production and cell surface expression of TLR2, TLR4, and CD14 were made using the Student t test or Mann-Whitney U test. Simple linear regression was performed with log-transformed TNF-α cytokine levels. Differences were considered significant when the P value was <0.05. Statistical analysis was performed using SPSS statistical software (version 10.0; SPSS, Inc., Chicago, IL).
RESULTS
Whole cord blood and CB CD14+ monocytes produce less LPS and LAM-induced TNF-α than AB cells.
To assess LPS-induced TNF-α production in cord (CB) compared to that in adult blood (AB), we first examined the responses in whole blood to various concentrations of LPS (Fig. 1A) following 6 h of activation (found to be the optimal time point for LPS-induced TNF-α for both CB and AB [data not shown]). CB secreted lower levels of TNF-α than AB in response to all concentrations of LPS tested (0.1 to 20 ng/ml). The CB mononuclear cell (MNC) fraction also produced less LPS-induced TNF-α than AB MNCs (Fig. 1B, left). Enriched CD14+ monocytes from CB produced less LPS- and LAM-induced TNF-α than those from AB (Fig. 1C). Of note, MNCs from adult and cord blood spontaneously released similar amounts of TNF-α (geometric means, 38.9 ng/ml and 21.9 ng/ml, respectively). Similarly, enriched CD14+ monocytes from adult and cord blood released similar amounts of TNF-α in the absence of stimulation (geometric means, 14.3 ng/ml and 10.4 ng/ml, respectively).
Fig 1.
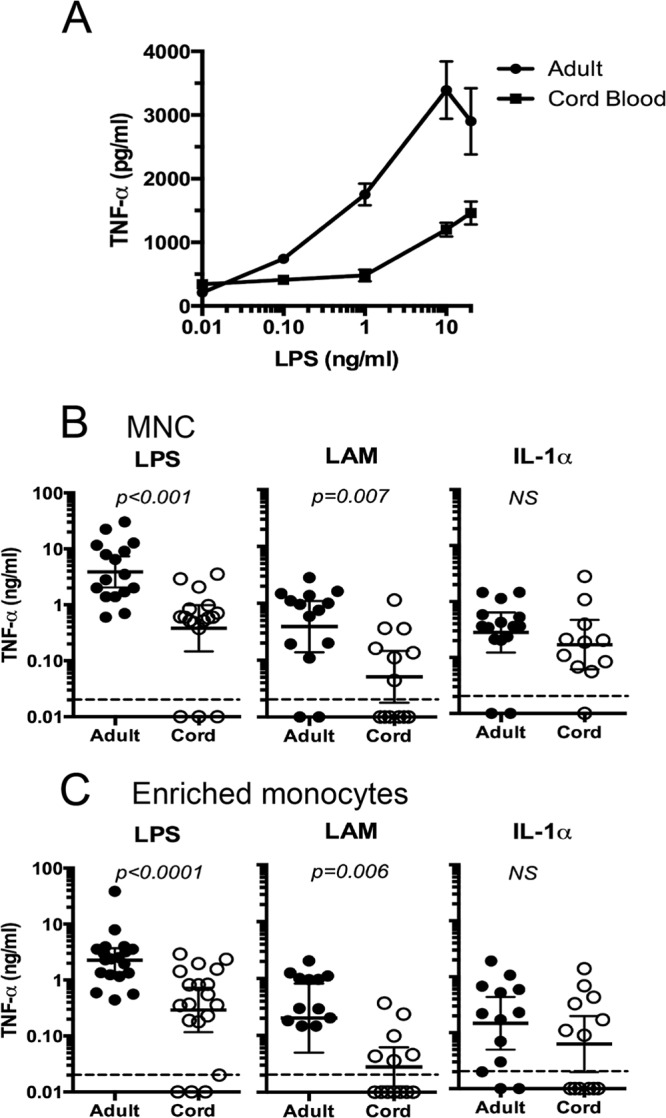
Cord blood (CB), CB MNCs, and CB enriched monocytes produced less TNF-α than adult blood (AB), AB MNCs, and AB enriched monocytes in response to LPS. (A) Mean net LPS-induced TNF-α production by whole cord (n = 6) or adult (n = 3) blood diluted 1:2 with RPMI in 6-h cultures. Values are means ± standard deviations (SD), and differences were significant at a P value of <0.05. (B, C) Results of LPS, LAM, and IL-1α stimulation of TNF-α production by cord blood mononuclear cells (CBMCs) and adult PBMCs (B) or enriched CD14+ monocytes (C). Net TNF-α production (antigen/cytokine-induced minus spontaneous) was measured 6 h after stimulation. Mononuclear cells at a concentration of 1 × 106 cells/ml or enriched monocytes (0.4 ×106/ml) were cultured in the presence of 1 ng/ml of LPS, 1 μg/ml Man-LAM, or 25 ng/ml rIL-1α. Each data point represents TNF-α production from one individual. Bars indicate geometric means. Values of <0.02 ng/ml were undetectable. Differences in TNF-α production by CB and AB cells were determined by Student's t test of log-transformed values.
To evaluate whether reduced production of TNF-α was a consequence of impaired signal transduction, we analyzed TNF-α production following stimulation of MNCs or CD14+ monocytes from CB and AB with IL-1α. IL-1 signals through IL-1R, which is distinct from the TLRs and yet shares a similar intracellular domain and common signaling pathways (37). Therefore, TNF-α production following IL-1α stimulation is TLR independent but MyD88 dependent. IL-1α induced similar TNF-α production between CB and adult MNCs and CD14+ monocytes (Fig. 1B and C). Thus, the low response of CB MNCs and monocytes to LPS and LAM was unlikely to be due to differences in signaling pathways.
A reduced frequency of CD14+ monocytes could also contribute to the reduced response to LPS seen in whole CB (Fig. 1A). We therefore analyzed the frequency of CD14+ monocytes in MNCs from CB and AB by flow cytometry. Although there was considerable variation in the number of CD14+ cells among samples, CB had equivalent numbers of CD14+ cells (median, 19.3%; range 2.5 to 28.5%) compared to adult MNCs (median, 12.5%; range 2.0% to 25.2%; P = 0.42). The CD14 mean fluorescence intensity (MFI) was also equivalent between monocytes isolated from CB (482 ± 178 [mean ± standard deviation) and AB (561 ± 295) (P = 0.39). Thus, differences between CB and AB in the levels of LPS-driven TNF-α cannot be attributed to differences in the proportion of CD14+ monocytes.
CB monocytes express less TLR4 but not less TLR2 than AB monocytes.
Because of the lower response to LPS in CB monocytes, there may be impaired formation of the TLR4 receptor complex in cord blood relative to its formation in adult blood monocytes, due to reduced expression of TLR4. Histograms of TLR2 and TLR4 surface staining of monocytes from representative CB and AB samples are shown in Fig. 2A. The MFI for TLR4-specific staining in CB monocytes was 13-fold lower than the TLR4-specific MFI for adult monocytes (Fig. 2B) (P < 0.001). The levels of TLR4 expression on enriched CB and AB monocytes combined correlated with the amount of LPS-induced TNF-α production (r2 = 0.43, P = 0.001; n = 12 CB and n = 9 AB samples).
Fig 2.
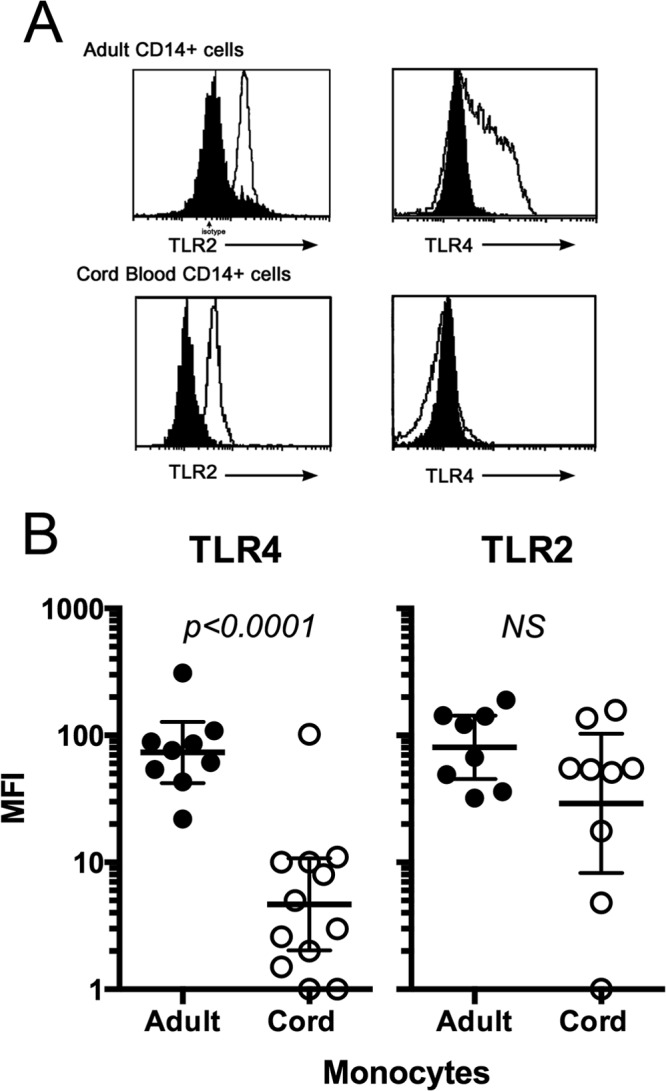
Ex vivo TLR4 and TLR2 expression on CD14+ cord blood versus adult blood monocytes, assessed by flow cytometry. (A) Representative flow cytometry histograms for TLR2 and TLR4 gated on CD14+ lymphocytes from freshly prepared PBMCs. The dark histogram represents the isotype control. (B) The relative mean fluorescence intensities (MFI) for TLR4 for individual adult (closed circles) and cord (open circles) blood samples are shown. Values were calculated as net MFI or MFITLR4 or MFITLR2 minus MFIisotype control. Bars indicate means ± SD. There was a significant difference in net MFI between adults and neonates for TLR4 (P < 0.001) but not for TLR2 (P = 0.12).
TLR2 tended to be lower on CB monocytes than on AB monocytes, but the difference was not statistically significant (P = 0.12) (Fig. 2B). TLR2 expression did not correlate with LAM-induced TNF-α production for CB and adult monocytes (r2 = 0.03, P = 0.5).
Lower concentrations of the TLR4-specific inhibitor RsDPLA are needed to inhibit LPS-induced TNF-α production in CB than in AB monocytes.
To confirm the decreased functional activity of TLR4 on monocytes, we used Rhodobacter sphaeroides diphosphoryl lipid A (RsDPLA), which is a specific competitive inhibitor of LPS-induced activation that engages TLR4/MD2 in the LPS receptor complex on the cell surface (38). Therefore, the concentration of RsDPLA needed to inhibit LPS-induced TNF-α production would be dependent on functional TLR4 expression. Enriched CD14+ monocytes from CB and AB were incubated with LPS in the absence or presence of various concentrations of RsDPLA, and the TNF-α concentrations in the supernatants were analyzed (Fig. 3). Ten- to 50-fold less RsDPLA was required by CB than by adult CD14+ monocytes to achieve greater than 90% inhibition of LPS-induced TNF-α production (Fig. 3). Thus, the ability to form a functional TLR4-dependent complex is reduced on CB compared to the TLR4 complex formation on AB monocytes and contributes to the lower LPS-induced TNF-α production by CB monocytes.
Fig 3.
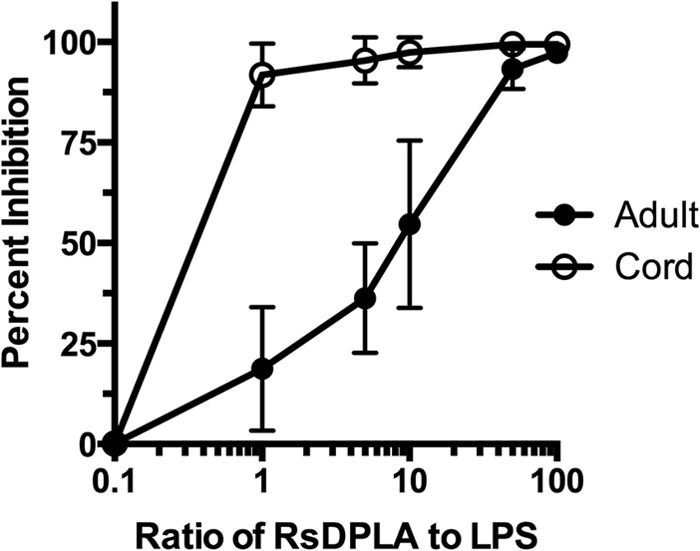
RsDPLA inhibition of LPS-induced TNF-α by enriched monocytes from cord (n = 5) and adult (n = 6) blood samples. Cells were preincubated for 30 min with various amounts of RsDPLA (0 to 100 ng, in ratios as shown) prior to the addition of 1 ng LPS, and TNF-α levels were measured in supernatants following 6 h of culture. The percent inhibition was calculated based on the level of cytokine production induced by LPS in the absence of RsDPLA. Each point represents the mean (±SD) percentage of inhibition of cytokines produced by monocytes obtained from the tested individual. Differences between cord and adult blood cells were significant (P < 0.05) at 1:1, 5:1, and 10:1 ratios.
Newborns have a lower frequency of CD14+ CD16+ monocytes than adults that contributes to lower LPS-induced TNF-α production.
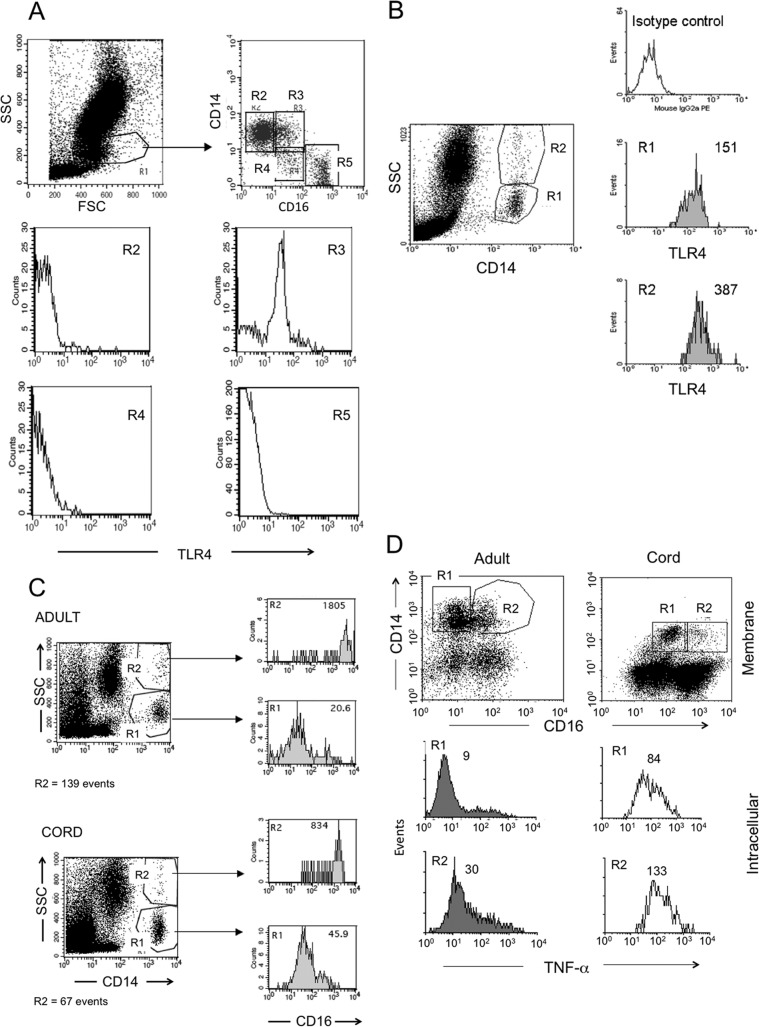
The expression of CD16+ on CD14+ monocytes has been associated with activation and increased capacity to produce TNF-α (16–18). Therefore, we explored the possibility that the reduced LPS responsiveness in CB may result from a decreased proportion of CD14+ CD16+ monocytes in CB compared to the amount in AB. As previously reported (39), TLR4 expression was higher on CD14+ CD16+ monocytes than on CD14+ CD16− monocytes (Fig. 4A). This pattern was consistent in both adult and cord blood monocytes. CD14+ CD16+ monocytes have intracellular pools of CD16 and show a granular appearance in flow cytometry (27, 40) that corresponds with increased expression of CD16+ (Fig. 4B and C, R2, high side scatter [SSC] and CD14+). These granular, CD14+ monocytes showed higher TLR4 expression (Fig. 4B) and produced more TNF-α (Fig. 4D, R2). No significant difference in MFI for intracellular TNF-α was observed when comparing cord and adult blood cells, indicating an equivalent production of cytokine per cell. Although the proportions of MNCs that are CD14+ are similar between cord and adult blood (see above), the percentage of CD14+ monocytes that were CD16+ was 2-fold lower in CB (mean, 8.7%; range, 4.3% to 22%) than in adult blood (mean, 19.3%; range, 7.7 to 27.1%; P < 0.001) (Fig. 5). Thus, the reduced LPS-induced TNF-α production in cord blood results, in part, from reduced numbers of CD14+ CD16+ monocytes.
Fig 4.
(A, B, D) CD14+ CD16+ (A) and CD14+ granular (B) cells express more TLR4 than CD14+ CD16− and lower granular cells and produce more LPS-induced TNF-α than CD14+ CD16− monocytes (D). (A) Fresh whole blood from an adult donor was stained with CD16-FITC, CD14-PE, and TLR4-TC, monocytes initially gated by morphology (forward scatter [FSC] versus SSC, R1) were plotted for CD16 and CD14 (upper right) to select distinct regions (R2, R3, R4, and R5), and each region was analyzed for TLR4 expression (lower histograms; the highest TLR4 expression was in R3). Similar staining patterns were found in cord blood cells, with higher TLR4 on CD14+ CD16+ monocytes. (B) TLR4 expression on low granular CD14+ (R1) and high granular CD14+ (R2) monocytes in fresh whole blood from an adult. (C) Granular CD14+ (R2) monocytes express higher levels of CD16+ than low granular CD14 (R1) monocytes from adult and cord whole blood. (D) Mononuclear cells were obtained from adult and cord blood, stimulated with 1 ng/ml LPS in the presence of brefeldin A, and incubated for 5 h; CD14 and CD16 were labeled on membranes and intracellularly for TNF-α, and cells were analyzed by flow cytometry. CD14+ CD16+ monocytes produced more TNF-α than CD14+ CD16− monocytes in response to LPS, as shown for adult and cord blood cells. Numbers on histograms represent geometric mean fluorescence intensity for the assessed molecule in the indicated region. Each of the flow cytometry experiments described above was performed on samples from at least 3 individuals with similar results.
Fig 5.
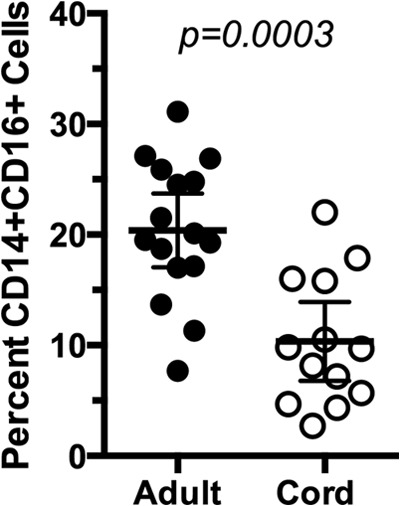
Cord blood has a lower percentage of CD14+ CD16+ cells than adult blood. Fresh blood samples from adult and cord blood were stained for CD14 and CD16 and analyzed by FACS as shown in Fig. 4D. The percentages of CD14+ CD16+ monocytes (corresponding to events in R2 in Fig. 4D) with respect to the total CD14 cells are shown. Each dot represents one individual, and horizontal lines correspond to the means of the groups, which are significantly different (P < 0.001, Mann-Whitney U test).
CB from newborns exposed to parasite antigens in utero has an increased proportion of CD14+ CD16+ monocytes and greater LPS-induced TNF-α production than CB from immunologically naive newborns.
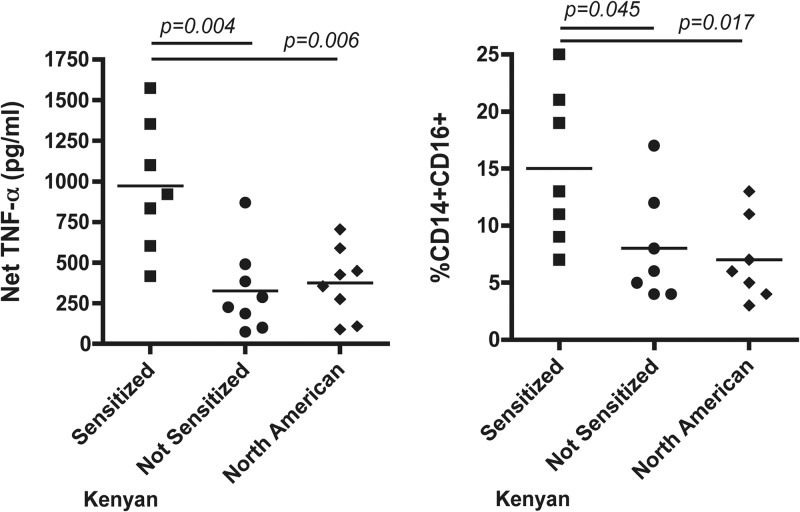
To examine the hypothesis that the low proportion of the CD14+ CD16+ monocyte subset in cord blood compared to the proportion of this subset in adult blood arises from a lack of in utero exposure to proinflammatory mediators, we examined LPS-induced TNF-α production in CB MNCs from Kenyan offspring whose mothers had been infected with malaria and/or intravascular helminth infections (schistosomiasis and/or lymphatic filariasis) during pregnancy and who became primed to soluble antigens corresponding to these parasites in utero by the production of antigen-driven IFN-γ, IL-13, IL-5, and/or IL-10, as previously described (36, 41). For comparison, CB MNCs from Kenyan newborns without known maternal infections were examined and failed to respond to parasite antigens in vitro (Table 1). Cord blood MNCs from North American newborns were also examined. LPS-induced TNF-α production was greater in cultures of CB from Kenyan newborns sensitized to parasite antigens than in unsensitized Kenyan CB MNCs and North American CB MNCs (Fig. 6). This corresponded to an increased proportion of CD14+ CD16+ cells in the CD14+ monocyte population compared to the proportions of this subset in unsensitized Kenyan and North American CB samples (Fig. 6). There was no correlation between the amount of LPS-induced TNF-α production and the percentage of CD14+ CD16+ cells (r2 = 0.13, P = 0.34); however, when the actual numbers of CD14+ CD16+ cells were calculated, the numbers correlated significantly with LPS-induced TNF-α (r2 = 0.53, P = 0.01). Thus, the impaired LPS-driven TNF-α response in many cord blood samples was due, in part, to smaller numbers of activated CD14+ CD16+ monocytes.
Table 1.
Presence or absence of parasite antigen-induced cytokine production by CB mononuclear cells from offspring of women infected or not with common intravascular parasitic infection during pregnancy
| Source of CB | Subject | Cytokine(s) induced by antigen toa: |
||
|---|---|---|---|---|
| Malariab | Schistosomec | Filariasisd | ||
| Kenya (sensitized) | 007 | IFN-γ/IL-13/IL-5 | ||
| 044 | IFN-γ | |||
| 061 | IFN-γ/IL-13 | IL-5/IL-13 | ||
| 195 | IL-13/IL-10 | |||
| 110 | IFN-γ/IL-13/IL-10 | IFN-γ/IL-13 | ||
| 211 | IFN-γ | IFN-γ/IL-10 | ||
| 024 | IFN-γ | |||
| 039 | IFN-γ/IL-13/IL-10 | IFN-γ/IL-13 | ||
| Kenya (not sensitized) | 016 | |||
| 049 | ||||
| 276 | ||||
| 082 | ||||
| 089 | ||||
| 055 | ||||
| 108 | ||||
| 067 | ||||
| North America | 1 | |||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
Positive response was assessed by ELISA and defined as cytokine production that was increased >2-fold over the background level.
The malaria antigens used were P. falciparum MSP1-42 and PfP0. If only one cytokine was detected, a positive response was defined as >2-fold production in response to two or more malaria blood stage antigens.
The schistosome antigen was crude soluble adult worm extract from Schistosoma mansoni (SWAP).
The filarial antigen was a crude soluble extract of Brugia malayi.
Fig 6.
(Left) LPS-induced TNF-α production in CBMCs from Kenyan newborns sensitized to parasite antigens in utero or not sensitized and from North American newborns. (Right) Frequency of the CD14+ CD16+ subset from the same cord blood samples. Aliquots of frozen CBMCs from the same Kenyan newborns were evaluated for priming to parasite antigen as shown in Table 1. Analysis of the LPS-induced TNF-α production was performed as described in the legend to Fig. 1, and flow cytometry for CD14+ CD16+ cells was performed similarly to that described in the legends to Fig. 4 and 5, with the addition of vital staining because cryopreserved CBMCs were used. Each spot indicates separate samples. Significance was determined with the Mann-Whitney U test.
DISCUSSION
Human neonates are particularly susceptible to a wide variety of infections. This susceptibility is considered to reflect inexperience or functional differences in innate and adaptive immunity, but the nature of these differences is not well understood. The innate immune responses direct the subsequent adaptive immune response after integrating information from TLRs, other pattern recognition receptors, and various environmental cues. The current results show that cord blood mononuclear cells have much lower LPS-induced TNF-α production than adult blood MNCs. This reduced response is associated with functionally lower TLR4 expression, particularly on activated or patrolling CD14+ monocytes, which also express FcγRIII or CD16+ and large amounts of TLR4 and whose numbers are lower in most newborns than in adults.
One potential reason for the lower frequency of CD14+ CD16+ monocytes and reduced LPS-induced TNF-α production is lack of prenatal exposure to proinflammatory mediators that might cross the placenta and/or stimulate the placental trophoblasts to produce proinflammatory cytokines released in the fetal circulation. This concept is supported by observations that offspring of mothers with chronic intravascular parasitic infections, such as malaria, schistosomiasis, and lymphatic filariasis, and whose fetal lymphocytes acquired antigen-specific recall responses to soluble antigens from these parasites (a potential marker for prenatal exposure to proinflammatory mediators), developed enhanced LPS responsiveness and expanded populations of CD14+ CD16+ monocytes. Malaria is a potent inducer of TNF-α (42), and chronic schistosomiasis results in elevation of plasma endotoxin (43).
Several studies have also shown lower TNF-α response to LPS in cord blood cells than in adult blood cells; however, the reasons for such different responses vary among studies (3–5). Some studies have reported lower levels of LPS binding protein (LBP; a soluble acute-phase protein produced by liver in response to infection that binds LPS and markedly enhances its affinity for the TLR4 complex) in cord blood than in adult blood (33, 44); however, this observation was not reproduced in another study (45). Lower levels of LBP or other soluble factors in cord blood are unlikely to be a dominant factor, since other authors have shown that LPS can active monocytes under serum-free conditions (46, 47). Indeed, a study by Kato and colleagues showed that, in human peripheral blood mononuclear cells (PBMCs), only the interferon regulatory transcription factor 3 (IRF-3)-inducible genes (but not the NF-κB-inducible genes that include the TNF-α gene) are strictly LBP dependent upon LPS stimulation (48).
Other investigators postulated that cord plasma contains suppressive factors, notably adenosine, that could account for the impaired LPS (and TLR ligand)-induced TNF-α production (31, 49). In the present work, we performed our LPS activation experiments with adult or cord blood mononuclear cells cultured in RPMI supplemented with 10% autologous plasma, so it is possible that some of the observed lower response to LPS in cord blood cells was due to the presence of soluble inhibitory factors. However, the ability for this LPS-induced hyporesponsiveness to be partially reversed in offspring exposed to parasite antigens and/or inflammatory mediators in utero suggests that this suppression may not be a dominant regulatory factor.
Other studies have proposed that the relatively low response to LPS in newborns arises from decreased expression of MyD88 in cord blood samples, affecting stimulation by several distinct TLR ligands (32, 50). Type I IL-1 receptor uses the same MyD88-dependent signal transduction pathway as TLR2 and TLR4 (51), and we found equivalent responses to IL-1α, in terms of TNF-α production (Fig. 1), by adult and cord blood cells, suggesting that other upstream differences between cord and adult blood monocytes must exist.
We also observed reduced LAM-induced TNF-α production by cord blood monocytes compared to that in adult blood monocytes; however, this reduced responsiveness did not correlate with lower TLR2 expression. This lack of correlation may occur because LAM is recognized by a receptor complex that includes TLR1, the mannose receptor, and DC-SIGN (12, 52, 53) and whose expression was not examined in the present study.
Previous studies have demonstrated equivalent TLR4 expression on CB cells and AB cells (31, 32), whereas others have found different TLR4 expression levels on the surfaces of CB monocytes from both term and preterm newborns compared to its expression on monocytes from adults (30). The anti-TLR4 monoclonal antibody HTA-125, used in the current study and most other studies for TLR staining, has fairly low affinity and may be partially inhibited by different unspecific mouse or human IgG isotypes (54). This may account for some of the variability observed in these studies. Because of this limitation, we performed LPS blocking experiments with RsDPLA, a specific inhibitor of the TLR4 complex, to independently evaluate functional recognition and activation with LPS. RsDPLA engages the complex TLR4/MD2-targeting MD2 molecule by the lipidic moiety of RsDPLA, which has 5 acyl chains in its structure (38) and is orthologous to LPS (with 6 acyl chains) but is nonactivating because it fails to induce the conformational change in TLR4 that is necessary to dimerize and trigger the intracellular signaling (55). The 10- to 50-fold greater inhibition by RsDPLA of LPS-induced TNF-α for newborns than for adults (Fig. 3) confirmed the importance of the impaired functional TLR4 receptor complex in newborns. We cannot exclude the possibility that there may be differences in the affinities of the TLR4 receptor complex to LPS that may be developmentally regulated; however, this seems unlikely, as there is no evidence of posttranslational modification of the receptor complex itself (56).
Different authors have shown that the response to LPS depends on the level of TLR4 expression on monocytes. For example, Kalis et al. (57) produced, by genetic engineering and backcrossing, different mouse strains expressing distinct levels of TLR4 (none, medium, and high) and found a direct correlation between the level of TLR4 expression and the degree of cytokine response to LPS. Similar findings are supported by other murine studies (58–60). In humans, it has been observed that a low response to LPS in terms of inflammatory cytokine production correlated with a lower expression of TLR4 in the blood monocytes of patients with chronic kidney disease (61).
Our studies show higher TLR4 expression on CD14+ CD16+ activated granular monocytes, and they are the main producers of TNF-α (Fig. 4), a finding consistent with some studies (17, 62) but not others (39, 63). This difference may depend on the gating, the fluorochrome used, and compensation, since both CD14+ and CD16+ expression can vary greatly and may not always form a clearly demarked group (64). This is the reason we also used granularity as a marker for CD14+ CD16+ monocytes. Importantly, we show that CD14+ CD16+ monocytes, which express higher TLR4 and produce more TNF-α, are reduced 2-fold in cord blood compared to their level in adult blood (Fig. 5). Similarly, the CD11c+ CD16+ subset of dendritic cells is reduced in cord blood compared to its proportion in adult blood (65).
In summary, we show a lower level of TLR4 expression on cord blood monocytes than on adult blood monocytes and that the CD14+ CD16+ monocyte subset expressing the highest levels of TLR4 is underrepresented in cord blood samples. We conclude that this reduced TLR4 expression contributes to the lower response to LPS observed in cord blood monocytes. Lower TLR4 surface expression on neonatal monocytes may modulate innate cellular immunity to endotoxin released from Gram-negative bacteria and account for the frequent lack of fever and other clinical symptoms often observed in neonates in the early stages of bacterial sepsis. Low TLR4 expression could make newborns more susceptible to infections but, at the same time, protect them from the deleterious effects of an overwhelming inflammatory reaction while they are acquiring adaptive immune responses to many new organisms. Increasing our knowledge about innate immunity in the human neonate may help to develop better adjuvants to augment the neonatal responses to vaccine antigens and understand the spectrum of disease during infancy and early childhood.
ACKNOWLEDGMENTS
We thank Doug Golenbock for generously providing repurified LPS, as well as the cell lines transfected with TLR2 and TLR4, Nilofer Qureshi for providing the diphosphoryl lipid A from Rhodobacter sphaeroides, and John Belisle for providing the Man-LAM reagent. We are also are grateful to Eric Pearlman and Alejandro Zentella-Dehesa for their support and critical review of the manuscript. Our acknowledgment to Janette Furuzawa for providing blood samples from healthy adult donors and to nurses from MacDonald Hospital in Cleveland and to Pablo Domínguez and Ambar López in Mexico for their help in collecting the cord blood samples.
Financial support was provided by National Institutes of Health grant AI33061.
Footnotes
Published ahead of print 17 April 2013
REFERENCES
- 1. Velilla PA, Rugeles MT, Chougnet CA. 2006. Defective antigen-presenting cell function in human neonates. Clin. Immunol. 121:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141–2146 [DOI] [PubMed] [Google Scholar]
- 3. Hebra A, Kramer R, Johnson JT, Wagner CL, Eicher DJ, Cook J. 1999. Detection of intracellular tumor necrosis factor alpha in stimulated fetal cells. J. Surg. Res. 82:300–304 [DOI] [PubMed] [Google Scholar]
- 4. Hodge S, Hodge G, Flower R, Han P. 2001. Cord blood leucocyte expression of functionally significant molecules involved in the regulation of cellular immunity. Scand. J. Immunol. 53:72–78 [DOI] [PubMed] [Google Scholar]
- 5. Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. 2000. Intracellular cytokine profile of cord blood T-, and NK- cells and monocytes. Haematologica 85:675–679 [PubMed] [Google Scholar]
- 6. Karlsson H, Hessle C, Rudin A. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688–6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berner R, Welter P, Brandis M. 2002. Cytokine expression of cord and adult blood mononuclear cells in response to Streptococcus agalactiae. Pediatr. Res. 51:304–309 [DOI] [PubMed] [Google Scholar]
- 8. Scott ME, Kubin M, Kohl S. 1997. High level interleukin-12 production, but diminished interferon-gamma production, by cord blood mononuclear cells. Pediatr. Res. 41:547–553 [DOI] [PubMed] [Google Scholar]
- 9. Takeda K, Akira S. 2003. Toll receptors and pathogen resistance. Cell. Microbiol. 5:143–153 [DOI] [PubMed] [Google Scholar]
- 10. Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752 [PubMed] [Google Scholar]
- 11. Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419–33425 [DOI] [PubMed] [Google Scholar]
- 12. Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748–6755 [PubMed] [Google Scholar]
- 13. Takeda K, Kaisho T, Akira S. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376 [DOI] [PubMed] [Google Scholar]
- 14. Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151 [DOI] [PubMed] [Google Scholar]
- 15. Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354–359 [DOI] [PubMed] [Google Scholar]
- 16. Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. 2002. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 168:3536–3542 [DOI] [PubMed] [Google Scholar]
- 17. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. 2010. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. 1996. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood 87:373–377 [PubMed] [Google Scholar]
- 19. Serbina NV, Cherny M, Shi C, Bleau SA, Collins NH, Young JW, Pamer EG. 2009. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J. Immunol. 183:2678–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziegler-Heitbrock L. 2007. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 81:584–592 [DOI] [PubMed] [Google Scholar]
- 21. Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H. 2002. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 46:2578–2586 [DOI] [PubMed] [Google Scholar]
- 22. Koch S, Kucharzik T, Heidemann J, Nusrat A, Luegering A. 2010. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin. Exp. Immunol. 161:332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merino A, Buendia P, Martin-Malo A, Aljama P, Ramirez R, Carracedo J. 2011. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J. Immunol. 186:1809–1815 [DOI] [PubMed] [Google Scholar]
- 24. Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. 1993. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 82:3170–3176 [PubMed] [Google Scholar]
- 25. Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. 1991. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J. Clin. Invest. 87:1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D. 2006. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344:267–276 [DOI] [PubMed] [Google Scholar]
- 27. Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. 1997. Unique monocyte subset in patients with AIDS dementia. Lancet 349:692–695 [DOI] [PubMed] [Google Scholar]
- 28. Chimma P, Roussilhon C, Sratongno P, Ruangveerayuth R, Pattanapanyasat K, Perignon JL, Roberts DJ, Druilhe P. 2009. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 5:e1000631. 10.1371/journal.ppat.1000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogonda LA, Orago AS, Otieno MF, Adhiambo C, Otieno W, Stoute JA. 2010. The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect. Immun. 78:2173–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, Pollak A, Spittler A. 2005. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr. Res. 58:121–124 [DOI] [PubMed] [Google Scholar]
- 31. Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173:4627–4634 [DOI] [PubMed] [Google Scholar]
- 32. Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. 2004. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 72:1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qing G, Howlett S, Bortolussi R. 1996. Lipopolysaccharide binding proteins on polymorphonuclear leukocytes: comparison of adult and neonatal cells. Infect. Immun. 64:4638–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ridings J, Weedon H, Ioannou C, Flego L, Macardle PJ, Zola H. 1996. Purification of cord blood lymphocytes. J. Immunol. Methods 195:43–48 [DOI] [PubMed] [Google Scholar]
- 35. Delude RL, Yoshimura A, Ingalls RR, Golenbock DT. 1998. Construction of a lipopolysaccharide reporter cell line and its use in identifying mutants defective in endotoxin, but not TNF-alpha, signal transduction. J. Immunol. 161:3001–3009 [PubMed] [Google Scholar]
- 36. Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL. 2006. Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J. Infect. Dis. 194:176–183 [DOI] [PubMed] [Google Scholar]
- 37. Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat. Rev. 1:135–145 [DOI] [PubMed] [Google Scholar]
- 38. Kutuzova GD, Albrecht RM, Erickson CM, Qureshi N. 2001. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J. Immunol. 167:482–489 [DOI] [PubMed] [Google Scholar]
- 39. Skinner NA, MacIsaac CM, Hamilton JA, Visvanathan K. 2005. Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin. Exp. Immunol. 141:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Webster NL, Kedzierska K, Azzam R, Paukovics G, Wilson J, Crowe SM, Jaworowski A. 2006. Phagocytosis stimulates mobilization and shedding of intracellular CD16A in human monocytes and macrophages: inhibition by HIV-1 infection. J. Leukoc. Biol. 79:294–302 [DOI] [PubMed] [Google Scholar]
- 41. Tobian AA, Mehlotra RK, Malhotra I, Wamachi A, Mungai P, Koech D, Ouma J, Zimmerman P, King CL. 2000. Frequent umbilical cord-blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J. Infect. Dis. 182:558–563 [DOI] [PubMed] [Google Scholar]
- 42. Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, Manogue KR, Cerami A, Brewster DR, Greenwood BM. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201–1204 [DOI] [PubMed] [Google Scholar]
- 43. Onguru D, Liang Y, Griffith Q, Nikolajczyk B, Mwinzi P, Ganley-Leal L. 2011. Human schistosomiasis is associated with endotoxemia and Toll-like receptor 2- and 4-bearing B cells. Am. J. Trop. Med. Hyg. 84:321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. 2006. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 60:205–209 [DOI] [PubMed] [Google Scholar]
- 45. Berner R, Furll B, Stelter F, Drose J, Muller HP, Schutt C. 2002. Elevated levels of lipopolysaccharide-binding protein and soluble CD14 in plasma in neonatal early-onset sepsis. Clin. Diagn. Lab. Immunol. 9:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chow JM, Lin HY, Shen SC, Wu MS, Lin CW, Chiu WT, Lin CH, Chen YC. 2009. Zinc protoporphyrin inhibition of lipopolysaccharide-, lipoteichoic acid-, and peptidoglycan-induced nitric oxide production through stimulating iNOS protein ubiquitination. Toxicol. Appl. Pharmacol. 237:357–365 [DOI] [PubMed] [Google Scholar]
- 47. Hochart H, Jenkins PV, Preston RJ, Smith OP, White B, O'Donnell J. 2008. Concentration-dependent roles for heparin in modifying lipopolysaccharide-induced activation of mononuclear cells in whole blood. Thromb. Haemost. 99:570–575 [DOI] [PubMed] [Google Scholar]
- 48. Kato A, Ogasawara T, Homma T, Saito H, Matsumoto K. 2004. Lipopolysaccharide-binding protein critically regulates lipopolysaccharide-induced IFN-beta signaling pathway in human monocytes. J. Immunol. 172:6185–6194 [DOI] [PubMed] [Google Scholar]
- 49. Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. 2006. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 177:1956–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor signaling. J. Infect. Dis. 195:296–302 [DOI] [PubMed] [Google Scholar]
- 51. Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, Di Marco F, French L, Tschopp J. 1998. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273:12203–12209 [DOI] [PubMed] [Google Scholar]
- 52. Doz E, Rose S, Nigou J, Gilleron M, Puzo G, Erard F, Ryffel B, Quesniaux VF. 2007. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 282:26014–26025 [DOI] [PubMed] [Google Scholar]
- 53. Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. 2008. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 83:692–701 [DOI] [PubMed] [Google Scholar]
- 54. Wang R, Stephens J, Lacy MJ. 2003. Characterization of monoclonal antibody HTA125 with specificity for human TLR4. Hybrid. Hybridomics 22:357–365 [DOI] [PubMed] [Google Scholar]
- 55. Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. 2005. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 175:4669–4676 [DOI] [PubMed] [Google Scholar]
- 56. Carpenter S, O'Neill LA. 2009. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem. J. 422:1–10 [DOI] [PubMed] [Google Scholar]
- 57. Kalis C, Kanzler B, Lembo A, Poltorak A, Galanos C, Freudenberg MA. 2003. Toll-like receptor 4 expression levels determine the degree of LPS-susceptibility in mice. Eur. J. Immunol. 33:798–805 [DOI] [PubMed] [Google Scholar]
- 58. Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164:3476–3479 [DOI] [PubMed] [Google Scholar]
- 59. De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. 2005. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 174:2037–2045 [DOI] [PubMed] [Google Scholar]
- 60. Chelvarajan RL, Collins SM, Doubinskaia IE, Goes S, Van Willigen J, Flanagan D, De Villiers WJ, Bryson JS, Bondada S. 2004. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J. Leukoc. Biol. 75:982–994 [DOI] [PubMed] [Google Scholar]
- 61. Ando M, Shibuya A, Tsuchiya K, Akiba T, Nitta K. 2006. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 70:358–362 [DOI] [PubMed] [Google Scholar]
- 62. Tsujimoto H, Ono S, Hiraki S, Majima T, Kawarabayashi N, Sugasawa H, Kinoshita M, Hiraide H, Mochizuki H. 2004. Hemoperfusion with polymyxin B-immobilized fibers reduced the number of CD16+ CD14+ monocytes in patients with septic shock. J. Endotoxin Res. 10:229–237 [DOI] [PubMed] [Google Scholar]
- 63. Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, Akashi S, Miyake K, Godowski PJ, Makino H. 2004. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 50:1457–1467 [DOI] [PubMed] [Google Scholar]
- 64. Baumgarth N, Roederer M. 2000. A practical approach to multicolor flow cytometry for immunophenotyping. J. Immunol. Methods 243:77–97 [DOI] [PubMed] [Google Scholar]
- 65. Drohan L, Harding JJ, Holm B, Cordoba-Tongson E, Dekker CL, Holmes T, Maecker H, Mellins ED. 2004. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum. Immunol. 65:1356–1369 [DOI] [PubMed] [Google Scholar]