Abstract
Current commercial prophylactic human papillomavirus (HPV) vaccines are based on virus-like particles assembled from the major capsid protein L1 and show excellent safety and efficacy profiles. Still, a major limitation is their rather narrow range of protection against different HPV types. In contrast, the minor capsid protein L2 contains a so-called major cross-neutralizing epitope that can induce broad-range protective responses against multiple HPV types. This epitope is conserved among different papillomaviruses (PV) and contains two cysteine residues that are present in the L2 proteins of all known PV types. The main challenge in developing L2-directed vaccines is to overcome the intrinsically low immunogenicity of the L2 protein. Previously, we developed a recombinant L2-based prototype vaccine by inserting peptide epitopes spanning the cross-neutralizing L2 sequence into a bacterial thioredoxin (Trx) scaffold. These antigens induced high-titer neutralizing antibodies in mice. Here, we address the question of whether Trx scaffold multimerization may further enhance the immunogenicity of the TrxL2 vaccine. We also demonstrate that the oxidation state of the conserved cysteine residues is not essential for vaccine functionality, but it contributes to immunogenicity.
INTRODUCTION
To date, at least 13 different types of human papillomaviruses (HPVs) have been defined as high-risk (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59) or probably high-risk (HPV-68), as they have been linked to cancer development (1). These HPV types are consistently detected in biopsy samples from invasive cervical cancers. Still, there is a great discrepancy between the number of cancer cases and the frequency of HPV infections, which are very common among adults. It is assumed that most infections are cleared by the immune system and, in fact, only a small fraction of benign HPV-positive lesions progress to cancer. Worldwide, the eight most-frequent high-risk HPV types associated with cervical cancer include HPV-16, HPV-18, HPV-45, HPV-31, HPV-33, HPV-35, HPV-52, and HPV-58 (2). Although other, albeit poorly understood, factors contribute to cervical cancer development, HPV infection is considered to be a key determinant of neoplastic progression (3, 4). Two commercial vaccines, Gardasil and Cervarix, were licensed in 2006 and 2007, respectively (5, 6). They are virus-like particle (VLP) vaccines based on the L1 major capsid protein. To date, >100 million doses have been administered, and both vaccines show impressive safety and efficacy profiles (7, 8). It is expected that each vaccine will reduce the rate of cervical cancer in vaccinated women by 70 to 80%.
Despite their clinical success, VLP vaccines have some important limitations, the major one being their rather narrow range of protection. The principle underlying VLP vaccines is the induction of neutralizing antibodies that block virus infection by binding to surface L1 protein loops that are highly heterogeneous among different HPV types (9–12).
For this reason, anti-L1 neutralizing antibodies are highly HPV-type specific. For example, anti-HPV-16 antibodies usually fail to neutralize any other HPV type besides HPV-16, although a limited degree of HPV-31 and HPV-33 protection is observed.
In contrast to L1, the minor capsid protein L2 contains a number of conserved epitopes that are targets for virus neutralization (13–15). One of these epitopes, spanning the amino acid (aa) region 17 to 38 of HPV-16 L2 (L217-38), has gained special attention, as antibodies recognizing this region show neutralizing activity against a broad range of different papillomavirus (PV) types (15–17). This major cross-neutralizing epitope, which we mapped to the aa 20 to 38 region of L2 (L220-38), contains two cysteine residues (positions 22 and 28) that are conserved in the L2 proteins of all known PVs. These cysteine residues are buried and disulfide bonded in mature HPV virions, and it has been suggested that disulfide-bond reduction, after viral entry, may be critical for endosomal escape and infectivity (18).
The main challenge in developing L2-directed vaccines is to overcome the intrinsically low immunogenicity of the L2 protein. Previously, we developed a recombinant L2-based prototype vaccine by inserting the cross-neutralizing L220-38 epitope into a bacterial thioredoxin (Trx) scaffold (15) (TrxL2). Despite the encouraging results obtained with the prototype TrxL2 vaccine, a detailed knowledge of all the factors (especially the higher-order multimerization and aggregation states of the antigen) that potentially influence immunogenicity and virus neutralization capacity is an important aspect to consider in further vaccine development. In fact, in various subunit vaccine settings, including L1-based vaccines, where VLPs are superior to pentameric L1 capsomeres in terms of immunogenicity (19, 20), antigen multiplicity and assembly states have been shown to be important determinants of vaccine immunogenicity and efficacy. Thus, the multimerization state of the L1 antigen is likely to be a major factor influencing immunogenicity, as has been observed with other antigens (21, 22).
Here, we investigate whether the effectiveness of the TrxL220-38 prototype vaccine can be enhanced by intermolecular multimerization of the Trx scaffold and whether the oxidation state of the L2 antigen influences immunogenicity. We also show that a neutralizing monoclonal antibody generated by using the L220-38 peptide grafted to Trx as the antigen preferentially binds to the oxidized L2 epitope.
MATERIALS AND METHODS
Construction of TrxL2 multimers.
The Trx-HPV-16 L2(20–38)3 (3-fold repeated) construct was generated as previously described (15). Briefly, the L2(20-38)3 DNA was inserted into a modified pET28 plasmid bearing the sequence for a dual 6×His-tagged version of Escherichia coli thioredoxin (pTrx) using the CpoI site of the Trx coding sequence as the cloning site. Phosphorylated oligonucleotides encoding the L220-38 sequence of HPV-16 were ligated to CpoI-digested pTrx. Following bacterial transformation, constructs bearing a tripeptide insertion of 20 to 38 L2 were isolated. For multimerization, an internal BglI site was removed from the Trx coding sequence of pTrx, while an SfiI site flanking the coding sequence was introduced via PCR. In a second PCR, a flanking BglI site was introduced. The product of this second PCR was blunt-end cloned into the StrataClone PCR cloning vector pSC-B-amp/kan (Agilent). The TrxL2 insert was excised from the pSC-B-amp/kan vector via an NdeI/XhoI double digestion and ligated into NdeI/XhoI-predigested and dephosphorylated pTrx. This resulted in a monomeric TrxL2 plasmid capable of multimerization (pTrx 1×). For dimer generation, pTrx 1× was first cleaved with SfiI, followed by SfiI-mediated TrxL2 excision from pSC-B-amp/kan and BglI digestion. Next, the linearized SfiI-cleaved pTrx 1× plasmid was combined with the SfiI/BglI-digested TrxL2 insert to generate the dimeric construct (pTrx 2×). A similar procedure was used to generate the trimer (pTrx 3×) and the tetramer (pTrx 4×).
Expression and purification of TrxL2 proteins.
TrxL2 protein expression was induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to E. coli Rosetta cells (Merck4Biosciences, Darmstadt, Germany), transformed with either the monomeric or the multimeric constructs (pTrx 1× to 4×), which were then allowed to grow for 12 to 16 h at 23 to 30°C. Following resuspension of the bacterial pellet (300 mM NaCl, 25 mM Tris, 0.16% Tween 20, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], and 0.1 mg/ml lysozyme [pH 8.0]) and lysis with EmulsiFlex (Avestin, Canada), His-tagged TrxL2 proteins were bound to Ni2+-substituted affinity columns (1 ml; Amersham, GE Healthcare, United Kingdom), purified as per the manufacturer's instructions, and dialyzed against phosphate-buffered saline (PBS) containing 300 mM NaCl. Protein concentration was determined with Coomassie blue G250 (Bio-Rad protein assay dye reagent) using bovine serum albumin as a standard, as well as by UV absorbance at 280 nm with the use of calculated extinction coefficients. The composition and purity of individual polypeptide preparations were assessed by electrophoretic analysis on 12.5% and 15% SDS polyacrylamide gels. The composition of the various TrxL2 protein preparations was further confirmed by immunoblotting using anti-Trx (K12Trx) and anti-L2 (K4L220–38 and K18L220–38) antibodies (17).
Chemical reduction, size exclusion chromatography, and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) assays of TrxL2 proteins.
Purified monomeric and multimeric TrxL2 proteins were adjusted to equal protein concentrations ranging from 0.5 to 1.0 mg/ml. For chemical reduction, DL-dithiothreitol (DTT) (BioUltra grade; Sigma-Aldrich) was added to the TrxL2 proteins either at a final concentration of 10 mM and incubated for 24 h at 4°C (“mild treatment”) or at a 20 mM concentration and incubated at 37°C for 48 h (“harsh treatment”). Following DTT treatment, protein samples either were analyzed immediately or were snap-frozen in liquid nitrogen.
Size exclusion chromatography (SEC) was performed with a Superdex 200 10/300 GL column using an Äkta fast protein liquid chromatography (FPLC) system, at a flow rate of 0.5 ml/min and a maximum pressure of 1.5 MPa, operated with Unicorn 5.0 (Amersham, GE Healthcare, United Kingdom). The running buffer was PBS for the untreated proteins, or PBS supplemented with DTT for the reduced (DTT-treated) proteins. Elution was carried out with 2 column volumes (48 ml) of running buffer; 1-ml fractions were collected, stored at 4°C, and analyzed for protein content and composition with the Coomassie dye-binding assay (see above) and SDS-PAGE.
The Ellman reagent [5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB); ε(412 nm) = 14,150 M−1 cm−1] was used to quantify free cysteine and SH groups (23, 25). The assay was conducted in a final volume of 150 μl containing 0.1 mM DTNB and 2% SDS in 50 mM sodium phosphate buffer (pH 8.0); a calibration curve was constructed with increasing concentrations of N-acetylcysteine (Sigma-Aldrich). The number of free cysteine or protein molecules was determined by dividing the concentration of DTNB-reactive SH groups (optical density at 412 nm [OD412 nm]/ε412 nm) by the concentration of protein utilized for the assay.
Enzyme-linked immunosorbent assay (ELISA) analysis of purified monomeric TrxL2.
Individual wells of a flexible 96-well plate (BD Falcon) were coated overnight at 4°C with 50 μl/well of purified monomeric or multimeric TrxL2 proteins (0.5 mg/ml) diluted 1:500 in PBS. After washing three times with PBS-0.3% Tween 20, the plates were blocked for 1 h at 37°C with 0.2% casein dissolved in PBS (50 μl/well). Next, monoclonal K18L220–38 (0.5 mg/ml) was serially diluted 1:2 on the plate (50 μl/well), starting from a 1:100 dilution. Incubation was allowed to proceed at 37°C for 1 h before washing with PBS-0.3% Tween 20. A secondary goat-anti-mouse horseradish peroxidase (HRP)-conjugated antibody (10 mg/ml; Dianova, Germany) was then added to the plate (50 μl/well) at a 1:3,000 dilution and was incubated for 1 h at 37°C. After washing with PBS-0.3% Tween 20 (100 μl/well), 1 mg/ml 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) substrate diammonium salt (Sigma-Aldrich) was added to the plate (100 μl/well), followed by the measurement of color development at 405 nm.
Immunization protocol.
Six- to eight-week-old female BALB/c mice were purchased from Charles River (Sulzfeld, Germany) and kept in the animal house facility of the German Cancer Research Center under specific-pathogen-free conditions. Mice were immunized subcutaneously, three to four times at biweekly intervals, with 2 to 50 μg of the various TrxL2 antigens adjuvanted with 50% (vol/vol) Montanide ISA 720 (Seppic, France). Doses used in the initial immunization experiments (50 μg and 25 μg) were higher than those employed in later experiments (2 μg). Within this window, similar antibody responses were induced, but comparisons were made only between groups that received the same dose. Intermediate blood samples were taken after the second and/or the third immunization by puncturing the submandibular vein. Eight weeks after the last immunization, final blood samples were collected by cardiac puncture, and neutralizing antibody titers were determined with an in vitro pseudovirion-based neutralization assay.
Pseudovirion-based neutralization assays.
Pseudovirions were prepared as described previously (34, 35), with some modifications (30). The absence of cell culture contamination was confirmed by the Multiplex cell contamination test (29). Neutralization assays were performed as described previously (30). Briefly, 50 μl of diluted polyclonal or monoclonal antibodies was combined with 50 μl of diluted pseudovirion stocks and incubated at room temperature for 30 min. Next, 50 μl of HeLa T cells (2.5 × 105 cells/ml) was added to the pseudovirion-antibody mixture and incubated for 48 h at 37°C (under a 5% CO2 atmosphere). The amount of secreted Gaussia luciferase was determined in 10 μl of cell culture medium using the coelenterazine substrate and Gaussia glow juice (PJK, Germany), according to the manufacturer's instructions. A microplate luminometer (Victor3, PerkinElmer) was used to measure culture medium-associated luminescence 15 min after substrate addition.
Statistical analysis.
The nonparametric Mann-Whitney test, performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA), was used to determine the statistical significance of the differences between the neutralization titers. Differences between groups were considered significant at a P value of <0.05.
RESULTS
Intermolecular multimerization of the TrxL2 antigen.
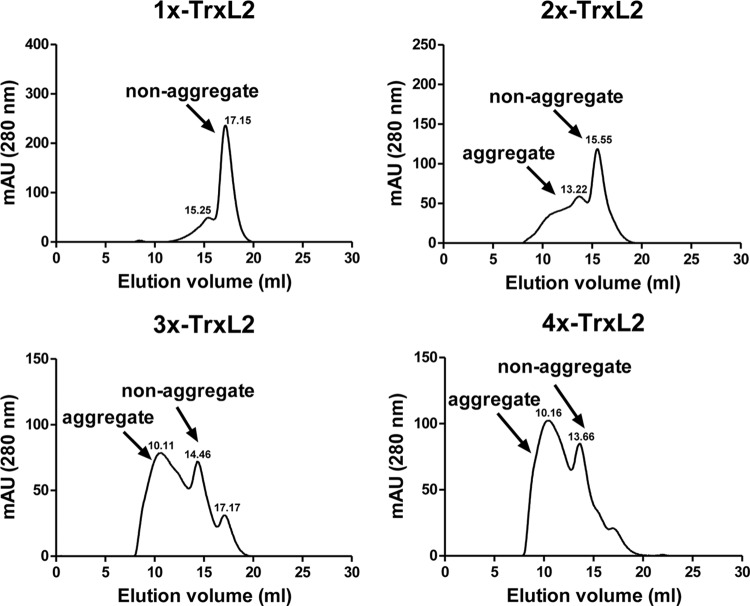
Our standard TrxL2 antigen already contained three intramolecular tandemly repeated copies of the L220–38 epitope, but we reasoned that additional intermolecular multimerization of TrxL2(20–38)3 might further increase anti-L2 immune responses. To test this hypothesis, we generated a set of multimerized TrxL2 proteins by covalently connecting individual TrxL2 building blocks to each other via a 15-amino-acid linker (Fig. 1A). Each building block comprised a Trx protein bearing three copies of the HPV-16 L220–38 epitope, with individual epitopes separated from each other by a GGP spacer sequence. The monomeric and multimeric TrxL2 proteins were all expressed at high levels in E. coli (Fig. 1B) and were purified by metal affinity chromatography. All multimer preparations contained degradation products migrating as monomers (2×-TrxL2, 3×-TrxL2, 4×-TrxL2), dimers (3×-TrxL2, 4×-TrxL2), and trimers (4×-TrxL2), probably resulting from spacer sequence cleavage. An attempt to remove these degradation products by size exclusion chromatography (SEC) (see Materials and Methods for details) was not successful due to the relatively small differences in size (and elution times) between cleavage products and the corresponding intact proteins. While performing these experiments, however, we found that the dimer, and to a greater extent, the trimer and the tetramer, were eluting much earlier than one would expect simply based on the predicted molecular weights of these proteins. In other words, multimerized TrxL2 proteins, but not the monomer, appeared to assemble into soluble high-molecular-weight aggregates, even though a fraction of the loaded multimeric proteins eluted at the expected volume, indicating that at least a subset of the protein is present in a nonaggregated form. We determined that these soluble aggregates are composed of cross-linked TrxL2 proteins with an average molecular mass of ≥600 kDa (Fig. 2).
Fig 1.
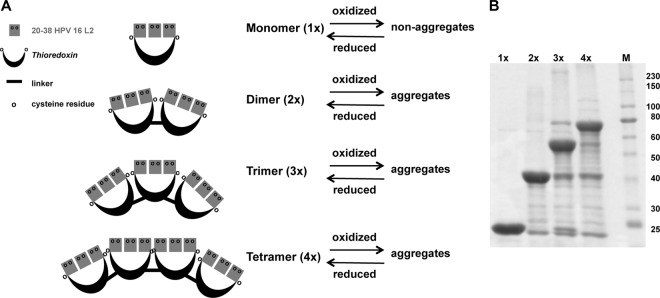
Monomeric and multimeric TrxL2 antigens. (A) Thioredoxin scaffolds containing a tripeptide of the HPV-16 L220–38 are multimerized and separated by a 15-aa linker (sequence, GAAGPPGGWPPRGSH). Intramolecular disulfide bridges (S—S) are mediated between L2 insert and/or scaffold cysteines within one molecule. Intermolecular S—S are mediated between L2 insert and/or scaffold cysteines of separate molecules and lead to aggregation. (B) Expression of mono- and multimers in E. coli resulted in soluble products. M, marker (in kDa).
Fig 2.
Size exclusion chromatography reveals aggregation of the soluble multimers. After Ni2+-chromatography, the purified TrxL2 proteins were analyzed via size exclusion chromatography using a Superdex 200 column. Except for the monomeric TrxL2, large portions of the multimerized antigens were present as soluble high-molecular-weight forms with an average of ∼600 kDa. The aggregate (∼600 kDa) and nonaggregate elution peaks are labeled, and the elution volumes are indicated above the peak. mAU, milli-absorbance units.
Are multimeric or aggregated TrxL2 antigens more immunogenic?
To address the question of whether multimeric or aggregated TrxL2 antigens are more immunogenic, different mice were immunized with monomeric or multimeric TrxL2. No significant differences were observed in the HPV-16 neutralization titers of the four immunization groups, although the immunogenicity of the dimeric (2×-TrxL2) TrxL2 antigen was apparently lower than those of the other groups (Fig. 3). The latter group had a mean titer of 390 (range, 50 to 800), while the 1×-TrxL2, 3×-TrxL2, and 4×-TrxL2 groups yielded mean titers of 3,080 (range, 200 to 12,800), 2,000 (range, 400 to 3,200), and 8,650 (range, 50 to 25,600), respectively.
Fig 3.
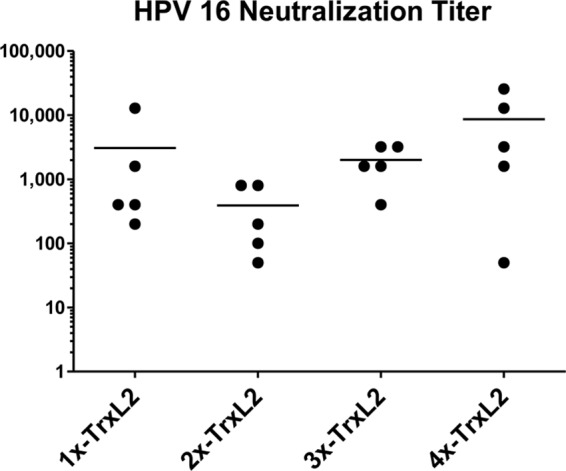
Comparable immunogenicities of TrxL2 mono- and multimers determined by in vitro neutralization assay. The HPV-16 neutralization titers (50% infective concentration [IC50]) of the 1×-TrxL2, 2×-TrxL2, 3×-TrxL2, and 4×-TrxL2 immune sera are shown. L2 polyclonal sera were serially diluted 1:2 starting with a 1:50 dilution. Each dot represents one mouse serum sample, with horizontal bars indicating the mean titers. Mice were immunized three times at biweekly intervals with 50 μg antigen adjuvanted with 50% (vol/vol) Montanide ISA 720. Final serum samples were collected 8 weeks after the third immunization.
Next, we wished to determine whether higher-order aggregation, on top of intermolecular multimerization, might influence TrxL2 immunogenicity. To this end, we set up a further immunization experiment based upon the following tetrameric antigens: (i) unfractionated 4×-TrxL2, i.e., the mixture of purified nonaggregated and aggregated proteins derived from bacterial lysates by metal affinity chromatography, (ii) SEC-fractionated 4×-TrxL2 aggregates, (iii) the purified nonaggregated 4×-TrxL2 antigen obtained upon SEC fractionation, and (iv) fractionated 4×-TrxL2 aggregates plus the corresponding nonaggregated antigen mixed together prior to immunization. As shown in Fig. 4, all forms of the 4×-TrxL2 antigen, whether aggregate, nonaggregate, or the two forms together, were found to be equally immunogenic, as indicated by the almost-identical mean titers of approximately 2,000. A somewhat-lower titer (800) was observed for the group that received the reconstituted aggregate-nonaggregate mix, but the difference was not statistically significant. Thus, under the presently examined conditions, the aggregation state of TrxL2 does not appear to influence immunogenicity.
Fig 4.
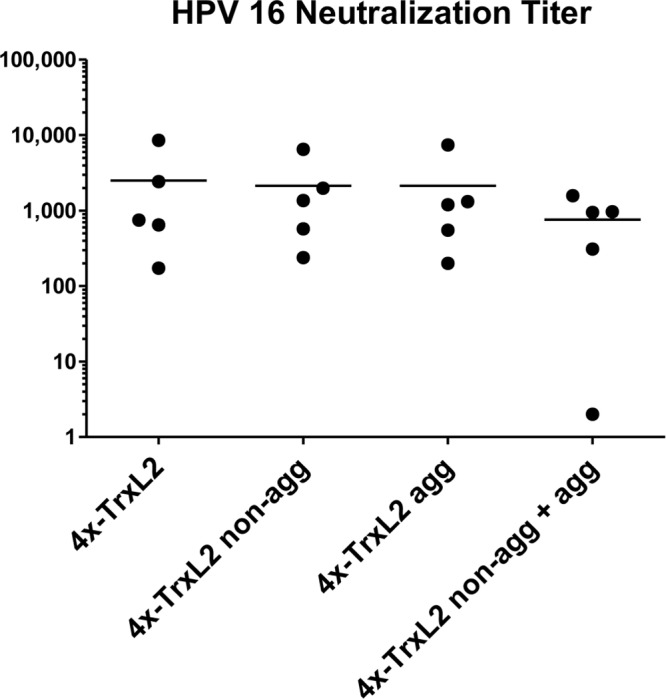
Aggregate and nonaggregate forms of tetrameric TrxL2 are equally immunogenic. The HPV-16 neutralization titers for the 4×-TrxL2, 4×-TrxL2 nonaggregate (non-agg), and/or aggregate (agg) immune sera are shown. Serum samples were serially diluted 1:3 starting with a 1:100 dilution. Each dot represents one mouse serum sample, with horizontal bars indicating the mean titers. Animals were immunized three times at biweekly intervals with 25 μg of antigen formulated with 50% (vol/vol) Montanide ISA 720. Final serum samples were collected 8 weeks after the third immunization.
Influence of the oxidation and aggregation states of the antigen on immune performance.
The above results (see the immune performance of the 4×-TrxL2 antigen in Fig. 3) hinted at a slight improvement in immunogenicity that is produced by TrxL2 multimerization. At the same time, however, multimerization is accompanied by the appearance of soluble high-molecular-weight aggregates, likely resulting from extensive disulfide bond-mediated intermolecular cross-linking of the multimeric TrxL2 antigens. Each L220–38 epitope contains two cysteine residues, for a total of 6 cysteine-tripeptide inserts, plus two Trx scaffold-associated cysteine residues flanking each L2 tripeptide insert. This corresponds to 8, 16, 24, and 32 cysteine residues for the 1×-TrxL2, 2×-TrxL2, 3×-TrxL2, and 4×-TrxL2 antigens, respectively.
We performed free SH-group titrations with DTNB to determine the degree of reduction of the various purified proteins. Untreated proteins extracted and purified from E. coli were found to be almost completely (>95%) oxidized. Only a prolonged (harsh) treatment with DTT led to a significant disulfide reduction (53 to 100% after incubation with 20 mM DTT for 48 h at 37°), thus indicating that the oxidized state is very stable (Fig. 5).
Fig 5.
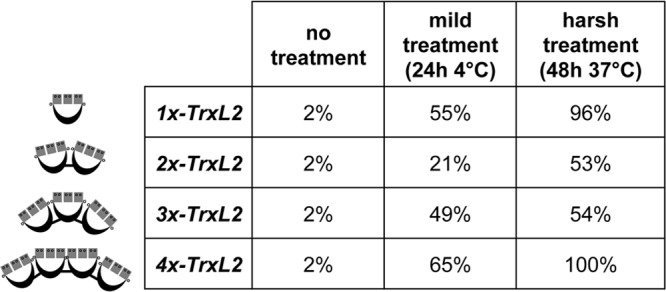
Oxidation status of purified monomer and multimer TrxL2 proteins. Each percentage indicates the degree of reduction as determined via DTNB assay. Untreated TrxL2 purified from E. coli is almost completely oxidized. Treatment of the samples with 10 to 20 mM DTT for different lengths of time and at different temperatures led to partial or complete reduction.
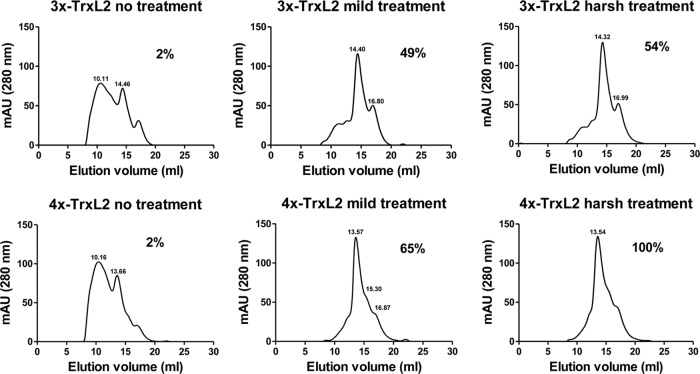
We then analyzed the impact of disulfide-bond reduction on the aggregation state of the proteins by SEC. As shown by the representative results of the 3×-TrxL2 and 4×-TrxL2 proteins presented in Fig. 6, most of the untreated protein was in the form of high-molecular-weight aggregates, but a mild DTT treatment (24 h at 4°C) led to a strong decrease of such aggregated species. This indicates that at least a fraction of the disulfide bonds (S—S) are in fact intermolecular, and that these bonds can be readily reduced and disrupted by relatively mild DTT treatment. Only the harsh DTT treatment (48 h at 37°C) led to a complete reduction of 4×-TrxL2 multimers. Although the SEC profiles were identical to those produced by the mild DTT treatment, intramolecular S—S bonds are also disrupted under these stronger conditions.
Fig 6.
Partial reduction of TrxL2 multimers leads to disappearance of high-molecular-weight aggregates. The percentages of cysteine residues in a reduced state are indicated.
Next, we tested the immunogenic capacity of 4×-TrxL2 proteins with different cysteine oxidation states using the fully oxidized monomeric protein as a reference antigen. As shown in Fig. 7, the HPV-16-type-specific titers in animals immunized with untreated oxidized 1×-TrxL2 and 4×-TrxL2 were similar (mean titers of approximately 10,000 and 5,000, respectively), even though there was a trend of higher titers in the 1×-TrxL2 group. Partial reduction of the 4×-TrxL2 protein led to a significantly reduced induction of anti-L2-neutralizing antibodies (mean titer of ∼2,000) compared to that of the untreated tetrameric TrxL2 antigen, and this difference became even more pronounced in the case of serum samples from mice treated with fully reduced 4×-TrxL2 antigen preparations (mean titer, ∼900). A similar trend was observed when the same serum samples (raised against the HPV-16 L2 epitope) were tested for their capacity to cross-neutralize heterologous HPV-58 and HPV-33 pseudovirions.
Fig 7.
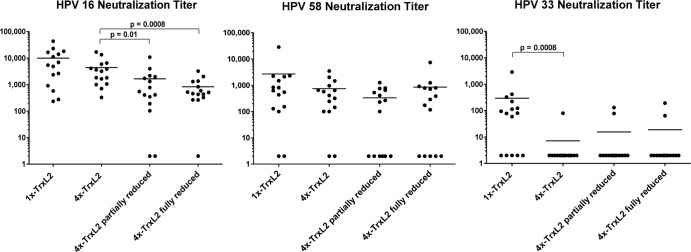
Oxidized tetrameric and monomeric TrxL2 elicit similar type-specific titers, but cross-protective titers are higher for the latter. Reduced tetrameric TrxL2 is less immunogenic than its oxidized counterpart. TrxL2 monomer and tetramer antigens were fully or partially reduced and then used for mouse immunization. The HPV-16-, HPV-58-, and HPV-33 neutralization titers were determined. For HPV-16 and HPV-58, serum samples were serially diluted 1:3 starting with a 1:100 dilution, while for HPV-33, serial 1:2 dilutions starting with a 1:50 dilution were employed. Each dot represents one mouse serum sample, with horizontal bars indicating the mean titers. Fifteen mice per group were immunized a total of four times at biweekly intervals with 2 μg antigen adjuvanted with 50% (vol/vol) Montanide ISA 720. Final serum samples were collected 8 weeks after the fourth immunization.
Most of the untreated oxidized 4×-TrxL2 protein is in the form of high-molecular-weight aggregates, and this aggregation state is disrupted by partial reduction. Thus, the observed decrease in immunogenicity might reflect the breakage of intermolecular disulfide bonds. In contrast, the monomeric TrxL2 protein contains only minor amounts of aggregates. To determine whether the observed decreased immunogenicity of reduced 4×-TrxL2 is a result of intermolecular disulfide bond breakage, we used oxidized and reduced monomeric TrxL2 proteins for immunization, applying the same immunization schedule as was done for the previous 1×-TrxL2 versus 4×-TrxL2 immunization. As shown in Fig. 8, in contrast to what we observed with the 4×-TrxL2 antigen, reduced and oxidized 1×-TrxL2 proteins were found to be equally immunogenic with regard to HPV-16-type-specific neutralization (mean titers of approximately 5,000 and 4,000, respectively). The same serum samples were also tested against other HPV types. As there are sequence differences in the aa 20 to 38 epitope between different HPV types, we reasoned that cross-protective responses are better indicators for the robustness of the induced immune responses. We selected HPV-58 and HPV-33 for this analysis, as we usually observed robust and impaired neutralization of these types using anti-HPV-16 L2 antisera, respectively. In fact, contrary to the finding for HPV-16 pseudovirions, the serum samples of mice that had received the reduced monomer neutralized HPV-58 pseudovirions with a lower efficiency than serum samples from mice immunized with the oxidized antigen. This difference in immunogenicity of a reduced versus an oxidized monomer became even more visible for the neutralization of HPV-33.
Fig 8.
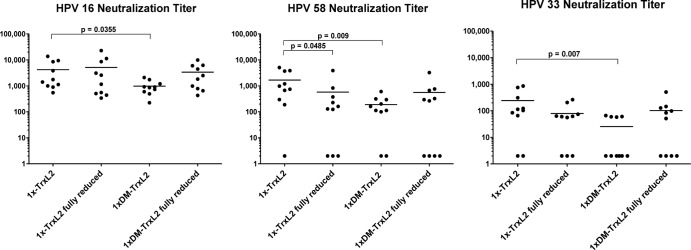
While HPV-16-type-specific titers are similar, the oxidized monomer surpasses its reduced counterpart in terms of cross-protective titers. The Cys → Ser exchange (1× DM-TrxL2) in the Trx scaffold lowers immunogenicity, but reduction restores it. Mice were immunized with monomeric TrxL2 proteins that were in an oxidized or reduced state. Shown here is a comparison of the induction of neutralizing antibodies by 1×-TrxL2 and 1× DM-TrxL2−, a protein in which both scaffold cysteines were exchanged for serines. For HPV-16 and HPV-58, serum samples were serially diluted 1:3 starting with a 1:100 dilution, while for HPV-33, serial 1:2 dilutions starting with a 1:50 dilution were employed. Each dot represents one mouse serum sample, with horizontal bars indicating the mean titers. Animals were immunized with 2 μg antigen adjuvanted with 50% (vol/vol) Montanide ISA 720, a total of four times at biweekly intervals. Final serum samples were collected 8 weeks after the fourth immunization.
The six cysteines of the 3-fold repeated L2 epitope are flanked by two cysteine residues provided by the Trx scaffold. In the oxidized form of the monomeric TrxL2 protein purified from E. coli, it can be assumed that these Trx-associated cysteines also form disulfide bonds, either with each other or with L2-epitope cysteines. The lower immunogenicity of the reduced TrxL2 antigen might, therefore, be a consequence of an altered and more relaxed structure of the Trx scaffold. To address this question, we generated monomeric TrxL2 proteins in which the two Trx cysteines were replaced by serine residues (1× DM-TrxL2). Immunization experiments indicated a significantly reduced immunogenicity of 1× DM-TrxL2 compared to the unmodified 1×-TrxL2 antigen, as reflected by the type-specific titers on HPV-16 (∼1,000 and ∼4,000, respectively), as well as by the further-reduced cross-protective titers measured on heterologous HPV pseudovirions (Fig. 8). In keeping with previous observations that the conformational quality (24), as well the immunogenicity (S. Ottonello and A. Bolchi, unpublished data), of multiepitope Trx fusion constructs tends to deteriorate above a certain insert-size threshold, this finding further indicates that intrascaffold S—S bond formation is critical for epitope presentation, as well as for the structural integrity and immunogenicity of Trx-based peptide antigens.
Interestingly, and most unexpectedly, reducing 1× DM-TrxL2 restored its immunogenicity to a large degree, at least when considering HPV-16 neutralization (mean titer, ∼3,500). However, when looking at the neutralization of HPV-58 and HPV-33 pseudovirions, four out of the 10 mice immunized with reduced 1× DM-TrxL2 failed to develop measurable responses, confirming the above-described observation that oxidized L2 epitopes are better immunogens in respect to cross-protective responses.
Preferential recognition of the oxidized L220–38 epitope by a neutralizing anti-L2 monoclonal antibody.
Using the monomeric TrxL2(20–38)3 antigen, we previously generated a monoclonal anti-L2 antibody (named K18L220–38) that recognizes the L220–38 epitope and thereby neutralizes a number of HPV types with high efficiency (17). To determine the preference of this antibody for either the oxidized or reduced form of the antigen, we performed ELISA analyses using different forms of the monomeric TrxL2 protein as capture antigens. As shown in Fig. 9A, the K18L220–38 monoclonal antibody (MAb) binds to the reduced and oxidized forms of Trx-HPV-16 L2 equally well. However, when we used TrxL2 antigens in which the natural HPV-16 L2 sequence was replaced by a tripeptide of a variant epitope (“HPV-16 L2 mimotope”) presenting a cysteine-containing but otherwise-suboptimal target sequence, K18L220–38 reacted more strongly with the oxidized than the reduced form of the antigen (Fig. 9B). In line with the results obtained with homologous and heterologous neutralization assays, this indicates that the intramolecular disulfide bond is not absolutely required for epitope recognition, and yet it contributes to the binding of HPV-neutralizing antibodies.
Fig 9.
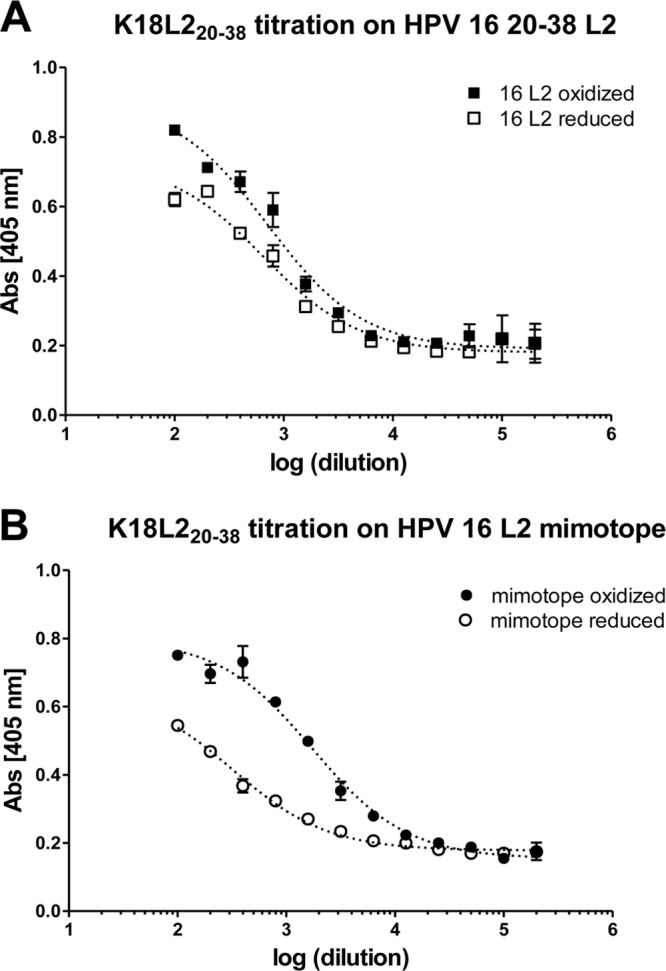
Binding of cross-neutralizing antibody K18L220–38 to a reduced or oxidized target sequence. K18L220–38 binding to a reduced or oxidized L220–38 epitope of HPV-16 (A) and to a mimotope that deviates from the HPV-16 L2 sequence (B). While reduction of the epitope does not influence K18L220–38 to the native sequence, it has a strong impact on the recognition of a not-perfect epitope, indicating that a disulfide bonds are not an absolute requirement for recognition but contribute to binding. Data points are reported as means of duplicates with the standard deviations (SD). HPV-16 L220–38: KTCKQAGTCPPDIIPKVEG; mimotope “18–32 L2”: TTMYCKSTDNCPSDV, where underlined are aa 20 to 32 contained in each peptide, and bold letters indicate identical amino acids between the two peptides; K18L220–38 binds to aa 22 to 30 of L2.
DISCUSSION
Commercial prophylactic HPV vaccines are based on virus-like particles, composed of 360 units of the L1 major capsid protein. Although it has been shown that L1 capsomeres also mount neutralizing antibody responses, albeit lower ones than those elicited by VLPs, it is clear that neutralizing L1 epitopes are highly conformation dependent. All epitopes identified so far are formed by at least two L1 loops displayed on the surface of L1 capsomeres or VLPs. In contrast to L1, all neutralizing epitopes associated with the minor capsid protein L2 seem to be linear epitopes, i.e., the neutralizing antibodies they elicit react with, and bind to, the denatured L2 protein, as well as to short synthetic L2 peptides. The possible existence of conformational neutralizing epitopes has not been demonstrated for the L2 protein so far, and this may explain the lack of a native and highly efficient L2 immunogen. While linear antigens are usually less immunogenic than conformational ones, they often come with the benefit of a greater ease of production. Of the several linear L2 epitopes that have been described as neutralizing (14–16), only a few are sufficiently conserved to provide a broad range of protection. In particular, there is a general consensus as to the presence of such a cross-neutralizing epitope within aa 17 to 38 of the L2 protein (15, 16). This region shows a remarkable conservation among papillomaviruses and contains two fully conserved cysteine residues. By forming disulfide bonds, these residues might promote the formation of a self-sustained epitope structure even in the absence of other L2 regions and outside of a capsid assembly context. Various strategies are being actively pursued by different groups to confer immunogenicity to L2-derived (poly)peptides of different lengths. With the exception of a monomeric L2 (aa 17 to 36) lipopeptide formulation (36), most of these strategies rely on displaying neutralizing L2 epitope-bearing regions, most notably the 17 to 36 cross-neutralizing epitope, as part of repetitive high-molecular-weight assemblies. Cross-protective responses have been elicited by a virus-like display of the L217–36 epitope on VLPs of bacteriophage PP7 (31), as well as on HPV-16 L1 VLPs (28) and on adeno-associated virus (AAV) particles (26). L2 (aa 13 to 47) has also been successfully incorporated into L1 capsomeres, although the elicited cross-protection was quite narrow (32). Heat-killed Lactobacillus casei cells displaying a larger portion of L2 (aa 1 to 224) were successful in inducing cross-protective antibodies even after oral administration (33). Another potent approach does not employ virus-like, capsomeric, or bacterial display assemblies, but instead relies on concatenated multitype L2 fusion proteins encompassing the aa 11 to 88 region of various HPV types (37). Unlike the high-molecular-weight approaches mentioned above, our first-generation TrxL2 antigens utilized the relatively small bacterial thioredoxin protein (109 aa) as a scaffold to display repetitive L220–38 sequences. Also, while most approaches include L2 sequence information spanning relatively large regions of the L2 N terminus (aa 1 to 120), TrxL2 contains repetitions of the shorter L220–38 epitope sequence. This has the advantage of eliciting targeted antibody responses focused on the conserved cross-protective L2 region. We recently found that the immunization of mice with a mix of TrxL2 antigens bearing L220–38 peptides from HPV-16, HPV-31, and HPV-51 elicits in vitro-detectable antibodies neutralizing 13 of 14 tested high-risk HPVs (Seitz et al., unpublished data). Further options to increase the cross-protection capacity of the TrxL2 prototype vaccine may rely on the incorporation of additional L2 peptides, such as the aa 56 to 75 peptide, which has recently been shown to represent another cross-protective epitope (27).
We reasoned that disulfide bond formation within the L220–38 epitope might be essential for vaccine efficacy and wished to determine its impact on immunogenicity. Our analysis was complicated by the fact that the thioredoxin scaffold itself structurally constrains the inserted (multi)peptide epitope via disulfide bond formation. In fact, heterologous peptides are interposed between two cysteine residues provided by the active (display) site of thioredoxin, and this guarantees proper surface exposure of the inserted peptide epitopes (38). These cysteine residues are disulfide bonded in native thioredoxin and, as revealed by the results of DTNB titrations carried out on untreated monomeric TrxL2 (>95% oxidized), the formation of this disulfide bond is apparently compatible with the insertion of the L220–38 tripeptide, and it presumably increases immunogenicity.
Multimerization of TrxL2 led to the aggregation of a substantial fraction of the multimeric proteins. This aggregation effect was not observed for monomeric TrxL2. Aggregate production was shown to be mediated by intermolecular disulfide (S—S) bond formation, and while these were easily disrupted by DTT treatment, intramolecular S—S bonds were much more resilient to reduction. This finding points to a marked stability and structural rigidity of the oxidized intramolecular multipeptide epitope, a feature of the TrxL2 antigen that may have important implications in terms of immunogenicity. In general, oxidized or reduced TrxL2 proteins proved to be extremely stable. Although this in vitro stability does not necessarily reflect the in vivo situation (i.e., administration of oxidized or reduced or adjuvanted antigens to mice), TrxL2 antigens maintained their oxidation state even after multiple freeze-thaw cycles and prolonged handling at the bench. Immunization experiments showed that neither the aggregates nor the nonaggregates of the multimers were more immunogenic than the nonaggregated monomeric TrxL2. The Trx multimerization strategy we describe, even though it did not appreciably increase immunogenicity in the case of the HPV-16 L220-38 epitope, is quite straightforward and may turn out to be useful as an immunogenicity enhancer in other recombinant peptide epitope contexts.
The reduced multimeric TrxL2 antigens, especially those that were fully reduced at the time of administration, were less effective than the oxidized antigens at eliciting neutralizing responses, especially with regard to cross-protection. A very similar behavior was observed for the reduced monomeric TrxL2 antigen, which elicited type-specific titers comparable to those of its oxidized counterpart on HPV-16, but had suboptimal performance in the case of more-stringent cross-protection titration analyses carried out on HPV-58 and HPV-33. One might even imagine that the fully reduced antigen at the time of administration may become partially oxidized over time after injection into the mouse, and that this oxidized antigen fraction may be the main contributor to the observed immunogenicity. In fact, the extracellular milieu is extremely oxidizing (39), and hence, antigen oxidation after administration seems likely. Regardless of what may happen in vivo, both the oxidized and reduced forms of TrxL2 elicited potent neutralizing responses. This indicates that the TrxL2 candidate vaccine is quite robust and intrinsically insensitive to uncontrolled changes in oxidation, as well as to the aggregation state.
Ultimately, nonaggregated fully oxidized monomeric TrxL2, which is what we purify from E. coli and is the easiest antigen form to produce with the least batch-to-batch variation, appears to be the best lead for the further development of a broadly cross-protective low-cost anti-HPV vaccine.
ACKNOWLEDGMENTS
We acknowledge financial support to S.O. by the Italian Association for Cancer Research (AIRC grant IG 12956).
We thank Lysann Schädlich for her expert help in protein purification and size exclusion chromatography.
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group 2009. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 10:321–322 [DOI] [PubMed] [Google Scholar]
- 2. Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518–527 [DOI] [PubMed] [Google Scholar]
- 4. zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342–350 [DOI] [PubMed] [Google Scholar]
- 5. Gissmann L. 2009. HPV vaccines: preclinical development. Arch. Med. Res. 40:466–470 [DOI] [PubMed] [Google Scholar]
- 6. Schiller JT, Lowy DR. 2010. Vaccines to prevent infections by oncoviruses. Annu. Rev. Microbiol. 64:23–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, Steben M, Jones RW, Bryan J, Taddeo FJ, Bautista OM, Esser MT, Sings HL, Nelson M, Boslego JW, Sattler C, Barr E, Paavonen J. 2007. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 369:1693–1702 [DOI] [PubMed] [Google Scholar]
- 8. Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, HPV PATRICIA Study Group 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314 [DOI] [PubMed] [Google Scholar]
- 9. Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557–567 [DOI] [PubMed] [Google Scholar]
- 10. Christensen ND, Cladel NM, Reed CA, Budgeon LR, Embers ME, Skulsky DM, McClements WL, Ludmerer SW, Jansen KU. 2001. Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types. Virology 291:324–334 [DOI] [PubMed] [Google Scholar]
- 11. Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz GS. 1996. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 224:477–486 [DOI] [PubMed] [Google Scholar]
- 12. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27 [DOI] [PubMed] [Google Scholar]
- 13. Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 81:11585–11592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kondo K, Ishii Y, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. 2007. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 358:266–272 [DOI] [PubMed] [Google Scholar]
- 15. Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Müller M, Ottonello S. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949–1956 [DOI] [PubMed] [Google Scholar]
- 16. Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RBS. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 81:13927–13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubio I, Seitz H, Canali E, Sehr P, Bolchi A, Tommasino M, Ottonello S, Müller M. 2011. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology 409:348–359 [DOI] [PubMed] [Google Scholar]
- 18. Campos SK, Ozbun MA. 2009. Two highly conserved cysteine residues in HPV16 L2 form an intramolecular disulfide bond and are critical for infectivity in human keratinocytes. PLoS One 4:e4463. 10.1371/journal.pone.0004463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schädlich L, Senger T, Gerlach B, Mücke N, Klein C, Bravo IG, Müller M, Gissmann L. 2009. Analysis of modified human papillomavirus type 16 L1 capsomeres: the ability to assemble into larger particles correlates with higher immunogenicity. J. Virol. 83:7690–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thönes N, Herreiner A, Schädlich L, Piuko K, Müller M. 2008. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J. Virol. 82:5472–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bachmann MF, Zinkernagel RM. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17:553–558 [DOI] [PubMed] [Google Scholar]
- 22. Ogun SA, Dumon-Seignovert L, Marchand JB, Holder AA, Hill F. 2008. The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria. Infect. Immun. 76:3817–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellman GL. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70–77 [DOI] [PubMed] [Google Scholar]
- 24. Moretto N, Bolchi A, Rivetti C, Imbimbo BP, Villetti G, Pietrini V, Polonelli L, Del Signore S, Smith KM, Ferrante RJ, Ottonello S. 2007. Conformation-sensitive antibodies against Alzheimer amyloid-beta by immunization with a thioredoxin-constrained B-cell epitope peptide. J. Biol. Chem. 282:11436–11445 [DOI] [PubMed] [Google Scholar]
- 25. Riddles PW, Blakeley RL, Zerner B. 1983. Reassessment of Ellman's reagent. Methods Enzymol. 91:49–60 [DOI] [PubMed] [Google Scholar]
- 26. Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Müller M, Kellner M, Horer M, Michaelis U, Roden RB, Gissmann L, Kleinschmidt JA. 2012. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One 7:e39741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakao S, Mori S, Kondo K, Matsumoto K, Yoshikawa H, Kanda T. 2012. Monoclonal antibodies recognizing cross-neutralization epitopes in human papillomavirus 16 minor capsid protein L2. Virology 434:110–117 [DOI] [PubMed] [Google Scholar]
- 28. Schellenbacher C, Roden R, Kirnbauer R. 2009. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J. Virol. 83:10085–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitt M, Pawlita M. 2009. High-throughput detection and multiplex identification of cell contaminations. Nucleic Acids Res. 37:e119. 10.1093/nar/gkp581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seitz H, Schmitt M, Böhmer G, Kopp-Schneider A, Müller M. 2013. Natural variants in the major neutralizing epitope of human papillomavirus minor capsid protein L2. Int. J. Cancer 132:E139–E148. 10.1002/ijc.27831 [DOI] [PubMed] [Google Scholar]
- 31. Tumban E, Peabody J, Peabody DS, Chackerian B. 2011. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 6:e23310. 10.1371/journal.pone.0023310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu WH, Gersch E, Kwak K, Jagu S, Karanam B, Huh WK, Garcea RL, Roden RBS. 2011. Capsomer vaccines protect mice from vaginal challenge with human papillomavirus. PLoS One 6:e27141. 10.1371/journal.pone.0027141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoon SW, Lee TY, Kim SJ, Lee IH, Sung MH, Park JS, Poo H. 2012. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 30:3286–3294 [DOI] [PubMed] [Google Scholar]
- 34. Buck CB, Thompson CD. 2007. Production of papillomavirus-based gene transfer vectors. Curr. Protoc. Cell Biol. Chapter 26:Unit 26.1. 10.1002/0471143030.cb2601s37 [DOI] [PubMed] [Google Scholar]
- 35. Kreider JW, Cladel NM, Patrick SD, Welsh PA, DiAngelo SL, Bower JM, Christensen ND. 1995. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J. Virol. Methods 55:233–244 [DOI] [PubMed] [Google Scholar]
- 36. Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, Zeng W, Jackson DC, Roden RB. 2008. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc. Natl. Acad. Sci. U. S. A. 105:5850–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RBS. 2009. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J. Natl. Cancer Inst. 101:782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colas P, Cohen B, Jessen T, Grishina I, McCoy J, Brent R. 1996. Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature 380:548–550 [DOI] [PubMed] [Google Scholar]
- 39. Holmgren A. 1989. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 264:13963–13966 [PubMed] [Google Scholar]