Abstract
We compared the serum levels of microRNA-155 (miR-155) between subjects with normal response to hepatitis B vaccine and nonresponders. Results showed that serum expression of miR-155 was significantly higher in nonresponders than in responders (6.40 versus 2.55; Z = 2.125, P = 0.034), suggesting that overexpression of miR-155 is correlated with nonresponsiveness to hepatitis B vaccine.
TEXT
Hepatitis B virus (HBV) remains a serious threat to public health. Hepatitis B vaccination is one of the most effective means of controlling HBV infection. However, among people who receive hepatitis B vaccination according to standard protocol, 5 to 10% are nonresponders, and most of these individuals have anti-HBs antibody titers below 10 mIU/ml (1); thus, they are susceptible to hepatitis B infection. Nevertheless, until now, the precise mechanism of nonresponsiveness to hepatitis B vaccine remained unclear.
MicroRNAs (miRNAs) are a type of evolutionarily conserved noncoding small RNA. They can either inhibit translation or induce degradation of mRNA via their specific interactions with those target genes (2). Studies have shown that multiple miRNAs are involved in the generation and differentiation of immune cells and in the innate and acquired immune responses that play important roles in maintaining a healthy immune system and preventing diseases of the immune system (3, 4). Thai et al. used transgenic technology to knock out the bic gene that encodes microRNA-155 (miR-155) in mice and found that an attenuated Salmonella vaccine did not offer immune protection against Salmonella in miR-155-knockout mice. This suggested that miR-155 plays a key role in regulating acquired immune response (5). All these findings suggest that miR-155 might be involved in the regulation of the immune response to hepatitis B vaccine. In the present study, to explore the relationship between miR-155 and nonresponsiveness to hepatitis B vaccine, we examined and compared the serum levels of miR-155 expression between subjects with normal response to hepatitis B vaccine and nonresponders. The study was approved by The Research Ethics Committee of Guangdong Medical College.
Among college freshmen recruited in 2010 and 2011, 1,481 completed hepatitis B vaccination as a 3-dose series on a 0-, 1-, and 6-month schedule (GlaxoSmithKline, Shanghai, China; recombinant hepatitis B vaccine, deltoid intramuscular injection). All of them had never received hepatitis B vaccination or combined hepatitis A and B vaccination. None of the subjects had any history of infection with human immunodeficiency virus, and none were immunodeficient. Liver function was evaluated by detecting the levels of serum alanine aminotransferase (ALT). Individuals with normal liver function who voluntarily received hepatitis B vaccination were tested for the five markers of hepatitis B and anti-hepatitis C virus (HCV) antibody; only the subjects who were negative for the five markers of hepatitis B and anti-HCV antibody were included in the study. Four to six weeks after the third dose, 5 ml peripheral venous blood was collected. The serum was separated for immediate testing of the anti-HBs antibody level by enzyme-linked immunosorbent assay (ELISA; the anti-HBs antibody quantitative detection kit was purchased from Da An Gene Co., Ltd., of Sun Yat-Sen University). Among these samples, 77 showed anti-HBs antibody levels below 10 mIU/ml (5.20%). These individuals were included in the nonresponder group. Of all nonresponders, the average age was 18.8 ± 0.7 years, and 45 (58.44%) were men. Seventy-seven age-, sex-, and body mass index (BMI)-matched normal controls were selected (Table 1). The anti-HBs antibody levels in the nonresponder group and the responder group were 1.13 ± 1.87 mIU/ml and 586.15 ± 286.02 mIU/ml, respectively.
Table 1.
Demographic characteristics of study cohort
| Characteristic | Result (mean ± SD) for: |
|
|---|---|---|
| Nonresponders (n = 77) | Responders (n = 77) | |
| Age (yr) | 19.32 ± 0.81 | 18.83 ± 1.03 |
| No. of males | 45 | 45 |
| BMI | 19.30 ± 1.79 | 19.63 ± 2.06 |
TRizol (Invitrogen) was used to extract total RNA from serum. Quantitative real-time PCR (qRT-PCR) was used to detect the expression of mature miR-155, and first-strand cDNA was generated with the RevertAidTM first-strand cDNA synthesis kit (Fermentas) using 100 ng of total RNA and miRNA-specific stem-loop reverse transcription primers (10). The reverse transcription primers for miRNA and U6 small nuclear RNA (snRNA) were as follows: miR-155, 5′-GCGAGGCGGTGGCAGTGGAAGCGTGATTTATTCACCGCCTCGCACCCCTAT-3′; U6 snRNA, 5′-AAAATATGGAACGCTTCACG-3′. All the sequences of mature miRNAs were from Sanger miRBase (http://microrna.sanger.ac.uk/sequences/).
qRT-PCR was done with a 7500 HT Fast real-time PCR system (Applied Biosystems, Foster City, CA) using SYBR green as the detection fluorophore. Forward (F) and reverse (R) primers were as follows: miR-155-F, 5′-CTCAGACTCGGTTAATGCTAATCGTGATAGG-3′; miR-155-R, 5′-GCTGTGGCAGTGGAAGCGTGATTTATT-3′; U6-F, 5′-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3′; U6-R, 5′-GCTTCACGAATTTGCGTGTCATCCTTGC-3′. The U6 snRNA was used as an internal control. Each SYBR green reaction was performed with 1.5 ml cDNA, 10 ml SYBR green mixture, 1.5 mM forward primer, 0.7 mM reverse primer, and water to adjust the final volume to 20 ml. All the reaction mixtures were incubated in a 96-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 40 s. Finally, at the end of the PCR cycles, melting curves were described to confirm the specificity of the expected PCR products. Each reaction was performed in triplicate for analysis.
miR-155 expression levels were calculated by the threshold cycle (ΔCT) method: ΔCT = mean value CT (miR-155) – mean value CT (reference U6 snRNA). The relative expression corresponded to the 2−ΔCT value and was presented as median and interquartile range (IQR). The statistical significance between the groups was determined using a Mann-Whitney U test (α = 0.05). Statistical analysis was done using the Statistical Program for Social Sciences (SPSS; version 15.0).
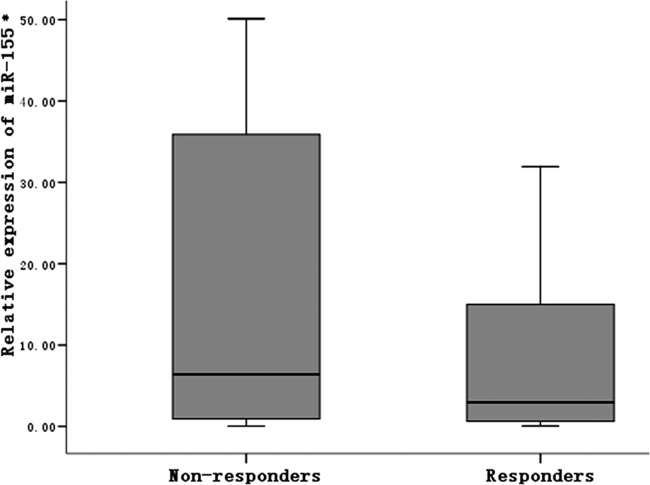
The relative expression level of serum miR-155 in the nonresponder group was 6.40 (IQR, 0.95, 35.95), whereas that in the responder control group was 2.55 (IQR, 0.61, 15.56). The difference between the two groups was statistically significant (Z = 2.125, P = 0.034) (Fig. 1).
Fig 1.
Serum miR-155 levels after HBV vaccination in nonresponder and normal control groups. *, relative expression level of serum miR-155: 2− ΔCT.
miR-155 is an miRNA with multiple functions. It is an indispensable regulatory factor in normal immune responses (5). The results of the present study show that the serum miR-155 level of hepatitis B vaccine nonresponders was higher than that of normal controls. Previous studies have suggested that the miR-155 plays a positive regulatory role in immune response (5, 6). However, in the current study, we observed that miR-155 may suppress the immune response to hepatitis B vaccine. Although the molecular mechanism through which miR-155 regulates the immune response to hepatitis B vaccine remains to be characterized, several published studies have provided some important clues for us. Mao et al. showed that, in the immune response of mice to DNA vaccine, miR-155 expression was induced during the maturation of dendritic cells (DCs), and it inhibited the expression of genes related to lymph node migration, antigen presentation, and T cell activation in DCs, thus inhibiting a T cell-mediated immune response (7). Ceppi et al. have shown that miR-155 can regulate TAB2 in a targeted manner. In the presence of lipopolysaccharide (LPS), miR-155 expression in monocyte-derived DCs (moDCs) is significantly elevated, inhibiting the production of inflammatory cytokines. This serves as a negative feedback loop during the late phase of DC activation (8). Charrier et al. reported that miR-155 was involved in a posttranscriptional downregulation of the Toll-like receptor 9/interferon regulatory factor 7 signaling pathway in plasmacytoid DCs (9). These evidences suggest that overexpression of miR-155 inhibits DC-mediated T cell activation and the production of inflammatory cytokines.
HBsAg is the main antigen component of hepatitis B vaccine. For it to activate immune response, Th cells and corresponding cytokines must be involved. We hypothesize that increased miR-155 expression may dampen the generation of T cell-mediated immune responses and result in failure to respond to hepatitis B vaccine.
In summary, our results provided the first evidence that overexpression of serum miR-155 is correlated with nonresponsiveness to hepatitis B vaccine. The data support the need for further studies focusing on the regulation of miRNAs on the immune response to human vaccination, and they may lead to new ways of immune modulation for nonresponsiveness to human vaccination.
ACKNOWLEDGMENTS
This work was partly supported by grants from the Natural Science Foundation of Guangdong (s2011040002978), Guangdong Medical Research Foundation (A2012422), and Science Foundation of Dongguan (201010815214).
We have no commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Roukens AH, Visser LG. 2011. Hepatitis B vaccination strategy in vaccine low and nonresponders: a matter of quantity of quality? Hum. Vaccin. 7:654–657 [DOI] [PubMed] [Google Scholar]
- 2. Filipowicz W, Bhattacharyya SN, Sonenberg N. 2008. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9:102–114 [DOI] [PubMed] [Google Scholar]
- 3. Chen CZ, Li L, Lodish HF, Bartel DP. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science 303:83–86 [DOI] [PubMed] [Google Scholar]
- 4. Gantier MP, Sadler AJ, Williams BR. 2007. Fine-tuning of the innate immune response by microRNAs. Immunol. Cell Biol. 85:458–462 [DOI] [PubMed] [Google Scholar]
- 5. Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. 2007. Regulation of the germinal center response by microRNA-155. Science 316:604–608 [DOI] [PubMed] [Google Scholar]
- 6. Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. 2009. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao CP, He L, Tsai YC, Peng S, Kang TH, Pang X, Monie A, Hung CF, Wu TC. 2011. In vivo microRNA-155 expression influences antigen-specific T cell-mediated immune responses generated by DNA vaccination. Cell Biosci. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. 2009. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 106:2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charrier E, Cordeiro P, Cordeau M, Dardari R, Michaud A, Harnois M, Merindol N, Herblot S, Duval M. 2012. Post-transcriptional down-regulation of Toll-like receptor signaling pathway in umbilical cord blood plasmacytoid dendritic cells. Cell. Immunol. 276:114–121 [DOI] [PubMed] [Google Scholar]
- 10. Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. 2008. Real-time PCR quantification of precursor and mature microRNA. Methods 44:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]