Abstract
Melioidosis is a severe infectious disease caused by Burkholderia pseudomallei. It is highly resistant to antibiotic treatment, and there is currently no licensed vaccine. Burkholderia thailandensis is a close relative of Burkholderia pseudomallei but is essentially avirulent in mammals. In this report, we detail the protective efficacy of immunization with live B. thailandensis E555, a strain which has been shown to express an antigenic capsule similar to that of B. pseudomallei. Immunization with E555 induced significant protection against a lethal intraperitoneal B. pseudomallei challenge in a mouse model of infection, with no mice succumbing to infection over the course of the study, even with challenges of up to 6,000 median lethal doses. By comparison, mice immunized with B. thailandensis not expressing a B. pseudomallei-like capsule had significantly decreased levels of protection. E555-immunized mice had significantly higher levels of IgG than mice immunized with noncapsulated B. thailandensis, and these antibody responses were primarily directed against the capsule.
INTRODUCTION
Burkholderia pseudomallei is a Gram-negative bacterium which can be readily isolated from soil and standing water in tropical regions. It is a facultative intracellular pathogen and is the etiological agent of melioidosis, a disease associated with a high case-mortality rate in regions where it is endemic. Diagnosis and treatment are complicated by the presentation of melioidosis in varied clinical forms, ranging from acute septicemic infection to skin abscesses (1). Chronic or latent infections are also common, with reactivation potentially occurring decades after exposure. Natural infection is primarily thought to be as a result of percutaneous inoculation or inhalation of aerosolized bacteria. The latter represents a route of infection leading to particularly acute disease, and it is primarily this which has prompted the listing of B. pseudomallei as a category B bioterrorism agent by the Centers for Disease Control and Prevention (2) and as a tier 1 select agent in the United States. Furthermore, B. pseudomallei is intrinsically insensitive to many antibiotics (3); even with appropriate therapy the mortality rate in areas of endemicity can be over 40% (4). The need for prevention in the form of a vaccine is thus of importance.
Significant effort has been expended in the identification of vaccine candidates for protection against melioidosis, but thus far none are nearing licensure (reviewed in reference 5). The most efficacious candidates to date have been live vaccines based on attenuated strains of B. pseudomallei, of which a number have been described in the literature (6–13). However, these vaccines have not induced sterilizing immunity in animal models and, more importantly, there are safety concerns with the use of live attenuated vaccines due to the potential for reversion to a fully virulent phenotype. An alternative approach that cannot lead to reversion to virulence would be to use the closely related species Burkholderia thailandensis. This species has a high degree of genetic and serological identity with B. pseudomallei but has demonstrably low virulence in animal models (14) and is rarely reported to cause disease in humans. When this does occur it is often associated with a traumatic event or reduced immunocompetence (15, 16). However, despite the obvious potential, to date the reported study that used live B. thailandensis as a vaccine did not represent a successful avenue of research; only 50% of animals were protected following immunization with B. thailandensis in a guinea pig model of melioidosis (17). It is probable that the lack of the manno-heptopyranose capsule, a key virulence factor and immunogenic molecule present in B. pseudomallei (18, 19) but absent from B. thailandensis, was a significant cause of the failure of B. thailandensis to induce protection in this model. Capsular polysaccharides represent good vaccine candidates and a number are used in licensed vaccines, including those protecting against infections caused by Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae (20).
In this report, we detail the use of B. thailandensis strains as live vaccines in a mouse model of melioidosis. Significant protection was induced by immunization with both of the B. thailandensis strains used. However, immunizations with the recently isolated B. thailandensis strain E555 (21), which has been shown by monoclonal antibody reactivity to express an antigenic capsule similar to the manno-heptose capsule of B. pseudomallei, induced significantly better protection than immunization with a noncapsulated B. thailandensis.
MATERIALS AND METHODS
Bacterial strains.
The bacteria used in this study were B. thailandensis strains E555 (21) and CDC2721121 (15), B. pseudomallei K96243 and K96243 ΔwcbH (22), and Francisella tularensis subsp. holarctica HN63. B. thailandensis and B. pseudomallei strains were routinely cultured in Luria broth (L broth) and enumerated on L agar with growth at 37°C. F. tularensis was cultured and enumerated on blood cysteine glucose agar plates supplemented with 0.1% histidine at 37°C. B. pseudomallei and F. tularensis were handled under Advisory Committee on Dangerous Pathogens (ACDP) containment level 3 conditions.
Genetic comparison.
The genetic relatedness of B. thailandensis strains (E264, E555, and CDC2721121) to B. pseudomallei K96243 was estimated based on the multilocus sequence typing (MLST) loci (http://bpseudomallei.mlst.net). Similarity metrics were generated by a k-mer (k = 10)-based approach (23).
Western blot analysis.
Strains were examined for antigen production using Western hybridization. Heat-killed extracts of the Burkholderia strains were prepared as previously described (24). Following this, samples were treated with 2 mg/ml proteinase K at 50°C for 90 min and separated on Novex 4 to 20% Tris-glycine SDS gels (Life Technologies, Paisley, United Kingdom) according to the manufacturer's instructions. The samples were next blotted to nitrocellulose membranes using the Novex electrotransfer system (Life Technologies) according to the manufacturer's instructions and probed as previously described (25) using capsule- and O-antigen-specific antibodies (detailed in Results) but using SigmaFast DAB (Sigma, Gillingham, United Kingdom) for development of the membranes.
Animal studies.
Animal studies were performed using 6- to 8-week-old female BALB/c mice (Charles River, United Kingdom). Animals were held in groups of five with free access to food and water under a 12-h light/dark cycle. Details of immunizations and challenges are in Results. After immunization with B. thailandensis via the intraperitoneal (i.p.) route, the mice were handled in primary containment under ACDP containment level 2 conditions within a flexible-film isolator. After challenge with B. pseudomallei or F. tularensis, animals were handled under ACDP containment level 3 conditions within a rigid-wall half-suit isolator. All investigations involving animals were carried out according to the requirements of the Animal (Scientific Procedures) Act 1986. For organ bacterial enumeration, animals were culled via cervical dislocation according to schedule 1 of the Animal (Scientific Procedures) Act 1986, and organs were removed. These were mashed through 40-μm sieves into phosphate-buffered saline (PBS), serially diluted, and plated onto L agar.
Analysis of antibody responses.
Approximately 100 μl of blood was removed from the tail veins of mice 5 weeks after immunization with B. thailandensis. After the blood was allowed to clot at 4°C the serum was removed and stored at −20°C. The responses directed against B. pseudomallei K96243 were assessed by an enzyme-linked immunosorbent assay (ELISA) as described previously (26), with wells coated with approximately 1 × 107 CFU of the appropriate heat-inactivated (>80°C for 4 h) B. pseudomallei strain. The concentrations of B. pseudomallei-specific antibodies were calculated by comparison to a standard curve generated using mouse IgG as previously described (26).
Data analysis.
All graphs were produced using the program GraphPad Prism V5.0. All analyses were performed using the statistical analysis package SPSS version 18. Survival data were analyzed using a log rank test stratified by bacterial challenge dose or experiment. Bacterial burden data were transformed to the logarithm of 10, to better fit the requirements for parametric analysis, and compared using a univariate general linear model with Bonferroni's posttests to compare vaccination groups and take account of experimental runs. Antibody data were transformed to the logarithm of 10, to better fit the requirements for parametric analysis, and compared using a univariate general linear model to compare vaccination groups and take account of experimental runs.
RESULTS
B. thailandensis E555 expresses capsular antigen.
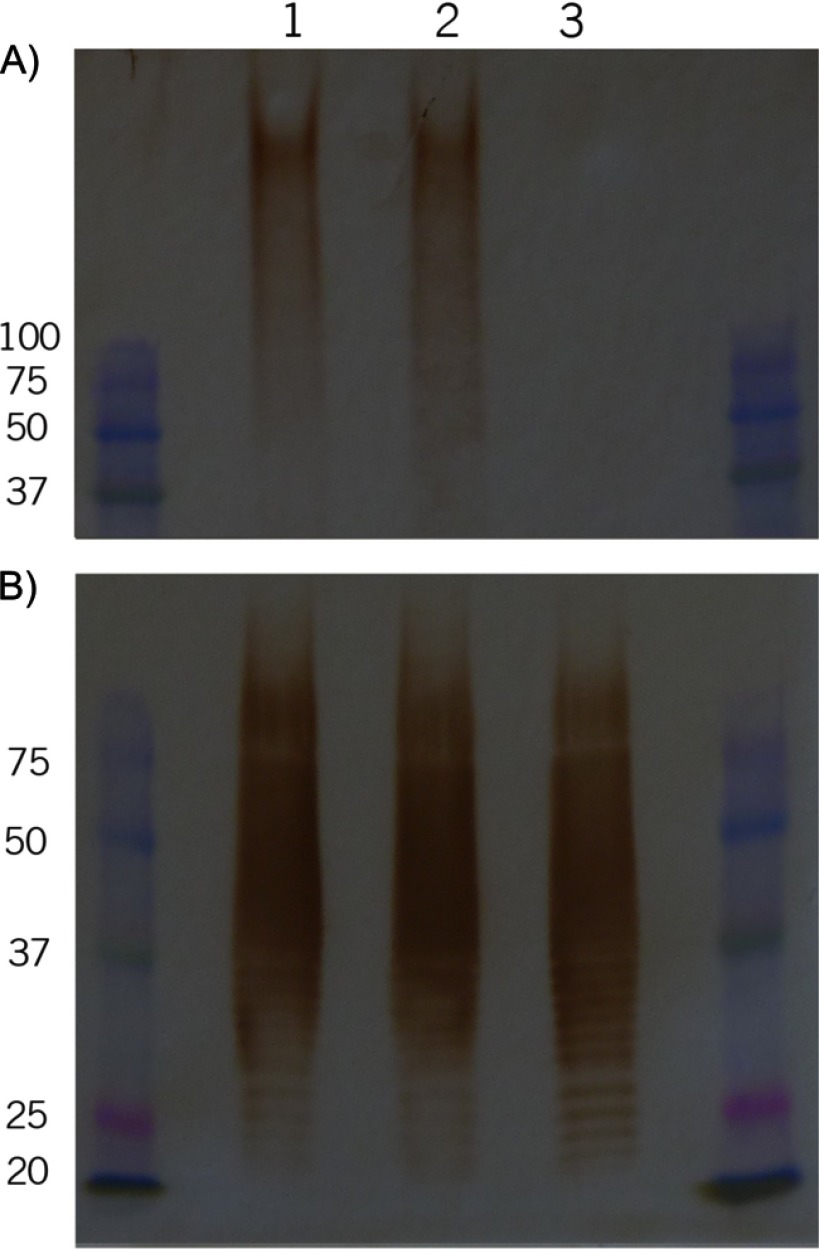
B. thailandensis E555 has previously been shown to express a capsular antigen which is similar to the manno-heptose capsule of B. pseudomallei (21). To confirm in vitro expression of this capsule prior to work involving animals, samples of heat-inactivated bacteria were examined using the capsule-specific monoclonal antibody 4VIH12 (27). Strain CDC2721121 was chosen to be the control noncapsulated B. thailandensis throughout this study. This strain is closely related to E555 and together these two strains are part of a subgroup which is genetically distinct from other B. thailandensis isolates (21). Similarity metrics generated using MLST loci indicate that strains E555 (68.89) and CDC2721121 (68.84) are both genetically more similar to B. pseudomallei K96243 than the B. thailandensis E264 reference strain (67.13). Immunodetection using monoclonal antibodies specific for the manno-heptose capsule (28) evidenced high-molecular-weight material in the B. pseudomallei K96243 and B. thailandensis E555 samples which was absent in noncapsulated B. thailandensis CDC2721121, indicating the production of manno-heptose antigen in B. thailandensis E555 (Fig. 1A).
Fig 1.
Expression of surface polysaccharide antigens. Heat-killed extracts of B. pseudomallei K96243 (lane 1), B. thailandensis E555 (lane 2), and B. thailandensis CDC2721121 (lane 3) following separation by SDS-PAGE, transfer to membranes, and hybridization with antibodies recognizing B. pseudomallei capsule (A) and B. pseudomallei type A lipopolysaccharide (LPS) (B). Marker sizes are in kDa.
It has been shown that, whereas there is only a single serotype of capsule, there are several serotypes of O antigen in B. pseudomallei (29) and slight modifications to O-antigen structure in B. mallei which affect antibody recognition (30). There is a robust immune response to the different O antigens which may complicate analysis of protection data if the vaccine strains express different O antigens, and as such the serotypes of the various strains in this study were assessed. The genome sequence of E555 indicates that it should express the “typical” O antigen of B. pseudomallei (type A according to the scheme of Anuntagool et al. [29]), which is found in the majority of B. pseudomallei isolates, including K96243 (29). This was confirmed through Western blot analysis using in-house-generated rabbit polyclonal antibodies recognizing B. pseudomallei O antigens type A and type B and monoclonal antibodies recognizing B. mallei O antigen (28). Strain CDC2721121 similarly expressed the type A O antigen (Fig. 1B), indicating that there are no identifiable antigenic differences between the O antigens of E555 and CDC2721121 which could complicate the study.
B. thailandensis E555 is rapidly cleared from BALB/c mice.
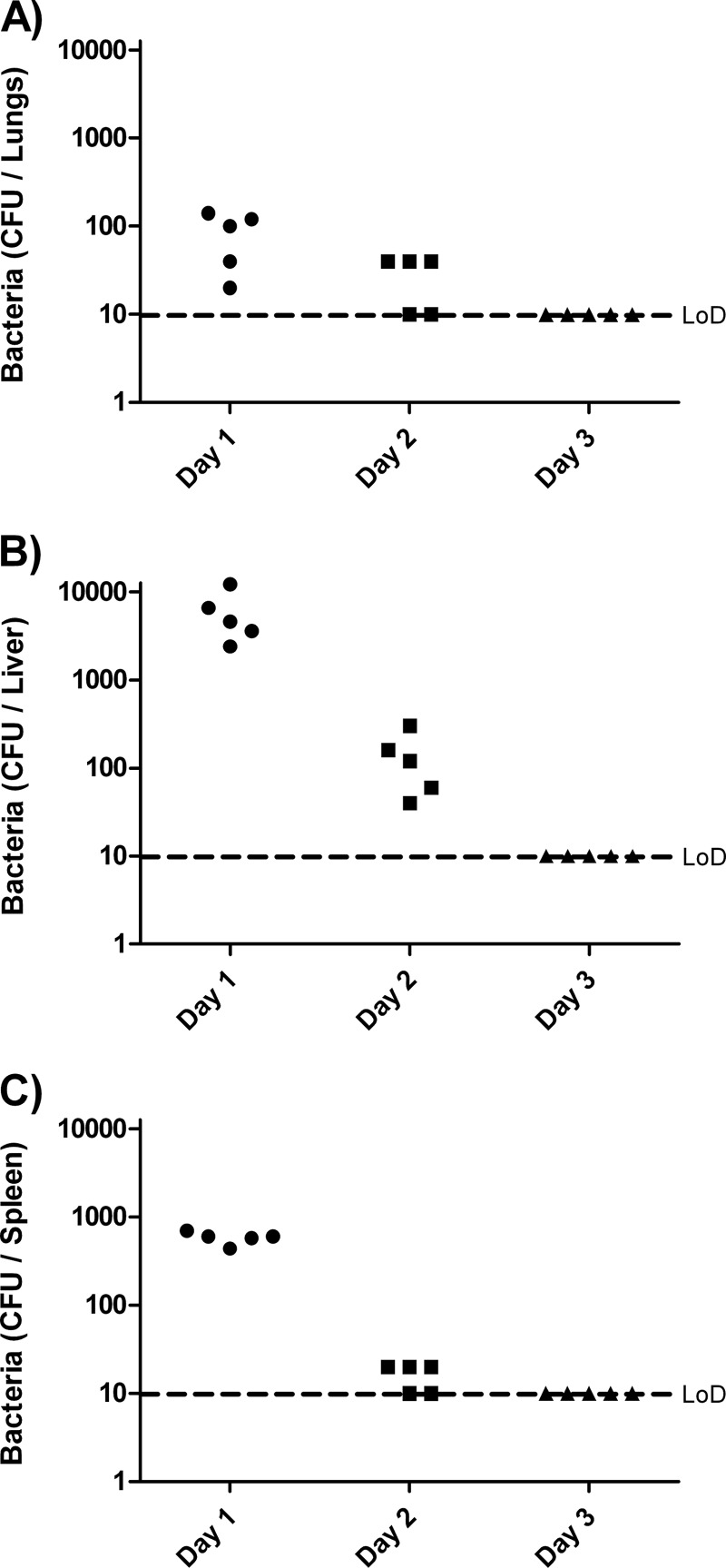
B. thailandensis E555 has previously been shown to be essentially avirulent in mice, with no mortality observed following inoculations of up to 106 CFU via the intranasal (i.n.) route (21). These mice had cleared the bacteria within 14 days. In order to examine the kinetics of B. thailandensis E555 survival following i.p. inoculation, groups of 5 mice were inoculated via the i.p. route with 1 × 106 CFU of B. thailandensis E555 and culled at selected time points thereafter, and organs were taken for bacterial enumeration (Fig. 2). Dissemination of B. thailandensis to organs was rapid; 24 h after inoculation bacteria were recovered from the lungs, liver, and spleen of all mice, with the liver being the most heavily contaminated organ, having counts of up to 1 × 104 CFU per organ. Clearance of the bacteria was equally rapid; by 48 h postinoculation bacterial counts had dropped by at least 2 logs in the liver and only isolated colonies were recovered from the lungs and spleen, and 72 h postinoculation and thereafter no bacteria were detected in any organs tested (our detection limit was ∼1 × 101 CFU/organ). Mice culled 35 days after inoculation similarly had no detectable B. thailandensis and visual examination revealed no obvious organ pathology which indicated an infection had developed.
Fig 2.
Clearance of B. thailandensis E555 from BALB/c mice. Mice were inoculated with ∼1 × 106 CFU of B. thailandensis E555 at day 0 and culled at selected points postinfection. Bacteria in the lungs (A), liver (B), and spleen (C) were enumerated. The limit of detection (LoD) is ∼10 CFU per organ.
Capsule-specific antibodies dominate humoral responses.
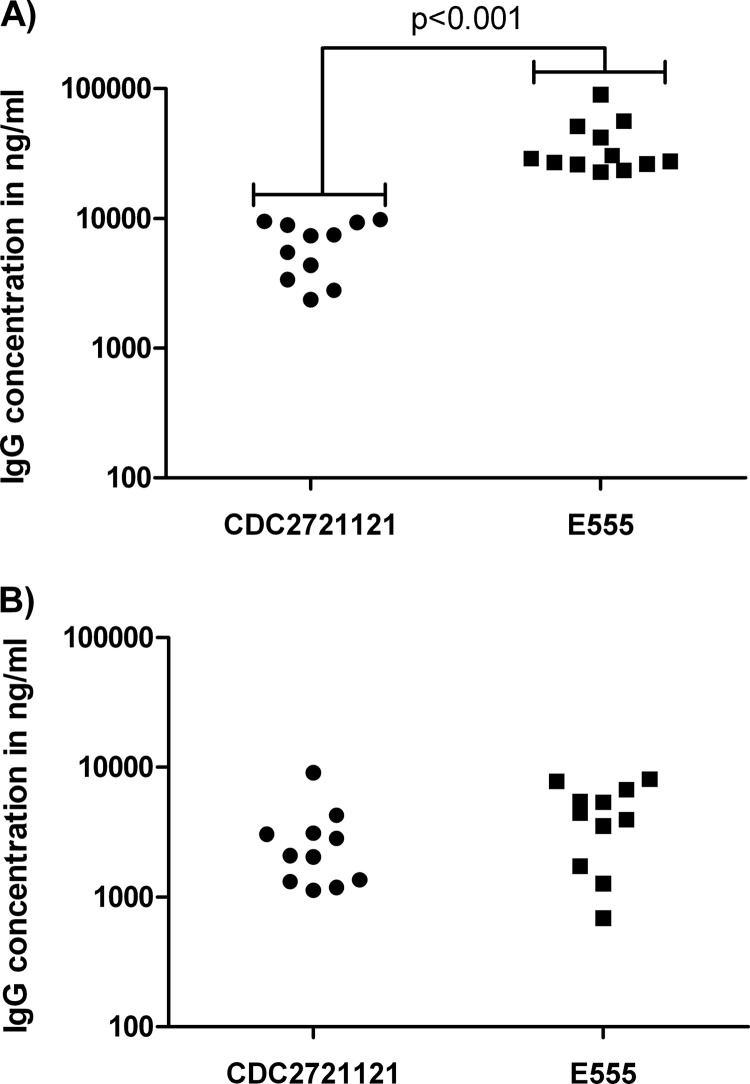
To examine the development of antibody responses in immunized mice, tail bleeds were performed 5 weeks after immunization and sera recovered to measure antigen-specific responses. Sera from PBS-immunized mice did not recognize heat-killed B. pseudomallei K96243, whereas sera from E555-immunized mice had a geometric mean B. pseudomallei-specific IgG concentration of 34,280 ng/ml. Interestingly, mice immunized with CDC2721121 had a geometric mean B. pseudomallei-specific IgG concentration of only 5,747 ng/ml, a statistically significant difference (P < 0.001) compared to the E555-induced IgG concentrations (Fig. 3A). In order to ascertain whether this difference was primarily due to capsule-specific antibodies generated only with E555 immunization, ELISAs were performed using capsule-deficient B. pseudomallei K96243 ΔwcbH as the antigen (Fig. 3B). The geometric mean IgG concentrations in the two vaccine groups using this antigen (2,316 ng/ml and 3,560 ng/ml for CDC2721121 and E555, respectively) were not significantly different from each other (P = 0.178).
Fig 3.
IgG concentration in immunized BALB/c mice. Serum was recovered from mice 35 days after immunization with B. thailandensis CDC2721121 or E555, and the levels of IgG antibodies specific for wild-type B. pseudomallei K96243 (A) and noncapsulated B. pseudomallei K96243 ΔwcbH (B) were determined by ELISA. Each point represents the pooled sera from a group of 5 mice. Significance levels are indicated.
Immunization with capsulated B. thailandensis elicits protective immunity.
Groups of mice were immunized with approximately 5 × 105 CFU of E555 or CDC2721121 or with PBS via the i.p. route and 42 days thereafter were challenged with 6 × 104 CFU (∼60 minimal lethal dose [MLD]), 6 × 105 CFU (∼600 MLD), or 6 × 106 CFU (∼6,000 MLD) of B. pseudomallei K96243 via the i.p. route. This route of infection leads to highly acute disease in BALB/c mice and, as expected, the mice immunized with PBS rapidly succumbed to the infection prior to the end of the experiment, with only a single survivor in the lowest-challenge group. Mice immunized with the noncapsulated B. thailandensis CDC2721121 were protected against the 60- and 600-MLD challenges but not against the 6,000-MLD challenge, where only one of five mice survived to the end of the study. In contrast, all of the mice immunized with B. thailandensis E555 survived to the end of the study irrespective of the challenge dose (Table 1). Analysis of these data using a stratified (by challenge) log rank test indicated that immunization with either CDC2721121 or E555 provided significant protection compared to the PBS-immunized mice (P < 0.0001). Furthermore, immunization with capsulated B. thailandensis E555 induced significantly better protection at day 35 than immunization with B. thailandensis CDC2721121 (P < 0.05).
Table 1.
Survival of immunized mice following challenge with B. pseudomallei K96243a
| Immunizing B. thailandensis strainb | % survival after challenge with B. pseudomallei at a dose (CFU) of: |
||
|---|---|---|---|
| 6 × 104 | 6 × 105 | 6 × 106 | |
| PBS (control) | 20 | 0 | 0 |
| CDC2721121 | 100 | 100 | 20c |
| E555 | 100 | 100 | 100 |
Survival at day 35 postchallenge (n = 5 mice for each group).
Immunizations via the i.p. route with ∼5 × 105 CFU per mouse.
The median time to death was significantly increased in this group compared to that in the PBS group (P = 0.0495).
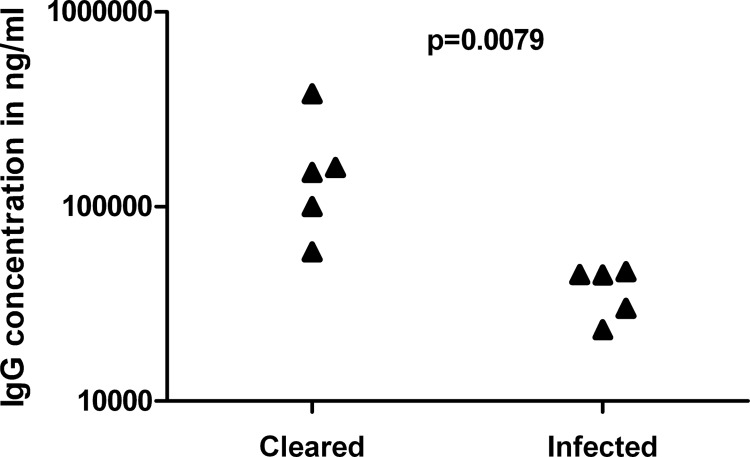
At the end of the study, the spleens of surviving immunized mice were recovered and bacterial burdens determined. The lowest challenge dose (6 × 104 CFU) was cleared in all groups, at least beyond our limit of detection (∼1 × 101 CFU/spleen). Higher challenge doses resulted in a mix of mice displaying clinical signs of infection, abscesses on the spleens, and high splenic burden (between 2.9 × 104 CFU/spleen and 3.5 × 107 CFU/spleen) and mice displaying no clinical signs, no obvious organ pathology on visual inspection, and no bacteria present. Fifty percent of the mice receiving E555 as a vaccine and a challenge of 6 × 105 or 6 × 106 CFU had no detectable bacteria in spleens compared with 30% for the surviving CDC2721121-immunized mice receiving the same challenges. There was a correlation between the ability of the E555-immunized mice to clear infection and the prechallenge anti-B. pseudomallei IgG concentration (Fig. 4).
Fig 4.
Correlation between IgG concentration and clearance of infection. Mice were immunized with B. thailandensis E555 and challenged with either 6 × 105 or 6 × 106 CFU of B. pseudomallei K96243. One week prior to challenge, sera were recovered and the concentration of anti-B. pseudomallei IgG was determined and correlated against clearance of the B. pseudomallei infection at day 35 postchallenge. Each point represents a mouse. The significance level is indicated.
Protection conferred by capsulated B. thailandensis is specific.
It is known that B. pseudomallei establishes persistent infections and, although no such phenotype has been documented for B. thailandensis and the clearance studies performed in this work detail rapid clearance of the E555 inoculation, it is conceivable that the protection observed against B. pseudomallei may have been due to low levels of B. thailandensis persisting in the host and stimulating an innate immune response. In order to fully discount this possibility, we immunized a group of 5 mice with B. thailandensis E555 as previously described and subsequently challenged the mice with approximately 1,000 CFU (1,000 × MLD) of F. tularensis HN63 by the i.p. route. It has been shown previously that administration of synthetic CpG DNA can induce nonspecific protective responses in mice that are capable of protecting against i.p. challenges with up to 1,000 × MLD of F. tularensis subsp. holarctica (31, 32). However, none of the mice in this study survived beyond day 4 postchallenge, and the mice immunized with E555 succumbed at the same rate as a group of 5 mice immunized with PBS rather than B. thailandensis (data not shown). In order to demonstrate that those mice receiving the E555 immunization did elicit an immune response against B. thailandensis E555, sera taken from the immunized mice was assessed for antibody levels. The IgG response of these mice was not significantly different from those of the mice surviving the B. pseudomallei challenge.
Immunization with capsulated B. thailandensis promotes early control of bacterial numbers.
To ascertain whether vaccine-induced immunity acted early in the infection process, immunized BALB/c mice were challenged with 6 × 104 CFU of B. pseudomallei and culled after 24 h, and spleens were removed to determine the bacterial burden (Fig. 5). Mice immunized with PBS had geometric mean splenic counts of 1.51 × 104 CFU/spleen. Mice immunized with CDC2721121 displayed lower counts with a geometric mean of 2.78 × 103 CFU/spleen, but these counts were not significantly lower than those from the mice receiving PBS as a vaccine. In contrast, those mice receiving E555 as a vaccine had a geometric mean count of 1.97 × 102 CFU/spleen, significantly lower than both the PBS-immunized and CDC2721121-immunized mice (P < 0.001 and P < 0.01, respectively), including four mice (40%) from which no bacteria were isolated at all.
Fig 5.
Splenic bacterial burden 24 h after challenge. Mice were immunized as indicated on the x axis, challenged with 6 × 104 CFU of B. pseudomallei K96243, and culled after 24 h, and the splenic bacterial burden was assessed. Each point represents a mouse. Significance levels are indicated. The limit of detection (LoD) was ∼10 CFU per spleen.
DISCUSSION
B. thailandensis strain E555 represents an important asset in the search for a vaccine against melioidosis. Live bacterial vaccines are in use today, notably the BCG vaccine protecting against tuberculosis; however, the appetite for licensing new live vaccines is low due to uncertainty regarding safety and production. B. thailandensis is a relatively harmless bacterium with very few instances of human infection reported, and in these cases it was generally associated with traumatic events or potentially reduced immunocompetence (15, 16). Similarly, animal models indicate that to establish disease with B. thailandensis requires considerable numbers of bacteria (14, 19, 21, 33–35). The data presented in this report suggest that this strain of B. thailandensis in particular seems to be less virulent than most B. thailandensis isolates; certainly it is cleared from the host rapidly. B. thailandensis E264 delivered to C57BL/6 mice via aerosol is found at high levels in distant organs for at least 3 days after challenge (33), and highly attenuated B. pseudomallei bacteria are able to persist in organs for several weeks after infection (7, 8, 11). Contrastingly, B. thailandensis E264 mutants lacking a fully functional bsa (Burkholderia secretion apparatus)-encoded type III secretion system is effectively cleared from organs within 3 days (33). Although B. thailandensis E555 does not have any obvious defects in its bsa type III secretion system, this comparison might suggest that E555 is attenuated compared to typical B. thailandensis isolates such as E264, which are themselves considered to be essentially avirulent, and that E555 might be a favorable option from a safety perspective.
The presence of capsular antigen appears to be important in the success of E555 as a vaccine for melioidosis. Noncapsulated B. thailandensis CDC2721121 elicits responses that provide a degree of protection, but the presence of capsular antigen is necessary for the superior protection seen with E555, although it must be made clear here that E555 and CDC2721121 are not isogenic strains and there could be other antigenic differences between them. These data are in line with previous observations regarding the importance of capsular antigen in a live vaccine setting; B. thailandensis lacking capsule (36) and B. pseudomallei mutants lacking capsule fail to provide full protection when used as live vaccines (27), whereas auxotrophic mutants retaining the capsule provide excellent levels of protection (7–9, 13). Analysis of antibody concentrations in the vaccinated mice in this study supports the importance of the capsule as an immuno-dominant protective antigen. The CDC2721121- and E555-immunized mice had similar levels of antibodies directed against noncapsule antigens, and yet the CDC2721121-immunized mice failed to adequately control infection with higher challenges. In contrast, more than half of the antibody responses in the E555-immunized mice were directed against the capsule and these mice were well protected against high challenges. The exact mechanism of protection observed in this study remains to be fully elucidated, and it is probable that this is multifactorial in nature, utilizing both cell-mediated and antibody responses. Antibodies are known to protect to a degree when used passively in animals (25, 37–41), and certain types of antibody are correlated with better clinical outcomes in humans (42). The complete protection induced by immunization with E555 precluded correlation between antibody levels and survival in this study, although it is of interest to note that the single surviving CDC2721121-immunized mouse in the group challenged with 6 × 106 CFU B. pseudomallei also had the highest concentration of anti-B. pseudomallei IgG in that group. There was a strong correlation between the ability of E555-immunized mice to clear the infection and their anti-B. pseudomallei IgG concentrations (P = 0.0079); the five highest antibody concentrations were in the five mice clearing infection. This points to an important role for antibodies in the protection observed in these studies and may suggest antibodies be examined in more detail as potential correlates of protection.
B. pseudomallei is well known to cause chronic infections, characterized by abscesses of the spleen in particular, so the ability of these immunizations to induce what is, at least to our ability to detect, a sterilizing immunity with no chronic infection in 50% of the mice challenged with at least 6 × 105 CFU of B. pseudomallei is encouraging. Previous studies using attenuated B. pseudomallei live vaccines have revealed that survivors are generally all colonized at the end of the study (9, 13). Clearly it is highly desirable for a vaccine to prompt clearance of infection from all individuals, and in this study that was not achieved. However, there is considerable scope to optimize this vaccine, through prime-boost strategies for example (6, 9), and to use the vaccine in combination with conventional antibiotic therapy to promote bacterial clearance, approaches which are currently being examined. The combination of antibiotics and vaccines may be a particularly promising approach. It should be noted that previous work has indicated that protection levels may vary depending on the route of challenge, with intravenous challenges overcoming immunity capable of protecting against intraperitoneal challenges (13). Future work will examine whether the E555-induced protection documented against an intraperitoneal challenge extends to inhalational challenges.
Early elimination of infecting bacteria is obviously beneficial since it will prevent disease from occurring, and melioidosis seems to progress fairly independently of any treatment once established. It is known that mice which have an innate resistance to melioidosis, such as C57BL/6 mice, are capable of clearing most bacteria within a few days of infection, whereas highly susceptible strains such as BALB/c fail to adequately control bacterial numbers early in the infection and thus develop melioidosis with lower bacterial challenges (43, 44). Our data from mice culled 24 h after challenge indicates that vaccination with E555 induced responses that showed significantly better limitation of infection within the critical first few hours than the CDC2721121-induced responses. Interestingly, although all of the E555-immunized mice survived challenges of >6 × 105 CFU B. pseudomallei, 50% of them did have high numbers of bacteria colonizing their spleens and would presumably therefore succumb to melioidosis at some point. The remaining 50% had cleared the infection, at least beyond our ability to detect. This figure is not dissimilar to the 40% clearance seen after 24 h and indicates that early clearance may be the key to longer-term sterilizing immunity.
In summary, our data indicate that the use of live B. thailandensis induces protective responses but that the best protection is observed with the use of B. thailandensis expressing capsular polysaccharide. This further demonstrates the importance of capsular polysaccharide in the generation of a protective immune response against B. pseudomallei. Furthermore, we have identified B. thailandensis strain E555 as a potentially safe and efficacious vaccine for use in protection against melioidosis.
ACKNOWLEDGMENT
This work was supported by funding from the United Kingdom Ministry of Defense.
Footnotes
Published ahead of print 15 May 2013
REFERENCES
- 1. Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4:272–282 [DOI] [PubMed] [Google Scholar]
- 4. Chaowagul W, Suputtamongkol Y, Dance DAB, Rajchanuvong A, Pattara J, White NJ. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181–1185 [PubMed] [Google Scholar]
- 5. Patel N, Conejero L, De RM, Easton A, Bancroft GJ, Titball RW. 2011. Development of vaccines against Burkholderia pseudomallei. Front. Microbiol. 2:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haque A, Chu K, Easton A, Stevens MP, Galyov EE, Atkins T, Titball R, Bancroft GJ. 2006. A live experimental vaccine against Burkholderia pseudomallei elicits CD4+ T cell-mediated immunity, priming T cells specific for 2 type III secretion system proteins. J. Infect. Dis. 194:1241–1248 [DOI] [PubMed] [Google Scholar]
- 7. Stevens MP, Haque A, Atkins T, Hill J, Wood MW, Easton A, Nelson M, Underwood-Fowler C, Titball RW, Bancroft GJ, Galyov EE. 2004. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150:2669–2676 [DOI] [PubMed] [Google Scholar]
- 8. Atkins T, Prior RG, Mack K, Russell P, Nelson M, Oyston PCF, Dougan G, Titball RW. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 70:5290–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norris MH, Propst KL, Kang Y, Dow SW, Schweizer HP, Hoang TT. 2011. The Burkholderia pseudomallei Δasd mutant exhibits attenuated intracellular infectivity and imparts protection against acute inhalation melioidosis in mice. Infect. Immun. 79:4010–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dannenberg AM, Scott EM. 1960. Melioidosis: pathogenesis and immunity in mice and hamsters. III. Effect of vaccination with avirulent strains of Pseudomonas pseudomallei on the resistance to the establishment and the resistance to the progress of respiratory melioidosis caused by virulent strains; all-or-none aspects of this disease. J. Immunol. 84:233–246 [PubMed] [Google Scholar]
- 11. Levine HB, Maurer RL. 1958. Immunization with an induced avirulent auxotrophic mutant of Pseudomonas pseudomallei. J. Immunol. 81:433–438 [PubMed] [Google Scholar]
- 12. Srilunchang T, Proungvitaya T, Wongratanacheewin S, Strugnell R, Homchampa P. 2009. Construction and characterization of an unmarked aroC deletion mutant of Burkholderia pseudomallei strain A2. Southeast Asian J. Trop. Med. Public Health 40:123–130 [PubMed] [Google Scholar]
- 13. Breitbach K, Kohler J, Steinmetz I. 2008. Induction of protective immunity against Burkholderia pseudomallei using attenuated mutants with defects in the intracellular life cycle. Trans. R. Soc. Trop. Med. Hyg. 102:S89–S94 [DOI] [PubMed] [Google Scholar]
- 14. DeShazer D. 2007. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol. Lett. 277:64–69 [DOI] [PubMed] [Google Scholar]
- 15. Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, Godoy D, Spratt BG, Clark TA, Wilkins PP. 2006. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J. Clin. Microbiol. 44:4601–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lertpatanasuwan N, Sermsri K, Petkaseam A, Trakulsomboon S, Thamlikitkul V, Suputtamongkol Y. 1999. Arabinose-positive Burkholderia pseudomallei infection in humans: case report. Clin. Infect. Dis. 28:927–928 [DOI] [PubMed] [Google Scholar]
- 17. Iliukhin VI, Senina TV, Plekhanova NG, Antonov VA, Merinova LK, Seimova IK. 2002. Burkholderia thailandensis: biological properties, identification and taxonomy. Mol. G. Mikrobiol. Virusol. 1:7–11. (In Russian.) [PubMed] [Google Scholar]
- 18. Reckseidler SL, DeShazer D, Sokol PA, Woods DE. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48(Pt 1):317–320 [DOI] [PubMed] [Google Scholar]
- 20. Arnon R. 2011. Overview of vaccine strategies, p 1–20 In Rappuoli R, Bagnoli F. (ed), Vaccine design: innovative approaches and novel strategies, 1st ed. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 21. Sim BMQ, Chantratita N, Ooi WF, Nandi T, Tewhey R, Wuthiekanun V, Thaipadungpanit J, Tumapa S, Ariyaratne P, Sung WK, Sem XH, Chua HH, Ramnarayanan K, Lin CH, Liu YC, Feil EJ, Glass MB, Tan G, Peacock SJ, Tan P. 2010. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 11:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott AE, Twine SM, Fulton KM, Titball RW, Essex-Lopresti AE, Atkins TP, Prior JL. 2011. Flagellar glycosylation in Burkholderia pseudomallei and Burkholderia thailandensis. J. Bacteriol. 193:3577–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goke J, Schulz MH, Lasserre J, Vingron M. 2012. Estimation of pairwise sequence similarity of mammalian enhancers with word neighbourhood counts. Bioinformatics 28:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, Titball RW. 2009. Protective efficacy of heat-inactivated B. thailandensis, B. mallei or B. pseudomallei against experimental melioidosis and glanders. Vaccine 27:4447–4451 [DOI] [PubMed] [Google Scholar]
- 25. Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. 2004. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J. Med. Microbiol. 53:1177–1182 [DOI] [PubMed] [Google Scholar]
- 26. Ngugi SA, Ventura VV, Qazi O, Harding SV, Kitto G, Estes D, Dell A, Titball RW, Atkins TP, Brown KA, Hitchen PG, Prior JL. 2010. Lipopolysaccharide from Burkholderia thailandensis E264 provides protection in a murine model of melioidosis. Vaccine 28:7551–7555 [DOI] [PubMed] [Google Scholar]
- 27. Atkins T, Prior R, Mack K, Russell P, Nelson M, Prior J, Ellis J, Oyston PCF, Dougan G, Titball RW. 2002. Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J. Med. Microbiol. 51:539–547 [DOI] [PubMed] [Google Scholar]
- 28. Ellis J. 2000. Antibody detection of Burkholderia pseudomallei and Burkholderia mallei. Ph.D. thesis Aston University Birmingham, United Kingdom [Google Scholar]
- 29. Anuntagool N, Wuthiekanun V, White NJ, Currie BJ, Sermswan RW, Wongratanacheewin S, Taweechaisupapong S, Chaiyaroj SC, Strisinha S. 2006. Lipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical origins. Am. J. Trop. Med. Hyg. 74:348–352 [PubMed] [Google Scholar]
- 30. Burtnick MN, Brett PJ, Woods DE. 2002. Molecular and physical characterization of Burkholderia mallei O antigens. J. Bacteriol. 184:849–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291–2298 [PubMed] [Google Scholar]
- 32. Klinman DM, Conover J, Coban C. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haraga A, West TE, Brittnacher MJ, Skerrett SJ, Miller SI. 2008. Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect. Immun. 76:5402–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith MD, Angus BJ, Wuthiekanun V, White NJ. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65:4319–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brett PJ, DeShazer D, Woods DE. 1997. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol. Infect. 118:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iliukhin VI, Kislichkin NN, Merinova LK, Riapis LA, Denisov II, Farber SM, Kislichkina OI. 1999. The efficacy and outlook for the study of live vaccines for the prevention of melioidosis. Zh. Mikrobiol. Epidemiol. Immunobiol. 49–51. (In Russian.) [PubMed] [Google Scholar]
- 37. Zhang S, Feng SH, Li B, Kim HY, Rodriguez J, Tsai S, Lo SC. 2011. In vitro and in vivo studies of monoclonal antibodies with prominent bactericidal activity against Burkholderia pseudomallei and Burkholderia mallei. Clin. Vaccine Immunol. 18:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones SM, Ellis JF, Russell P, Griffin KF, Oyston PCF. 2002. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 51:1055–1062 [DOI] [PubMed] [Google Scholar]
- 39. Brett PJ, Woods DE. 1996. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect. Immun. 64:2824–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho M, Schollaardt T, Smith MD, Perry MB, Brett PJ, Chaowagul W, Bryan LE. 1997. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect. Immun. 65:3648–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. AuCoin DP, Reed DE, Marlenee NL, Bowen RA, Thorkildson P, Judy BM, Torres AG, Kozel TR. 2012. Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS One 7:e35386. 10.1371/journal.pone.0035386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charuchaimontri C, Suputtamongkol Y, Nilakul C, Chaowagul V, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Brett PJ, Woods DE. 1999. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin. Infect. Dis. 29:813–818 [DOI] [PubMed] [Google Scholar]
- 43. Hoppe I, Brenneke B, Rohde M, Kreft A, Haussler S, Reganzerowski A, Steinmetz I. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan GYG, Liu Y, Sivalingam SP, Sim SH, Wang D, Paucod JC, Gauthier Y, Ooi EE. 2008. Burkholderia pseudomallei aerosol infection results in differential inflammatory responses in BALB/c and C57BI/6 mice. J. Med. Microbiol. 57:508–515 [DOI] [PubMed] [Google Scholar]