Abstract
Diarrhea is one of the most important bovine diseases. Enterotoxigenic Escherichia coli (ETEC) and bovine viral diarrhea virus (BVDV) are the major causes of diarrhea in calves and cattle. ETEC expressing K99 (F5) fimbriae and heat-stable type Ia (STa) toxin are the leading bacteria causing calf diarrhea, and BVDV causes diarrhea and other clinical illnesses in cattle of all ages. It is reported that maternal immunization with K99 fimbrial antigens provides passive protection to calves against K99 fimbrial ETEC and that BVDV major structural protein E2 elicits antibodies neutralizing against BVDV viral infection. Vaccines inducing anti-K99 and anti-STa immunity would protect calves more effectively against ETEC diarrhea, and those also inducing anti-E2 neutralizing antibodies would protect calves and cattle against diarrhea caused by both ETEC and BVDV. In this study, we used the ETEC K99 major subunit FanC as a backbone, genetically embedded the STa toxoid STaP12F and the most-antigenic B-cell epitope and T-cell epitope predicted from the BVDV E2 glycoprotein into FanC for the multivalent antigen FanC-STa-E2, and examined immunogenicity of this multivalent antigen to assess vaccine potential against bovine diarrhea. Mice intraperitoneally (i.p.) immunized with this multivalent antigen developed anti-K99, anti-STa, and anti-BVDV antibodies. Moreover, elicited antibodies showed neutralization activities, as they inhibited adherence of K99 fimbrial E. coli, neutralized STa toxin, and prevented homologous BVDV viral infection in vitro. Results from this study suggest that this multiepitope fusion antigen can potentially be developed as a vaccine for broad protection against bovine diarrhea and that the multiepitope fusion strategy may be generally applied for multivalent vaccine development against heterogeneous pathogens.
INTRODUCTION
Diarrhea is economically one of the most important diseases in calves and cattle (1–3). Enterotoxigenic Escherichia coli (ETEC) and bovine viral diarrhea virus (BVDV) are among the major causes of bovine diarrhea. ETEC expressing K99 (F5) and/or F41 adhesin and enterotoxins, particularly heat-stable toxin type Ia (STa), is the primary bacterial cause of diarrhea for calves (4–7) and also often for lambs and piglets (8). BVDV, including mainly type 1 (BVDV-1) and type 2 (BVDV-2), causes diarrhea in cattle at all ages and is also responsible for clinical illnesses, such as reproductive loss, respiratory disease, and fetal infections (9–11). The key virulence factors of ETEC in bovine diarrhea are K99 adhesin and STa enterotoxin (12). K99 adhesins mediate attachment and colonization of ETEC at calf small intestines, and STa toxin disrupts fluid homeostasis in host small-intestinal epithelial cells to cause fluid and electrolyte hypersecretion through activation of intracellular guanylate cyclase (13). BVDV is an enveloped and single-stranded RNA virus (14), with its major structural glycoprotein of virus envelope (E2) shown to be most immunogenic and to carry antigenic epitopes that elicit antibodies neutralizing against viral infection (15). K99 and E2 antigens have been the targets in vaccine development against ETEC- and BVDV-associated bovine diarrheal diseases.
K99 and E2 antigens have been demonstrated to induce protective immune responses against ETEC and BVDV, respectively. Pregnant cows immunized with extracted K99 pili had anti-K99 antibodies present in serum, colostrum, and milk; moreover, suckling calves born by the immunized cows were protected against challenge of E. coli strains expressing K99 fimbria (16–19). However, STa antigen has never been included in bovine ETEC vaccine development due to its potent toxicity and poor immunogenicity. In order to be broadly effective, an ETEC vaccine needs to induce anti-adhesin immunity to block bacterial adherence but also antitoxin immunity to neutralize enterotoxicity (20, 21). Recently, studies demonstrated that an STa toxoid, when genetically fused to a strongly immunogenic carrier protein, such as a heat-labile toxoid or E. coli fimbria, elicited protective anti-STa antibodies (22–24). That indicates that STa antigen can be included as an ETEC vaccine component to induce protective antitoxin immunity. Vaccines against BVDV, mainly whole-cell vaccines, including modified live and inactivated vaccines, were developed over half a century ago (25). However, modified live vaccine strains can potentially be sources of in utero infections and cause host immunosuppression, and inactivated vaccines usually do not carry a sufficient amount of viral antigens to induce high titers of antibodies. Alternatively, subunit vaccines, especially with E2 antigens, have become the new target to protect against BVDV (26–28).
In this study, we applied the genetic fusion strategy to use the K99 fimbrial major subunit FanC as the backbone, embed an STa toxoid and a BVDV E2 protein B-cell epitope and T-cell epitope into FanC to construct a multiepitope antigen, FanC-STa-E2, examined this antigen for immunogenicity in a murine model, and assessed its potential as a subunit vaccine against ETEC- and BVDV-associated bovine diarrhea.
MATERIALS AND METHODS
Bacterial and viral strains and plasmids.
E. coli and BVDV strains and plasmids used in this study are listed in Table 1. Recombinant E. coli strain I297, which carries the K99 Fan operon to express K99 fimbriae (29, 30), was used to amplify the fanC gene, to extract K99 fimbriae (as enzyme-linked immunosorbent assay [ELISA] coating antigens to titrate anti-K99 antibodies), and also to serve as the challenge strain for an in vitro antibody adherence inhibition assay. E. coli BL21 (GE Healthcare, Piscataway, NJ) and vector pET28α (Novagen, Madison, WI) were used to express the target multiepitope fusion protein. Plasmid pMAL-p5X (New England BioLabs, Ipswich, MA) was used to clone the BVDV E2 gene for expression of a maltose binding protein (MBP)-E2 fusion protein as an ELISA coating agent to titrate anti-BVDV E2-specific antibodies. The BVDV NADL strain (ATCC VR-534) was used to amplify the BVDV E2 gene and also as a challenge strain in viral neutralizing activity assays.
Table 1.
Bacterial and viral strains and plasmids used in this study
| Strain or plasmid | Relevant property(ies)a | Reference |
|---|---|---|
| E. coli strains | ||
| I297 | Recombinant E. coli strain expressing K99 fimbria | 29 |
| BL21 | B F− ompT hsdS(rB− mB−) gal dcm | GE Healthcare |
| 9196 | BL21/pFanC-STa-E2 | This study |
| 9210 | BL21/pMAL-E2 | This study |
| BVDV strain | ||
| NADL | Bovine viral diarrhea virus 1; NADL strain | ATCC VR-534 |
| Plasmids | ||
| pK99 | pBR322 with a cloned 7.1-kb K99 BamHI fragment | 29 |
| pET28a | Expression vector, His-tagged, T7, lacI, Kanr | Novagen |
| pMAL-p5X | Expression vector with MBP, tac, IacIq, Ampr | New England Biolabs |
| pFanC-STa-E2 | pET28a with a cloned FanC-STa-E2 chimeric fusion | This study |
| pMAL-E2 | pMAL-p5X with a cloned BVDV E2 gene | This study |
Kanr, kanamycin resistant; Ampr, ampicillin resistant.
FanC-STa-E2 chimeric gene construction.
The K99 major structural subunit gene fanC was amplified in a PCR with primers FanCNheI-F2 (5′-ATGATGGCTAGCACACTCCTAGCTATTATCTTAGGT-3′; NheI restriction site underlined) and FanCEagI-R (5′-TCATCGATACGGCCGCAATGTAA-3′; EagI site underlined) and pK99 plasmid as the DNA template. Amplified PCR products were purified through gel electrophoresis, digested with NheI and EagI restriction enzymes (New England BioLabs), and cloned into the expression vector pET28α.
To embed nucleotides encoding the STa toxoid STaP12F (22) and two BVDV E2 epitopes into the fanC gene, we first applied basic antigenic domain prediction algorithms (31–33) and web-based epitope prediction programs (34–36) to find epitopes of the K99 major subunit FanC and the most-antigenic B-cell and T-cell epitopes of the BVDV E2 protein; then we replaced the nucleotide fragment encoding the surface-exposed but less-antigenic FanC epitopes (while the strongly antigenic epitopes were retained to induce anti-K99 immunity) with nucleotides encoding the STa toxoid and the two BVDV E2 epitopes for a FanC-STa-E2 chimeric gene. As described previously (22, 24, 37), splice overlapping extension (SOE) PCRs using specifically designed PCR primers (Table 2) were conducted to generate the chimeric gene. Briefly, we first overlapped two PCR products, one amplified with primers FanCNheI-F and STa12/FanC107-R and the other amplified with primers STa12/FanC91-F and FanCEagI-R, with pK99 plasmid as a DNA template, to replace nucleotides encoding amino acid residues 91 to 107 of FanC with those of STaP12F toxoid for a FanC-STa chimeric gene. To replace nucleotides encoding residues 116 to 126 of FanC with nucleotides encoding the most-antigenic B-cell epitope of BVDV E2 protein, we overlapped another set of two PCR products, one with primers FanCNheI-F and BVDV/FanC126-R and the other with primers BVDV/FanC116-F and FanCEagI-R, with the FanC-STa overlapped product as the DNA template. To replace nucleotides encoding residues 154 to 162 of FanC with those encoding the most-antigenic T-cell epitope of BVDV E2 and to generate a FanC-STa-E2 chimeric gene, we overlapped two PCR products, which were amplified using FanCNheI-F paired with BVDV/FanC162-R and BVDV/FanC154-F paired with FanCEagI-R (Fig. 1A). The final overlapped chimeric gene was digested with NheI and EagI enzymes, cloned into the expression vector pET28α, and verified with DNA sequencing.
Table 2.
PCR primers designed to amplify the fanC gene and to construct the chimeric FanC-STa-E2 genea
| Primer | Nucleotide sequence (5′–3′) |
|---|---|
| FanCNheI-F2 | ATGATGGCTAGCACACTCCTAGCTATTATCTTAGGT |
| FanCEagI-R | TCATCGATACGGCCGCAATGTAA |
| STa12/FanC91-F | GAACTTTGTTGTAATTTTGCCTGTGCTGGATGTGGAAATACTGCTGCTAAAGGATACCAT |
| STa12/FanC107-R | GGCAAAATTACAACAAAGTTCACAGCAGTAAAATGTGTTGTTAGACCAGTCAATACGAGC |
| BVDV/FanC116-F | GCCTTGCCGACCAGTGTGGTATTCGCTAATATTAATACTTCATTCACTACG |
| BVDV/FanC126-R | ACTGGTCGGCAAGGCTCTTGTATGCAAAGTCATATGGTATCCTTTAGCAGC |
| BVDV/FanC154-F | CTACTATACAAAGGGGGCTCTGGTGGATATAAAGCTGGCGTATT |
| BVDV/FanC162-R | GCCCCCTTTGTATAGTAGTTGGTCCAGCTGGGCTGAATAGTTAAATGACT |
| BVD-E2Nde1-F* | ATTTCACATATGCACTTGGATTGAAAACCTGAA |
| BVD-E2BamH1-R* | TCTGTAGGATCCAGGCATAGGTCCGAGTTTGGT |
PCR primers marked with an asterisk were used to produce the MBP-E2 chimeric gene for the recombinant MBP-E2 protein as coating antigens. Underlined nucleotides indicate restriction sites, and italicized nucleotides are of the inserted STaP12F or E2 epitope.
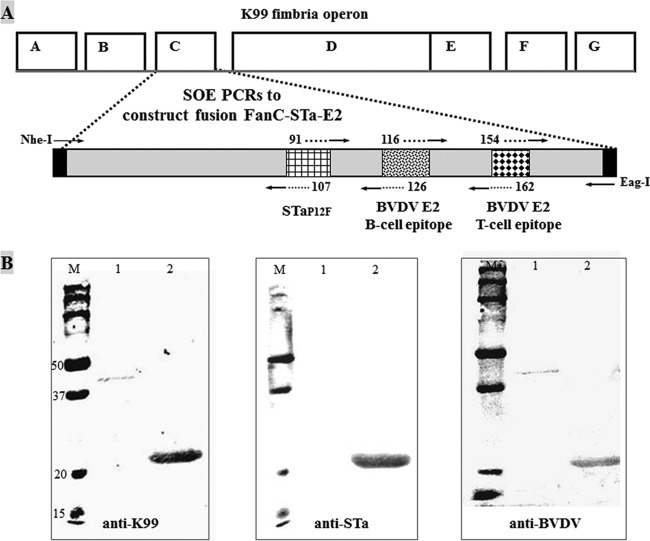
Fig 1.
Construction and detection of the multiepitope FanC-STa-E2 fusion protein. (A) Illustration of genetic structure of the K99 fimbrial operon and the FanC-STa-E2 fusion. The K99 fimbrial major subunit FanC was used as the backbone, with nucleotides encoding the surface-exposed but less-antigenic epitopes replaced with nucleotides encoding STaP12F, the most-antigenic B-cell epitope of BVDV E2, and the most-antigenic T-cell epitope of E2 to construct the multiepitope FanC-STa-E2 fusion at an expected size of 22.5 kDa (with a short peptide of expression vector pET28α). (B) Expressed 6×His-tagged multiepitope FanC-STa-E2 fusion protein was detected in 10 to 12% SDS-PAGE gel using anti-K99 (1:1,000), anti-STa (1:2,500), and anti-BVDV (1:1,000) antibodies. IRDye-labeled goat anti-rabbit IgG (1:5,000; LI-COR, Lincoln, NE) was used as the secondary antibody. M, molecular marker (kDa); 1, E. coli BL21; 2, 9196 (BL21/pFanC-STa-BVDV).
In addition, the E2 gene was PCR amplified with primers BVD-E2NdeI-F and BVD-E2BamH1-R (Table 2). Amplified products were digested with NdeI and BamHI enzymes and ligated into plasmid pMAL-p5X for MBP-E2 fusion protein expression by following the manufacturer's protocol.
Expression and detection of the FanC-STa-E2 fusion protein.
E. coli strain BL21 was transformed with the pET28α plasmid carrying the FanC-STa-E2 chimeric gene for expression of the multiepitope fusion protein. This strain was grown in 5 ml Luria Bertani broth (LB) supplemented with kanamycin (30 μg/ml) at 37°C overnight on a shaker (200 rpm). Overnight growth was added to 500 ml LB medium for continuous incubation until the optical density at 600 nm (OD600) reached 0.5. The culture was then induced with isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) and incubated for 4 more hours. The bacterial culture was centrifuged at 5,000 × g for 20 min, and pellets were suspended in 10 ml bacterial protein extraction reagent (B-PER; in phosphate buffer) (Pierce, Rockford, IL) for total insoluble protein (inclusion body fraction) extraction. Recombinant 6×His-tagged FanC-STa-E2 fusion protein was further extracted from total insoluble protein extracts to a purity of greater than 90% with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Valencia, CA). Purified 6×His-tagged protein was refolded using a protein-refolding kit by following the manufacturer's protocol (Novagen, Madison, WI). Refolded protein was dialyzed in 20 mM Tris-HCl buffer overnight at 4°C and was concentrated (to 1 to 2 mg/ml) using Spectra/Por molecular porous membrane tubing (Spectrum Laboratories Inc., Rancho Dominquez, CA) and polyethylene glycol compound (PEG; Sigma, St. Louis, MO) as previously described (24, 37).
Ten microliters of the refolded protein was analyzed in 10 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assay. Immunoblots were incubated with anti-K99 (lot 092790; 1:1,000 dilution), anti-STa (a gift of D. Robertson at Kansas State University; 1:2,500 dilution), and anti-BVDV (from C. Chase; 1:1,000 dilution) polyclonal antibodies. IRDye-labeled goat anti-rabbit IgG (1:5,000; LI-COR, Lincoln, NE) was used as the secondary antibody. Bound proteins were detected using a LI-COR Odyssey premium infrared gel imaging system (LI-COR).
Mouse immunization with the multiepitope FanC-STa-E2 protein.
Sixteen 6- to 8-week-old female C57BL/6 mice (Charles River Laboratories International, Inc., Wilmington, MA), divided into two groups, were used for the immunization study. Two hundred micrograms of refolded protein (in 200 μl phosphate-buffered saline [PBS]), in an equal amount of Freund's complete adjuvant (Sigma), was injected intraperitoneally (i.p.) into each mouse of the immunization group. For mice of the control group, 200 μl Freund's complete adjuvant and 200 μl 0.02 M Tris-HCl were i.p. injected into each of the 8 mice. Mice received two booster injections with the same dose but with Freund's incomplete adjuvant at a 2-week interval.
Blood and fecal pellets were collected from each mouse before the immunization and weekly after each immunization. Fecal pellets were suspended in fecal reconstitution buffer supplemented with the protease inhibitor phenylmethylsulfonyl fluoride (Sigma) at a ratio of 1:6 (1 g feces to 5 ml buffer) (37). Fecal suspensions were centrifuged, and supernatants were collected. Intestines were collected from each mouse at necropsy, minced with surgical scissors in fecal reconstitution buffer (1 g intestine tissue in 2.5 ml buffer, a 1:3.5 dilution), vortexed, and centrifuged at 10,000 × g for 10 min to collect supernatants as intestinal wash samples. Collected serum, fecal suspension, and intestinal wash samples were stored at −80°C until use. The mouse immunization study complied with the Animal Welfare Act by following the 1996 National Research Council guidelines (38) and was approved and supervised by a state veterinarian and by the South Dakota State University's Institutional Animal Care and Use Committee.
Mouse antibody titration.
Serum, fecal suspension, and intestinal wash samples from each mouse were examined for anti-K99, anti-STa, and anti-E2 IgG and IgA antibodies in ELISAs. To titrate anti-FanC antibodies, heat-extracted K99 fimbriae were used as ELISA coating antigens. K99 fimbriae were extracted based on methods previously described (37, 39). Briefly, K99 fimbrial E. coli strain I297 bacteria, grown on sheep blood agar plates for 18 h at 37°C, were harvested and suspended gently in PBS. The suspension was incubated in a water bath shaker for 40 min at 65°C. While still warm, the suspension was bladed in a grinder for 2 to 3 min. The suspension was then centrifuged for 30 min at 15,000 × g and dialyzed in PBS overnight at 4°C. Fimbriae were precipitated by pH adjustment with acetic acid, collected with centrifugation, and concentrated with PEG. Five hundred nanograms of extracted K99 fimbriae, in 100 μl antigen coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3, pH 9.6), was used to coat each well of a 2HB Immunolon plate (Thermo Scientific, Rochester, NY) for 1 h at 37°C, followed by incubation overnight at 4°C. Coated plates were washed (3×) with PBS with 0.05% Tween 20 (PBST) and blocked with 200 μl 10% nonfat milk-PBST for 1 h at 37°C. After 3 washes with PBST, each well was incubated with serum samples (1:80, diluted in 2.5% milk-PBST), fecal suspension (1:18), or intestinal suspension (1:20) of each immunized or control mouse in a binary dilution for 1 h at 37°C. Wells were washed (5×) with PBST, and 100 μl of 1:3,000-diluted horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma) or 1:1,000-diluted IgA (Sigma, St. Louis, MO) was added at 1 h at 37°C. Wells were washed (3×) with PBST and incubated with a 100-μl TMB Microwell peroxidase substrate system (2-C; KPL, Gaitherburg, MD) for 30 min at room temperature. Optical density (OD) was measured on a plate reader with a 405-nm wavelength.
To titrate anti-STa antibodies, STa-ovalbumin conjugates (as coating agents) and Costar microtiter plates (Corning Inc., Corning, NY) were used in an ELISA as described previously (22, 24). In this study, 10 ng of each STa-ovalbumin conjugate was used to coat each well of an ELISA plate. To titrate anti-BVDV E2 antibodies, 500 ng of each recombinant MBP-E2 fusion protein was used to coat each well of a 2HB plate. Similar to ELISAs to titrate anti-K99 antibodies, serum, fecal suspensions, and intestinal washes of each mouse (in triplicate) were examined for anti-STa- and anti-E2-specific antibodies. HRP-conjugated goat anti-mouse IgG (1:3,000) and IgA (1:1,000) were used as secondary antibodies, and the OD values in each well were measured after 30 min of incubation with TMB peroxidase substrates. OD readings of greater than 0.3 from a highest dilution, after subtraction of background readings, were calculated to antibody titers in a log10 scale as described previously (22) or were directly compared between the immunized group and the control group if the OD was below 0.3 after subtraction of the background reading.
Anti-adhesin antibody adherence inhibition assay.
The human intestine cell line INT-407 (ATCC, CCL-6) and the porcine small intestine cell line IPEC-J1 can both be adhered by K99 fimbrial E. coli bacteria (40), and K99 fimbrial E. coli strain I297 was used to examine elicited antibodies in a bacterial adherence inhibition assay. Similar to the method described previously (23), INT-407 and IPEC-J1 cells (1 × 105) were seeded at each well of a 12-well tissue culture plate containing Dulbecco's modified Eagle's medium (DMEM) with 20% fetal bovine serum (FBS). E. coli I297 cells grown overnight at 37°C on sheep blood agar plates were harvested and gently suspended in sterile PBS. The multiplicity of infection (MOI) ratio was set to 5 bacteria to 1 cell. One hundred microliters of the E. coli bacterial suspension (5 × 105 bacteria) was incubated with 20 μl of serum or fecal suspension pooled from each group at room temperature for 1 h on a shaker at 50 rpm. The bacterial and serum or fecal mixture was added to each well that had 1 × 105 cells seeded (in 750 μl cell culture medium) and incubated for 1 h at 37°C in a CO2 incubator (5% CO2). Each well was gently washed (3× with PBS) to remove nonadherent bacteria and then incubated with 200 μl 0.25% trypsin for 30 min at 37°C in a CO2 incubator to dislodge INT-407 or IPEC-J1 cells. Dislodged cells were collected, centrifuged (15,000 × g for 10 min), and resuspended in 1 ml PBS. The suspension was serially diluted and plated on LB plates at 37°C, and overnight-grown E. coli bacteria (CFU) were counted.
Antibody neutralization activity against STa toxin.
T-84 cells (ATCC, CCL-248) and an intracellular cyclic GMP (cGMP) enzyme immunoassay (EIA) kit (Assay Design, Ann Arbor, MI) were used to examine antibody neutralization activity against STa toxin (a gift from D. Robertson at Kansas State University) as described previously (22–24). Briefly, 2 × 105 T-84 cells were grown in 700 μl DMEM and Ham's F12 medium (Invitrogen, CA) supplemented with 5% FBS in each well of a 12-well tissue culture plate. Each well had a preincubated (1 h at room temperature) mixture of 2 ng of STa toxin (in 150 μl of DMEM/F12) and 150 μl of serum or fecal suspension (1:10 dilution in DMEM/F12) added and was incubated for 2 h at 37°C in a CO2 incubator. Incubated cells were washed and lysed. Lysates were collected and used in a cGMP EIA ELISA by following the manufacturer's instructions.
Antibody neutralization against BVDV viral infection.
A BVDB viral neutralization study was conducted based on the assay method described previously (41), with minor modifications. Madin Darby bovine kidney (MDBK; ATCC, CCL22) cells and cytopathic BVDV-1a strain NADL were used to examine viral antibody neutralization activity. Briefly, 2.5 × 105 MDBK cells were seeded to a 96-well tissue culture plate with Eagle minimum essential medium (MEM) (Sigma) supplemented with 20 μg/ml gentamicin and 10% FBS. Heat-inactivated (56°C for 30 min) serum samples or fecal suspension samples, pooled from the immunized group or the control group, respectively, in a binary dilution (started at a 1:40 dilution and ended at a 1:5,120 dilution), were mixed with equal volumes of virus containing 0.1 median tissue culture infectious doses (TCID50) and incubated for 1 h at 37°C in a 5% CO2 incubator. The mixture was added to monolayers of BVDV-free MDBK cells cultured in the 96-well plate and incubated for 3 days at 37°C in a 5% CO2 incubator. The highest dilution of serum which gave complete neutralization of virus, as determined by the absence of cytopathic effect (CPE), was recorded as the serum neutralization (SN) titer. All experiments were performed in triplicate.
Statistical analysis.
Data were analyzed by using SAS for Windows, version 8 (SAS Institute, Cary, NC). Results are expressed as means and standard errors. Student's t test was used to compare the different treatment groups. Calculated P values of less than 0.05 were regarded as significant when treatments were compared with a two-tailed distribution and two-sample equal or unequal variance.
RESULTS
The constructed fusion protein FanC-STa-E2 carried representative epitopes.
Epitope prediction programs suggested that peptides of residues 91 to 107, 116 to 126, and 154 to 162 of the K99 major subunit FanC are surface exposed but less antigenic and that peptides of residues 70 to 80 and 193 to 201 of the BVDV E2 protein are the most-antigenic B-cell epitope and T-cell epitope. Replacement of peptides of residues 91 to 107, 116 to 128, and 154 to 162 of FanC with STaP12F (NTFYCCELCCNFACAGC), the BVDV E2 B-cell epitope (70HTRALPTSVVF80), and the E2 T-cell epitope (193DQLLYKGGS201), respectively, resulted in the FanC-STa-E2 fusion protein (Fig. 1A). DNA sequencing revealed that the chimeric gene had the right nucleotides embedded and stayed in a correct reading frame. The 6×His-tagged fusion protein, at an expected size of 22.5 kDa, was purified and refolded and was detected by anti-K99, anti-STa, and anti-BVDV antibodies (Fig. 1B).
The multiepitope FanC-STa-E2 fusion protein was safe and immunogenic.
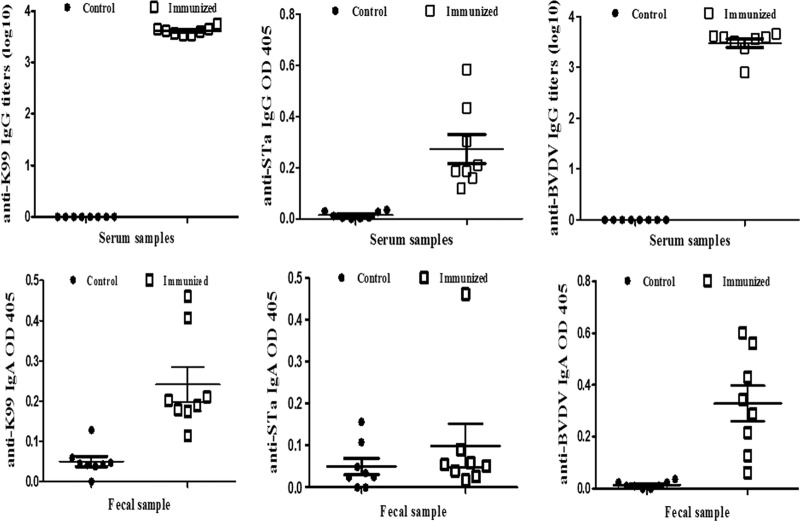
Mice did not display any noticeable distress or adverse effects after being i.p. immunized with this 6×His-tagged multiepitope FanC-STa-E2 fusion protein. Specific anti-K99, anti-STa, and anti-BVDV E2 IgG antibodies were detected from the serum samples of the immunized mice, but no IgG antibodies were detected from the serum samples of the control mice (Fig. 2). Anti-K99 and anti-BVDV E2 IgG were detected at titers of 3.61 ± 0.026 and 3.48 ± 0.088 (log10), respectively. Anti-STa IgG was also detected in the serum of immunized mice, with an OD of 0.273 ± 0.056, which differed significantly from the OD at wells incubated with serum samples of the control mice (0.014 ± 0.005; P < 0.01).
Fig 2.
Detection of anti-K99, anti-STa, and anti-BVDV E2 antibodies from mouse serum and fecal samples. Anti-K99, anti-STa, and anti-BVDV IgG (top panels) and IgA (bottom panels) antibodies were titrated from serum and fecal suspension samples of the immunized mice (solid diamonds) and control mice (blank squares). Five hundred nanograms of heat-extracted K99 fimbriae, 10 ng of STa-ovalbumin conjugates, and 500 ng of MBP-E2 fusion proteins were used to coat each well in an ELISA to titrate anti-K99, anti-STa, and anti-BVDV E2 antibodies, respectively. HRP-conjugated goat anti-mouse IgG (1:3,000) and IgA (1:1,000) were used as the secondary antibodies. Optical densities of greater than 0.3 (after subtracting the background reading) from the greatest dilution were used to calculate antibody titers (in log10), or OD values less than 0.3 after subtraction of the background were directly compared between groups. Bars are means and standard errors.
Anti-K99 and anti-BVDV E2 IgA antibodies were detected in the fecal samples of the immunized mice but not in the control mice (Fig. 2). In an ELISA to titrate anti-K99 IgA antibodies, the OD in the wells incubated with the fecal suspension samples of the immunized mice was 0.242 ± 0.122, which was significantly greater than the readings in the wells incubated with the fecal samples from the control mice (0.050 ± 0.036; P < 0.001). Similarly, in an ELISA to titrate anti-BVDV E2 IgA antibodies from the fecal samples, the OD for the immunized group was 0.329 ± 0.07, whereas the OD for the control group was 0.015 (P < 0.001). For anti-STa IgA antibodies in a fecal sample, the OD for the immunized group was measured at 0.100 ± 0.148, which was not significantly different from that for the control group (0.05 ± 0.055; P = 0.38). Intestinal wash samples were also examined, but due to high background, data were not included in final analyses.
Antibodies in serum samples of the immunized mice neutralized STa toxin.
tk;4Serum of the immunized mice prevented STa toxin from stimulation of intracellular cGMP in T-84 cells (Fig. 3A). The cGMP level in the T-84 cells incubated with 2 ng STa toxin premixed with pooled serum samples of the immunized group was 0.543 ± 0.07 pmol/ml. That was not different from the cGMP level in T-84 cells cultured with the culture medium but was significantly lower than that in cells incubated with the premixture of 2 ng STa toxin with pooled serum samples of the control mice (4.10 ± 0.761; P < 0.01). Fecal samples were also used to examine antibody neutralization activities; however, no significant differences were detected in cGMP levels in T-84 cells incubated with the STa toxin premixed with a fecal suspension from the immunized group or the control group.
Fig 3.
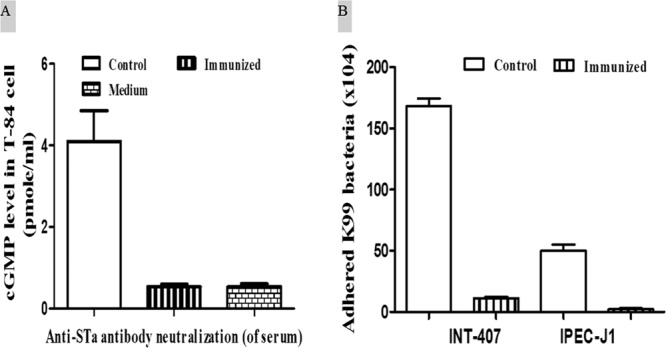
Antibody neutralization against STa toxin and adherence inhibition against K99 fimbrial E. coli bacteria. (A) Antibody neutralization against STa toxin using T-84 cells and an EIA cGMP kit. Serum samples of immunized mice prevented STa toxin from stimulating intracellular cGMP in T-84 cells. (B) Antibody inhibiting adherence of K99 fimbrial E. coli I297 to INT-407 or IPEC-J1 cells. INT-407 and IPEC-J1 cells had significantly fewer I297 bacteria adhered when pretreated with the serum samples from the immunized mice. Bars are means and standard errors.
Antibodies in serum samples of the immunized mice significantly inhibited adherence of K99 fimbrial E. coli bacteria to INT-407 and IPEC-J2 cells.
Antibodies in serum samples of the immunized mice showed bacterium adherence-inhibiting activities (Fig. 3B). INT-407 cells (1 × 105 cells per well), after incubation with I297 (5 × 105 CFU) E. coli bacteria premixed with the pooled serum samples of the immunized mice, had 11.33 × 103 ± 0.88 × 103 E. coli cells adhered. That was significantly lower than the number of E. coli bacteria adhered to INT-407 cells when the pooled serum sample from the control mice was used (168.3 × 103 ± 6.01 × 103; P < 0.01). Similarly, IPEC-J1 cells, when incubated with premixed I297 E. coli and the pooled serum sample from the immunized mice, had 2.33 × 103 ± 0.667 × 103 bacteria adhered. That was significantly lower than for IPEC-J1 cells incubated with the same amount of E. coli bacteria pretreated with the pooled serum sample of the control mice (50.00 × 103 ± 5.00 × 103; P < 0.01).
Antibodies in serum samples of the immunized mice showed neutralizing activities against BVDV infection.
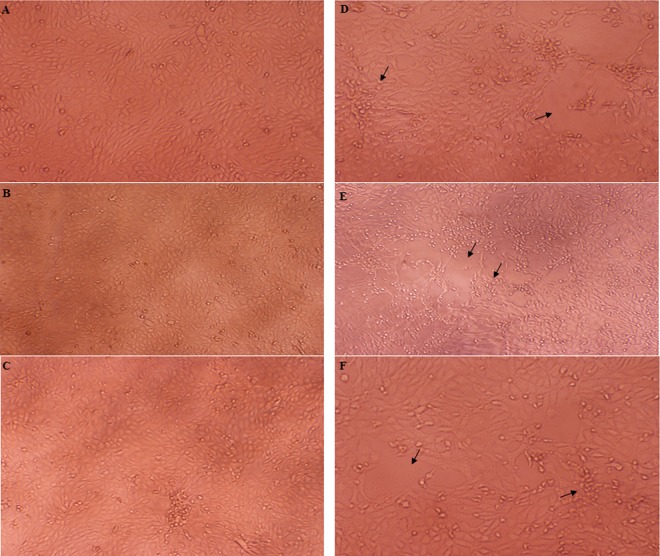
Antibodies in serum samples of the immunized mice prevented BVDV from causing cytopathic effects in MDBK cells (Fig. 4). The BVDV NADL strain mixed with the serum sample pooled from the immunized mice (up to a 1:640 dilution), when used to infect MDBK cells, did not cause cytopathic effects to the MDBK cells. There was no noticeable difference between the cells without treatment (Fig. 4A) and those incubated with a mixture of BVDV and the serum sample of the immunized mice until the serum sample of the immunized mice was diluted to over 1:640 (Fig. 4B and C). In contrast, BVDV NADL virus, when premixed with the serum sample pooled from the control mice and then used to infect MDBK cells, caused cell cluster forming and detachment (Fig. 4E and F). Similar cytopathic effects were also observed in cells incubated with the virus alone (Fig. 4D).
Fig 4.
Antibody neutralization against BVDV viral infection. (A) Normal MDBK cells grown in cell culture medium. (B) MDBK cells incubated with BVDV NADL virus pretreated with the serum sample (1:320 dilution) pooled from mice immunized with the FanC-STa-E2 fusion. (C) MDBK cells incubated with BVDV NADL virus pretreated with the serum sample (1:640 dilution) pooled from mice immunized with the FanC-STa-E2 fusion. (D) MDBK cells incubated with BVDV NADL virus alone. (E) MDBK cells incubated with BVDV NADL virus pretreated with the serum sample (1:320) pooled from the control mice. (F) MDBK cells incubated with BVDV NADL virus pretreated with the serum sample (1:640) pooled from the control mice. Arrows point to MDBK cell clustering and cell detachment (cytopathic effects).
DISCUSSION
ETEC is the most common bacterial cause of neonatal diarrhea, and BVDV is the major viral cause of bovine diarrhea; vaccines effectively against these two types of pathogens may provide effective protection. The current vaccine strategy against calf ETEC diarrhea focuses on passive protection. By immunizing pregnant cows, we can protect born suckling calves against ETEC infection. However, maternal antibodies in milk drop rapidly in the first few days; as acquired antibodies decrease, calves often develop neonatal diarrhea after ETEC infection within the first month of their lives. Moreover, antigens used to immunize pregnant cows are limited on K99 pili, whereas toxin antigens, especially antigens derived from STa, are not included. Toxins indeed elevate intracellular cyclic AMP or GMP in host intestinal epithelial cells to cause hypersecretion of electrolyte-rich fluid that leads to diarrhea (13); thus, they are the key virulence determinants in ETEC diarrhea and need to be targeted primarily in vaccine development. Recently, it was suggested that only vaccines inducing immunity to block bacteria adherence but also to neutralize enterotoxins (such as STa) can provide effective protection against ETEC diarrhea (20, 21, 42). Current immunization practices using purified pili or inactivated K99 fimbrial E. coli bacteria do not induce any antitoxin immunity to protect against enterotoxicity. As a small-size and poorly immunogenic molecule, STa alone does not stimulate anti-STa immunity. Therefore, even inactivated K99 fimbrial bacteria expressing STa toxin cannot stimulate immunity protecting against STa toxin. In contrast, since STa is potently toxic, any products carrying native STa are considered to be not safe. Data from this study indicated that the fusion antigen carrying the K99 antigen FanC and STa toxoid STaP12F elicited both anti-K99 and anti-STa antibodies. Moreover, antibodies in serum of the immunized mice exhibited activities to inhibit adherence of K99 fimbrial E. coli bacteria and to neutralize STa toxin.
It is noted that greater titers of anti-K99 and anti-BVDV E2 IgG antibodies were detected in the serum samples of the immunized mice. But the anti-STa immune response was detected as low in the immunized mice. Indeed, only two immunized mice had anti-STa IgG detected above the arbitrarily set-up OD cutoff point (0.3 after subtraction of background readings) from the initially diluted serum samples. However, the OD readings from the immunized mice differed significantly from those from the control mice. That clearly indicated that the immunized mice developed anti-STa immune responses (Fig. 2). Moreover, serum samples from the immunized mice showed clear neutralizing activity against STa toxin (Fig. 3). The low anti-STa titers detected from the immunized mice most likely resulted from the nature of low immunogenicity from STa or its derived toxoid antigens. Fusing additional copies of the STa toxoid (in heat-labile [LT]-STa toxoid fusion antigens) was shown to facilitate overall anti-STa immunogenicity (our unpublished data). Future studies to fuse two or more STaP12F copies into this FanC-STa-E2 fusion will likely induce greater titers of anti-STa antibodies. It was also noticed that IgA antibodies in the fecal samples were not always detected. Only 1, 2, and 5 immunized mice had anti-STa, anti-FanC, and anti-E2 IgA antibodies detected above the OD cutoff point, respectively. The nature of low antibody contents present in feces certainly contributed to the low detection of IgA antibodies among the immunized mice. In addition, fecal suspension samples used in this study likely were too diluted (1:18 dilution). That further limited detection of IgA antibodies in fecal samples. Future studies to collect more fecal pellets and to use less-diluted suspension samples will likely enhance detection of IgA antibodies specific to each antigen.
Knowing that BVDV isolates are heterogeneous and that such heterogeneity poses significant challenges in developing broadly effective vaccines, we carried out this study to explore an alternative approach to develop vaccines against BVDV-associated bovine diarrhea. Heterogeneity (genetically and, more importantly, antigenically) makes it nearly impossible for any live-attenuated or inactivated vaccines derived from a single BVDV strain to provide broad protection. A cocktail of live-attenuated strains can improve protective efficacy, but it increases the risk of virulence reversal of the product and also viral infections in utero. For inactivated vaccines, mixing of multiple strains becomes less desirable due to difficulties in carrying sufficient antigens from individual strains. Subunit vaccines can overcome the above-mentioned disadvantages, but they likely will not provide broad protection against heterogeneous strains, particularly if a single protein, typically E2, is the only antigen included (43, 44). While the B-cell epitope used in this study is relatively conserved among BVDV-1 and BVDV-2 strains, the most-antigenic T-cell epitope is not among strains from different groups (44). A chimeric E2 protein that is derived from hybridization of multiple E2 species from divergent groups or that carries representative epitopes from strains of different groups may induce neutralizing antibodies broadly against heterogeneous strains. Data from this study indicated that selective E2 epitopes induced antibodies neutralizing BVDV viral infection in homologous challenge, but future studies are needed to determine whether the elicited antibodies also neutralize against other BVDV-1 strains or BVDV-2 strains. In future studies, we would like to also include multiple E2 epitopes selected from BVDV strains of other subgroups for a fusion antigen to induce broad anti-BVDV immunity for effective protection. In addition, such fusion antigens can be expressed by a nonpathogenic bovine E. coli strain at a high expression level. That can potentially be developed as a live-attenuated vaccine strain to be easily differentiated from BVDV field strains or as an inactivated vaccine to carry sufficient target antigens.
It should be pointed out that this study examined mainly the immunogenicity of the constructed FanC-STa-E2 fusion antigen. Examination of specific host immune responses induced by this fusion antigen may better characterize the selected BVDV B-cell and T-cell epitopes. Although data showed that this fusion antigen elicited specific antibodies in mice and that the antibodies showed neutralizing activity against ETEC and BVDV viral infection, its candidacy as a subunit vaccine against ETEC neonatal diarrhea in calves and BVDV diarrhea at other ages can be determined only in large-sample and well-designed field trials. Future studies will also be needed for development of a broad-spectrum vaccine against bovine diarrhea.
ACKNOWLEDGMENTS
We thank Don Robertson (Kansas State University) and Eric Nelson (South Dakota State University) for providing reagents for anti-STa and anti-K99 assays.
Financial support for this study was provided by the South Dakota Agricultural Experiment Station.
Footnotes
Published ahead of print 22 May 2013
REFERENCES
- 1. USDA Beef 2007-08. Part IV. Reference of beef cow-calf management practices in the United States, 2007-08. U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, Fort Collins, CO [Google Scholar]
- 2. Smith DR. 2012. Field disease diagnostic investigation of neonatal calf diarrhea. Vet. Clin. North Am. Food Anim. Pract. 28:465–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanchard PC. 2012. Diagnostics of dairy and beef cattle diarrhea. Vet. Clin. North Am. Food Anim. Pract. 28:443–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altmann K, Pyliotis NA, Mukkur TK. 1982. A new method for the extraction and purification of K99 pili from enterotoxigenic Escherichia coli and their characterization. Biochem. J. 201:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagy B, Fekete PZ. 1999. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 30:259–284 [PubMed] [Google Scholar]
- 6. Foster DM, Smith GW. 2009. Pathophysiology of diarrhea in calves. Vet. Clin. North Am. Food Anim. Pract. 25:13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jay CM, Bhaskaran S, Rathore KS, Waghela SD. 2004. Enterotoxigenic K99+ Escherichia coli attachment to host cell receptors inhibited by recombinant pili protein. Vet. Microbiol. 101:153–160 [DOI] [PubMed] [Google Scholar]
- 8. Gaastra W, de Graaf FK. 1982. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol. Rev. 46:129–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker JC. 1995. The clinical manifestations of bovine viral diarrhea infection. Vet. Clin. North Am. Food Anim. Pract. 11:425–445 [DOI] [PubMed] [Google Scholar]
- 10. Evermann JF, Barrington GM. 2005. Clinical features, p 105–120 In Goyal SM, Ridpath JF. (ed), Bovine viral diarrhea virus: diagnosis, management, and control. Wiley-Blackwell, Ames, IA [Google Scholar]
- 11. Moerman A, Straver PJ, de Jong MC, Quak J, Baanvinger T, van Oirschot JT. 1994. Clinical consequences of a bovine virus diarrhoea virus infection in a dairy herd: a longitudinal study. Vet. Q. 16:115–119 [DOI] [PubMed] [Google Scholar]
- 12. Smit H, Gaastra W, Kamerling JP, Vliegenthart JF, de Graaf FK. 1984. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect. Immun. 46:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collett MS, Anderson DK, Retzel E. 1988. Comparisons of the pestivirus bovine viral diarrhoea virus with members of the flaviviridae. J. Gen. Virol. 69(Part 10):2637–2643 [DOI] [PubMed] [Google Scholar]
- 15. Donis RO, Corapi W, Dubovi EJ. 1988. Neutralizing monoclonal antibodies to bovine viral diarrhoea virus bind to the 56K to 58K glycoprotein. J. Gen. Virol. 69(Part 1):77–86 [DOI] [PubMed] [Google Scholar]
- 16. Acres SD, Isaacson RE, Babiuk LA, Kapitany RA. 1979. Immunization of calves against enterotoxigenic colibacillosis by vaccinating dams with purified K99 antigen and whole cell bacterins. Infect. Immun. 25:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagy B. 1980. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxic colibacillosis. Infect. Immun. 27:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Myers LL. 1980. Passive protection of calves against experimentally induced and naturally occurring enteric colibacillosis. Am. J. Vet. Res. 41:1952–1956 [PubMed] [Google Scholar]
- 19. Snodgrass DR, Nagy LK, Sherwood D, Campbell I. 1982. Passive immunity in calf diarrhea: vaccination with K99 antigen of enterotoxigenic Escherichia coli and rotavirus. Infect. Immun. 37:586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Ind. J. Med. Res. 133:188–196 [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines 11:677–694 [DOI] [PubMed] [Google Scholar]
- 22. Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78:316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C, Zhang W. 2010. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin. Vaccine Immunol. 17:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTRG-STaPF) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect. Immun. 79:4002–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beregt D. 2005. Introduction and history, p 3–34 In Goyal SM, Ridpath JF. (ed), Bovine viral diarrhea virus: diagnosis, management, and control. Wiley-Blackwell, Ames, IA [Google Scholar]
- 26. Pecora A, Aguirreburualde MS, Aguirreburualde A, Leunda MR, Odeon A, Chiavenna S, Bochoeyer D, Spitteler M, Filippi JL, Dus Santos MJ, Levy SM, Wigdorovitz A. 2012. Safety and efficacy of an E2 glycoprotein subunit vaccine produced in mammalian cells to prevent experimental infection with bovine viral diarrhoea virus in cattle. Vet. Res. Commun. 36:157–164 [DOI] [PubMed] [Google Scholar]
- 27. Aguirreburualde MS, Gomez MC, Ostachuk A, Wolman F, Albanesi G, Pecora A, Odeon A, Ardila F, Escribano JM, Dus Santos MJ, Wigdorovitz A. 2013. Efficacy of a BVDV subunit vaccine produced in alfalfa transgenic plants. Vet. Immunol. Immunopathol. 151:315–324 [DOI] [PubMed] [Google Scholar]
- 28. Loy JD, Gander J, Mogler M, Vander Veen R, Ridpath J, Harris DH, Kamrud K. 2013. Development and evaluation of a replicon particle vaccine expressing the E2 glycoprotein of bovine viral diarrhea virus (BVDV) in cattle. Virol. J. 10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isaacson RE, Start GL. 1992. Analysis of K99 plasmids from enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 69:141–146 [DOI] [PubMed] [Google Scholar]
- 30. Lee JH, Isaacson RE. 1995. Expression of the gene cluster associated with the Escherichia coli pilus adhesin K99. Infect. Immun. 63:4143–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levitt M. 1978. Conformational preferences of amino acids in globular proteins. Biochemistry 17:4277–4285 [DOI] [PubMed] [Google Scholar]
- 32. Hopp T, Woods KR. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. U. S. A. 78:3824–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zimmerman JM, Eliezer N, Simha R. 1968. The characterization of amino acid sequences in proteins by statistical methods. J. Theor. Biol. 21:170–201 [DOI] [PubMed] [Google Scholar]
- 34. Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, Ben-Tal N. 2003. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics 19:163–164 [DOI] [PubMed] [Google Scholar]
- 35. Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. 2010. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38:W529–W533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saha S, Raghava GPS. 2007. Prediction methods for B-cell epitopes. Methods Mol. Biol. 409:387–394 [DOI] [PubMed] [Google Scholar]
- 37. Ruan X, Liu M, Casey TA, Zhang W. 2011. A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection. Clin. Vaccine Immunol. 18:1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington DC [Google Scholar]
- 39. Morris JA, Stevens AE, Sojka WJ. 1977. Preliminary characterization of cell-free K99 antigen isolated from Escherichia coli B41. J. Gen. Microbiol. 99:353–357 [DOI] [PubMed] [Google Scholar]
- 40. Koh SY, George S, Brozel V, Moxley R, Francis D, Kaushik RS. 2008. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 130:191–197 [DOI] [PubMed] [Google Scholar]
- 41. Kalaycioglu AT, Russell PH, Howard CR. 2012. The characterization of the neutralizing bovine viral diarrhea virus monoclonal antibodies and antigenic diversity of E2 glycoprotein. J. Vet. Med. Sci. 74:1117–1120 [DOI] [PubMed] [Google Scholar]
- 42. Boedeker EC. 2005. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 21:15–19 [PubMed] [Google Scholar]
- 43. Bruschke CJ, van Oirschot JT, van Rijn PA. 1999. An experimental multivalent bovine virus diarrhea virus E2 subunit vaccine and two experimental conventionally inactivated vaccines induce partial fetal protection in sheep. Vaccine 17:1983–1991 [DOI] [PubMed] [Google Scholar]
- 44. Deregt D, Bolin SR, van den Hurk J, Ridpath JF, Gilbert SA. 1998. Mapping of a type 1-specific and a type-common epitope on the E2 (gp53) protein of bovine viral diarrhea virus with neutralization escape mutants. Virus Res. 53:81–90 [DOI] [PubMed] [Google Scholar]