Abstract
Antimicrobials administered postexposure can reduce the incidence or progression of anthrax disease, but they do not protect against the disease resulting from the germination of spores that may remain in the body after cessation of the antimicrobial regimen. Such additional protection may be achieved by postexposure vaccination; however, no anthrax vaccine is licensed for postexposure prophylaxis (PEP). In a rabbit PEP study, animals were subjected to lethal challenge with aerosolized Bacillus anthracis spores and then were treated with levofloxacin with or without concomitant intramuscular (i.m.) vaccination with anthrax vaccine adsorbed (AVA) (BioThrax; Emergent BioDefense Operations Lansing LLC, Lansing, MI), administered twice, 1 week apart. A significant increase in survival rates was observed among vaccinated animals compared to those treated with antibiotic alone. In preexposure prophylaxis studies in rabbits and nonhuman primates (NHPs), animals received two i.m. vaccinations 1 month apart and were challenged with aerosolized anthrax spores at day 70. Prechallenge toxin-neutralizing antibody (TNA) titers correlated with animal survival postchallenge and provided the means for deriving an antibody titer associated with a specific probability of survival in animals. In a clinical immunogenicity study, 82% of the subjects met or exceeded the prechallenge TNA value that was associated with a 70% probability of survival in rabbits and 88% probability of survival in NHPs, which was estimated based on the results of animal preexposure prophylaxis studies. The animal data provide initial information on protective antibody levels for anthrax, as well as support previous findings regarding the ability of AVA to provide added protection to B. anthracis-infected animals compared to antimicrobial treatment alone.
INTRODUCTION
Anthrax is an acute infectious disease caused by the spore-forming bacterium Bacillus anthracis. Depending on the route of exposure, the disease can occur in cutaneous, gastrointestinal, inhalation, and injectional forms. Inhalation anthrax is considered highly lethal, with the fatality rate approaching 100% when untreated (1). The primary virulence factors of B. anthracis include the polyglutamate capsule, which prevents phagocytosis of the bacterium, and three polypeptides, protective antigen (PA), lethal factor (LF), and edema factor (EF), which form two binary toxins. PA and LF combine to produce anthrax lethal toxin (LT), and PA and EF combine to produce edema toxin (ET). PA binds to the host cell and oligomerizes, forming a pore that allows for the translocation of EF and LF into the cytosol (2–4). Neutralizing antibodies generated against PA, the common component of anthrax toxins, have been shown to confer protection against anthrax disease (5–10).
The bioterrorism events of 2001 highlighted the need for the development of postexposure prophylaxis (PEP) medical countermeasures (MCMs) for public health emergency use. Although several antimicrobials are currently approved and indicated for reduction of the incidence or progression of disease following exposure to aerosolized B. anthracis, they do not protect against the potential for disease caused by the germination of residual spores that may remain after the recommended 60-day antimicrobial regimen is completed (11–13). Such additional protection may be achieved by postexposure vaccination.
The use of vaccines for PEP is well documented. A classic example is the rabies vaccine, which in the postexposure setting is administered via a series of immunizations, starting as soon as possible after exposure, in combination with a single dose of rabies immune globulin (14, 15). Postexposure prophylaxis of hepatitis B involves a combination of a single active immunization using a vaccine and concomitant passive immunization using an immune globulin (16, 17). Prophylaxis against tetanus in wound management involves a single dose of tetanus toxoid and may include immune globulin administration, depending on the immunization history of the exposed individual (18). In the case of anthrax, the available vaccine, anthrax vaccine adsorbed (AVA) (BioThrax; Emergent BioDefense Operations Lansing LLC, Lansing, MI), is indicated only for preexposure prophylaxis in persons at high risk of exposure (19), including military personnel, veterinarians, processors of animal hair and hides, and laboratory personnel (20, 21). No anthrax vaccine is currently licensed for PEP use, and the availability of such a vaccine remains an unmet medical need.
Licensure of vaccines is typically achieved based on direct demonstration of clinical efficacy in large field trials or based on an established surrogate endpoint, such as a serological correlate of protection (22, 23). The latter approach has been employed for licensure of vaccines against influenza (24), smallpox (25), meningococcal disease (26), and other diseases. In these cases, new vaccines may be approved based on the demonstration of the ability of human subjects to reach a threshold of antibody response that has been shown in prior clinical efficacy trials to be protective against disease. The approval process for biodefense MCMs, such as vaccines against anthrax, presents a number of challenges. It is not ethical to evaluate the efficacy of anthrax vaccines in humans, and field trials are no longer feasible due to the rarity of naturally occurring anthrax in the United States. Therefore, the licensure of an anthrax vaccine for PEP must be achieved using the U.S. Food and Drug Administration (FDA) “Animal Rule” (27, 28), and serological correlates of protection for such vaccines must be derived entirely from immunogenicity and efficacy studies in animal models.
The ability of AVA, when administered in conjunction with antimicrobials following lethal anthrax challenge, to improve the survival outcome compared to antimicrobial treatment alone has been demonstrated in rabbits (29) and nonhuman primates (NHPs) (12, 30), which are the preferred animal models of inhalation anthrax (28, 31). Building upon these data, a series of nonclinical and clinical studies were conducted in order to (i) assess the ability of AVA (administered postexposure concomitantly with antimicrobial treatment) to significantly increase survival, compared with antimicrobial treatment alone, in the rabbit anthrax PEP model (29, 32), (ii) identify a protective vaccine-induced antibody titer based on the level associated with a specific probability of survival in the rabbit preexposure model and confirmed in the NHP preexposure model (5, 10), and (iii) evaluate in a clinical study the ability of human subjects to achieve this antibody titer at a time point after antimicrobial treatment would have been discontinued. This strategy was based on the approach recently described by Burns (27) and discussed at a meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) (33).
MATERIALS AND METHODS
Experimental animals.
Animal studies were conducted at the Battelle Biomedical Research Center (Battelle, West Jefferson, OH). The work was performed in compliance with the Animal Welfare Act and followed the principles outlined in the National Research Council's Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC). The studies in rabbits were conducted in accordance with the FDA guidelines on good laboratory practices (GLP). Specific-pathogen-free New Zealand white (NZW) rabbits (Oryctolagus cuniculus), weighing between 2.1 and 2.7 kg, were procured from Harlan Laboratories (Indianapolis, IN). Cynomolgus macaques (Macaca fascicularis) of Vietnamese origin, weighing between 1.5 and 2.6 kg (2.3 to 3.5 years old) were procured from Covance Research Products (Alice, TX). The monkeys were screened for anti-PA immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA), and only seronegative animals were placed in the study. Only healthy animals of the specified weight range that were free of obvious clinical signs of disease or malformations were placed in each study, and equal numbers of male and female animals were assigned to each treatment group.
Clinical study subjects.
The clinical study was conducted in accordance with the principles of the International Conference on Harmonization (ICH) E6 Guideline on Good Clinical Practices (GCP) and the principles of the Declaration of Helsinki, and all aspects of this study were conducted in accordance with all national, state, and local laws or regulations. Prior to the study onset, the protocol, informed consent, advertisements used for subject recruitment, and any other information regarding the study provided to a subject or a subject's legal guardian was approved by the institutional review board (IRB). The study was conducted as an open-label multicenter immunogenicity and safety study. A total of 210 healthy male and female subjects, 18 to 65 years of age, were screened, and 150 subjects were enrolled and included in the intent-to-treat (ITT) population. Enrollment was stratified (1:1) by gender to allow for efficient testing of a possible difference in the immune response in females compared to males.
Test and control articles.
AVA (19, 34) was provided by Emergent Biodefense Operations Lansing (Lansing, MI). Aluminum hydroxide gel 2% adjuvant (Alhydrogel) was procured from Brenntag Biosector (Frederikssund, Denmark). Levofloxacin (Levaquin oral solution, 25 mg/ml; Ortho-McNeil, New Brunswick, NJ) was purchased from a licensed pharmacy.
Experimental design.
Three nonclinical anthrax challenge studies (a PEP and a preexposure prophylaxis study in rabbits, as well as a preexposure study in NHPs) and one clinical immunogenicity and safety study in healthy human volunteers were conducted.
(i) Rabbit PEP study.
NZW rabbits (90 males and 90 females) were randomly assigned to seven groups of 24 rabbits each (groups 1 to 5, 7, and 8) and one group of 12 rabbits (group 6). Equal numbers of male and female animals were randomized to each group. Because of the limited number of animals that can be challenged with B. anthracis spores in a given day, the rabbits were also randomized to one of five challenge days.
On day 0, rabbits in groups 1 to 6 were challenged via inhalation with aerosolized B. anthracis (Ames strain) spores at a target dose exceeding the median lethal dose (LD50) by 200-fold (200 LD50), or ∼2.1 × 107 spores (35). Animals in groups 1 to 5 were dosed once a day (SID) for 7 days with levofloxacin at 50 mg/kg via oral gavage, with the first dose administered within 6 to 12 h postchallenge. The antibiotic regimen used was based on the results of the model development work conducted by Hewitt and coworkers (29) and was designed to afford partial protection due to the truncated treatment schedule, while maintaining a therapeutic dose during the 7-day treatment. Animals treated with antibiotic also received two intramuscular (i.m.) immunizations with 0.5 ml of AVA at 6 to 12 h postchallenge and 7 days later. Group 1 animals were immunized with the human dose of the vaccine; groups 2, 3, and 4 were immunized with a 1:4, 1:16, and 1:64 dilution of the human dose, respectively. Vaccine dilutions were prepared in sterile saline, and all vaccines were administered in a volume of 0.5 ml. Group 5 received adjuvant alone (saline with 650 μg of aluminum hydroxide gel). Animals in group 6 were dosed orally for 7 days (or until death) with sterile water for injection (WFI). Rabbits in groups 7 and 8 were not challenged with B. anthracis spores but were included in the challenge-day randomization. These animal groups received two i.m. immunizations with 1:4 and 1:16 dilutions of AVA, respectively, as well as the same levofloxacin treatment regimen as the animals in groups 1 to 5, starting at the same time as in the challenge group animals. The rabbits were monitored for 30 days postchallenge.
Blood was collected at selected time points to measure serum neutralizing antibody levels (by toxin-neutralizing antibody [TNA] assay, described below) and blood plasma levofloxacin levels in all animals (groups 1 to 8), as well as to assess the presence or absence of bacteremia in the infected animals (groups 1 to 6 only). Complete gross necropsy was performed on any animal that was found dead or euthanized due to moribund condition. Histopathology analysis was performed as needed to confirm death due to anthrax disease.
(ii) Rabbit preexposure prophylaxis study.
NZW rabbits (54 males and 54 females) were randomly assigned to four groups of 24 and one group of 12 with an equal number of male and female animals per group. All animals were also randomized to one of four challenge days. On days 0 and 28, the rabbits in groups 1 to 4 were vaccinated i.m. with serial 4-fold dilutions, ranging from 1:4 to 1:256, of the human dose of AVA. The group 5 rabbits received two immunizations with adjuvant alone (saline with 650 μg of aluminum hydroxide gel). On day 70, all animals were challenged via inhalation with aerosolized B. anthracis (Ames strain) spores at a target dose of 200 LD50, which was chosen based on published LD50 calculations (35).
Blood was collected from all animals at selected time points to measure prechallenge serum neutralizing antibody levels by TNA assay, as well as to assess the presence or absence of bacteremia. Complete gross necropsy was performed on any animal found dead or that was euthanized due to moribund condition. Histopathology analysis was performed as needed to confirm death due to anthrax disease.
(iii) NHP preexposure prophylaxis study.
Cynomolgus macaques (24 males and 24 females) were randomly assigned to six groups of eight animals each, with an equal number of male and female animals per group. All animals were also randomized to one of three challenge days. On days 0 and 28, the monkeys in groups 1 to 5 were vaccinated i.m. with serial 4-fold dilutions of the human dose of AVA, ranging from the undiluted human dose to 1:256. The monkeys in group 6 received two i.m. injections of phosphate-buffered saline (PBS). On day 70, all animals were challenged via inhalation with aerosolized B. anthracis (Ames strain) spores at a target dose of 200 LD50, which was chosen based on published LD50 calculations (36).
Blood was collected from all animals at selected time points to measure serum neutralizing antibody levels (by TNA assay) pre- and postchallenge, as well as to assess the presence or absence of bacteremia. Complete gross necropsy was performed on any animal found dead or that was euthanized due to moribund condition. Histopathology analysis was performed as needed to confirm death due to anthrax disease.
(iv) Exploratory clinical immunogenicity and safety study.
Subjects received 0.5 ml of AVA administered as a 0.5-ml subcutaneous (s.c.) injection in the deltoid region (alternating arms) on days 0, 14, and 28, using a 5/8-in., 25- to 27-gauge needle and syringe. Safety and immunogenicity data were collected at screening and on days 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 84, and 100. The primary endpoints for this study were antibody titers on days 35, 42, 49, and 56 as measured by TNA assay, assessed by seroconversion rates (4-fold increase in antibody titer from screening), and geometric mean titers (GMTs) in the per protocol (PP) population, defined as subjects that received all three vaccinations within the protocol-specified time windows of ±2 days for day 14 and day 28 and had follow-up visits with evaluations on days 35, 42, 49, and 56 within the protocol-specified time windows (±1 day on days 35 and 42, and ±2 days on days 49 and 56). The primary analysis of immunogenicity was in the PP population. Serum samples from a subset of 30% (n = 45) of the subjects, balanced by gender, were tested by anti-PA IgG ELISA.
Subjects were required to fill out a daily diary for 7 days after each vaccination to assess the presence and severity of injection site and systemic symptoms. Baseline safety data collected at screening consisted of subjects' medical history, laboratory data (hematology, chemistry, urinalysis, history of human immunodeficiency virus [HIV], hepatitis B virus [HBV], hepatitis C virus [HCV], and pregnancy test), physical examination (complete), vital signs, adverse events (AEs), and concomitant medications. Safety data collected during or following the vaccination period included medical history, laboratory data (hematology, chemistry, urinalysis, pregnancy test), physical examination (complete and symptom-directed), vital signs, AEs, and concomitant medications.
Analysis of immune response. (i) TNA assay.
Anthrax toxin-neutralizing antibody levels in the serum samples of laboratory animals and human subjects were measured using a validated TNA assay (37, 38). The assay colorimetrically determines the viability of cells exposed to LT using a tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), as the reporter or signal system. The serum-mediated neutralization of LT manifests as a suppression of cytotoxicity, and hence, preservation of cell viability. A human serum reference standard, AVR801 (BEI Resources, Manassas, VA), was used for all human and animal TNA analyses, and the data analysis was conducted using a SAS platform and the analysis algorithm developed by the CDC (37, 38). All TNA analyses were conducted at Battelle.
Briefly, 96-well microtiter cell plates were seeded with J774A.1 cells, and the cells were allowed to adhere. In separate microplates (“prep plates”), serial dilutions of the test samples and controls were prepared. LT was added to the prep plate and incubated to allow for LT neutralization by neutralizing antibodies. The contents of the prep plate were then transferred to the cell plate and incubated to allow intoxication to proceed. MTT was then added to the cell plates to assess cell viability, as only viable cells are able reduce the MTT dye. The cells were then lysed using a sodium dodecyl sulfate (SDS)-based solubilization buffer to release the reduced MTT, and optical density (OD) values for each plate were determined using a microplate reader (BioTek Instruments, Winooski, VT) at a wavelength of 570 nm with a 690-nm reference wavelength. The raw OD data were exported into the SAS software (SAS Institute, Cary, NC), and the CDC TNA SAS program (37) was used to fit a four-parameter logistic-log (4PL) function to the seven-point serial dilutions of the reference serum standard and the test sample OD values. This 4PL curve was in turn used to calculate the reportable values.
The primary assay endpoints were the 50% effective dilution (ED50) and the 50% neutralization factor (NF50). The ED50 was defined as the reciprocal of the dilution of a serum sample that results in 50% neutralization of anthrax lethal toxin and was calculated as the reciprocal of the dilution corresponding to the inflection point (“c” parameter) of a 4PL curve. The NF50 was defined as the quotient of the ED50 of the test sample and the ED50 of the reference serum. The mean ED50 of the AVR801 reference standard was 656 (38). The limit of quantitation (LOQ) values for ED50 were 39, 55, and 33 for the rabbit, NHP, and human serum, respectively. The LOQ values for NF50 were 0.086, 0.105, and 0.064 for the rabbit, NHP, and human serum, respectively. Values below the LOQ were replaced with the LOQ (in the animal studies) or 1/2 LOQ (in the clinical study) for geometric mean titer (GMT) calculation and statistical analysis.
(ii) Anti-PA IgG ELISA.
Serum anti-PA IgG levels were measured in 30% of clinical study subjects using ELISA based on a method originally developed and validated by the CDC (39). The assay is designed to quantify IgG antibodies against anthrax PA using purified recombinant PA (rPA) as the solid-phase immobilized antigen and an enzyme-conjugated anti-gamma chain secondary antibody as the reporter system. The human serum reference standard AVR801 (BEI Resources), with the anti-PA IgG concentration of 109.4 μg/ml (38), was used.
Briefly, microtiter plates were coated with purified rPA. Test samples, anti-PA IgG reference standard serum, and positive-control sera were added to the microtiter plate, and the plate was incubated to allow the PA-specific antibodies to bind to the rPA coated on the plate. After washing, the bound anti-PA antibodies were detected by a species-specific anti-gamma chain IgG-horseradish peroxidase (HRPO) conjugate, followed by the addition of a peroxidase substrate. The OD values were determined using a microplate reader (BioTek) at a wavelength of 405 nm, with a reference wavelength of 490 nm.
The anti-PA IgG levels were determined by taking the average of the acceptable concentrations from the 8-point dilution of the test sample back-calculated from the standard curve. Results were reported in μg/ml of anti-PA IgG, and the assay endpoint was reported as the median serum concentration (μg/ml). The LOQ of the assay was 3.7 μg/ml.
Analysis of blood plasma levofloxacin levels.
For blood plasma levofloxacin level determination in the rabbit PEP study, blood was collected 6 days before (baseline) and following levofloxacin or WFI administration on days 1, 2, 7, 8, and 11. Blood samples collected on days 1 and 7 were for peak levofloxacin levels (collected 1 ± 0.5 h after dosing). Blood samples collected on days 2 and 8 were for trough levofloxacin levels (collected 24 ± 0.5 h after the previous dose). Blood collection on day 11 was not a timed collection. At each time point, approximately 2 ml of blood was collected from each rabbit into a chilled ethylenediaminetetraacetic acid (EDTA)-containing tube, inverted 2 to 3 times to mix the blood with the anticoagulant, and placed on wet ice or cold packs. Blood samples were centrifuged at ∼1,300 relative centrifugal force for 10 to 15 min at 4°C, within 1 h of collection, to separate the plasma. The plasma was filtered through 0.2-μm filters, transferred into labeled storage tubes, and stored at or below −20°C until assayed by high-performance liquid chromatography (HPLC). HPLC calibration standards and quality-control (QC) standards were prepared by spiking naïve rabbit plasma with levofloxacin.
Chromatography was performed on an autosampler-equipped (injection volume, 10 μl) Waters 2690/2790 separation module (Waters, Milford, MA) using an Inertsil ODS-2 C18 column (5 μm, 4.6 by 250 mm; Phenomenex, Torrance, CA). Mobile phase A consisted of 5 mM copper(II) sulfate and 10 mM l-isoleucine in water; mobile phase B consisted of methanol. Isocratic elution mode was used. Detection was performed by fluorescence at the excitation wavelength of 335 nm and emission wavelength of 475 nm. Levofloxacin retention time was approximately 7.7 min.
Statistical analysis.
Survival rates were compared between groups using Fisher's exact tests. A Bonferroni-Holm adjustment was used to control the overall error rate at 0.05 within each study. For the PEP study, the survival rates in the vaccinated groups (groups 1 through 4) and the naïve control group (group 6) were compared to the survival rate in the group that received antimicrobial treatment alone (group 5) using a one-sided test. For the preexposure prophylaxis studies, the survival rate in each vaccinated group was compared to the survival rate in the control group using a two-sided test.
Logistic regression analysis was performed on the data from the preexposure prophylaxis studies to estimate the relationship between survival and immunogenicity in both animal species. The models were fitted to the survival data with an effect for the base-10 log-transformed TNA NF50 titers on day 69 from the rabbit study and day 70 from the NHP study. These models included only vaccinated animals, and individual values below the LOQ were replaced with the LOQ value (0.086 for rabbit samples and 0.105 for NHP samples).
Predictive vaccine efficacy (VE) was evaluated based on the continuous relationship method developed by Kohberger (29), which can be described by the equation VE = 100 (1 − pv/pu)%, where pv is the population probability of death in a vaccinated individual, and pu is the probability of death for an unvaccinated individual.
Applying this relationship to the use of AVA for postexposure prophylaxis of anthrax, pv (the population probability of death at a specific time point in a vaccinated subject) can be estimated using equation 1:
| (1) |
where f(x) is probability of death, given x = log10(NF50); this function is estimated by logistic regression modeling using survival data from the animal preexposure prophylaxis studies; and g(x) is probability density function of log10(NF50); the distribution g(x) is assumed to be normal, with an adjusted mean and observed standard deviation from the clinical immunogenicity data.
RESULTS
PEP efficacy of AVA in rabbits.
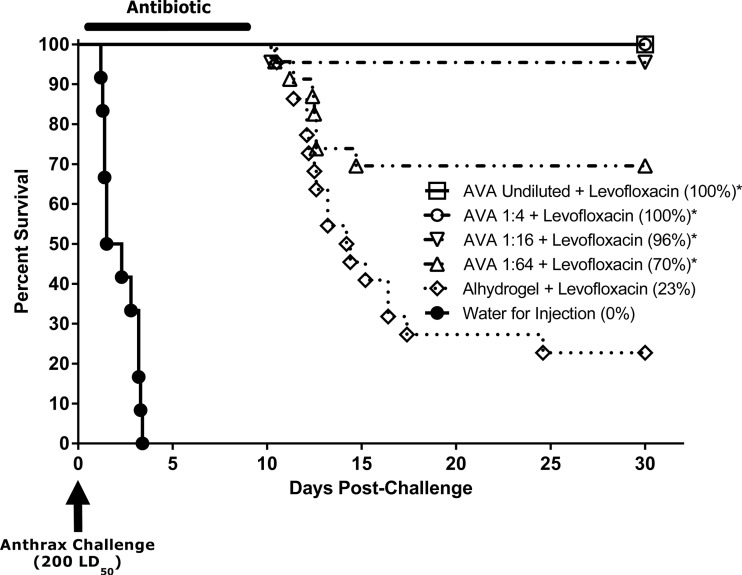
The purpose of the PEP study in rabbits was to determine whether AVA vaccination postchallenge increased survival after the cessation of the antibiotic treatment compared with antibiotic treatment alone. The actual exposure dose received by the animals was 260 ± 45 (mean ± standard deviation) LD50 of aerosolized B. anthracis Ames spores. The subsequent postexposure vaccination with AVA (groups 1 to 4) increased survival rates in a dose-dependent manner (Fig. 1, Table 1). Survival enhancement induced by undiluted AVA (100% survival), as well as the 1:4, 1:16, and 1:64 dilutions (100%, 96%, and 70% survival, respectively), was statistically significant (P < 0.004) compared with antimicrobial treatment alone (Table 1). In contrast, animals that received the adjuvant and antibiotic (group 5) began to die on day 10, and ultimately, 77% of these animals succumbed to the disease, including one animal that died as late as day 25 postinfection (17 days after the cessation of antimicrobial treatment); all untreated controls (group 6) succumbed to the disease by day 4 (Fig. 1).
Fig 1.
Survival of NZW rabbits following lethal B. anthracis spore challenge and levofloxacin treatment with or without concomitant AVA vaccination (rabbit PEP study). Rabbits were challenged via inhalation with aerosolized B. anthracis spores on day 0 and treated once daily for 7 days with levofloxacin at 50 mg/kg via oral gavage, starting at 6 to 12 h postchallenge; untreated controls were treated with WFI. Concomitantly, animals received two i.m. immunizations with AVA at 6 to 12 h postchallenge (day 1) and on day 8. Antibiotic-only controls received adjuvant alone (saline with 650 μg of aluminum hydroxide gel). Animals were monitored for morbidity and mortality for 30 days postchallenge. Overall group survival rates at the time of study termination are shown in parentheses. *, Survival rate was significantly greater than that in the control group (P < 0.004).
Table 1.
Survival of NZW rabbits following lethal B. anthracis spore challenge and levofloxacin treatment with or without concomitant AVA vaccination (rabbit PEP study)
| Group | Vaccine dose | Levofloxacin treatment | Spore challenge | Survival rate |
Pa | |
|---|---|---|---|---|---|---|
| No. alive/total no. | % | |||||
| 1 | Human dose | + | + | 24/24 | 100 | <0.0001 |
| 2 | 1:4 | + | + | 24/24 | 100 | <0.0001 |
| 3 | 1:16 | + | + | 21/22b | 95.5 | <0.0001 |
| 4 | 1:64 | + | + | 16/23b | 69.6 | 0.0038 |
| 5 | Adjuvant | + | + | 5/22b | 22.7 | |
| 6 | None | − | + | 0/12 | 0 | |
| 7 | 1:4 | + | − | 23/23b | 100 | |
| 8 | 1:16 | + | − | 24/24 | 100 | |
Compared to group 5.
Two rabbits each in groups 3 and 5, and one rabbit each in groups 4 and 7, died during the oral gavage levofloxacin administration. The death of these animals was not related to anthrax infection (which was confirmed by histopathology for the challenged animals), and these animals were excluded from the statistical analyses.
Two rabbits each in groups 3 and 5 and one rabbit each in groups 4 and 7 died during the administration of levofloxacin via oral gavage (Table 1). The death of these animals was determined to have resulted from the complications of the gavage procedure and was not related to anthrax infection (confirmed by histopathology for the challenge group animals; data not shown). These animals were, therefore, excluded from the statistical analyses.
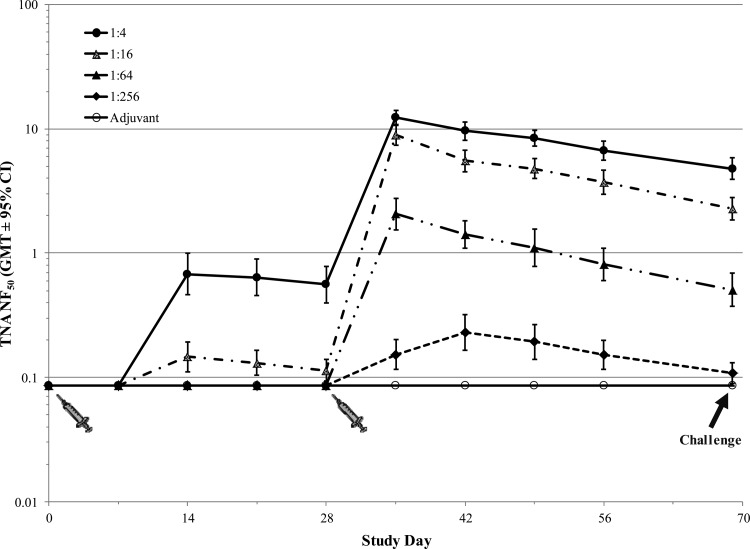
The TNA data indicated that postexposure vaccination with AVA induced a dose-dependent neutralizing antibody response (Table 2). In vaccinated animals (groups 1 to 4, 7, and 8), the TNA NF50 levels increased rapidly between days 11 and 22 and then started to decline. The TNA results also showed that a significant component of the antibody response in the challenge groups was a result of the response to infection, as indicated by the pronounced differences between the TNA NF50 levels observed in challenge group animals receiving 1:4 and 1:16 dilutions (groups 2 and 3, respectively) of the vaccine compared to animals that were not challenged and that were administered the same dose levels of AVA (groups 7 and 8, respectively).
Table 2.
TNA NF50 GMTs in NZW rabbits following lethal B. anthracis spore challenge and levofloxacin treatment with or without concomitant AVA vaccination (rabbit PEP study)
| Group | Vaccine dose | Levofloxacin treatment | Spore challenge | Survival rate (%) | TNA NF50 GMTa by postchallenge day: |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 13 | 15 | 18 | 22 | 30 | |||||
| 1 | Undiluted | + | + | 100 | 3.84 | 4.28 | 5.49 | 7.99 | 8.00 | 7.48 |
| 2 | 1:4 | + | + | 100 | 2.03 | 3.93 | 4.73 | 7.45 | 7.66 | 7.01 |
| 3 | 1:16 | + | + | 95.5 | 0.31 | 2.38 | 3.19 | 3.20 | 3.91 | 3.87 |
| 4 | 1:64 | + | + | 69.6 | 0.10 | 0.81 | 2.74 | 4.15 | 3.65 | 2.69 |
| 5 | Adjuvant | + | + | 22.7 | 0.09 | 0.09 | 0.09 | 0.19 | 0.25 | 0.26 |
| 6 | WFIc | − | + | 0 | —b | —b | —b | —b | —b | —b |
| 7 | 1:4 | + | − | 100 | 0.69 | 2.14 | 2.30 | 3.63 | 4.44 | 4.28 |
| 8 | 1:16 | + | − | 100 | 0.13 | 0.82 | 1.38 | 1.51 | 1.72 | 1.82 |
For calculation of the geometric mean titers (GMTs), individual TNA NF50 values below the LOQ were replaced with the LOQ value (0.086; rounded to 0.09 for this table).
All animals in group 6 died prior to the day 11 blood collection.
WFI, water for injection.
Preexposure efficacy of AVA in rabbits and NHPs.
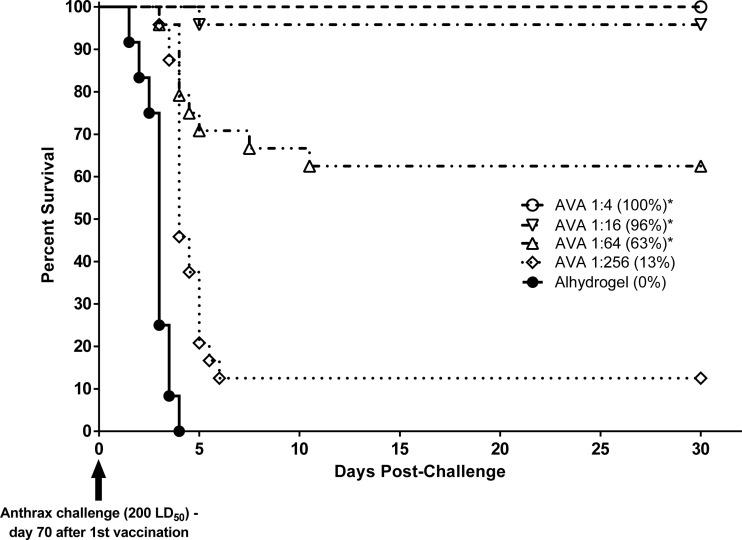
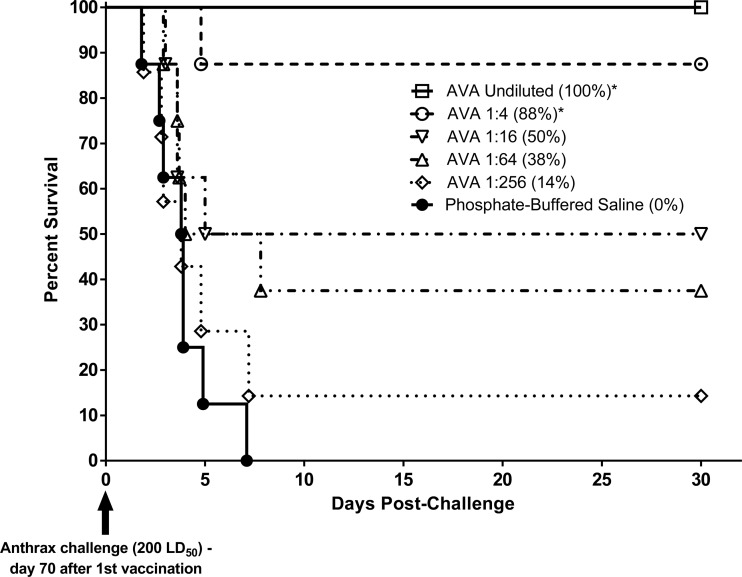
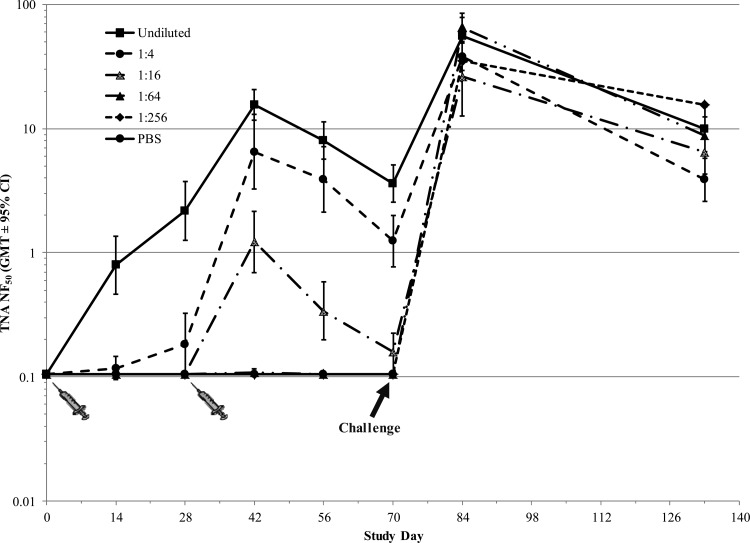
In the preexposure prophylaxis studies, the actual exposure doses received by the rabbits and NHPs were 254 ± 38 and 252 ± 65 LD50 of aerosolized B. anthracis Ames spores, respectively. In both rabbits (Fig. 2) and NHPs (Fig. 3), AVA conferred protection against lethal aerosol challenge with B. anthracis spores in a dose-dependent manner, with higher vaccine dose levels affording a statistically significant (P < 0.006) increase in survival compared to the control group. A dose-dependent prechallenge immune response was also observed in both animal models, as measured by TNA ED50 (data not shown) and NF50 (Fig. 4 and 5) levels. Postchallenge TNA titers measured in surviving NHPs (Fig. 5) were between 10- and 250-fold higher than the prechallenge titers, suggesting a strong anamnestic immune response to the infection following vaccination-induced priming.
Fig 2.
Survival and time to death of NZW rabbits following vaccination with AVA and subsequent lethal B. anthracis spore challenge (rabbit preexposure prophylaxis study). Animals were immunized with serial dilutions of the human dose of AVA on days 0 and 28. Control animals were immunized with adjuvant alone (saline with 650 μg of aluminum hydroxide gel). On day 70, all rabbits were challenged via inhalation with 200 LD50 of aerosolized B. anthracis spores. Animals were monitored for morbidity and mortality for 30 days postchallenge. Overall group survival rates at the time of study termination are shown in parentheses. *, Survival rate was significantly greater than that in the control group (P < 0.0005).
Fig 3.
Survival and time to death of cynomolgus macaques following vaccination with AVA and subsequent lethal B. anthracis spore challenge (NHP preexposure prophylaxis study). Animals were immunized with serial dilutions of the human dose of AVA on days 0 and 28. Control animals were immunized with PBS. On day 70, all monkeys were challenged via inhalation with 200 LD50 of aerosolized B. anthracis spores. Animals were monitored for morbidity and mortality for 63 days postchallenge (survival up to 30 days postchallenge shown in the figure; no additional deaths occurred after that period). Overall group survival rates at the time of study termination are shown in parentheses. *, The survival rate was significantly greater than that in the control group (P < 0.0006).
Fig 4.
Immune response to vaccination with AVA in NZW rabbits (rabbit preexposure prophylaxis study). Animals were immunized with serial dilutions of the human dose of AVA on days 0 and 28. Control animals were immunized with adjuvant alone (saline with 650 μg of aluminum hydroxide gel). On day 70, all rabbits were challenged via inhalation with 200 LD50 of aerosolized B. anthracis spores. Each TNA NF50 value was calculated as the ratio of the ED50 of the test sample to the ED50 of the reference standard. Individual values below the LOQ were replaced with the LOQ value (0.086) for statistical analysis purposes.
Fig 5.
Immune response to vaccination with AVA in cynomolgus macaques (NHP preexposure prophylaxis study). Animals were immunized with serial dilutions of AVA on days 0 and 28. Control animals were immunized with PBS. On day 70, all monkeys were challenged via inhalation with 200 LD50 of aerosolized B. anthracis spores. Each TNA NF50 value was calculated as the ratio of the ED50 of the test sample to the ED50 of the reference standard. Individual values below the LOQ were replaced with the LOQ value (0.105) for statistical analysis purposes. Postchallenge TNA NF50 GMTs (days and 133) were calculated using group sizes based on surviving animals: 8 (group 1), 7 (group 2), 4 (group 3), 3 (group 4), 1 (group 5). Pre- and postchallenge TNA responses are separated by a vertical dashed line.
Safety and immunogenicity of AVA in humans.
A total of 210 subjects were screened for this study. Of those, 150 subjects were enrolled. Of the 150 enrolled subjects, 121 (80.7%) were included in the PP population. All enrolled subjects received at least one 0.5-ml s.c. dose of AVA, and 138 (92%) completed the study. AVA was well tolerated at the dose of 0.5 ml administered s.c. three times over 4 weeks (study days 0 ± 1, 14 ± 2, and 28 ± 2). There were no clinically significant changes in vital signs or safety laboratory analyses, including chemistry, hematology, and urinalysis parameters. Clinically significant abnormalities were not found during physical examinations. In addition, no serious adverse events or withdrawals due to adverse events occurred during this study.
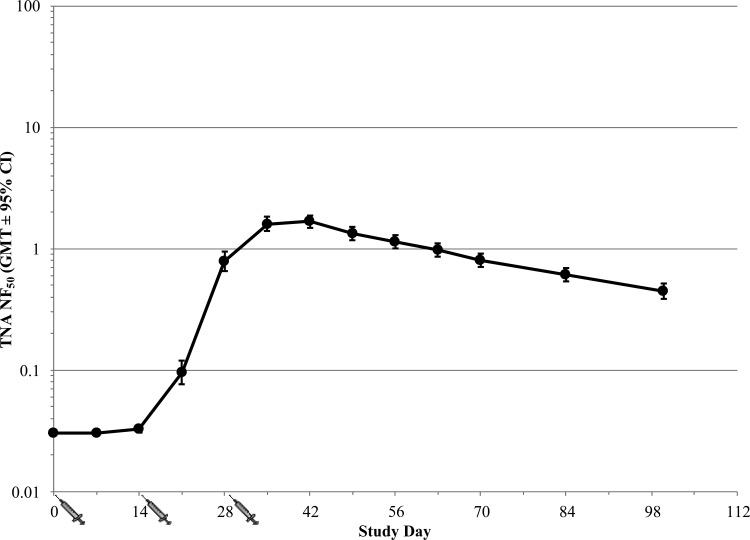
Subjects that were vaccinated with AVA on days 0, 14, and 28 via the s.c. route demonstrated 100% seroconversion by day 42, or 14 days following the third vaccination (Fig. 6). Seroconversion was defined as a ≥4-fold rise in antibody titers compared to those observed at screening. At day 42, 90% of the subjects had a TNA NF50 value of ≥0.788 and an anti-PA IgG concentration (measured by ELISA) of ≥217.4 μg/ml. After day 42, slow declines in both the TNA NF50 levels (Fig. 6) and anti-PA IgG concentrations (data not shown) were observed. The peak TNA NF50 geometric mean titer (GMT) was 1.67, and the peak anti-PA IgG geometric mean concentration (GMC) by ELISA was 454.3 μg/ml. A strong correlation between TNA titers and PA-specific IgG concentrations measured by ELISA was observed, with Pearson's correlation coefficients of 0.993 and 0.996 for the ED50 and NF50 values, respectively.
Fig 6.
Immune response to vaccination with AVA in healthy human subjects (human immunogenicity study). Subjects were immunized with 0.5 ml of AVA via the s.c. route on days 0, 14, and 28. The figure illustrates the TNA NF50 profile over time (GMT ± 95% confidence interval [CI]) in the PP population (n = 121). Individual values below the LOQ (0.064) were replaced with the 1/2 LOQ value (0.032) for statistical analysis purposes.
TNA values associated with survival in animals and predictive vaccine efficacy.
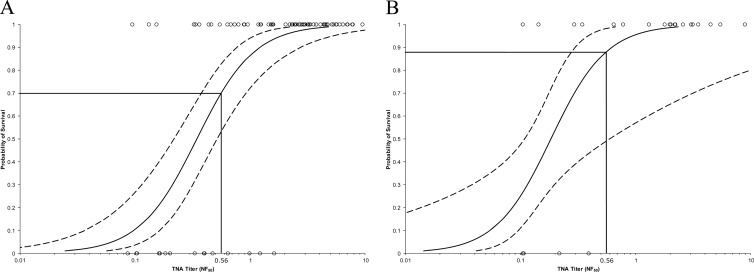
The relationship between antibody levels (as measured by TNA NF50) and the survival of animals vaccinated with AVA was investigated using logistic regression analysis (Fig. 7). A statistically significant (P < 0.05) relationship was observed between animal survival and the TNA levels (NF50) measured at every time point starting 1 week after the second immunization (i.e., at day 35). In particular, a strong correlation was observed between TNA levels just prior to challenge (at day 69 in rabbits or day 70 in NHPs) and survival following challenge. Thus, serum neutralizing antibody levels measured at the time of challenge, which corresponds to the time point shortly after the recommended 60-day antimicrobial regimen would be discontinued in a PEP scenario, was predictive of the animal survival outcome following challenge.
Fig 7.
Relationship between prechallenge AVA-induced TNA levels and probability of animal survival following lethal challenge with B. anthracis spores in rabbit (A) and NHP (B) preexposure prophylaxis studies. Logistic regression models were fitted to the survival curve and either day 69 TNA NF50 levels in rabbits (A) (n = 96) or day 70 TNA NF50 levels in NHPs (B) (n = 39) in order to assess antibody level immediately prior to anthrax challenge as a predictor of survival in the vaccinated animals. Individual values below the LOQ were replaced with the LOQ value (0.086 for rabbit samples and 0.105 for NHP samples). The graphs represent the logistic curves fitted to the data (solid lines) and 95% confidence intervals (dashed lines). Individual animal data points are represented by open-circle symbols (○); survival = 1; death = 0.
Based on these findings, a logistic regression model was fitted to the TNA and survival data, which allowed for the prediction of the animal survival probability based on the neutralizing antibody level observed. For example, a preexposure TNA NF50 value of 0.56 (at day 69 or 70) corresponded to a 70% probability of survival (Fig. 7A) in rabbits and 88% probability of survival in NHPs (Fig. 7B). The data collected in the exploratory clinical immunogenicity study indicated that approximately 82% of human subjects vaccinated on days 0, 14, and 28 via the s.c. route met or exceeded this TNA level on day 63, i.e., 3 days after the recommended 60-day antimicrobial PEP regimen would be discontinued.
An alternative approach, based on the Kohberger methodology described previously in Materials and Methods (29), was also employed. The population probability of death from anthrax in a vaccinated individual was estimated using equation 2:
| (2) |
where f(x), probability of death, is estimated by logistic regression modeling based on the data from the animal preexposure prophylaxis studies and g(x), the distribution of human immune responses in the exploratory clinical study, is assumed to be normal.
Using this methodology, predictive VE would be estimated to be 83%.
DISCUSSION
The purpose of the work described here was to evaluate the ability of AVA to improve survival in vaccinated animals following exposure to aerosolized B. anthracis spores, compared to antimicrobial treatment alone, and to provide a mechanism for bridging animal and human data by identifying an antibody level associated with survival in animals and assessing the ability of human subjects to achieve this antibody level.
The PEP study in rabbits described here supports previous findings by Hewitt and coworkers (29) and demonstrates increased survival with postexposure vaccination with AVA compared with antimicrobial treatment alone. The preexposure efficacy studies in rabbits and NHPs provided the means of identifying the level of immune response that is associated with a specific probability of survival in animals. This level of immune response was based on prechallenge TNA titers, which are a reliable predictor of animal survival following lethal anthrax challenge (5–10), and could then be compared with TNA values observed in humans at day 63, i.e., a time point shortly after the recommended 60-day antimicrobial PEP regimen would be discontinued and the risk of infection resulting from the germination of any residual spores would increase. The appropriateness of comparing the animal TNA levels achieved at day 69 or 70 following two i.m. immunizations (in the preexposure model) and human TNA levels achieved at day 63 following three s.c. immunizations (the proposed PEP vaccination regimen) is supported by the fact that TNA titers in animals at day 69 or 70 correlate with survival following challenge, and circulating antibody levels at the time of exposure contribute to survival. The TNA titer associated with protection in animals may be conservative, considering that the animals were challenged with a very high dose of B. anthracis spores (31). The challenge dose of 200 LD50 used to determine the threshold of protection in immunized rabbits is likely a higher exposure than that which is expected to occur from residual spores after a recommended 60-day course of antimicrobials has been completed.
It should be noted that circulating neutralizing antibody is probably not the sole mechanism of protection against lethal anthrax challenge at day 70 in the preexposure efficacy model. The rapidly inducible immunological memory response generated after two vaccinations likely contributes to the protection of animals. This hypothesis is supported by the long-term protection study in NHPs conducted by Quinn and coworkers (40). In that study, animals vaccinated with three doses of AVA (at 0, 1, and 6 months) were protected against inhalation anthrax for up to 4 years after the initial vaccination, despite the fact that the circulating anti-PA IgG and TNA levels immediately prior to challenge were near or below the LOQ. The authors concluded that long-term protection was afforded by PA-specific B- and T-memory cells that are capable of mounting a rapid protective anamnestic response following challenge with aerosolized B. anthracis Ames spores (40). The contribution of memory response to protection is further supported by observations from passive immunization studies, in which animals were exposed to 200 LD50 of aerosolized anthrax spores and treated pre- or postexposure by intravenous infusion with purified immune globulin derived from blood plasma samples of human subjects who were vaccinated with AVA (41, 42, 43). While those studies indicated that neutralizing antibody alone could protect animals against lethal anthrax challenge, the level of circulating antibody associated with 70% survival in rabbits was >10-fold higher than that noted in active immunization studies (43). This observation suggests that in the case of active immunization, the anamnestic response leads to a rapid increase in the level of circulating antibody (33), thereby bridging the gap between the level of circulating antibody available at the time of exposure and that which is required for protection.
The studies described here provide initial information on the neutralizing antibody levels associated with the protection of animals from lethal anthrax challenge, as well as support previous findings regarding the ability of AVA to provide added protection to B. anthracis-infected animals compared to those that receive antimicrobial treatment alone.
ACKNOWLEDGMENTS
We thank the staff at Battelle Biomedical Research Center (West Jefferson, OH) and SRI International (Menlo Park, CA) for excellent technical support in the execution of animal studies, Sukjoon Park for a critical review of the manuscript, Judith A. Hewitt, Ed Nuzum, Nicholas J. Vietri, Arthur M. Friedlander, and Conrad P. Quinn for valuable scientific discussions and advice, William C. Blackwelder and Diane Sweeney for statistical support, and Peter T. Rogge, Serena M. Mraz, Scott D. Parker, Richard Greenberg, Frank C. Hampel, Jr, Tyler D. Laudenslager, Laureen E. Little, Desiree Bland, Mark J. Lyons, Elisha N. Morrison, Subhendu Basu, Christine Valencia, Bryan Fortson, Jamie Gillette, Marjana Marinac, Tarl Spangler, Pamela Duchars, Sarah Sweeny, Nancy Daczkowski, Devinder Poonian, Martina Brunhuber, and David Main for technical and programmatic support.
This project has been funded in part with federal funds from the Biomedical Advanced Research and Development Authority, Department of Health and Human Services, under contract no. HHSO-100-2007-00037C.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K, Working Group on Civilian Biodefense 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 (Erratum, 288:1849.) [DOI] [PubMed] [Google Scholar]
- 2. Brossier F, Mock M. 2010. Toxins of Bacillus anthracis. Toxicon 39:1747–1755 [DOI] [PubMed] [Google Scholar]
- 3. Bann JG, Hultgren SJ. 2004. Structural biology: anthrax hijacks host receptor. Nature 430:843–844 [DOI] [PubMed] [Google Scholar]
- 4. Kintzer AF, Sterling HJ, Tang II, Williams ER, Krantz BA. 2010. Anthrax toxin receptor drives protective antigen oligomerization and stabilizes the heptameric and octameric oligomer by a similar mechanism. PLoS One 5:e13888. 10.1371/journal.pone.0013888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768–4773 [DOI] [PubMed] [Google Scholar]
- 6. Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, Kobiler D, Shafferman A, Velan B. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beedham RJ, Turnbull PC, Williamson ED. 2001. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine 19:4409–4416 [DOI] [PubMed] [Google Scholar]
- 8. Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422–430 [DOI] [PubMed] [Google Scholar]
- 9. Little SF, Ivins BE, Webster WM, Fellows PF, Pitt MLM, Norris SLW, Andrews GP. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530–2536 [DOI] [PubMed] [Google Scholar]
- 10. Fay MP, Follmann DA, Lynn F, Schiffer JM, Stark GV, Kohberger R, Quinn CP, Nuzum EO. 2012. Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci. Transl. Med. 4:151ra126. 10.1126/scitranslmed.3004073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henderson DW, Peacock S, Belton FC. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. (Lond.) 54:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedlander AM, Welkos SL, Pitt ML, Ezzell JW, Worsham PL, Rose KJ, Ivins BE, Lowe JR, Howe GB, Mikesell P, Lawrence WB. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239–1243 [DOI] [PubMed] [Google Scholar]
- 13. Kao LM, Bush K, Barnewall R, Estep J, Thalacker FW, Olson PH, Drusano GL, Minton N, Chien S, Hemeryck A, Kelley MF. 2006. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob. Agents Chemother. 50:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Novartis Vaccines and Diagnostics 2012. RabAvert rabies vaccine package insert. Novartis Vaccines and Diagnostics, Inc. Cambridge, MA: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM312931.pdf [Google Scholar]
- 15. Sanofi Pasteur. 2012. Imovax rabies vaccine package insert. Sanofi Pasteur, Inc; Swiftwater, PA: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM133484.pdf [Google Scholar]
- 16. GlaxoSmithKline 2012. ENGERIX-B hepatitis B vaccine (recombinant) package insert. GlaxoSmithKline, Rixensart, Belgium: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM224503.pdf [Google Scholar]
- 17. Merck & Co 2011. RECOMBIVAX HB® hepatitis B vaccine (recombinant) package insert. Merck & Co., Inc, Whitehouse Station, NJ: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM110114.pdf [Google Scholar]
- 18. Aventis Pasteur. 2005. Tetanus toxoid adsorbed package insert. Aventis Pasteur, Swiftwater, PA: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142732.pdf [Google Scholar]
- 19. Emergent BioSolutions. 2012. BioThrax (anthrax vaccine adsorbed) package insert. Emergent BioDefense Operations Lansing LLC, Lansing, MI: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/UCM074923.pdf [Google Scholar]
- 20. Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. 1962. Field evaluation of a human anthrax vaccine. Am. J. Public Health Nations Health 52:632–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL. (ed). 2002. The anthrax vaccine: is it safe? Does it work? National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 22. Code of Federal Regulations 2012. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter F. Biologics. Part 601. Review procedures to determine that licensed biological products are safe, effective, and not misbranded under prescribed, recommended, or suggested conditions of use. 21 CFR 601.25. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=601.25. [Google Scholar]
- 23. Code of Federal Regulations 2012. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter F. Biologics. Part 601. Approval based on a surrogate endpoint or on an effect on a clinical endpoint other than survival or irreversible morbidity. 21 CFR 601.41. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=601.41. [Google Scholar]
- 24. FDA 2007. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. U.S. Department of Health and Human Services. Food and Drug Administration. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091990.pdf. [Google Scholar]
- 25. Vaccines and Related Biological Products Advisory Committee 17 May 2007. Transcript of Vaccines and Related Biological Products Advisory Committee Meeting. U.S. Department of Health and Human Services, Food and Drug Administration; Gaithersburg, MD: http://www.fda.gov/ohrms/dockets/ac/07/transcripts/2007-4292t2.pdf [Google Scholar]
- 26. Vaccines and Related Biological Products Advisory Committee 6–7 April 2011. Vaccines and Related Biological Products Advisory Committee meeting summary minutes. U.S. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm254361.htm [Google Scholar]
- 27. Burns DL. 2012. Licensure of vaccines using the Animal Rule. Curr. Opin. Virol. 2:353–356 [DOI] [PubMed] [Google Scholar]
- 28. CDER and CBER 2009. Guidance for industry animal models: essential elements to address efficacy under the Animal Rule. U.S. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078923.pdf [Google Scholar]
- 29. CBER 8 November 2007. Anthrax vaccines: bridging correlates of protection in animals to immunogenicity in humans. U.S. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM054424.pdf [Google Scholar]
- 30. Vietri NJ, Purcell BK, Tobery SA, Rasmussen SL, Leffel EK, Twenhafel NA, Ivins BE, Kellogg MD, Webster WM, Wright ME, Friedlander AM. 2009. A short course of antibiotic treatment is effective in preventing death from experimental inhalational anthrax after discontinuing antibiotics. J. Infect. Dis. 199:336–341 [DOI] [PubMed] [Google Scholar]
- 31. CBER 23 April 2002. Anthrax vaccines: efficacy testing and surrogate markers of immunity. U.S. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM054606.pdf [Google Scholar]
- 32. Leffel EK, Bourdage JS, Williamson ED, Duchars M, Fuerst TR, Fusco PC. 2012. Recombinant protective antigen anthrax vaccine improves survival when administered as a postexposure prophylaxis countermeasure with antibiotic in the New Zealand white rabbit model of inhalation anthrax. Clin. Vaccine Immunol. 19:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaccines and Related Biological Products Advisory Committee 16 November 2010. Transcript of FDA Advisory Committee Meeting. Vaccines and Related Biological Products Advisory Committee Meeting Materials. U.S. Department of Health and Human Services, Food and Drug Administration; http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm237045.htm [Google Scholar]
- 34. Brachman PS, Friedlander AM, Grabenstein JD. 2008. Anthrax vaccine, p 111–126 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 5th ed Saunders, Philadelphia, PA [Google Scholar]
- 35. Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982–992 [PubMed] [Google Scholar]
- 36. Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 83(8):1201–1209 [DOI] [PubMed] [Google Scholar]
- 37. Li H, Soroka SD, Taylor TH, Jr., Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. 2008. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods 333:89–106 [DOI] [PubMed] [Google Scholar]
- 38. Omland KS, Brys A, Lansky D, Clement K, Lynn F, the Participating Laboratories 2008. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin. Vaccine Immunol. 15:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt DS, Mothershed E, Pruckler J, Schwartz S, Benson RF, Helsel LO, Holder PF, Johnson SE, Kellum M, Messmer T, Thacker WL, Besser L, Plikaytis BD, Taylor TH, Jr, Freeman AE, Wallace KJ, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa JR, Martin SK, Walls J, Bronsdon M, Carlone GM, Bajani-Ari M, Ashford DA, Stephens DS, Perkins BA. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 10:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quinn CP, Sabourin CL, Niemuth NA, Li H, Semenova VA, Rudge TL, Mayfield HJ, Schiffer J, Mittler RS, Ibegbu CC, Wrammert J, Ahmed R, Brys AM, Hunt RE, Levesque D, Estep JE, Barnewall RE, Robinson DM, Plikaytis BD, Marano N, AVRP Laboratory Working Group 2012. A three-dose intramuscular schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin. Vaccine Immunol. 19:1730–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chakrabarti A, Hopkins R, Malkevich N, Ionin B, Skiadopoulos M, Smith J, Nabors G. 2009. Recent advances in the development of a human anthrax intravenous immune globulin (AIGIV) for treatment of inhalation anthrax disease, abstr. 119C. Bacillus-ACT 2009: the International Bacillus anthracis, B. cereus, and B. thuringiensis Conference, Santa Fe, NM [Google Scholar]
- 42. Basu S, Mytle N, Van Zandt KE, Meister GT, Ionin B, Chakrabarti AC, Nabors GS, Skiadopoulos MH. 2011. Postexposure efficacy of human anthrax immune globulin, intravenous (AIGIV) in rabbits, abstr. 119-Mo. Bacillus-ACT 2011: the International Bacillus anthracis, B. cereus, and B. thuringiensis Conference, Bruges, Belgium [Google Scholar]
- 43. Nuzum E. 16 November 2010. DMID/NIAID efforts that support the pathway to licensure for protective antigen-based anthrax vaccines for a post-exposure indication using the animal rule. Vaccines and Related Biological Products Advisory Committee Meeting Presentations (PPTX-895KB). http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm239733.htm