Abstract
Aspergillus lentulus was described in 2005 as a new species within the A. fumigatus sensu lato complex. It is an opportunistic human pathogen causing invasive aspergillosis with high mortality rates, and it has been isolated from clinical and environmental sources. The species is morphologically nearly identical to A. fumigatus sensu stricto, and this similarity has resulted in their frequent misidentification. Comparative studies show that A. lentulus has some distinguishing growth features and decreased in vitro susceptibility to several antifungal agents, including amphotericin B and caspofungin. Similar to the once-presumed-asexual A. fumigatus, it has only been known to reproduce mitotically. However, we now show that A. lentulus has a heterothallic sexual breeding system. A PCR-based mating-type diagnostic detected isolates of either the MAT1-1 or MAT1-2 genotype, and examination of 26 worldwide clinical and environmental isolates revealed similar ratios of the two mating types (38% versus 62%, respectively). MAT1-1 and MAT1-2 idiomorph regions were analyzed, revealing the presence of characteristic alpha and high-mobility-group (HMG) domain genes, together with other more unusual features such as a MAT1-2-4 gene. We then demonstrated that A. lentulus possesses a functional sexual cycle with mature cleistothecia, containing heat-resistant ascospores, being produced after 3 weeks of incubation. Recombination was confirmed using molecular markers. However, isolates of A. lentulus failed to cross with highly fertile strains of A. fumigatus, demonstrating reproductive isolation between these sibling species. The discovery of the A. lentulus sexual stage has significant implications for the management of drug resistance and control of invasive aspergillosis associated with this emerging fungal pathogen.
INTRODUCTION
Aspergillus lentulus is an opportunistic human pathogenic fungus from the Aspergillus section Fumigati (1) that can cause invasive aspergillosis in immunocompromised patients (2). It was first discovered in 2004, as an unexpected result from a U.S. study examining the susceptibility of isolates of A. fumigatus to azole antifungal drugs (3). Four A. lentulus isolates that caused fatal infections in hematopoietic stem cell transplant patients between the years 1995 and 2000 had been misidentified as poorly sporulating variants of A. fumigatus because their 18S rRNA sequences matched that of A. fumigatus. However, all of the isolates had distinct random amplified polymorphic DNA (RAPD) fingerprint patterns and mitochondrial cytochrome b sequences, and several exhibited in vitro antifungal susceptibility profiles with significantly higher resistance than normal for A. fumigatus. A follow-up study examined the phylogenetic relationships of these isolates to A. fumigatus using multilocus sequence typing (2). The analysis revealed that the four isolates formed a separate clade clearly distant from A. fumigatus, and it was concluded that these represented a new species, which was named A. lentulus (2). A subsequent phylogenetic study of Japanese clinical isolates showed that along with A. fumisynnematus, A. lentulus forms a sister clade to A. fumigatus (4).
Similar to other members of the section Fumigati that are closely related to A. fumigatus, such as A. fumigatiaffinis, many of the phenotypic characters of A. lentulus overlap with A. fumigatus. This makes identification difficult when based solely on morphological grounds, which often leads to misdiagnoses in clinical laboratories (5). However, a variety of methods have since been found that can differentiate between the two species. Phenotypic dissimilarities include their conidial ornamentation (4), conidiophore architecture, growth characteristics (1), and mycotoxin profiles—most notably the inability of A. lentulus to produce gliotoxin (1, 6). The most obvious growth characteristics that distinguish A. lentulus from A. fumigatus are its delayed onset of sporulation and inability to grow at 48°C, although it should be noted that these two features are common to several other species in the section Fumigati (7). Species-specific molecular methods are also available to identify A. lentulus and include the use of a microsphere-based Luminex assay (8), RAPD patterns (1), a multiplex PCR assay (9), and restriction fragment length polymorphisms (10).
Despite the wide geographic distribution and presence of the species in common environmental niches (3–5, 7, 11–13), opportunistic A. lentulus infections appear rare. To date, only six studies have reported cases of invasive aspergillosis in which A. lentulus was confirmed as the probable or causal agent (3, 11, 12, 14–16). Two further cases involved the colonization of cystic fibrosis patients (13, 17). This apparent low incidence rate may be due to the misidentification problems described previously (5), because A. lentulus isolates have been recovered in several retrospective studies of clinical fungal samples (4, 7).
The pathology of invasive infections caused by A. lentulus appears to mirror that of A. fumigatus (3). However, of medical significance is the fact that most isolates exhibit an increased natural resistance to several antifungal agents currently in clinical use compared to A. fumigatus, namely, itraconazole, voriconazole, caspofungin, and amphotericin B (3, 4, 7). The molecular mechanisms underlying these resistance mechanisms have recently started to be elucidated (18, 19), but further work is required to fully understand the genetic basis of resistance. Of particular interest are the mechanisms governing echinocandin resistance, as A. lentulus is highly unusual in being simultaneously resistant to caspofungin yet highly sensitive to anidulafungin and micafungin (7, 19, 20). The discovery of A. lentulus has also proven beneficial to industry, as an A. lentulus isolate has been found with activity as a biosorbent for the removal of toxic compounds. A. lentulus strain AML05, which was recovered from industrial textile effluent in India by a chromium enrichment process, can very successfully remove Cr(VI) from electroplating industry effluent (21, 22) and dyes from textile effluent (23, 24).
A. lentulus is currently known to reproduce only by asexual means, through the production of conidia (2). Hong and coworkers (1) previously tried unsuccessfully to mate strains of A. lentulus, but crucially, this was before recent reports of the discovery of a sexual cycle in A. fumigatus (teleomorph Neosartorya fumigata) (25) and other related Aspergillus and Penicillium species which were previously considered asexual (26–31). In most of these cases, the discovery of a sexual state was preceded by the identification of mating-type (MAT) genes within the species, such genes acting as key regulators of sexual identity in filamentous ascomycete fungi (32, 33). In heterothallic (obligate outbreeding) species, these MAT genes are contained within a region of the genome termed the MAT locus, with highly divergent forms of this locus, known as “idiomorphs,” present in isolates of sexually compatible MAT1-1 and MAT1-2 genotypes. In contrast, homothallic (self-fertile) species may contain one or more MAT loci within the same genome rather than dissimilar idiomorphs (33, 34).
The close phylogenetic relationship of A. lentulus to A. fumigatus suggested that it might be possible to induce sexual reproduction in A. lentulus using conditions similar to those required for A. fumigatus. The aims of this study were, therefore, first, to investigate the presence of MAT genes in A. lentulus to see if isolates with putative sexual compatibility could be identified; second, to determine if a sexual cycle could be induced in A. lentulus; third, to use molecular analysis of offspring to determine if the breeding system was heterothallic or homothallic in nature; and, finally, to see if any gene flow was possible between A. lentulus and A. fumigatus via sexual crossing. The ability to perform sexual crosses in A. lentulus would provide a valuable tool for the genetic analysis of traits relating to pathogenicity, antifungal drug resistance, and industrial processes in this species.
MATERIALS AND METHODS
Strains, growth conditions, and DNA extraction.
Twenty-six isolates of A. lentulus from clinical and environmental sources were used in the study, comprising most isolates of the species previously reported in the scientific literature (see Table S1 in the supplemental material). Sixteen of the clinical isolates were kindly donated by Kieren Marr and Edmond Byrnes (John Hopkins School of Medicine, Maryland). Strains were maintained on Aspergillus complete medium (ACM) (35) at 28°C and are stored in 10% glycerol under liquid nitrogen at the School of Biology, University of Nottingham, United Kingdom (BDUN [Botany Department, University of Nottingham] culture collection). Genomic DNA was extracted using a DNeasy plant minikit (Qiagen) in accordance with the manufacturer's instructions. Cultures were grown in liquid ACM at 28°C for 5 days. The resulting mycelia were harvested, flash frozen, and ground under liquid nitrogen prior to DNA extraction.
Multiplex mating-type PCR assay and PCR amplification of the mating-type idiomorphs.
The mating-type genotype of all A. lentulus isolates and ascospore progeny was determined with the A. fumigatus multiplex mating-type PCR diagnostic of Paoletti et al. (35), using primers AFM1, AFM2, and AFM3 (see Table S2 in the supplemental material). Attempts were then made to amplify the entire MAT idiomorph regions of A. lentulus isolates 78-2 and 78-3 with the primer pair Loc1 and AFM3 (see Table S2) using the conditions described by Paoletti et al. (35); these primers had previously been used to successfully amplify the idiomorph regions of A. fumigatus (35). Resulting amplicons were purified using a Geneflow Q-Spin PCR purification kit (according to the manufacturer's instructions) and were sequenced using primers AL31-AL34 and AL51-AL53 (see Table S2) at the DNA Sequencing Facility of the School of Biomedical Sciences, University of Nottingham, United Kingdom. Arising sequences were analyzed and aligned using MacVector 11 (MacVector Inc.).
Sexual crosses.
Sixteen representative A. lentulus isolates of MAT1-1 and MAT1-2 genotype (see Table S1 in the supplemental material) were chosen for sexual crossing experiments and inoculated in all possible pairwise combinations by following the protocol of O'Gorman et al. (25). Briefly, crosses were set up on oatmeal agar (pinhead oatmeal, Odlums, Ireland [36]) in triplicate, sealed with one layer of Nescofilm, and incubated at 25, 28, or 30°C in the dark. Four A. fumigatus crosses known to reliably produce cleistothecia and ascospores (AfRB2 × AfIR928, AfRB2 × AfIR964, AfIR974 × AfIR928, and AfIR974 × AfIR964) (25) were set up in parallel as controls. Control “selfed” A. lentulus crosses were also tested on oatmeal agar at 28°C using one representative isolate of each mating type (78-2 [MAT1-2] and 78-3 [MAT1-1]). In addition, crosses were set up on oatmeal agar at 28°C and 30°C between representative A. lentulus MAT1-1 (78-3, 78-6, and 78-20) and MAT1-2 (78-2 and 78-8) isolates (see Table S1 in the supplemental material) and known highly fertile isolates of A. fumigatus (AFB62 [MAT1-1] and AfIR928 [MAT1-2]) (37) to assess reproductive isolation. All crosses were examined periodically for the presence of cleistothecia for up to 7 weeks using a Nikon-SMZ-2B dissection microscope and then, finally, after 4 and 12 months of incubation where cleistothecia were not detected in the initial growth period.
Preparation of single-ascospore cultures.
Mature cleistothecia from the cross 78-2 × 78-3 were removed and cleaned as described previously (25), with the modification that 4% water agar was used to clean the cleistothecia instead of a drop of sterile water. Five cleistothecia were added to 50 μl of 0.05% Tween 80 (BDH Chemicals) under sterile conditions and ruptured by squashing with a needle tip. The solution was brought up to 500 μl with 0.05% Tween 80 and vortex mixed for 1 min to release the ascospores. The suspension was then heat treated at 80°C for 30 min, this temperature being sufficient to kill any contaminating conidia without damaging the ascospores (data not shown). One hundred microliters of a 5 × 105-ascospore ml−1 heat-treated suspension was spread inoculated on three defined areas of an ACM plate. Triplicates were prepared and incubated at 37°C for 14 h. Single-spore cultures were established on ACM by transferring individual germinating ascospores with a LaRue lens cutter attached to a Nikon-Optiphot microscope.
Analysis of recombination.
The segregation of five genetic markers (four RAPD bands and the mating-type genotype) in the ascospore progeny was examined for evidence of recombination. RAPD-PCR fingerprinting was performed by following the protocol of O'Gorman et al. (25). Four primers (OMT1, R108, R151, and OPWO8 [see Table S2 in the supplemental material]) from an initial screen of 12 were found to yield suitable polymorphisms for genotyping.
SEM.
Cleistothecia were collected from 6-month-old crosses of 78-2 × 78-3 that had been incubated at 28°C. Representative intact and crushed cleistothecia were transferred onto 0.2-μm filter discs (Whatman) and fixed in 2% osmium tetroxide (Sigma) for 2 h at room temperature. The fixed samples were then mounted onto aluminum stubs, dried at 37°C overnight, and sputter coated with gold. Scanning electron microscopy (SEM) micrographs were taken using a JSM-840 JEOL scanning electron microscope.
Statistical analysis.
The hypothesis of a 1:1 ratio of mating types in the worldwide sample population and ascospore progeny was tested using χ2 and contingency χ2 tests. Where expected frequencies were <5, Fisher's exact test was used instead (38).
Accession numbers.
The sequences for the two mating-type loci amplified from isolates 78-3 (MAT1-1) and 78-2 (MAT1-2) have been deposited in GenBank under accession numbers KC876046 and KC876047. The diagnosis of the Aspergillus lentulus (neosartorya-morph) has been deposited in MycoBank under accession number MB356679 (see the supplemental material).
RESULTS AND DISCUSSION
Sex is thought to have evolved in early eukaryotic microbes and is now widespread throughout the Eukaryota (39). The ability to undergo sexual reproduction is considered to be of major importance given the many benefits it confers. These include the potential to purge harmful mutations and improve the fitness of offspring, which, in turns, allows them to better resist adverse environmental conditions (40–43). It is therefore surprising that the kingdom Fungi appears to contain a disproportionally large number of supposedly asexual species, with an estimated 20% having no known sexual stage. Many are members of the phylum Ascomycota that are of medical or economic significance (44). Some, such as A. oryzae, have been shown to possess MAT genes and other “sexual machinery,” yet their sexual cycle remains elusive (45). While there are clear advantages to asexual over sexual reproduction, such as the relatively lower metabolic cost and ability to produce spores under a wider range of environmental conditions, the rewards of sexual reproduction appear to be much greater (46).
The discovery of a heterothallic sexual cycle in A. fumigatus, which had long been considered to be reproduce purely by mitotic means, confirmed suspicions that at least some of these supposed “asexuals” do in fact have the potential to reproduce sexually (25, 43). Significantly, a “sexual revolution” has since followed, with the reporting of functional sexual cycles in several other related filamentous fungi (26–31, 47). The aim of this work was to determine whether it was possible to induce a sexual cycle in A. lentulus, given its close phylogenetic relationship to A. fumigatus (1) and its increasing importance as a human pathogen with resistance to drugs in several antifungal classes relative to A. fumigatus (2).
Presence, distribution, and characterization of the MAT idiomorphs.
The A. lentulus genome was first examined for the presence of the master regulatory MAT genes that are transcription factors common to all heterothallic fungi and which determine cell sexual identity (32). It was found that the previously described multiplex PCR mating type diagnostic for A. fumigatus (35) produced corresponding amplicons of the predicted size (ca. 834 bp for MAT1-1 and 438 bp for MAT1-2) in different isolates of A. lentulus (see Fig. S1 in the supplemental material). This indicated a heterothallic (obligate outcrossing) arrangement in the species and confirmed the phylogenetic affinity with A. fumigatus, given that this was designed as a species-specific diagnostic test. The worldwide collection of A. lentulus isolates was then screened to determine the ratio of complementary MAT1-1 and MAT1-2 genotypes (see Table S1 in the supplemental material). Amplicons were generated for all isolates, and the overall mating-type distribution did not deviate significantly from a 1:1 ratio (38.5% MAT1-1 and 61.5% MAT1-2; χ2 = 1.38; n = 26), consistent with a sexually reproducing species. When isolates were grouped according to geographic origin, there was also no significant difference in the MAT distribution (data not shown).
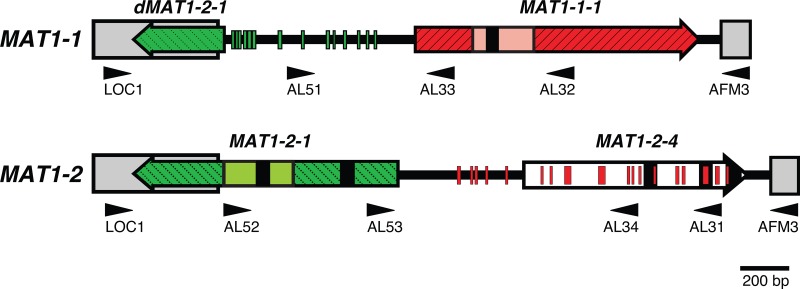
It was then found that the entire MAT1-1 and MAT1-2 idiomorph regions of A. lentulus could be amplified using the primers Loc1 and AMF3, which had previously been used to amplify MAT regions from A. fumigatus (35). This yielded amplicons of 2,524 and 2,731 bp, respectively. Figure 1 shows the complete sequenced idiomorph structure of the two mating-type loci amplified from isolates 78-3 (MAT1-1) and 78-2 (MAT1-2). The idiomorphs showed the same overall structural organization as those previously reported from A. fumigatus (35). The MAT1-1 idiomorph of 78-3 contains a 1,157-bp putative open reading frame (ORF), predicted to encode a 368-amino-acid protein with a characteristic α1 domain, and was therefore termed MAT1-1-1 (33). The ORF is interrupted by a 50-bp intron in the conserved position found in other ascomycetes, and the overall protein and α1 domain region share 93% and 98% amino acid identity to A. fumigatus, respectively (see Fig. S2A in the supplemental material). The MAT1-2 idiomorph of 78-2 contains two putative ORFs. The first is a 1,077-bp ORF predicted to encode a 322-amino-acid protein with a characteristic high-mobility-group (HMG) box, which was therefore termed MAT1-2-1 (33). This ORF contains two introns (53 and 55 bp), and the overall protein and HMG domain share 89% and 92% amino acid identity to A. fumigatus, respectively (see Fig. S2B). The second is a putative ORF of 903 bp with three introns (46, 46, and 58 bp), predicted to encode a 242-amino-acid product which was found to share 95% and 87% amino acid identity to the putative MAT1-2-4 proteins from A. fumigatus and Neosartorya fischeri, respectively (see Fig. S2C) (48, 49; C. Eagle and P. S. Dyer, unpublished data). A related MAT1-2-4 family gene has also been described for Talaromyces (Penicillium) marneffei (50). However, similar MAT1-2-4 genes are absent from many other aspergilli, and their expression and possible functionality remain to be determined. A MAT1-2-4 gene has also been reported for Coccidioides immitis and C. posadasii (51, 52), but this shares little sequence conservation with the MAT1-2-4 proteins from the aspergilli and is absent from the MAT loci of many other heterothallic eurotiomycete species, such as those recently described for Blastomyces dermatitidis (53).
Fig 1.
MAT locus of Aspergillus lentulus. The arrangement of the A. lentulus idiomorph region shows the difference in organization between isolates 78-3 (MAT1-1) and 78-2 (MAT1-2). Colored block arrows indicate MAT1-1-1 (red), MAT1-2-1 (green), and MAT1-2-4 (white) sequences. Colored boxes indicate the α1 domain (salmon pink), HMG domain (light green), and nearly identical flanking regions (gray). Introns are represented by black boxes; lines extending between boxes and arrows represent idiomorph sequence. Smaller red and green segments represent regions between 10 and 29 bp in length with ≥70% MAT1-1-1 and MAT1-2-1 nucleotide conservation, respectively. Black arrowheads (direction indicates 5′ to 3′ sequence) show the positions of primers (see Table S2 in the supplemental material) used for amplification of the idiomorph region.
Similar to the previous report for A. fumigatus (35), although the MAT1-2-1 gene commenced within the MAT1-2 idiomorph, a terminal 374-bp region was found to lie within the flanking sequence bordering both idiomorphs (Fig. 1). However, the fragment bordering the MAT1-1 idiomorph appeared nonfunctional, as it lacked the HMG domain region and any start codon and contained a deletion, giving rise to a frameshift mutation. Therefore, the fragment was termed dMAT1-1-1 in recognition of the disabled ORF (48). Intriguingly, further analysis of the MAT1-1 idiomorph revealed an additional 14 regions between 10 and 15 bp in length with ≥70% nucleotide conservation compared to the A. lentulus MAT1-2-1 gene. These extended directly upstream from the 374-bp dMAT1-2-1 fragment, with seven of the regions present within the predicted HMG domain (Fig. 1). Similarly, further analysis of the MAT1-2 idiomorph revealed 18 regions between 10 and 29 bp in length within the idiomorph which exhibited ≥70% nucleotide conservation compared to A. lentulus MAT1-1-1 gene, including 13 within the MAT1-2-4 gene itself (Fig. 1). These data indicate a complex evolutionary history for the idiomorphs, possibly signifying the presence of an ancestral homothallic MAT locus containing both MAT1-1-1 and MAT1-2-1 genes, which has since undergone accelerated mutation and evolution in the transition to heterothallism (54). It has been suggested that homothallism might be the ancestral state within the Aspergillus section Fumigati, with the overwhelming majority of teleomorphic Neosartorya species exhibiting homothallic breeding systems (55, 56). It can also be speculated that the MAT1-2-4 gene, whose origins are obscure, might have arisen from sequence derived from the MAT1-1-1 gene.
Sexual crosses.
Crosses were set up between isolates of complementary mating type using the conditions that had successfully induced sex in A. fumigatus (25), with one modification. Both 25°C and 28°C were tested in addition to the 30°C required for A. fumigatus mating, given that A. lentulus has a lower growth temperature range than A. fumigatus (1). Nine isolates of clinical and environmental origin from North America and Asia (see Table S1 in the supplemental material) were crossed in all pairwise combinations (Table 1). Significantly, after 3 weeks of incubation at both 28°C and 30°C, certain crosses were found to be fertile, producing cleistothecia that contained viable ascospores (Table 1 and Fig. 2; see supplemental material for species diagnosis). In contrast, no cleistothecia were observed in crosses incubated at 25°C. Cleistothecia also failed to develop on single MAT cultures, indicating that A. lentulus is a heterothallic species.
Table 1.
Mean numbers of cleistothecia produced by 14 Aspergillus lentulus crosses on oatmeal agar at 28°C or 30°C in the dark after 3 weeksa
| MAT1-2 strain | Score for cleistothecium production of cross with MAT1-1 strain |
|||
|---|---|---|---|---|
| 28°C |
30°C |
|||
| 78-3 | 78-6 | 78-3 | 78-6 | |
| 78-1 | − | − | − | − |
| 78-2 | > | + | ++++ | +++ |
| 78-4 | − | − | − | − |
| 78-5 | − | − | − | − |
| 78-7 | − | − | − | − |
| 78-8 | + | + | + | − |
| 78-9 | − | − | − | − |
Ratings indicate the mean number of cleistothecia produced from three replicate crosses on oatmeal agar in 9-cm-diameter petri dishes after incubation in the dark, as follows: −, none; +, 1 to 19; +++, 40 to 59; ++++, 80 to 100; >, more than 100.
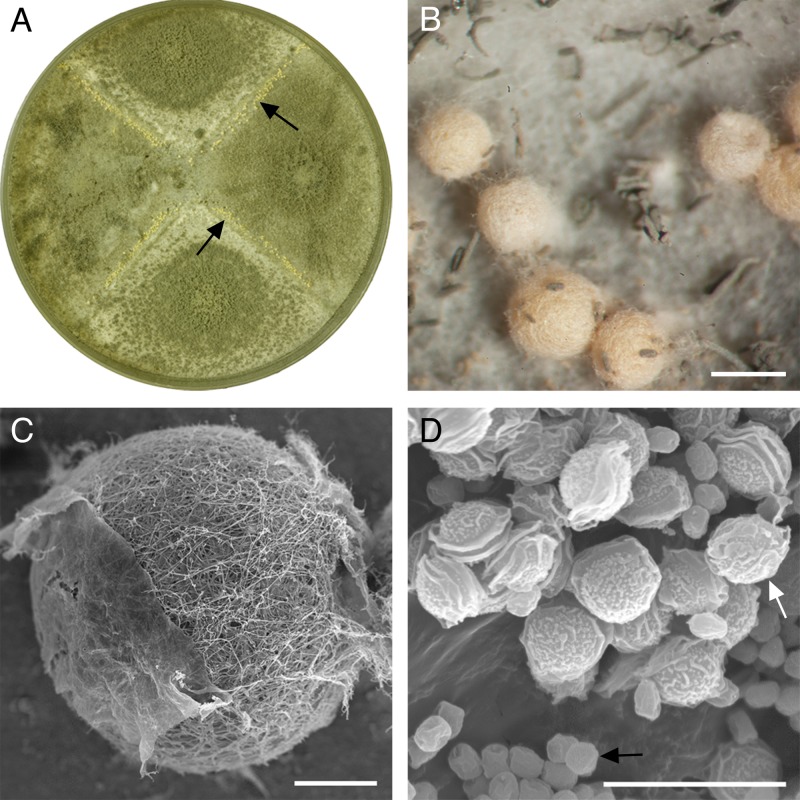
Fig 2.
Sexual reproduction in Aspergillus lentulus. (A) Paired cultures of isolates 78-2 (MAT1-2) × 78-3 (MAT1-1) on oatmeal agar (9-cm-diameter petri dish) with cleistothecia (arrows) along the barrage zones following 3 weeks of incubation at 28°C. (B) Close-up of a barrage zone showing pale yellow cleistothecia. Scale bar, 400 μm. (C) SEM micrograph of a cleistothecium showing the interwoven hyphae that form the peridial wall. Scale bar, 100 μm. (D) SEM micrograph of lenticular ascospores (white arrow) and smaller globose conidia (black arrow). Scale bar, 10 μm.
In crosses where cleistothecia were produced, they formed along the barrage zones between isolates of opposite mating type (Fig. 2A) and were pale yellow-orange in color (Fig. 2B). All crosses forming cleistothecia produced viable ascospores. The ascospores were heat resistant (57), capable of surviving at 80°C for at least 30 min, as is typical for other members of the genus Neosartorya (56). This might reflect selection in a common ancestor of Neosartorya for survival in ecological niches where high temperatures might be encountered, such as composting vegetation (58). Ascospore progeny from a cross between isolates 78-2 and 78-3 were then assessed for evidence of recombination. Distinct segregation patterns were clearly observed between four RAPD-PCR markers and the MAT genotype in 12 ascospore progeny, with Fisher's exact test confirming 1:1 Mendelian segregation of the markers due to independent assortment, confirming a heterothallic sexual breeding system (Table 2 and Fig. 3). Unique genotypes were found in 92% of the progeny, with only one of the offspring identical to its parent (based on the markers examined).
Table 2.
Genotypes of parental isolates and 12 ascospore progeny from a cross between A. lentulus isolates 78-2 and 78-3, based on mating type and RAPD-PCR bands
| Isolate | Mating type | RAPD banda |
Genotypeb | |||
|---|---|---|---|---|---|---|
| OMT1 | R108 | OPW08 | R151 | |||
| 78-3 | MAT1-1 | − | + | + | − | P1 |
| 78-2 | MAT1-2 | + | − | − | + | P2 |
| 3-2-1 | MAT1-2 | − | − | − | − | A |
| 3-2-2 | MAT1-2 | − | − | + | + | B |
| 3-2-3 | MAT1-1 | − | + | + | − | P1 |
| 3-2-4 | MAT1-1 | − | − | + | − | C |
| 3-2-6 | MAT1-1 | − | − | − | − | D |
| 3-2-7 | MAT1-2 | − | − | + | − | E |
| 3-2-8 | MAT1-2 | − | + | + | − | F |
| 3-2-9 | MAT1-1 | − | − | − | + | G |
| 3-2-12 | MAT1-2 | − | + | − | − | H |
| 3-2-13 | MAT1-2 | − | − | + | − | E |
| 3-2-15 | MAT1-1 | + | − | + | − | I |
| 3-2-16 | MAT1-1 | + | − | + | + | J |
RAPD-PCR bands amplified using primers OMT1, R108, OPW08, and R151. “+” and “−” denote presence and absence, respectively, of a particular amplicon. P values (two-tailed) for OMT1, R108, OPW08, and R151 were 0.06, 0.24, 0.57, and 0.24, respectively. Fisher's exact test was conducted to check for deviation from the null hypothesis of independent assortment of mating-type and RAPD markers in the progeny (i.e., a 1:1:1:1 MAT1-1+:MAT1-1−:MAT1-2+:MAT1-2− ratio for each RAPD marker). Fisher's exact test was used instead of the χ2 test because the expected frequencies were <5. A contingency χ2 test was conducted to check for deviation from the null hypothesis of independent assortment of mating-type and RAPD markers in the progeny (i.e., an overall 1:1:1:1 MAT1-1+:MAT1-1−:MAT1-2+:MAT1-2− ratio for the sum of the RAPD markers). It showed a value of 0.375 with 1 degree of freedom.
The genotype of each progeny isolate, defined by unique combinations of mating-type and RAPD markers as distinct from those of the parental isolates (designated P1 and P2), is identified by a different letter of the alphabet.
Fig 3.
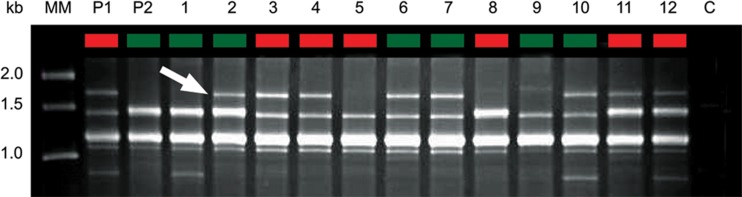
Evidence for meiotic recombination. The gel shows segregation patterns of a RAPD-PCR amplicon in A. lentulus parental isolates (P1 and P2) and 12 ascospore progeny (lanes 1 to 12) from the cross 78-3 (P1) × 78-2 (P2), using primer OPW08. MM, molecular size marker; C, water control. Arrow indicates the diagnostic RAPD band. Red and green lane headings indicate MAT1-1 and MAT1-2 genotypes, respectively.
In accordance with the “One Fungus = One Name” proposal (59), the newly discovered sexual state (teleomorph) of A. lentulus will not be assigned a separate Latin name, as was formerly the case under “dual nomenclature” (60). This follows the recent taxonomic move to simplify the naming of pleomorphic fungi. In the future, the teleomorph of A. lentulus should be referred to as A. lentulus (neosartorya-morph) where appropriate (59), given the phylogenetic link between the teleomorph genus Neosartorya and the Aspergillus section Fumigati (47, 56).
As shown in Table 1, three of the four fertile crosses (fertility is here defined as the production of cleistothecia with viable ascospores) yielded sexual offspring at both 28°C and 30°C, suggesting that A. lentulus may not be as fastidious as A. fumigatus in its temperature requirement for mating (61). Crosses were then reincubated for a further 4 weeks and reexamined for the presence of cleistothecia (see Table S3 in the supplemental material). The longer incubation period resulted in a further 7 and 14% of crosses reaching sexual maturity at 28°C and 30°C, respectively. Thus, a total of 35% of the crosses were fertile at both temperatures. A second series of 20 crosses was then set up at 28°C for 3 weeks to test the fertility of an additional seven isolates (see Table S4 in the supplemental material). Six of the seven isolates were fertile with at least one mating partner, although overall fertility was still only 35%. These results illustrate the importance of having multiple isolates of opposite mating type in close proximity in the environment, to ensure compatibility with at least one complementary strain.
Finally, crosses were attempted between representative MAT1-1 and MAT1-2 isolates of A. lentulus and highly fertile “supermater” isolates of A. fumigatus (37). Hyphal aggregations resembling immature cleistothecia were formed very occasionally in such crosses (see Fig. S3 in the supplemental material). However, despite prolonged incubation, for up to 12 months, at both 28°C and 30°C, these never matured to form ascospores. This indicates that the species are true sibling species, being phylogenetically closely related but without gene flow via sexual means (62). This has important implications for the evolution of resistance to antifungal drugs in A. fumigatus, as it suggests, fortunately, that transmission of genes conferring such resistance is unlikely to occur between A. lentulus and A. fumigatus. This result is consistent with the phylogenetic divergence reported between the species (2, 4), although it is cautioned that crosses were attempted only between a subset of A. lentulus and A. fumigatus isolates. It has also been reported that abortive cleistothecia can be produced in crosses between Neosartorya fennelliae and A. fumigatus (63). These crossing results compare with data for some other ascomycete species, such as within the genus Neurospora, where interspecies crossing is possible, albeit with reduced fertility and ascospore viability (64, 65).
The large variation in fertility of A. lentulus isolates depending on the mating partner is similar to observations reported for A. fumigatus by both O'Gorman et al. (25) and Sugui et al. (37) and for Neosartorya udagawae by Sugui et al. (66). For example, the A. lentulus cross 78-3 × 78-8 produced fewer than 20 cleistothecia per plate (Table 1). However, when 78-3 was crossed with 78-2 it was the most fertile pairing at both temperatures, consistently producing ≥80 cleistothecia per plate (Table 1). The pairing of 78-3 × 78-2 is therefore recommended for community use. It should be noted that these two isolates came from agricultural soils within 50 mi of each other in the Republic of Korea. Their similar geographic origins may hint at a close genetic relationship and lack of reproductive barriers, this being one of a variety of factors suggested to influence fertility of fungal sexual crosses (67–69). Genome relatedness is thought to be an important factor for fertility, as illustrated for A. fumigatus by the “supermater” pair (37). These isolates share 99% genome similarity based on a CGH (comparative genomic hybridization) analysis, and the cross is the most successful A. fumigatus pairing to date.
It is important to note that only 25% of the A. lentulus crosses at both 28°C and 30°C were fertile after 3 weeks. This figure is extremely low in comparison to that for A. fumigatus, for which the study by Sugui et al. (37) found that 80% (n = 50) of crosses were fertile after 4 weeks. O'Gorman et al. (25) reported 94% of their A. fumigatus crosses to be fertile (n = 36), but this was after 6 months of incubation, which can be considered to represent maximum maturity. The large disparity in fertility between A. lentulus and A. fumigatus is surprising given their nearly identical MAT idiomorph structures (Fig. 1) and conditions required for the sexual cycle. It is conceivable that either part or all of the global A. lentulus population is undergoing a slow decline in fertility (44). Alternatively, it could suggest a difference in the population dynamics of the two species due to an unknown underlying fertility or incompatibility mechanism in A. lentulus. Natural A. fumigatus populations have been well studied and have yielded evidence of recombination, confirming that sexual reproduction is taking or has recently taken place (35, 70, 71). Similar studies have yet to be conducted for A. lentulus, for which many fewer isolates are available for study. Defining its population structure will inform future studies to determine how widespread sexual reproduction is in nature and whether the low fertility seen in this study is representative of the global population.
Conclusions.
The discovery of a sexual cycle in A. lentulus is important both for the biology of the species and for future efforts to control this pathogen. It also shows that yet another supposedly asexual pathogenic fungus possesses a functional sexual cycle, thereby harboring the potential to evolve rapidly in the face of selective pressures (43). Sexuality in its close relative A. fumigatus can now be directly compared to that of A. lentulus, and future studies may shed light on their different evolutionary paths. However, of most significance is the fact that the sexual cycle will provide an invaluable tool for classical genetic analysis to facilitate research into the genetic basis of pathogenicity and drug resistance in this emerging agent of aspergillosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kieren Marr and Edmond Byrnes for providing A. lentulus isolates and Tim Smith for technical assistance with scanning electron microscopy.
S.S.S. was supported by a Government of Iraq PhD scholarship. C.M.O. and P.S.D. are funded by The Wellcome Trust (United Kingdom).
Footnotes
Published ahead of print 6 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00040-13.
REFERENCES
- 1.Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA. 2005. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97:1316–1329 [DOI] [PubMed] [Google Scholar]
- 2.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. 2005. Aspergillus lentulus sp nov. , a new sibling species of A. fumigatus. Eukaryot. Cell 4:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balajee SA, Weaver M, Imhof A, Gribskov J, Marr KA. 2004. Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob. Agents Chemother. 48:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaguchi T, Horie Y, Tanaka R, Matsuzawa T, Ito J, Nishimura K. 2007. Molecular phylogenetics of multiple genes on Aspergillus section Fumigati isolated from clinical specimens in Japan. Jpn. J. Med. Mycol. 48:37–46 [DOI] [PubMed] [Google Scholar]
- 5.Balajee SA, Nickle D, Varga J, Marr KA. 2006. Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot. Cell 5:1705–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen TO, Smedsgaard J, Nielsen KF, Hansen MA, Samson RA, Frisvad JC. 2007. Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med. Mycol. 45:225–232 [DOI] [PubMed] [Google Scholar]
- 7.Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne KA, Gade L, Lockhart SR, Diekema DJ, Messer SA, Pfaller MA, Balajee SA. 2009. Screening of a large global Aspergillus fumigatus species complex collection by using a species-specific microsphere-based Luminex assay. J. Clin. Microbiol. 47:4171–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano R, Gusmão L, Amorim A, Araujo R. 2011. Rapid identification of Aspergillus fumigatus within section Fumigati. BMC Microbiol. 11:82. 10.1186/1471-2180-11-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staab JF, Balajee SA, Marr KA. 2009. Aspergillus section Fumigati typing by PCR-restriction fragment polymorphism. J. Clin. Microbiol. 47:2079–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhambra A, Catalan M, Moragues MD, Brena S, Ponton J, Montejo JC, del Palacio A. 2008. Isolation of Aspergillus lentulus in Spain from a critically ill patient with chronic obstructive pulmonary disease. Rev. Iberoam. Micol. 25:246–249 [DOI] [PubMed] [Google Scholar]
- 12.Montenegro G, Sánchez Puch S, Jewtuchowicz VM, Pinoni MV, Relloso S, Temporitti E, Iovannitti CA, Mujica MT. 2009. Phenotypic and genotypic characterization of Aspergillus lentulus and Aspergillus fumigatus isolates in a patient with probable invasive aspergillosis. J. Med. Microbiol. 58:391–395 [DOI] [PubMed] [Google Scholar]
- 13.Symoens F, Haase G, Pihet M, Carrere J, Beguin H, Degand N, Mely L, Bouchara JP. 2010. Unusual Aspergillus species in patients with cystic fibrosis. Med. Mycol. 48(Suppl 1):S10–S16 [DOI] [PubMed] [Google Scholar]
- 14.Balajee SA, Kano R, Baddley JW, Moser SA, Marr KA, Alexander BD, Andes D, Kontoyiannis DP, Perrone G, Peterson S, Brandt ME, Pappas PG, Chiller T. 2009. Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 47:3138–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubka V, Kubatova A, Mallatova N, Sedlacek P, Melichar J, Skorepova M, Mencl K, Lyskova P, Sramkova B, Chudickova M, Hamal P, Kolarik M. 2012. Rare and new etiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med. Mycol. 50:601–610 [DOI] [PubMed] [Google Scholar]
- 16.Zbinden A, Imhof A, Wilhelm MJ, Ruschitzka F, Wild P, Bloemberg GV, Mueller NJ. 2012. Fatal outcome after heart transplantation caused by Aspergillus lentulus. Transpl. Infect. Dis. 14:E60–E63 [DOI] [PubMed] [Google Scholar]
- 17.Mortensen KL, Johansen HK, Fuursted K, Knudsen JD, Gahrn-Hansen B, Jensen RH, Howard SJ, Arendrup MC. 2011. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 30:1355–1363 [DOI] [PubMed] [Google Scholar]
- 18.Mellado E, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2011. Role of Aspergillus lentulus 14-α sterol demethylase (Cyp51A) in azole drug susceptibility. Antimicrob. Agents Chemother. 55:5459–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staab JF, Kahn JN, Marr KA. 2010. Differential Aspergillus lentulus echinocandin susceptibilities are Fksp independent. Antimicrob. Agents Chemother. 54:4992–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart SR, Zimbeck AJ, Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Pappas PG, Chiller T. 2011. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob. Agents Chemother. 55:3944–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik A, Sharma S, Satya S, Mishra A. 2011. Development of a biological system employing Aspergillus lentulus for Cr removal from a small-scale electroplating industry effluent. Asia Pac. J. Chem. Eng. 6:55–63 [Google Scholar]
- 22.Sharma S, Malik A, Satya S. 2009. Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J. Hazard. Mater. 164:1198–1204 [DOI] [PubMed] [Google Scholar]
- 23.Kaushik P, Malik A. 2010. Effect of nutritional conditions on dye removal from textile effluent by Aspergillus lentulus. World J. Microbiol. Biotechnol. 26:1957–1964 [Google Scholar]
- 24.Kaushik P, Malik A. 2011. Process optimization for efficient dye removal by Aspergillus lentulus FJ172995. J. Hazard. Mater. 185:837–843 [DOI] [PubMed] [Google Scholar]
- 25.O'Gorman CM, Fuller HT, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474 [DOI] [PubMed] [Google Scholar]
- 26.Arabatzis M, Velegraki A. 2013. Sexual reproduction in the opportunistic human pathogen Aspergillus terreus. Mycologia 105:71–79 [DOI] [PubMed] [Google Scholar]
- 27.Böhm J, Hoff B, O'Gorman CM, Wolfers S, Klix V, Binger D, Zadra I, Kürnsteiner H, Pöggeler S, Dyer PS, Kück U. 2013. Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. U. S. A. 110:1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn BW, Moore GG, Carbone I. 2009. Sexual reproduction in Aspergillus flavus. Mycologia 101:423–429 [DOI] [PubMed] [Google Scholar]
- 29.Horn BW, Ramirez-Prado JH, Carbone I. 2009. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 46:169–175 [DOI] [PubMed] [Google Scholar]
- 30.Horn BW, Moore GG, Carbone I. 2011. Sexual reproduction in aflatoxin-producing Aspergillus nomius. Mycologia 103:174–183 [DOI] [PubMed] [Google Scholar]
- 31.Houbraken J, Frisvad JC, Samson RA. 2010. Sex in Penicillium series Roqueforti. IMA Fungus 1:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debuchy R, Turgeon BG. 2006. Mating-type structure, evolution, and function in euascomycetes, p 293–323 Kües U, Fischer R. (ed), The Mycota: growth, differentiation, and sexuality, 2nd ed, vol 1 Springer-Verlag, Berlin, Germany [Google Scholar]
- 33.Debuchy R, Berteaux-Lecellier V, Silar P. 2010. Mating systems and sexual morphogenesis in ascomycetes, p 501–535 Borkovich KA, Ebbole DJ. (ed), Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC [Google Scholar]
- 34.Paoletti M, Seymour FA, Alcocer MJC, Kaur N, Calvo AM, Archer DB, Dyer PS. 2007. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr. Biol. 17:1384–1389 [DOI] [PubMed] [Google Scholar]
- 35.Paoletti M, Rydholm C, Schwier EU, Anderson MJ, Szakacs G, Lutzoni F, Debeaupuis JP, Latgé JP, Denning DW, Dyer PS. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15:1242–1248 [DOI] [PubMed] [Google Scholar]
- 36.Robert V, Groenewald M, Epping W, Boekhout T, Smith M, Poot G, Stalpers J. 2007. CBS yeasts database. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands [Google Scholar]
- 37.Sugui JA, Losada L, Wang W, Varga J, Ngamskulrungroj P, Abu-Asab M, Chang YC, O'Gorman CM, Wickes BL, Nierman WC, Dyer PS, Kwon-Chung KJ. 2011. Identification and characterization of an Aspergillus fumigatus “supermater” pair. mBio 2:e00234–11. 10.1128/mBio.00234-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher RA. 1938. Statistical methods for research workers, 7th ed. Oliver & Boyd, Edinburgh, United Kingdom [Google Scholar]
- 39.Dacks J, Roger AJ. 1999. The first sexual lineage and the relevance of facultative sex. J. Mol. Evol. 48:779–783 [DOI] [PubMed] [Google Scholar]
- 40.Hurst LD, Peck JR. 1996. Recent advances in understanding of the evolution and maintenance of sex. Trends Ecol. Evol. 11:46–52 [DOI] [PubMed] [Google Scholar]
- 41.Milgroom MG. 1996. Recombination and the multilocus structure of fungal populations. Annu. Rev. Phytopathol. 34:457–477 [DOI] [PubMed] [Google Scholar]
- 42.Normark BB, Judson OP, Moran NA. 2003. Genomic signatures of ancient asexual lineages. Biol. J. Linn. Soc. 79:69–84 [Google Scholar]
- 43.Heitman J. 2010. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe 8:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyer PS, Paoletti M. 2005. Reproduction in Aspergillus fumigatus: sexuality in a supposedly asexual species? Med. Mycol. 43(Suppl 1):S7–S14 [DOI] [PubMed] [Google Scholar]
- 45.Wada R, Maruyama J, Yamaguchi H, Yamamoto N, Wagu Y, Paoletti M, Archer DB, Dyer PS, Kitamoto K. 2012. Presence and functionality of mating type genes in the supposedly asexual filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 78:2819–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoustra S, Rundle HD, Dali R, Kassen R. 2010. Fitness-associated sexual reproduction in a filamentous fungus. Curr. Biol. 20:1350–1355 [DOI] [PubMed] [Google Scholar]
- 47.Dyer PS, O'Gorman CM. 2011. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr. Opinion Microbiol. 14:649–654 [DOI] [PubMed] [Google Scholar]
- 48.Rydholm C, Dyer PS, Lutzoni F. 2007. DNA sequence characterization and molecular evolution of MAT1 and MAT2 mating-type loci of the self-compatible ascomycete mold Neosartorya fischeri. Eukaryot. Cell 6:868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fedorova ND, Khaldi N, Joardar VS, Maiti R, Amedeo P, Anderson MJ, Crabtree J, Silva JC, Badger JH, Albarraq A, Angiuoli S, Bussey H, Bowyer P, Cotty PJ, Dyer PS, Egan A, Galens K, Fraser-Liggett CM, Haas BJ, Inman JM, Kent R, Lemieux S, Malavazi I, Orvis J, Roemer T, Ronning CM, Sundaram JP, Sutton G, Turner G, Venter JC, White OR, Whitty BR, Youngman P, Wolfe KH, Goldman GH, Wortman JR, Jiang B, Denning DW, Nierman WC. 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. 10.1371/journal.pgen.1000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo PCY, Chong KTK, Tse H, Cai JJ, Lau CCY, Zhou AC, Lau SKP, Yuen KY. 2006. Genomic and exeprimental evidence for a potential sexual cycle in the pathogenic thermal dimorphic fungus Penicillium marneffei. FEBS Lett. 580:3409–3416 [DOI] [PubMed] [Google Scholar]
- 51.Mandel MA, Barker BM, Kroken S, Rounsley SD, Orbach MJ. 2007. Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot. Cell 6:1189–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraser JA, Stajich JE, Tarcha EJ, Cole GT, Inglis DO, Sil A, Heitman J. 2007. Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryot. Cell 6:622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Sullivan TD, Walton E, Averette AF, Sakthikumar S, Cuomo CC, Klein BS, Heitman J. 2013. Identification of the mating-type (MAT) locus that controls sexual reproduction of Blastomyces dermatitidis. Eukaryot. Cell 12:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyer PS. 2007. Sexual reproduction and significance of MAT in the aspergilli, p 123–142 Heitman J, Kronstad JWTaylor, JW, , Casselton LA. (ed), Sex in fungi: molecular determination and evolutionary principles. ASM Press, Washington, DC [Google Scholar]
- 55.Geiser DM, Frisvad JC, Taylor JW. 1998. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia 90:831–845 [Google Scholar]
- 56.Samson RA, Hong S, Peterson SW, Frisvad JC, Varga J. 2007. Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 59:147–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houbraken J, Samson RA. 2006. Standardization of methods for detecting heat resistant fungi. Adv. Exp. Med. Biol. 571:107–111 [DOI] [PubMed] [Google Scholar]
- 58.O'Gorman CM. 2011. Airborne Aspergillus fumigatus conidia: a risk factor for aspergillosis. Fung. Biol. Rev. 25:151–157 [Google Scholar]
- 59.Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, Taylor JW, Wingfield MJ, Abaci O, Aime C, Asan A, Bai FY, de Beer ZW, Begerow D, Berikten D, Boekhout T, Buchanan PK, Burgess T, Buzina W, Cai L, Cannon PF, Crane JL, Damm U, Daniel HM, van Diepeningen AD, Druzhinina I, Dyer PS, Eberhardt U, Fell JW, Frisvad JC, Geiser DM, Geml J, Glienke C, Gräfenhan T, Groenewald JZ, Groenewald M, de Gruyter J, Guého-Kellermann E, Guo LD, Hibbett DS, Hong SB, de Hoog GS, Houbraken J, Huhndorf SM, Hyde KD, Ismail A, Johnston PR, Kadaifciler DG, Kirk PM, Kõljalg U, Kurtzman CP, Lagneau PE, Lévesque CA, Liu X, Lombard L, Meyer W, Miller A, Minter DW, Najafzadeh MJ, Norvell L, Ozerskaya SM, Oziç R, Pennycook SR, Peterson SW, Pettersson OV, Quaedvlieg W, Robert VA, Ruibal C, Schnürer J, Schroers HJ, Shivas R, Slippers B, Spierenburg H, Takashima M, Taşkın E, Thines M, Thrane U, Uztan AH, van Raak M, Varga J, Vasco A, Verkley G, Videira SI, de Vries RP, Weir BS, Yilmaz N, Yurkov A, Zhang N. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett JW. 2010. An overview of the genus Aspergillus, p 1–17 Machida M, Gomi K. (ed), Aspergillus molecular biology and genomics. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 61.Kwon-Chung KJ, Sugui JA. 2009. Sexual reproduction in Aspergillus species of medical or economical importance: why so fastidious? Trends Microbiol. 17:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burnett J. 2003. Fungal populations and species. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 63.Takada M, Udagawa SI, Norizuki K. 1986. Isolation of Neosartorya fennelliae and interspecific pairings between N. fennelliae, N. spathulata, and Aspergillus fumigatus. Trans. Mycol. Soc. Jpn. 27:415–423 [Google Scholar]
- 64.Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW. 2003. Reproductive isolation and phylogenetic divergence in Neurospora—comparing methods of species recognition in a model eukaryote. Evolution 57:2721–2741 [DOI] [PubMed] [Google Scholar]
- 65.Turner E, Jacobson DJ, Taylor JW. 2011. Genetic architecture of a reinforced, postmating, reproductive isolation barrier between Neurospora species indicates evolution via natural selection. PLoS Genet. 7:e1002204. 10.1371/journal.pgen.1002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugui JA, Vinh DC, Nardone G, Shea YR, Chang YC, Zelazny AM, Marr KA, Holland SM, Kwon-Chung KJ. 2010. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson JB, Kohn LM, Leslie JF. 1992. Genetic mechanisms in fungal adaptation, p 73–98 Carroll GC, Wicklow DT. (ed), The fungal community: its organization and role in the ecosystem. Marcel Dekker, New York, NY [Google Scholar]
- 68.Dyer PS, Ingram DS, Johnstone K. 1992. The control of sexual morphogenesis in the Ascomycotina. Biol. Rev. 67:421–458 [Google Scholar]
- 69.Fisher MC, Henk DA. 2012. Sex, drugs and recombination: the wild life of Aspergillus. Mol. Ecol. 21:1305–1306 [DOI] [PubMed] [Google Scholar]
- 70.Varga J, Toth B. 2003. Genetic variability and reproductive mode of Aspergillus fumigatus. Infect. Genet. Evol. 3:3–17 [DOI] [PubMed] [Google Scholar]
- 71.Pringle A, Baker DM, Platt JL, Wares JP, Latge JP, Taylor JW. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886–1899 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.