Abstract
The cochlear implant (CI) is one of the great success stories of modern medicine. A high level of function is provided for most patients. However, some patients still do not achieve excellent or even good results using the present-day devices. Accumulating evidence is pointing to differences in the processing abilities of the “auditory brain” among patients as a principal contributor to this remaining and still large variability in outcomes. In this chapter, we describe a new approach to the design of CIs that takes these differences into account and thereby may improve outcomes for patients with compromised auditory brains.
Keywords: cochlear implant, cochlear prosthesis, auditory prosthesis, brain–machine interface, brain plasticity, neural prostheses, hearing, deafness, central auditory processing, auditory cortex
Introduction
The cochlear implant (CI) is the most successful neural prosthesis developed to date. More than 220,000 people have received a CI or bilateral CIs as of this writing (early 2011). This number exceeds by orders of magnitude the number for all other types of neural prostheses combined. According to the most recent NIH Consensus Statement on CIs in adults and children (National Institutes of Health, 1995), “A majority of those individuals with the latest speech processors for their implants will score above 80% correct on high-context sentences even without visual cues.” This restoration of function is remarkable and far surpasses that of any other neural prosthesis.
Although the average and top performances of the present-day CIs are spectacular, a large variability in outcomes remains. Patients using the same type of implant device may score almost anywhere in the range of possible scores in tests of speech reception that are more difficult than high-context sentences. Also, a small proportion of patients have low scores even for the relatively easy tests. Accumulating evidence is pointing to differences in brain function among patients as a principal contributor to this remaining variability.
The main purpose of this chapter is to describe a new “top-down” or “cognitive neuroscience” approach to the design of CIs that takes such differences into account. The chapter includes (1) a brief review of the design and performance of the present-day CIs, (2) a summary of the evidence indicating the importance of the brain in determining outcomes with CIs, (3) the description of the new approach, and (4) a list of questions that are raised by the approach.
Present-day cochlear implants
All CI systems now in widespread use include multiple channels of sound processing and multiple sites of stimulation along the length of the cochlea. The aim of these systems is to mimic at least to some extent the “place” or “tonotopic” representation of frequencies in the normal cochlea, that is, by stimulating electrodes near the basal end of the cochlea to indicate the presence of high-frequency sounds and by stimulating electrodes closer to the apical end to indicate the presence of sounds at lower frequencies.
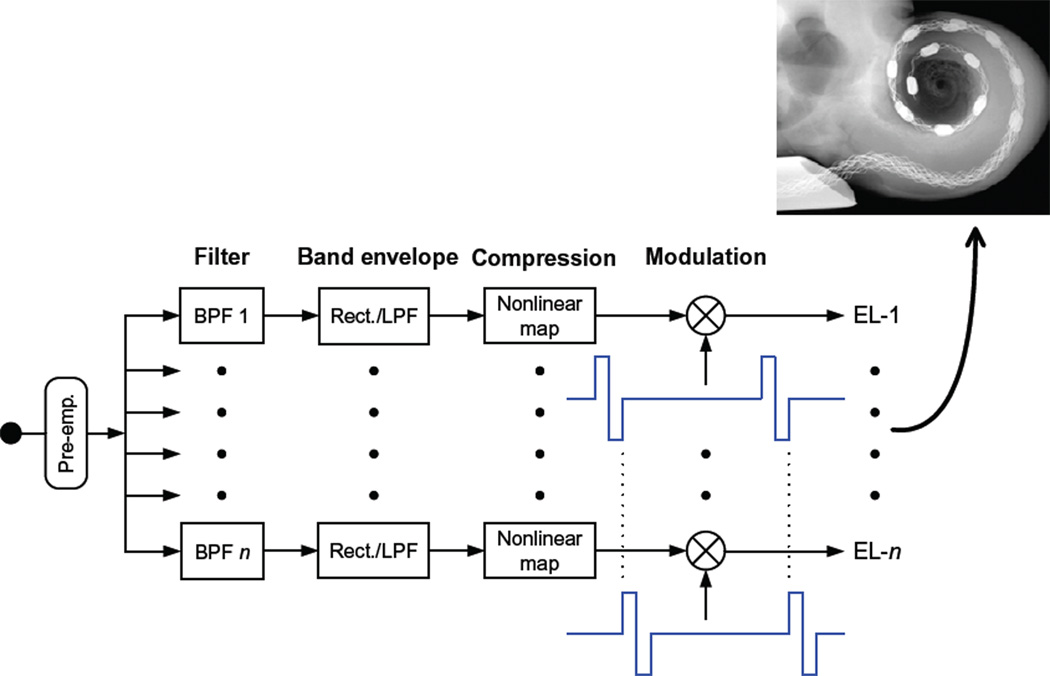
One of the simpler and most effective designs for achieving this aim, illustrated in Fig. 1, is called the continuous interleaved sampling (CIS) strategy for CIs (Wilson et al., 1991). Other strategies also are highly effective and are described in Wilson and Dorman (2009). As noted there, most of those other strategies are based on CIS or are variations of CIS.
Fig. 1.
Block diagram of the continuous interleaved sampling (CIS) strategy. The input to the strategy is indicated by the filled circle in the left-most part of the diagram. Next, a pre-emphasis filter (Pre-emp.) is used to attenuate strong components in speech below 1.2 kHz. This filter is followed by multiple channels of processing. Each channel includes band-pass filtering (BPF), envelope detection, compression, and modulation. The envelope detectors typically use a rectifier (Rect.) followed by a low-pass filter (LPF). A Hilbert transform or a half-wave rectifier without the LPF also may be used. Carrier waveforms for two of the modulators are shown immediately below the two corresponding multiplier blocks (the circles with an “X” within them). The outputs of the multipliers are directed to intracochlear electrodes (EL-1 to EL-n), as illustrated by the X-ray micrograph in the inset. (Block diagram is adapted from Wilson et al. (1991) with the permission of the Nature Publishing Group. Inset is reproduced from Hüttenbrink et al. (2002) with the permission of Lippincott Williams and Wilkins.)
The CIS strategy filters input sounds into bands of frequencies with a bank of band-pass filters. Envelope variations in the different bands are represented at corresponding electrodes in the cochlea with modulated trains of biphasic electrical pulses. The envelope signals extracted from the band-pass filters are compressed with a nonlinear mapping function prior to the modulation, to map the wide dynamic range of audible sounds in the environment (about 100 dB) into the much narrower dynamic range of electrically evoked hearing (stimulus levels needed for eliciting loud percepts typically are only 10 dB higher than the levels needed for eliciting threshold percepts). The output of each band-pass channel is directed to a single electrode, with channels with low-to-high center frequencies assigned to apical-to-basal electrodes, to mimic at least the order, if not the precise locations, of frequency mapping in the normal cochlea. The pulse trains are interleaved in time, so that the pulses across channels and the associated electrodes are nonsimultaneous. This eliminates a principal component of electrode interaction, which otherwise would be produced by direct summation of the electric fields from different (simultaneously stimulated) electrodes. The corner or “cutoff” frequency of the low-pass filter in each envelope detector typically is set at 200 Hz or higher, so that the fundamental frequencies of voiced speech sounds are represented in the modulation waveforms. CIS gets its name from the continuous sampling of the (compressed) envelope signals by rapidly presented pulses that are interleaved across electrodes. As many as 22 channels have been used in CIS implementations to date, although speech reception scores generally do not increase with increases in the number of channels beyond 4–8, for the CIS and the other strategies used in conjunction with the present-day CIs (e.g., Friesen et al., 2001).
Patterns of activity in the auditory nerve produced by the CIS and the other strategies are far different from the patterns found in normal hearing. For example, only a small number of (broadly overlapping and only somewhat independent) sites of stimulation are provided with the strategies, up to a maximum of 22 sites, whereas the number of sites in normal hearing approximates 3500, corresponding to the number of rows of sensory hair cells distributed along the length of the cochlea. Many additional differences between normal and electrically elicited hearing are described in Wilson and Dorman (2007, 2009).
Despite the differences between the peripheral representations of sound produced by electric versus acoustic stimulation, most CI users are able to understand everyday speech with their restored hearing alone and without the aid of lipreading, although it often requires rather focused attention. Indeed, some patients have speech reception scores that approach—and in many tests match—the scores obtained by subjects with normal hearing (e.g., Wilson and Dorman, 2007). This result is an existence proof that the present-day CIs can provide enough information at the periphery for excellent understanding of speech in many circumstances. In addition, the result is a testament to the brain, in that the brain is somehow able to reconstruct speech from a decidedly sparse and otherwise unnatural input.
However, a large variability in outcomes remains with the present-day CIs, with widespread distributions of scores, from very low scores to very high scores, for the relatively difficult tests such as recognition of monosyllabic words or of sentences presented in competition with noise. In addition, some patients still have great difficulty even with the easier tests.
A further aspect of the results with the presentday and prior CIs is a general tendency toward improvement in speech reception scores over time, as patients gain experience with their implants. Scores typically continue to improve during the first 3–12 months of implant use, although the period can be even longer for some patients and for difficult tests. In the study by Helms et al. (1997), for example, the average scores for 55 CI patients improved significantly out to 12 months after the first use of the implant, and then plateaued thereafter. These long time courses also indicate a role of the brain in determining outcomes with CIs, in that the times needed to reach asymptotic performance far exceed the times of any possible changes at the periphery and must instead reflect plastic changes in brain organization and function, as the brain “reconfigures” itself over the months to make progressively better use of the impoverished input.
The results reviewed above generally apply to postlingually deafened patients (i.e., patients who lost their hearing after they had acquired language). Results for prelingually deafened patients may be different, depending on the age of implantation. If the implant occurs at an early age, for example, 18 months or less, then the results for the prelingually deafened population are as good as the better outcomes for the postlingually deafened population (e.g., Niparko et al., 2010). In contrast, implantations after the second or third birthday for the prelingual patients are associated with outcomes that are usually worse than those for the postlingual patients, and the odds for a good outcome are very poor for prelingually deafened persons implanted after 4–6 years of age.
Importance of brain function in determining outcomes
Two positive aspects of brain function have been mentioned: the brain’s ability in some cases to make outstanding use of a sparse input from the periphery, and the brain’s ability in most cases to adapt at least partially to the input over a long time span. These aspects clearly appear to contribute to the present high levels of performance with CIs.
However, damage to the brain can impair performance. Such damage may be produced by a prolonged period of auditory deprivation (Shepherd and Hardie, 2001; Shepherd et al., 2006); cross-modal plasticity in the brains of congenitally or otherwise prelingually deafened children who are implanted after age 3 or thereabouts (Buckley and Tobey, 2010; Giraud and Lee, 2007; Lee et al., 2001); missing the “sensitive period” for normal development of the “auditory brain” for congenitally deaf persons who are implanted after 3.5 years of age (Sharma et al., 2002); various genetic defects; and various diseases such as meningitis and demyelinating disorders. (Cross-modal plasticity in the congenitally deafened cases is the encroachment of the auditory cortical areas by another sensory modality, e.g., vision, and the sensitive period refers to the time window in which especially rapid and large plastic changes in brain function may occur.) The resulting deficits in brain function may underlie at least in part (1) the observed negative correlation between outcomes with CIs and the duration of deafness or severe hearing loss prior to implantation for postlingually deafened patients (e.g., Blamey et al., 1996) and (2) the poor outcomes for congenitally or otherwise prelingually deafened persons who are not implanted early in life. In addition, differences in brain function among patients, at least vis-à-vis the functional interaction with the implant, may explain or help to explain the remaining variability in outcomes for the postlingually deafened patients. Results may be good in the fortuitous cases of early implantation for prelingually deafened persons and implantation within a year or so of the first substantial hearing loss for postlingually deafened persons. For all other cases, however, outcomes may be affected by deficits in the ability of the brain to make functional use of the input provided by the implant. These latter cases constitute the majority of CI recipients.
These and many other findings indicating the importance of brain function in determining outcomes with CIs are described in detail in the recent reviews by Fallon et al. (2008), Giraud and Lee (2007), Kral et al. (2006), Kral and Eggermont (2007), Moore and Shannon (2009), Musiek and Daniels (2010), and Wilson and Dorman (2009). In addition, further information about cross-modal plasticity in various brain systems is presented in the review by Bavelier and Neville (2002), and extended discussion about sensitive periods in various brain systems, and on the persistence of some brain plasticity following a sensitive period, is presented in the reviews by Chen et al. (2002), Irvine et al. (2006), Jäncke (2009), and Knudsen (2004).
A “top-down” or “cognitive neuroscience” approach to implant designs
The evidence indicating the importance of the brain in determining outcomes with implants has led to a new way of thinking about the design of CIs. To date, a “bottom-up” approach has been used, in which the goal is to reproduce in so far as possible the normal patterns of neural activity at the auditory periphery. Great progress has been made with that approach, and further substantial progress is certainly possible, given the large gaps that remain between the normal patterns and the patterns produced by the present-day CIs.
Despite the successes of the bottom-up approach, however, some or even most patients may be underserved by it. In particular, this traditional approach ignores the brain as a part of the overall prosthesis system and, while this may not matter (or matter much) when the brain is perfect or is in the “clean slate” state of the sensitive period and prior to any effects of cross-modal plasticity, ignoring the brain is likely to be detrimental for most other cases.
Concept
An alternative approach is to include the perspective of the brain instead of focusing exclusively on the periphery. This top-down approach asks what the (usually compromised) brain needs as an input in order to perform optimally, and/or to learn how to perform optimally. The answer to this question almost certainly would vary from patient to patient according to the functional abilities of each patient’s auditory brain. In addition, the inputs may depart dramatically from those produced with the bottom-up approach, especially for patients with severe deficits in brain function.
At the outset, one might anticipate that the stimuli prescribed by the top-down approach could be far slower or far sparser or both for many patients, compared with the stimuli derived from the bottom-up approach. In particular, the compromised auditory brain may have greatly degraded abilities to “follow” or otherwise process temporal information from the periphery (e.g., Fu, 2002; Walton, 2010), or to distinguish among stimulus sites in the cochlea or otherwise process spectral information from the periphery (e.g., Fallon et al., 2008). These likely deficits may well intrinsically limit the effective processing of the inputs to the brain provided by the present-day CIs. Instead, a simplification of the inputs may be needed to produce a match between the inputs and the brain’s capabilities to process them. Possible simplifications include reductions in (1) the rate of stimulation at each electrode in the implant, along with conjoint reductions in the cutoff frequency of the envelope detectors for each channel and associated electrode (see Fig. 1) and (2) the number of activated electrodes. The choices for the reductions might be guided by psychophysical measures of temporal and spectral acuities for each patient, for example, gap detection, forward masking, or modulation detection for the temporal acuities, and electrode or spectral ripple discrimination for the spectral acuities.
Fortunately, some simplifications could be made while still preserving important information about speech and other sounds. For example, much of the information about speech is contained in envelope variations below 16 Hz in a relatively small number of bands (e.g., Xu and Zheng, 2007). This means that the envelope cutoff frequency could be reduced from the typical 200–400 Hz down to as low as 16 Hz before removing absolutely essential information about speech. Fundamental frequency variations would be discarded in the modulation waveforms, but those variations convey little phonetic information and instead provide information about the speaker’s gender and age, declarative versus interrogative intent, and emotion in speech. Except in the cases of tone languages, intelligibility of speech is largely retained when fundamental frequencies are removed from the representation. Thus, the essential aspects for western and other nontonal languages would be preserved using the lower cutoff frequencies for the envelope detectors.
Such sharp reductions in the cutoff frequencies could be accompanied by similarly sharp reductions in the pulse rate at each of the activated electrodes, up to the same proportional amount. These large reductions in the pulse rate could greatly reduce the likely deleterious effects of prolonged forward masking or otherwise “sluggish” central auditory processing, by allowing the central system to follow inputs that could not be followed before. (The reductions also could improve sensitivity to modulation; see, e.g., Pfingst et al., 2007.) The low frequencies of the envelope variations, and the low pulse rates at each electrode, might fit within or at least approach the temporal processing abilities of a compromised auditory brain, as may be indicated, for example, by one or more of the psychophysical measures mentioned above.
In addition, for speech presented in quiet, as few as four band-pass processing channels are sufficient for high levels of speech recognition (Shannon et al., 1995). Thus, the number of channels and associated active electrodes could be reduced to four in a CI system and still allow the representation of important speech information. As many as 12, 16, or 22 channels and active electrodes are used in the present-day CIs, depending on the particular device and processing strategy. A lower number might fit within or at least approach the spectral (or, more precisely, the tonotopic) processing abilities of a compromised auditory brain.
Of course, higher pulse rates, higher cutoff frequencies for the envelope detectors, and higher numbers of channels could be better for patients who can utilize the additional represented information. The advantages of including information about the fundamental frequency variations have been mentioned. Those advantages would only accrue with the use of the higher pulse rates and cutoff frequencies. In addition, higher numbers of channels may be needed for useful speech reception in other than quiet conditions (Dorman et al., 1998, 2002; Friesen et al., 2001; Shannon et al., 2004), for example, for speech reception in typically noisy environments. More channels are required as well for music reception (Shannon et al., 2004), and information about the fundamental frequency variations may be essential for conveying critical phonemic contrasts in tone languages. The point here, however, is that great simplifications are possible, before all of the important information about speech is eliminated. In addition, the simplifications could be made using the presentday implant systems and processing strategies, with adjustments in the parameter values for the strategies. Other implant systems or processing strategies could be envisioned, that also would produce marked reductions in the rates of stimulation, the number of sites stimulated, or both, but a useful first step may be made with informed manipulations in the parameter values for the present-day systems and strategies.
The goal in the top-down approach is to provide a good match between what the implant provides and what the brain can most effectively process or “handle,” while still presenting the maximum amount of information possible. Maintaining the quality of the match most likely would require adjustments in the stimuli from time to time, as the brain is not static and can reconfigure itself through plastic changes when presented with stimuli within its processing abilities and with enough experience with those stimuli (Irvine et al., 2006). Such changes are possible for adults as well as children, although the maximum speed and magnitude of the changes during the sensitive period (generally before 2–3 years of age for auditory processing by humans) may far exceed those maxima later in life (e.g., Knudsen, 2004).
Good matches may require simplifications in the stimuli, and the patterns of activity in the auditory nerve evoked by those stimuli, compared with the stimuli derived with the bottom-up approach and used in the present-day CIs. (Certainly, an increase in the complexity of the stimuli would seem to be going in the wrong direction for CI users with compromised auditory brains, both because the stimuli of the present-day CIs include enough information for some users to achieve high levels of speech reception and because psychophysical and other results indicate that the compromised brains are less able than fully intact brains to process temporal and spectral information.) The simplifications if needed should not be overdone, of course, but should instead be just enough for a match or a helpful approximation to the brain’s abilities, both to preserve the maximum amount of information included in the peripheral representations, and to challenge the brain to work at its limits and thereby encourage desired plastic changes in brain organization and function. Ultimately, with continued adjustments of the stimuli and with continued development of the brain’s processing abilities, both the brain and the stimuli may approach normality. That is, the previously compromised brain may ultimately more closely resemble the fully intact brain in function, and the stimuli may resemble those delivered by the present-day CIs. The critical step may be the first one, in giving the compromised brain an input it can process and thereby start it on a path to (at least partial) recovery.
In some cases, the brain may be so damaged that a good match cannot be achieved, even with huge simplifications in the stimuli. Such brains may be beyond remediation. However, training aimed at improving performance on a basic psychophysical task—involving stimuli that are far simpler than even the simplest of the stimuli described in the preceding paragraphs—may help to “nudge” the highly compromised brain into a position where stimuli of greater complexity could be processed, perhaps up to a point at which a match could then be achieved. Possible psychophysical tasks include electrode discrimination or gap or modulation detection. The idea again is to drive the brain at its limits to invoke desired plastic changes in function. Alternatively, certain drug therapies have promise for “reopening” sensitive periods (Castrén and Rantamäki, 2010; Maya Vetencourt et al., 2008; Thiel, 2007), in which large plastic changes in brain function could be induced, in the directions of increased temporal and spectral processing abilities. (In addition, Tobey et al. (2005) have suggested a pharmacological enhancement of brain plasticity that does not necessarily involve a reopening of a sensitive period.) Such therapies could reestablish at least temporarily a favorable milieu for rapid and large changes in brain organization and function, and may even allow a partial or full recovery from a preceding cross-modal encroachment by vision or another sense in the areas of cortex normally utilized primarily for the processing of auditory inputs and information.
Measures of brain function
Another important feature of the new approach being proposed here is that measures of brain function might inform or guide the design of processing strategies using consideration of top-down factors. For example, and as described above, psychophysical measures could inform choices for the rate of stimulation, the cutoff frequency of the envelope detectors, and the number of sites to be stimulated in CIs. In addition, electrophysiological or brain imaging measures could be used. Ideally, the selected measures should be simple and inexpensive to apply, and such measures should be predictive of outcomes with CIs.
Many of the psychophysical measures mentioned in the preceding subsection would fulfill these criteria. All of the measures could be made relatively quickly and in clinical settings. In addition, many of them are correlated or strongly associated with outcomes with CIs, including modulation detection (Fu, 2002), gap detection (Hochmair-Desoyer et al., 1985), forward masking (Nelson and Donaldson, 2002), and spectral ripple discrimination (Henry et al., 2005).
In addition, various electrophysiological measures would fulfill the criteria. For example, the latency of the short-latency P1 wave of the cortical evoked potential to a brief stimulus such as a speech syllable may indicate the functional status of the auditory pathways from the cochlear nucleus to the primary auditory cortex, at least for children (Sharma et al., 2002). The P1 latencies are associated with outcomes in that the latencies enter the normal range for prelingually deafened persons implanted at or before 3.5 years of age, but do not typically do so for prelingually deafened persons implanted after their seventh birthday. (Variable results are obtained for the intermediate ages.) As noted previously, CI outcomes are much better for the earlyimplanted group than for the late-implanted group.
Further electrophysiological measures that may be helpful are the mismatch negativity (MMN) response to a feature “oddball” stimulus or the adaptation of longer-latency cortical responses to repeated stimuli. Zhang et al. (2011) have found, for example, that (1) the MMN response to an oddball tone stimulus is significantly larger in high-performing CI subjects than for CI subjects with moderate or low levels of performance and (2) adaptation in late auditory evoked potentials (the N1-P2 wave of the cortical evoked potential) to a tone or speech stimulus is significantly greater in the good versus the moderateto-poor performers. The MMN response to a tone with a different frequency from the non-oddball stimuli is a measure of the brain’s spectral processing abilities, and MMN responses to oddball speech stimuli are measures of speech discrimination. These MMN and adaptation data require more time to collect and more sophisticated paradigms than the P1 latencies. However, it is conceivable that, with refinement, these additional tools could be brought within the realm of clinical settings.
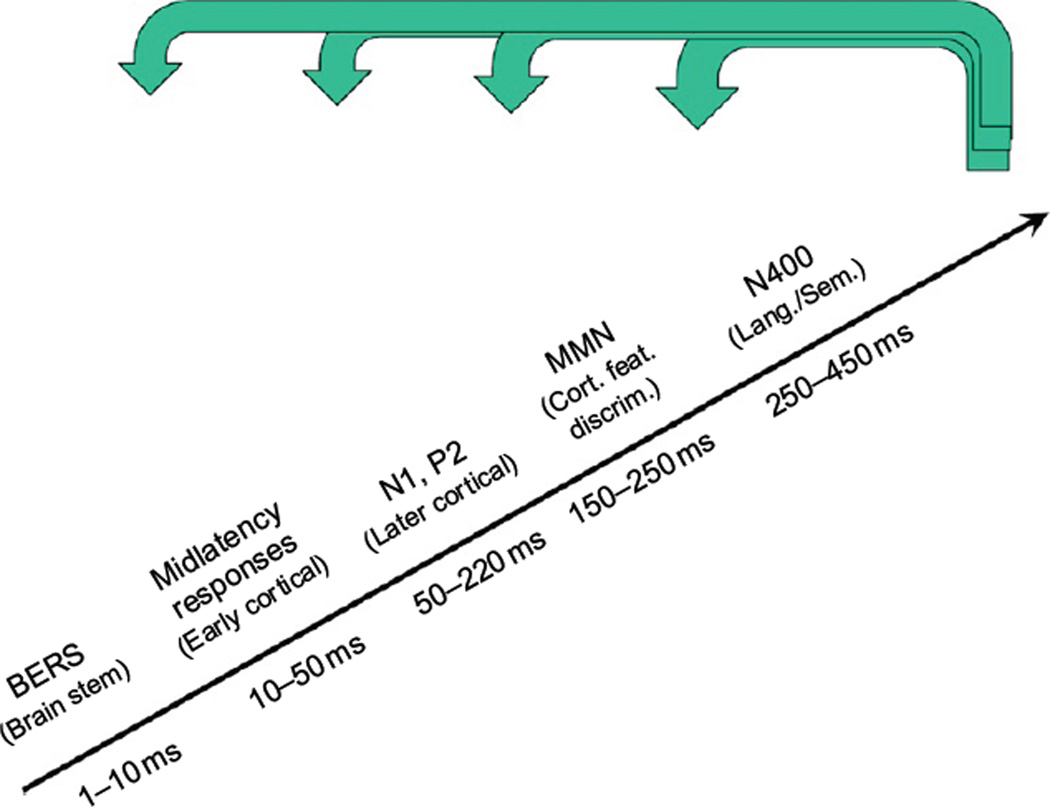
For research studies, in which time is not always such a pressing issue, a cascade of electrophysiological measures, such as the one shown in Fig. 2, could be helpful. These various measures probe the functions of different structures in the auditory pathways. The electrically evoked auditory brainstem responses (BERs in the figure) probe the functions of the auditory nuclei in the brainstem up to the inferior colliculus and thalamus, and the different waves in the responses can reflect the functional integrities of the different nuclei. At the other end of the cascade, the MMN can be used to assess feature discrimination at the cortical level, and the still longerlatency N400 response the cortical processing of language and semantic information.
Fig. 2.
Hierarchy or cascade of electrophysiological measures (event-related potential (ERP) components) to assess the functional integrity of the auditory pathways and cortices. Abbreviations in the figure include BERs for brainstem evoked responses; MMN for mismatch negativity responses; Cort. feat. discrim. for Cortical feature discrimination; and Lang./Sem. for Language and Semantic processing. Measures of other auditory ERP components could also be included for additional noninvasive assessment of the functional processing in the auditory pathways. The thick arrows coming in across the top of the figure reflect the potential influence of top-down factors, such as attention, on these various processing stages.
All or a subset of these measures could be made for individual CI subjects and compared with their speech-reception outcomes and also with the same measures collected for normal-hearing and age-matched controls. (CI subjects with the best performances also could serve as control subjects.) Significant differences between results for the CI subjects versus the control subjects at any point in the cascade would indicate a likely problem for the CI subjects on a site-specific basis. This in turn could be a powerful diagnostic for targeting both training and design efforts to improve results for that site or those sites. Such targeting would not be possible with the psychophysical measures, inasmuch as they are all “global” measures that only indicate the ability of the auditory brain as a whole to perform certain tasks, as opposed to identifying a particular structure that may be deficient in its processing abilities.
An important aspect of the measures beyond the BERs is that effects of attention or vigilance can be strong (e.g., Woldorff et al., 1991, 1993). Such effects are indicated in the figure by the arrows at the top. Fortunately, attention and vigilance can be measured and engendered, and thereby used to better understand processes and to facilitate the development of improved training protocols.
The sites at which problems may occur in the ascending auditory pathways and cortices also might be identified with other brain imaging measures. Some brain imaging measures may have sufficient spatial resolution to provide a crude indication of tonotopic processing at the auditory cortices. In general, however, brain imaging measures are far more time consuming and expensive than the psychophysical and electrophysiological measures described previously. Also, functional magnetic resonance imaging (fMRI) cannot be used in conjunction with CI stimulation for the vast majority of CI patients, because the radio-frequency fields of the MRI machine would interfere with the correct operation of the radio-frequency communication links used in all present-day CI devices (Seghier et al., 2005).
Feedback in the design process
Measures of brain function also could provide useful feedback in the design process. For example, parameter values for a processing strategy for CIs could be adjusted toward greater and greater simplicity, or otherwise differing parameters, until oddball speech or other stimuli elicit an MMN response. Also, the use of psychophysical measures has been mentioned in the context of setting parameter values.
Once the inputs produced by the CI are simple or suitable enough for the compromised brain to process, then plastic changes in brain function may be anticipated, as experience is gained with the inputs. Such changes could be monitored with periodic psychophysical or electrophysiological measures. A detected improvement in brain function would prompt a new adjustment in the parameter values or even a new choice in the processing strategy, depending on the magnitude of the change. A “closed-loop” process for design could include (1) assessment of the functional abilities of each patient’s auditory brain at the outset, (2) prescription of the stimuli to be delivered by the implant based on the assessment, (3) repeated measures of brain function at periodic intervals or when a change in speech reception performance is noticed by a patient or his or her clinician(s), and (4) when the measures indicate an improvement in brain function, looping back to step 2 and repeating the process from there. In this way, a good match between the implant and the brain could be maintained at all times, and the expectation would be for continued and linked improvements in the brain’s abilities and the performance of the CI until high performance is achieved or until no further changes in brain function are possible.
Training to facilitate desired plastic changes in brain function
Improvements over time also might be facilitated with directed training. The training could be aimed at discriminating basic psychophysical stimuli at the boundaries of a patient’s abilities. For example, the training could be directed at improving the sensitivity to modulation, which reflects temporal processing in the brain and additionally is strongly correlated with implant outcomes. Improvements in modulation detection could generalize to better speech reception scores and further could indicate the need for repeated adjustments in the parameter values for the CI processing strategy.
The training also could be aimed at improving gap detection, or electrode or spectral ripple discrimination. An improvement in the first of these measures might generalize to better temporal processing abilities in the brain, and an improvement in either of the two remaining measures might generalize to better spectral (or tonotopic) processing abilities.
Alternatively, training aimed at improving speech reception directly may be even more effective, as it uses meaningful stimuli and as the training objective is the “end point” of CI performance. Further, training using complex stimuli such as speech may facilitate desired plastic changes in both temporal and spectral processing abilities of the brain simultaneously. Training using speech as stimuli has been shown to be more effective than training using simpler stimuli for patients using the present-day CIs and standard parameter values for the processing strategies (Fu and Galvin, 2008). The same may be true for at least some patients using CIs based on the top-down approach to implant designs. However, it is also possible that beginning with simpler discrimination tasks, and then building up to tasks involving more-complex linguistic stimuli, would be more effective.
Training may accelerate possible improvements in brain function and implant performance. In addition, the ultimate asymptotic performance level may be higher with training than without it. Any of multiple training options might be used; the most important aspect may be to force the brain to work at its limits during the training, building appropriately on increasing levels of successes, as this is the most efficient if not the only way to drive the desired plastic changes.
Questions raised by the top-down approach
Development of the top-down approach is in its nascent stages. Questions that are raised by the approach and still not fully answered include the following:
How can a sensory prosthesis be best matched to a brain that has been compromised through years of sensory deprivation or other causes?
How can brain function be measured in ways that would be helpful for achieving the match?
Can a well-designed training procedure accelerate or enhance learning with (or accommodation to) a sensory prosthesis?
If so, how can the device be adapted to the desired plastic changes in brain function, to continually provide good matches across time?
Can the effects of cross-modal plasticity be reversed, to allow a “reconnection” between a cortical area and its original primary sensory input?
Can sensitive periods be reopened, to make the previously damaged brain a “clean slate” for possible full recovery, perhaps even following cross-modal changes in brain organization?
How would the stimuli specified by the topdown and bottom-up approaches differ, and for which population(s) of patients would those differences be the greatest?
These are general questions that relate not only to CIs but also to other neural prostheses. Tentative answers in the context of CIs have been presented in this chapter. Each of the questions can of course be restated as a hypothesis or a set of connected hypotheses. Studies are now underway to evaluate some of the various hypotheses, in tests with CI users and control subjects. In addition, questions 5 and 6 are important in contemporary neuroscience, so those questions are receiving considerable attention from a large number of research teams worldwide. Further information in response to all of the questions listed above may well help to shepherd in a new era in neural prosthesis designs, in which the brain is regarded as a key part of the system.
Acknowledgments
This chapter is based on an invited lecture given at the symposium on Brain machine interfaces— implications for science, clinical practice, and society, held in Ystad, Sweden, August 26–29, 2010. Parts of the chapter are drawn or adapted from prior publications (Wilson, 2011; Wilson and Dorman, 2009). B. S. W. is a consultant for MED-EL GmbH of Innsbruck, Austria, one of the three major manufacturers of CI systems. None of the statements in this chapter favor that or any other company.
Abbreviations
- BER
brainstem evoked response
- CI
cochlear implant
- CIS
continuous interleaved sampling
- ERP
event related potential
- fMRI
functional magnetic resonance imaging
- MMN
mismatch negativity (as in MMN responses)
References
- Bavelier D, Neville HJ. Cross-modal plasticity: Where and how? Nature Reviews. Neuroscience. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology & Neuro-Otology. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- Buckley KA, Tobey EA. Cross-modal plasticity and speech perception in pre- and postlingually deaf cochlear implant users. Ear and Hearing. 2010;32:2–15. doi: 10.1097/AUD.0b013e3181e8534c. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental Neurobiology. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallet M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6–20 channels. Journal of the Acoustical Society of America. 1998;104:3583–3585. doi: 10.1121/1.423940. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Spahr AJ, Maloff E. A comparison of the speech understanding provided by acoustic models of fixed-channel and channel-picking signal processors for cochlear implants. Journal of Speech, Language, and Hearing Research. 2002;45:783–788. doi: 10.1044/1092-4388(2002/063). [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DRF, Shepherd RK. Cochlear implants and brain plasticity. Hearing Research. 2008;238:110–117. doi: 10.1016/j.heares.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. Journal of the Acoustical Society of America. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fu QJ. Temporal processing and speech recognition in cochlear implant users. NeuroReport. 2002;13:1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ., III Maximizing cochlear implant patients’ performance with advanced speech training procedures. Hearing Research. 2008;242:198–208. doi: 10.1016/j.heares.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Lee HJ. Predicting cochlear implant outcome from brain organization in the deaf. Restorative Neurology and Neuroscience. 2007;25:381–390. [PubMed] [Google Scholar]
- Helms J, Müller J, Schön F, Moser L, Arnold W, et al. Evaluation of performance with the COMBI 40 cochlear implant in adults: A multicentric clinical study. ORL; Journal for Oto-Rhino-Laryngology and Its Related Specialties. 1997;59:23–35. doi: 10.1159/000276901. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. Journal of the Acoustical Society of America. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Hochmair-Desoyer IJ, Hochmair ES, Stiglbrunner HK. Psychoacoustic temporal processing and speech understanding in cochlear implant patients. In: Schindler RA, Merzenich MM, editors. Cochlear implants. New York: Raven Press; 1985. pp. 291–304. [Google Scholar]
- Hüttenbrink KB, Zahnert T, Jolly C, Hofmann G. Movements of cochlear implant electrodes inside the cochlea during insertion: An x-ray microscopy study. Otology & Neurotology. 2002;23:187–191. doi: 10.1097/00129492-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Fallon JB, Kamke MR. Plasticity in the central auditory system. Acoustics Australia. 2006;34:13–17. [PMC free article] [PubMed] [Google Scholar]
- Jäncke L. The plastic human brain. Restorative Neurology and Neuroscience. 2009;27:521–538. doi: 10.3233/RNN-2009-0519. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kral A, Eggermont JJ. What’s to lose and what’s to learn: Development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Research Reviews. 2007;56:259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Klinke R, Hartmann R. Cochlear implants: Cortical plasticity in congenital deprivation. Progress in Brain Research. 2006;157:283–313. doi: 10.1016/s0079-6123(06)57018-9. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepres-sant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: Awakening the deafened brain. Nature Neuroscience. 2009;12:686–691. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- Musiek FE, Daniels SB. Central auditory mechanisms associated with cochlear implantation: An overview of selected studies and comment. Cochlear Implants International. 2010;11(Suppl. 1):15–28. doi: 10.1179/146701010X12671178390753. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Cochlear implants in adults and children. NIH Consensus Statement. 1995;13(2):1–30. (This statement also is available in the JAMA, 274, 1955–1961.) [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS. Psychophysical recovery from pulse-train forward masking in electric hearing. Journal of the Acoustical Society of America. 2002;112:2932–2947. doi: 10.1121/1.1514935. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. Journal of the American Medical Association. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Effects of carrier pulse rate and stimulation on modulation detection by subjects with cochlear implants. Journal of the Acoustical Society of America. 2007;121:2236–2246. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Boëx C, Lazeyras F, Sigrist A, Pelizzone M. FMRI evidence for activation of multiple cortical regions in the primary auditory cortex of deaf subjects users of multichannel cochlear implants. Cerebral Cortex. 2005;15:40–48. doi: 10.1093/cercor/bhh106. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Fu Q-J, Galvin J., III The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Oto-Laryngologica. 2004;2004(Suppl):50–54. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear and Hearing. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: Implications for cochlear implants. Audiology & Neuro-Otology. 2001;6:305–318. doi: 10.1159/000046843. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Meltzer NE, Fallon JB, Ryugo DK. Consequences of deafness and electrical stimulation on the peripheral and central auditory system. In: Waltzman SB, Roland JT Jr., editors. Cochlear implants. 2nd ed. New York: Thieme Medical Publishers; 2006. pp. 25–39. [Google Scholar]
- Thiel CM. Pharmacological modulation of learninginduced plasticity in human auditory cortex. Restorative Neurology and Neuroscience. 2007;25:435–443. [PubMed] [Google Scholar]
- Tobey EA, Devous MD, Sr, Buckley K, Overson G, Harris T, Ringe W, et al. Pharmacological enhancement of aural habilitation in adult cochlear implant users. Ear and Hearing. 2005;26(Suppl. 4):45S–56S. doi: 10.1097/00003446-200508001-00007. [DOI] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: Temporal processing deficits in the aged auditory brainstem. Hearing Research. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS. A ‘top down’ or ‘cognitive neuroscience’ approach to cochlear implant designs and fittings. Cochlear Implants International. 2011;12:S35–S39. doi: 10.1179/146701011X13001035753272. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. The surprising performance of present-day cochlear implants. IEEE Transactions on Biomedical Engineering. 2007;54:969–972. doi: 10.1109/TBME.2007.893505. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. The design of cochlear implants. In: Niparko JK, Kirk KI, Mellon NK, Robbins AM, Tucci DL, Wilson BS, editors. Cochlear implants: Principles & practices. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 95–135. [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, et al. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology. 1991;28:30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Zheng Y. Spectral and temporal cues for phoneme recognition in noise. Journal of the Acoustical Society of America. 2007;122:1758–1764. doi: 10.1121/1.2767000. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hammer T, Banks H-L, Benson C, Xiang J, Fu Q-J. Mismatch negativity and adaptation measures of the late auditory evoked potential in cochlear implant users. Hearing Research. 2011;275:17–29. doi: 10.1016/j.heares.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]