Abstract
We used daptomycin plus antistaphylococcal β-lactams (ASBL) to clear refractory MRSA bacteremia. In vitro studies showed enhanced daptomycin bactericidal activity, increased membrane daptomycin binding, and decrease in positive surface charge induced by ASBLs against daptomycin nonsusceptible MRSA. Addition of ASBLs to daptomycin may be of benefit in refractory MRSA bacteremia. (Although the official designation is “daptomycin nonsusceptiblity,” we will use the term “daptomycin-resistance” in this paper for facility of presentation.)
Methicillin–resistant Staphylococcus aureus (MRSA) has emerged as one of the most important pathogens both in health care and community-onset infections [1]. MRSA infection is associated with higher morbidity and mortality and prolonged hospital stay, compared with similar infections caused by methicillin-susceptible S. aureus [2]. Patients with prolonged MRSA bacteremia (>7 days) are at high risk for metastatic complications and death [3]. These infections are challenging to treat and often require a combined medical-surgical intervention for successful outcome. However, in cases of prolonged bacteremia in which a removable focus is not definable, clinicians are often confronted with limited therapeutic options.
Prior in vitro studies suggested an enhanced activity of daptomycin (DAP) against MRSA when combined with anti-staphylococcal β-lactams (ASBLs) [4–6]. Recently, a see-saw effect has been demonstrated between DAP and ASBLs, whereby the development of DAP resistance (DAP-R) in vitro was accompanied by increased ASBL susceptibility in MRSA in a mecA-independent manner [7]. Faced with some very difficult clinical cases of refractory MRSA bacteremia, we used combined DAP–ASBL therapy with salutary outcomes in 7 recent patients.
METHODS
We identified 7 consecutive clinical cases from 3 different institutions in which combined DAP-ASBL therapy was used successfully to eradicate persistent MRSA bacteremia (summarized in Table 1). Isolates were available for detailed in vitro analysis from 3 cases (3–5). DAP and vancomycin minimum inhibitory concentrations (MICs) were determined using E-test in antibiotic-free Mueller-Hinton agar plates and in plates containing varying concentrations of ASBLs. MICs to methicillin and nafcillin were determined using broth microdilution in accordance with Clinical Laboratory Standards Institute methods. Time-kill curves were performed on selected isolates at 37°C in calcium-supplemented (50 mg/L) Mueller-Hinton broth containing DAP (10 mg/L) with or without oxacillin (20 mg/L). These concentrations were chosen to reflect approximations of clinically-relevant serum concentrations of these antibiotics [8–10]. Experiments were done 3 times on 3 different days with use of starting inocula of 106–107 cfu/mL (to represent high-inoculum infections, such as infective endocarditis).
Table 1.
Clinical Summary of Cases Where NAF or OXA Were Added to High-Dose DAP to Clear Persistent or Relapsing MRSA Bacteremia
| Age/sex | Underlying conditions | Diagnostic findings | Primary source | 1st-line therapy | 2nd-line therapy | 3rd-linetherapy | 4th-linetherapy | Comments/outcome |
| 1. 33/F | IVDA | Pulmonary Nodules; TV 2x3 mm Vegetation | TV IE | VAN d 1–21 relapsed 17 | DAP 10 d 22–50 relapse d 42 | DAP 10 + OXA clear 24 h d 51–123 | N/A | TEE d 53, 2.1 cm TV veg TV repair on d 81 cure |
| 2. 47/M | DM | Chest wall phlegmon; empyema; pulmonary nodules TEE neg | Abscess cSSSI | VAN d 1–2 | DAP 10 + GEN d 3–5 | DAP 10 + NAF clear 24 h days 6–20 | Linezolid d 21–42 | Abscess I&D d 11 absolute Eos = 3100/mm3 change to linezolid d 20 cure |
| 3. 55/M | DM, HTN, COPD, ITP steroid | TEE neg CT neg MRI spine neg | Unknown | VAN d 1–11 VAN MIC 1 DAP MIC 0.5 | DAP 6 d 12–16 | DAP 8 + GEN d 17–20 VAN MIC 4 DAP MIC 2–4b | DAP 10 + NAF* clear 24 h d 21–76 | Sacral OM d 28 cure |
| 4. 53/M | DM, HTN, COPD, pancreatitis | TEE neg CT neg MRI spine neg | Unknown | VAN d 1-7 VAN MIC 1.5 DAP MIC 0.5 | DAP 6 d 8–13 | DAP 8 + GEN d 14–19 VAN MIC 2 DAP MIC 1 | DAP 10 + NAF* clear 48 h d 20–61 | Lumbar OM d28 cRelapse 12 wk post-therapy treated 4 wk DAP+NAF cure relapse isolate: VAN MIC 2, DAP MIC 1 |
| 5. 87/M | DM, HTN ESRD on HD AVG AICD aortic stenosis | TEE neg CT neg NM neg | Unknown | VAN d 1–4 VAN MIC 2 DAP MIC 0.75 | DAP 6 d 5–11 | DAP 8 + GEN d 12–15 VAN MIC 2 DAP MIC 0.75 | DAP 10 + NAFa clear 24 h d 16–25 | DAP+ Cefazolin after HD days 26–67 to complete lumbar OM d 21 crelapse8 wk post-therapy with PV IE, died Relapse isolate: VAN MIC 3, DAP MIC 1.5 |
| 6. 48/F | DM renal failure | TEE Neg CT neg MRI spine neg | Unknown | VAN d 1–2 | DAP 8–10 +GEN d 3–6 | DAP 10 + NAF clear 24 h d 7–21 | N/A | DAP 10 d 22–42 cure |
| 7. 60/M | DM renal failure | TEE neg CT neg MRI spine neg | Unknown | VAN d 1–10 | DAP 8 d 11–14 | DAP 8 +NAF clear 24 h d 15–28 | N/A | MRI-Lumbar epidural phlegmon d12 DAP 8 d 29–56 to complete cure |
NOTE. AICD, implantable cardioverter defibrillator; AVG, arteriovenous graft; COPD, chronic obstructive pulmonary disease; cSSI, complicated skin and skin structure infection; d, day; DAP, daptomycin; DM, diabetes mellitus; ESRD, end stage renal disease; GEN, gentamicin; HTN, hypertension; IE, infective endocarditis; IVDA, intravenous drug abuse; MRI, magnetic resolution imaging; NAF, nafcillin; NM, WBC nuclear medicine scan; OM, osteomyelitis; OXA, oxacillin; PV, pulmonic valve; TEE, transesophageal echocardiogram; TV, tricuspid valve; VAN, vancomycin.
Concomitant GEN overlap with DAP+NAF for 3–5 days.
Daptomycin nonsusceptibility was determined during the retrospective analysis of follow up blood culture samples.
The relapse isolate was not available for genotyping; therefore a small possibility of reinfection with a different strain was not ruled out.
To determine whether ASBLs are able to impact the ability of DAP to bind to the MRSA membrane, a DAP-R strain from patient 3 was grown in Lurea-Bertoni (LB) broth at 37°C in the presence or absence of nafcillin (40 mg/mL), then incubated for 10 minutes with Bodipy-fluorescein–labeled DAP (16 μg/mL; supplied courtesy of Cubist Pharmaceuticals). Excess unincorporated label was removed by washing the cells 3 times in LB broth. The cells were counterstained with FM 4–64 to visualize the membrane [11] and DAPI to visualize the nucleoid and then were imaged using a Delta Vision Deconvolution microscope (Applied Precision) [11].
The binding of the fluorescein isothiocyanate–labeled cationic poly-L-lysine (PLL) probe to the staphylococcal surface was used to quantify relative surface charge with use of a flow cytometric assay, as detailed elsewhere [12]. PLL-binding assays were performed on selected isolates grown up to stationary phase in Mueller-Hinton broth in the presence or absence of oxacillin (40 mg/L). Data were expressed in terms of relative fluorescent units (±standard deviation [SD]), and statistics were determined from a minimum of 3 experiments.
RESULTS
Clinical Descriptions
Seven cases of prolonged and persistent (7–22 day duration), non–catheter-related MRSA bacteremia, despite a variety of conventional therapies, were identified in which DAP plus ASBLs eventually achieved a microbiologic and clinical cure (Table 1). One case was associated with bacteremic relapse during therapy (case 1), and the remaining 6 cases exhibited persistent bacteremia (cases 2–7). Conventional therapy included vancomycin or DAP alone and/or in combination with gentamicin. Blood cultures were monitored daily until the result was negative for 72 hours. When vancomycin was used, serum trough concentrations of 15–20 mg/L were maintained.
All patients were thoroughly evaluated for an endovascular focus of infection and metastatic complications of infection (ie, abscesses) with use of transthoracic echocardiography and transesophageal echocardiography, abdominal CT, spinal MRI, and indium-111 labeled white blood cell scans.
In 5 of the cases (3–7), there was no obvious focus of infection initially identified, although such foci were subsequently identified. In one instance (case 2), a chest wall abscess that developed from a deep soft tissue phlegmon was surgically drained. In the case involving tricuspid valve endocarditis (case 1), the patient refused surgical intervention initially but eventually underwent valve replacement surgery.
Patient 4 experienced relapse 12 weeks after conventional therapy; the patient was then cured after treatment with an additional 4 weeks of DAP plus nafcillin. Patient 5 experienced relapse 8 weeks after discontinuation of therapy, was identified with pulmonic valve endocarditis, and died. In case 3, a vancomycin-intermediate S. aureus (VISA) isolate with concomitant DAP-R emerged during first- and second-line therapies. One patient (2) developed peripheral eosinophilia without rash or pneumonitis on day 18 of daptomycin therapy, with a peak absolute eosinophil count of 3100 cells/mm3 (normal count, <500 cells/mm3) 4 days after discontinuation and subsequent decrease and normalization 10 days after discontinuation of daptomycin.
Susceptibility Testing
In all 7 cases, the initial MRSA bloodstream isolates were susceptible to DAP and vancomycin at the onset of illness, according to clinical microbiology reports. In case 3, during therapy with vancomycin, the MRSA isolate developed the VISA phenotype by MIC testing (MIC, 3 mg/L) with concomitant DAP-R (MIC = 2–4). Both strains were identical by pulse-field gel electrophoresis (data not shown). We examined the vancomycin and DAP MICs by E test and the MICs to selected ASBLs with use of broth dilution techniques. The VISA-DAP-R isolate from case 3 did not show any evidence of the DAP-MRSA see-saw effect (ie, increasing DAP MIC associated with decreasing ASBL MICs [7]). Of note, in media containing DAP plus ASBLs, we observed reductions in DAP MICs for this isolate. For example, in the presence of nafcillin (80 mg/mL; comparable to serum level 1 hour after treatment in patients [8]), the DAP MIC of this DAP-R isolate was reduced to the susceptible range (MIC, 0.38 mg/L).
Table 2.
Antimicrobial Susceptibilities (MIC, mg/L) of Select MRSA Isolates
| VAN | MET | NAF | DAPTOMYCIN E-test in agar containing: |
AMP 100 mg/L | |||
| No antibiotic | NAF 20 mg/L | NAF 80 mg/L | |||||
| Case 3 MRSA | 2.0 | >512 | 256 | 0.5–1.0 | 0.38 | 0.25 | 0.5 |
| Case 3 VISA | 3.0 | >512 | 256 | 3.0–4.0 | 2.0 | 0.38 | 1.5 |
| ATCC 29213 | 1.0 | 1.0 | 0.50 | 0.19 | NOT PERFORMED | ||
| ATCC 33591 | 1.0 | 256 | 128 | 0.19 | NOT PERFORMED | ||
NOTE. MET, Methicillin; MRSA, Methicillin resistant Staphylococcus aureus; NAF, nafeillin; VAN, vancomycin; VISA, vancomycin intermediate resistant Staphylococcus aureus.
Kill Curves
Time-kill curves were performed on isolates from cases 3, 4, and 5. For DAP-susceptible isolates from cases 4 and 5, DAP exhibited rapid bactericidal activity that was not further enhanced by the addition of oxacillin (data not shown). Kill curves from case 3 are shown in Figure 1. Figure 1A shows a representative assay from the initial DAP-susceptible isolate, revealing rapid bactericidal activity of DAP alone, with modest enhancement of activity with the addition of oxacillin. Figure 1B shows the result of similar studies with the later VISA-DAP-R isolate from this case (3). After exposure to DAP alone, this latter isolate demonstrated significant regrowth at 24 hours, whereas the combination of DAP plus oxacillin resulted in reduction in counts below the level of detection at 24 hours.
Figure 1.
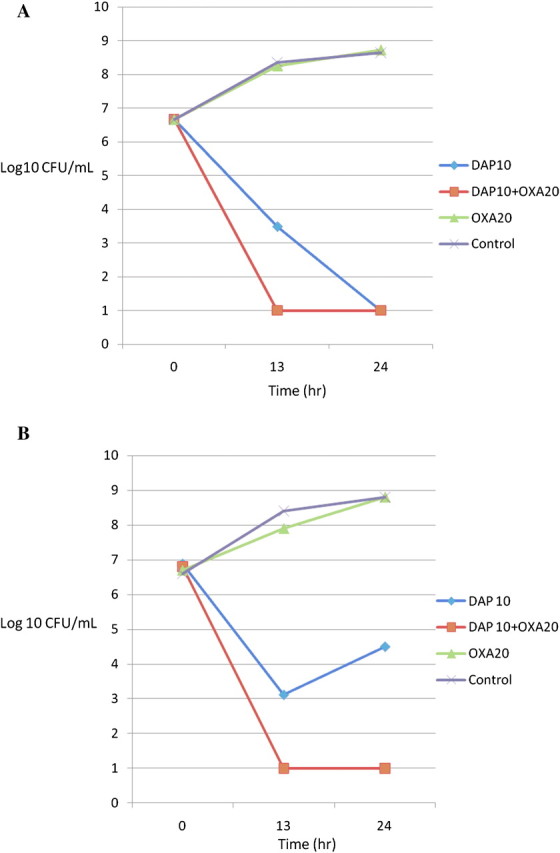
One representative experiment of kill curves of isogenic MRSA strains from case 3 as follows: daptomycin 10 mg/L (diamond); daptomycin 10 mg/L plus oxacillin 20 mg/L (square); oxacillin 20 mg/L (triangle); growth control (X). Panel A shows a representative kill curve assay using DAP-susceptible parental strain, and panel B shows a similar analysis against a subsequent DAP-R-VISA isolate that emerged on therapy. Note enhanced DAP killing in the presence of oxacillin for the VISA strain, even though the organism remained highly resistant to ASBLs and showed minimal growth inhibition by oxacillin 20 mg/L alone.
Daptomycin Binding Studies
Using the DAP-R VISA isolate from case 3, we examined DAP binding in the presence or absence of nafcillin with use of fluorescent-labeled DAP. Results are shown in Figure 2. DAP bound poorly to this strain in the absence of concomitant exposures to ASBLs (Figure 2A–C). However, in the presence of nafcillin (40 mg/L), significant DAP binding was detected (Figure 2D–F). Of interest, this enhanced binding occurred focally on the organism’s surface. In addition, pixel intensity quantifications confirmed this increased surface binding.
Figure 2.
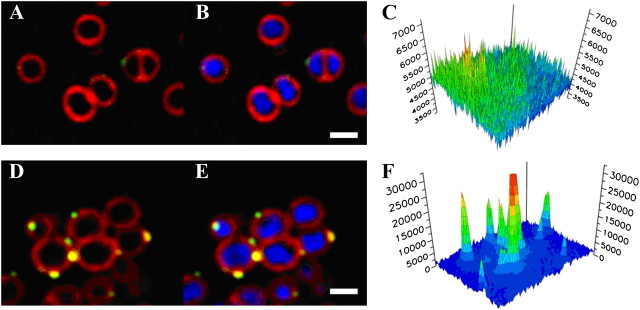
Incorporation of fluorescently-labelled DAP by DAP-R-VISA grown in the presence or absence of nafcillin (case 3). The strain was labeled with 16mg/L bodipy-labelled DAP (green; A, B, D, E) for 10 minutes at 37°C after growth to log phase in either antibiotic-free LB broth (A–C) or in LB broth containing nafcillin 40 mg/L (D–F). Cells were stained with FM 4–64 (red; A, B, D, E) and DAPI (blue; B & E). Scale bar equals 1 micron. Panels C and F show a quantitative comparison of DAP-bodipy incorporation, demonstrating increased DAP labeling in the presence of nafcillin (yellow-green surface deposits). Three dimensional graphs show the pixel intensities for DAP-bodipy fluorescence for cells grown without (C) or with (F) 40 mg/L nafcillin.
Bacterial Surface Charge Studies
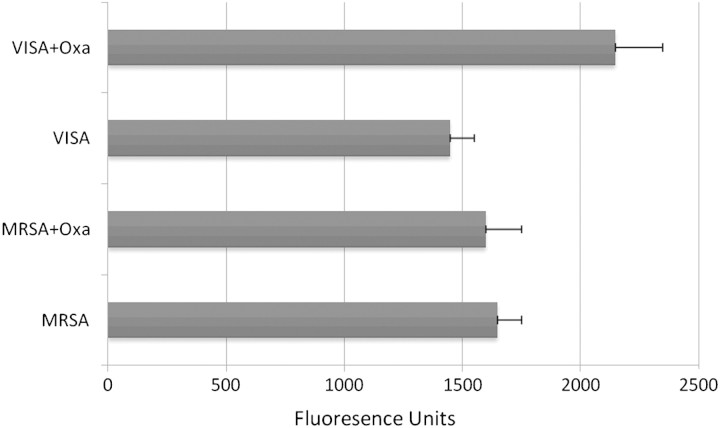
To examine the potential mechanisms for enhanced DAP binding, PLL-binding assays were performed on the DAP-susceptible isolate and the VISA-DAP-R strain pair from case 3. Figure 3 demonstrates that growth in ASBL resulted in reduction in net positive surface charge (versus non–ASBL-exposed cells) that was more pronounced against the VISA-DAP-R strain, versus the DAP-susceptible parental strain (P = .00017 and P = .014, respectively).
Figure 3.
Surface charge evaluation using fluorescein isothiocyanate (FITC)-labeled cationic poly-L-lysine (PLL) binding to the staphylococcal surface of initial MRSA and subsequent VISA-DAP resistant isolate from case 3 determined after growth in Mueller-Hinton broth with or without oxacillin 40 mg/L. Units are expressed in relative fluorescence units, with higher fluorescence indicative of decrease in net positive charge. Note the significantly increase in PLL binding by VISA when grown in oxacillin compared with growth in antibiotic-free media (P = .00002). Also noteworthy is the decreased PLL binding accompanying VISA as compared with the parent MRSA strain.
DISCUSSION
In this report, 7 cases of MRSA bacteremia are presented that were refractory to a number of vancomycin-based and DAP-based regimens. In all patients, the addition of high-dose ASBLs (eg, nafcillin or oxacillin; 2 g intravenously every 4 h) to high-dose DAP (8–10 mg/kg/day) resulted in rapid bacteremia clearance. In vitro studies showed several important observations: (1) restoration of in vitro DAP susceptibility of 1 DAP-R-VISA strain that emerged during therapy in ASBL-containing media, (2) enhanced killing of this isolate by DAP plus ASBLs, (3) notable increases in DAP membrane binding after organism growth in ASBLs, and (4) reduction in net positive membrane surface charge by ASBLs that was more pronounced in the DAP-R strain, compared with the DAP-susceptible parent.
The enhancement of DAP membrane binding and activity by ASBLs through ASBL-mediated reduction in surface charge may be linked to release of wall lipo-teichoic acid [13]. Our DAP binding assays are compatible with this latter paradigm. With use of a fluorescently labeled DAP, microscopic examination revealed a clear increase in DAP binding to the surface of ASBL-treated cells (Figure 2). Of interest, the prior treatment with vancomycin selecting VISA and concomitant DAP-R may have established a “priming” scenario in which the ASBL-mediated reduction in surface charge was more pronounced.
Our laboratories are actively studying these potential mechanisms. In addition, more structured clinical studies are required to examine DAP-ASBL combination therapy in cases of refractory MRSA bacteremic infections. Finally, additional in vitro studies are needed to examine the effect of DAP plus other β-lactam derivatives, such as cephalosporins, carbapenems, and β-lactam/β-lactamase inhibitor combinations against MRSA.
Acknowledgments
We thank Jared Silverman (Cubist Pharmaceuticals) for providing bodipy-labeled daptomycin.
Financial support. This work was supported by Sharp Healthcare Foundation.
Potential conflicts of interest. A. D. has served on the speakers bureau and received speaking honoraria from Cubist, Pfizer, and Merck. A. S. B. has received research grants from Cubist Pharmaceuticals and Astellas Pharmaceuticals. G. S. has received research grants from Cubist and Pfizer Pharmaceuticals; speaking honoraria from Cubist, Pfizer, and Astellas Pharmaceuticals; and consulting fees from Cubist, Astellas, and Ortho-McNeil Pharmaceuticals. J. P. has received research grants and consulting fees from Cubist Pharmaceuticals.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove S, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Mortality related to methicillin-resistant Staphylococcus aureus compared to to methicillin-susceptible S. aureus: a meta-analysis. Clin Infect Dis. 2003;36:53–9. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–72. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 4.Rand KH, Houck HJ. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:2871–5. doi: 10.1128/AAC.48.8.2871-2875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snydman DR, McDermott LA, Jacobus NV. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and Enterococci by FIC index and timed-kill curves. J Chemother. 2005;17:614–21. doi: 10.1179/joc.2005.17.6.614. [DOI] [PubMed] [Google Scholar]
- 6.Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. Effect of combination of oxacillin and non-beta-lactam antibiotics on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1994;33:1155–63. doi: 10.1093/jac/33.6.1155. [DOI] [PubMed] [Google Scholar]
- 7.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”) Antimicrob Agents Chemother. 2010;54:3169–9. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz CR, Kane JG, Parker RH, Pelsor FR. Pharmacokinetics of nafcillin in patients with renal failure. Antimicrob Agents Chemother. 1977;12:98–101. doi: 10.1128/aac.12.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxacillin package insert. Princeton, NJ: Sandoz, Inc; 2009. [Google Scholar]
- 10.Daptomycin package insert. Lexington, MA: Cubist Pharmaceuticals; 2008. [Google Scholar]
- 11.Pogliano J, Osborne N, Sharp MD, et al. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–59. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones T, Yeaman MR, Sakoulas G, et al. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother. 2008;52:269–78. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.al-Obeid S, Gutmann L, Williamson R. Correlation of penicillin-induced lysis of Enterococcus faecium with saturation of essential penicillin-binding proteins and release of lipoteichoic acid. Antimicrob Agents Chemother. 1990;34:1901–7. doi: 10.1128/aac.34.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]