Abstract
Background
Heavy alcohol intake may exacerbate gastrointestinal (GI) symptoms in adults with irritable bowel syndrome (IBS); however, the role of alcohol in IBS is unclear.
Objectives
We investigated prospective associations between daily patterns of alcohol intake and next day's GI symptoms using daily diaries.
Method
In an observational study of women aged 18–48 years with IBS and healthy controls, participants recorded daily GI symptoms, alcohol intake, caffeine intake, and cigarette smoking for approximately one month. GI symptoms included abdominal pain, abdominal bloating, intestinal gas, diarrhea, constipation, nausea, stomach pain, heartburn, and indigestion. Binge drinking was defined as 4+ alcohol-containing drinks/ day.
Results
Patterns of alcohol intake did not differ between IBS patients and controls. While patterns of drinking were associated with GI symptoms among women with IBS, this was not the case with healthy controls. The strongest associations for IBS patients were between binge drinking and the next day's GI symptoms (e.g., diarrhea , p=0.006; nausea, p=0.01; stomach pain, p=0.009; and indigestion, p=0.004), whereas moderate and light drinking either were not associated or weakly associated with GI symptoms. Associations between alcohol intake and GI symptoms were stronger for women with IBS-diarrhea than for IBS-constipation or IBS-mixed. Effects of binge drinking on GI symptoms were strongest when comparing between individuals (rather than within individuals).
Conclusion
Our findings indicate that IBS symptoms differ according to the pattern of alcohol intake among IBS patients, suggesting that the pattern of drinking may in part explain the inconsistent findings between alcohol and IBS symptoms.
Keywords: Irritable bowel syndrome, alcohol consumption, female, gastrointestinal symptoms
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder that is defined by the occurrence of abdominal pain and discomfort associated with stool consistency and frequency. It is estimated to affect 5% to 10% of the population worldwide, with women being disproportionately affected.1 In a survey of 1,242 IBS patients, the majority of people felt that dietary modifications could reduce IBS symptoms,2 including 69% who had tried eating small, frequent meals to lessen their IBS symptoms. In addition, the majority of individuals with IBS had tried avoiding certain foods to reduce their symptoms, including avoidance of foods with high fat content (64%), high fiber content (58%), dairy (54%), caffeine (41%), and to a lesser extent alcohol (27%).2
Prior studies have had inconsistent findings on the role of alcohol in IBS. Alcohol consumption was not found to be associated with IBS in population-based studies.3-5 However, studies investigating perceived diet-related GI symptoms have reported a role of alcohol in GI symptoms among IBS patients.2,6,7 In addition, results from exclusion and reintroduction diets involving alcohol have been mixed .1 In the present study, our objectives were to describe the pattern of alcohol use among IBS patients and to investigate whether specific patterns of alcohol intake were associated with the next day's GI symptoms using a daily diary within a prospective study.
Methods
Study design
This prospective investigation of alcohol consumption and GI symptoms included women aged 18 to 48 years with IBS and without. Study participants were recruited through community advertisements from 1995 to 1999. To be eligible as an IBS patient, women had to have a medical diagnosis of IBS and report symptoms consistent with the Rome I criteria; to enrich the study with IBS patients, 40% women with IBS were also recruited from a local health maintenance organization (in addition to the 60% from the community).8 Control women were recruited as comparison group, and as such, had to be free of GI symptoms by self-report at the phone screening. Women were excluded if they reported a history of GI pathology (e.g., inflammatory bowel disease), were currently taking medications specific for IBS, had a history of cardiovascular, renal, or gynecological pathology, or had any known cardiac arrhythmia (due to the aims of the original study).9
Study participants were seen in person for their initial interview, at which time they provided informed consent. All women began a daily diary after this initial visit starting with the first evening of their next menses and continuing until 5 days after the following menses. The IBS patients went on to participate in a randomized trial of a comprehensive self-management intervention.8 This study was approved by the Institutional Review Board at the University of Washington.
Measures
The daily diary included questions regarding GI symptoms, number of drinks of alcohol and caffeine consumed, number of cigarettes smoked, and level of stress.
GI Symptoms
Each GI symptom was rated on a scale from 0 to 4, with 0 (not present), 1 (minimal), 2 (mild), 3 (moderate), and 4 (extreme). GI pain/discomfort symptoms in the daily diary included abdominal pain, abdominal bloating, intestinal gas, nausea, stomach pain, heartburn, and indigestion. GI symptoms related to stool characteristics were diarrhea and constipation. In addition, number of stools and their consistency was recorded.
Alcohol intake, caffeine intake, and cigarette use
The instructions given to each study participants included a definition of one drink of alcohol: a 4-oz glass of wine, an 8-oz beer, or 1 shot of liquor. The daily diary also collected information on the number of cigarettes participants smoked /day, and the number of servings of caffeine/day.
Data from the Bowel Disease Questionnaire (BDQ) were used to classify patients in IBS subtypes and assess for this analysis which study participants met Rome III criteria. For IBS subtypes, patients were classified according to bowel pattern as IBS-diarrhea (IBS-D), IBS-constipation (IBS-C), or IBS-mixed (IBS-M) based on Rome III definitions of predominant bowel patterns (more than 25% of time). IBS-M was defined to be report of each diarrhea and constipation more than 25% of the time. Because the current data were collected under the Rome I criteria, we determined the number of participants who met Rome III criteria for IBS diagnosis (i.e. participants who reported at least 3 days/month of abdominal pain or discomfort, and improvement of symptoms with defecation; however, we could not adequately assess the onset of pain with constipation). Subset analyses were conducted limiting our analysis to those who met Rome III criteria (n = 112).
Statistical analysis
For the dependent variables, we dichotomized GI symptoms as none versus any GI symptoms (collapsing categories 1, 2, 3, and 4, representing minimal, mild, moderate, and extreme severity, respectively). We imputed GI symptoms using prior and next day's GI symptoms for days on which participants had missing data. For alcohol variables, daily intake was categorized by the pattern of alcohol intake, defining binge drinking according to the standard definition of binge drinking for women (i.e., 4 or more drinks of alcohol on one occasion for women).10 Moderate drinking was categorized as 2-3 drinks/day; and light drinking as 1 drink/day. Subsequently, alcohol intake was further collapsed as ≥ 4 drinks in a day versus <4 drinks in a day as a binary variable comparing binge drinking to no binge drinking on a given day.
We used generalized estimating equations (GEE) models to assess the relationship of daily intake of alcohol, caffeine, and cigarettes with the presence of next day's GI symptoms. The GEE models were set up with independent correlations and robust standard errors, and used the logit command resulting in the coefficient being the log odds of developing the dependent variable (GI symptoms). In order to examine separately the within-person and between-person comparisons of alcohol use, we conducted additional analyses. For this, the GEE model included 2 variables for alcohol intake: (1) a term to estimate the between-person effect of alcohol consumption (xit); and (2) a term to estimate the within-person effect of alcohol consumption (xit –mean[ xi]).11
All GEE models adjusted for age as a confounding variable. In addition, in order to explore the potential impact of variables, such as stress and cigarettes smoked, on associations between alcohol intake and GI symptoms, we adjusted for additional variables in the GEE models in secondary analyses. Stress-related variables were derived from questions asking about stress in the daily diary (i.e. ‘how stressful was your day?’) and perceptions of coping (i.e. ‘how well were you able to cope with your life today?’), ranked from 0 (not at all) to 6 (extremely). Also, participants were asked to report feelings of anxiety, depression, desire to be alone, tension, and hopelessness, rating their response on a scale from 0 (not present) to 4 (extreme).
Results
Characteristics of our study population are presented separately for IBS patients and controls in Table 1. There were no differences in demographic characteristics between IBS patients and controls. The mean age was 32 years. The majority of our population had a college or graduate degree; approximately half were married or partnered; and our population was predominantly White.
Table 1.
Demographic Characteristics of Study Participants
| Characteristic | IBS patients (n = 166) | Controls (n = 48) | p-valuea |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (yrs) | 32.3 (7.6) | 32.2 (7.5) | 0.91 |
| n (%) | n (%) | ||
| Age Categories (yrs) | |||
| 18-25 | 35 (21%) | 11 (23%) | 0.61 |
| 26-30 | 41 (25%) | 10 (21%) | |
| 31-35 | 28 (17%) | 6 (12%) | |
| 36-39 | 29 (18%) | 13 (27%) | |
| 40-46 | 33 (20%) | 8 (17%) | |
| Married or partnered | 95 (57%) | 23 (48%) | 0.45 |
| College or graduate degree | 105 (64%) | 31 (65%) | 0.94 |
| White Race | 146 (88%) | 38 (79%) | 0.12 |
Calculated as t-test for continuous variables; chi-square for categorical variables
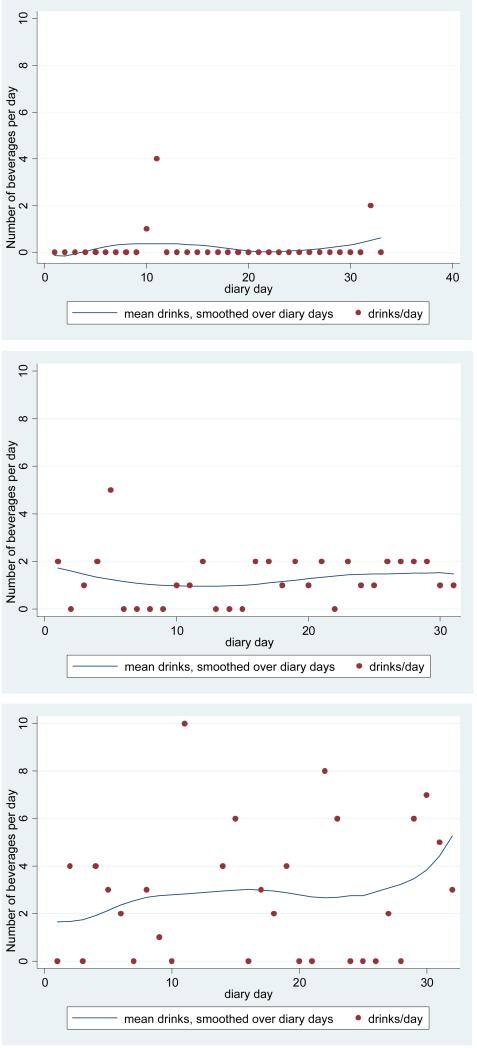
Patterns of alcohol consumption did not differ between IBS patients and controls (Table 2). Women in our study reported drinking a mean of 0.52 alcohol-containing drinks per day. Approximately one-fifth of women reported never drinking during the study period, while 62% reported drinking less than a drink per day, and approximately one-fifth reported a mean of more than 1 drink per day. Approximately one-third of all women reported binge drinking on one or more occasions during the study period. During a binge drinking episode, the mean amount consumed was 4.9 drinks. Figure 1 provides three exemplars of patterns of drinking among IBS women who reported binge drinking during the study, with one each exemplifying light, moderate, and heavy drinking overall.
Table 2.
Alcohol Patterns among IBS Patients and Controls
| Description | IBS patients (n = 166) | Controls (n = 48) | p-valuea |
|---|---|---|---|
| Number of drinks/day of alcohol, mean (SD) | 0.51 (1.2) | 0.53 (1.1) | 0.64 |
| Number (%) of people reporting the following average daily consumption of alcohol during the study diary | 0.99 | ||
| None | 33 (20%) | 8 (17%) | |
| 0.01-0.99 | 103 (62%) | 30 (62%) | |
| ≥ 1.0 | 30 (18%) | 10 (21%) | |
| Number (%) of people reporting any binge drinking | 53 (32%) | 19 (40%) | 0.32 |
| Among people who binge drink | |||
| Number of drinks of alcohol consumed on average | 1.3 (1.1) | 1.0 (0.6) | 0.19 |
| Number of drinks consumed during a binge episode, mean (SD) | 4.9 (1.1) | 4.9 (0.9) | 0.81 |
Calculated as t-test for continuous variables; chi-square for categorical variables
Figure 1. Exemplars of Patterns of Alcohol Consumption.
Three exemplars of patterns of alcohol consumption among participants who reported binge drinking
Not surprisingly, the mean reported level of GI symptoms differed between IBS patients and control women, with p-values comparing GI symptoms between the groups consistently less than 0.0001. For IBS patients, the GI symptom with the highest mean reported level was intestinal gas (mean= 1.57), indicating the mean reported level of intestinal gas was between minimal (level 1) and mild (level 2). For control women, the mean level of all GI symptoms was 0.19 (on a scale of 0 to 4).
Table 3 presents associations between patterns of alcohol intake and GI symptoms. We observed binge drinking to be strongly associated with the next day's symptoms of diarrhea (OR = 2.1 [95% CI: 1.2-3.5]), nausea (OR = 2.4 [95% CI: 1.2-4.8]), stomach pain (OR = 2.1 [95% CI: 1.2-3.7]), and indigestion (OR = 2.0 [95% CI: 1.1-3.6]). For moderate and light drinking, we observed either no association or a weaker association with next day's GI symptoms. We observed that the associations between binge drinking and GI symptoms were still significant after adjustment for the prior day's cigarette smoking or stress (including prior day's self-reported stress, coping, anxiety, depressed feelings, desire to be alone, hopeless feelings or tension). Results were unchanged when we limited the sample to women who met Rome III criteria for IBS diagnosis. We further investigated the relationship between alcohol consumption and GI symptoms in study participants without IBS. Here we did not observe a pattern of alcohol intake associated with GI symptoms. While we observed decreased odds of heartburn for moderate drinking, these results are limited by a smaller sample size.
Table 3.
Age-adjusted Associations between Pattern of Alcohol Intake and GI Symptoms in IBS Patients and Controls
| GI symptom | Prior Day's Alcohol Intakea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS patients (n = 143) | Controls (n = 43) | |||||||||||
| 1 | 2-3 | 4+ | 1 | 2-3 | 4+ | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Abdominal Pain | 0.9 | 0.7-1.1 | 1.0 | 0.7-1.3 | 1.3 | 0.8-2.2 | 0.8 | 0.3-2.1 | 0.7 | 0.2-1.8 | 0.6 | 0.2-1.5 |
| Bloating | 0.9 | 0.7-1.2 | 1.2 | 0.9-1.8 | 1.2 | 0.7-2.2 | 0.9 | 0.5-1.7 | 0.8 | 0.4-1.7 | 0.9 | 0.4-2.2 |
| Intestinal gas | 1.1 | 0.8-1.4 | 1.5 | 0.9-2.5 | 1.3 | 0.8-2.1 | 0.9 | 0.5-1.9 | 1.0 | 0.4-2.5 | 0.7 | 0.2-2.4 |
| Constipation | 1.1 | 0.8-1.5 | 1.2 | 0.8-1.6 | 1.2 | 0.6-2.1 | 1.2 | 0.6-2.4 | 1.2 | 0.5-2.8 | 0.4 | 0.1-1.3 |
| Diarrhea | 1.0 | 0.7-1.4 | 1.3 | 0.9-1.9 | 2.1 | 1.2-3.5 | 0.9 | 0.5-1.5 | 0.6 | 0.2-1.4 | 1.2 | 0.4-4.2 |
| Nausea | 0.8 | 0.6-1.0 | 1.2 | 0.9-1.7 | 2.4 | 1.2-4.8 | 0.7 | 0.3-1.6 | 0.8 | 0.3-1.7 | 0.5 | 0.1-2.4 |
| Stomach pain | 1.1 | 0.8-1.3 | 1.4 | 1.0-1.9 | 2.1 | 1.2-3.7 | 1.0 | 0.4-2.4 | 0.5 | 0.2-1.4 | 0.4 | 0.1-1.2 |
| Heartburn | 1.0 | 0.8-1.4 | 1.4 | 1.0-2.1 | 1.1 | 0.6-2.1 | 0.4 | 0.1-1.2 | 0.2 | 0.1-0.8 | 0.5 | 0.1-2.3 |
| Indigestion | 1.1 | 0.8-1.5 | 1.5 | 1.1-2.3 | 2.0 | 1.1-3.6 | 1.2 | 0.5-3.1 | 0.9 | 0.3-2.8 | 1.0 | 0.1-6.5 |
Categories of alcohol use in drinks/day; referent category of 0 alcohol-containing drinks
The data in Table 3, however, combine the within- and between-person variation and thus are difficult to interpret at the individual level. Thus, we present results of the analysis testing associations for GI symptoms with the prior day's alcohol intake separating the between- and within-person effect (Table 4). For this analysis, we collapsed alcohol intake to compare drinking 4+ drinks on one occasion (binge drinking) to <4 drinks on an occasion due to the lack of an association for light and moderate drinking. We observed between-person variation to be strongly associated with alcohol intake and specific GI symptoms, including diarrhea, nausea, stomach pain, and indigestion. However, within-person variation was not associated with GI symptoms. Additionally, the mean level of symptoms (diarrhea, nausea, stomach pain, and indigestion) differed between women who binge drank and women who didn't (p <0.001 for each symptom). When limited to women with IBS diagnosis according to Rome III criteria, the results were unchanged.
Table 4.
Age-adjusted Association between Prior Day's Alcohol Intake and GI Symptoms Presented Separately for Between- and Within-Person Variation among Women with IBS
| GI symptoms | Prior Day's Alcohol Intakea (n = 143) | |||
|---|---|---|---|---|
| Between-Person Variation | Within-Person Variation | |||
| Coefficient (SE)b | p-value | Coefficient (SE)b | p-value | |
| Abdominal Pain | 1.07 (0.62) | 0.08 | -0.07 (0.15) | 0.64 |
| Bloating | 0.57 (0.08) | 0.48 | 0.01 (0.15) | 0.99 |
| Intestinal gas | 0.76 (0.64) | 0.24 | -0.08 (0.12) | 0.53 |
| Constipation | 0.31 (0.90) | 0.73 | 0.01 (0.15) | 0.96 |
| Diarrhea | 1.46 (0.59) | 0.01 | 0.26 (0.18) | 0.16 |
| Nausea | 2.35 (0.57) | <0.001 | 0.10 (0.17) | 0.54 |
| Stomach pain | 1.98 (0.65) | 0.002 | 0.05 (0.15) | 0.76 |
| Heartburn | 0.21 (0.90) | 0.82 | -0.04 (0.15) | 0.76 |
| Indigestion | 2.16 (0.71) | 0.002 | -0.16 (0.14) | 0.25 |
Alcohol intake comparing 4+ vs. <4 drinks in a day
Coefficients are the log odds of developing the GI symptom
When IBS subtypes of IBS-C, IBS-D, and IBS–M were examined separately, we observed the strongest associations between alcohol intake and IBS symptoms for women with IBS-diarrhea. For the IBS-C group, there were no appreciable associations; for the IBS-M group, we observed some associations between alcohol intake and GI symptoms. This was the case for analysis examining binge drinking in which binge drinking was associated with a worsening of five GI symptoms (abdominal pain, intestinal gas, nausea, stomach pain, and indigestion) for the IBS-diarrhea group (Supplemental Table 1). A similar pattern emerged in the analysis examining within-persons and between-person for binge drinking within IBS subtypes (Supplemental Table 2). When examining the within- and between-person variability for control women, there was little association between GI symptoms and alcohol intake (Supplemental Table 3).
Because the biologic impacts of caffeine or cigarettes on the gut could occur within several hours (rather than on next day's GI symptoms), we tested whether the same day caffeine intake affected GI symptoms within IBS patients (Table 5). For cigarette smoking, we observed within-person associations between smoking and constipation (p = 0.001), and between-person associations between bloating and cigarette smoking. We did not observe any associations in our dataset between caffeine intake and GI symptoms. Almost all participants (95%) drank some type of caffeine beverage during the study period with a mean of 1.7 servings/day (SD 1.2). Among the 16% of people who reported smoking cigarettes, the mean number of cigarettes that women smoked/day was 3.3. The majority of smokers in our study were regular smokers but still reported slight variation in their smoking (varying the number of cigarettes by at least 1 cigarette/day on more than one occasion).
Discussion
In our study population, we observed that drinking patterns did not differ significantly between women with and without IBS. Among women with IBS, 80% reported consuming alcohol on at least one occasion. Prior studies have reported that approximately 12-17% of adults with IBS reported perceived intolerances to alcohol.6,7 In contrast, another study observed that 48% of IBS patients abstained from drinking alcohol (based on a questionnaire asking about past alcohol consumption) ; however, this higher percentage may be due to differences in definitions of alcohol intake with the latter study defining abstention as drinking less than 12 drinks per year, as well as their retrospective ascertainment of alcohol consumption.12
We observed that binge drinking was associated with GI symptoms among women with IBS, but that moderate to light drinking was not associated with GI symptoms to any appreciable degree. Our interpretation of these results is complicated by our finding that associations of alcohol consumption and GI symptoms were strongest when comparing between persons. Taken together, this would indicate that IBS patients who binge drink experience greater GI symptoms in general as indicated by the higher mean level of GI symptoms among women who reported binge drinking; however, our data also suggest that an individual's symptoms don't necessarily correlate with specific episodes of binge drinking. Thus, it may be that individuals who binge drink experience chronic changes in their gut that are associated with increases in GI symptoms. In addition, this association persisted after controlling for an individual's stress, indicating that the influence of alcohol on gut symptoms was not confounded by a person's emotional state. In further analysis, we report that the associations between alcohol intake and GI symptoms were strongest among the IBS-D group. We did not observe evidence of a role of alcohol intake on GI symptoms for women with IBS-C within our study population. Nor did we observe an association between pattern of alcohol use and GI symptoms among control women.
In the literature, it has thus far been unclear whether alcohol intake affects GI symptoms in IBS patients.1 Several reasons may exist for the lack of consistency in the published research. First, there are many study designs employed to investigate alcohol and IBS. The prior studies that have reported a link between alcohol and IBS have primarily focused on IBS patients’ perceptions of alcohol's impact on GI symptoms.6,7 On the other hand, asking participants to report past alcohol consumption (for example in case-control studies) may not be sensitive enough to examine associations between alcohol and GI symptoms in IBS patients.3-5,12 Secondly, investigating patterns of alcohol use may be required to elucidate the role of alcohol intake in GI symptoms. In two prior studies where adults were asked about weekly patterns of drinking using questionnaires asking about typical, past alcohol consumption, researchers reported no association between patterns of alcohol intake and IBS.5,12 However, to the extent that amount of alcohol consumed matters in IBS symptoms, detailed prospective assessment of daily alcohol consumption may be required.
Alcohol and its metabolites are known to affect the GI tract motility, absorption, and permeability. Alcohol decreases gastric motility in mice.13 In the small intestine, alcohol decreases the impedance wave motility (muscles that retain food for further digestion) but does not affect the propulsive wave motility (movements that propel food within the intestine) contributing to diarrhea seen in individuals who chronically consume alcohol.14,15 Alcohol has also been shown to increase intestinal permeability in both animals and humans.14,16 By measuring urine sugars, increased intestinal permeability in both the small intestine and colon has been found in patients with IBS.17 Chronic alcohol users demonstrate malabsorption of carbohydrates, fat, proteins, and xylose compared with non-alcohol abusers.14,15,18 These effects either individually or combined can worsen IBS symptoms. For example as alcohol decreases motility and absorption of carbohydrates, stasis can increase the effects of carbohydrates such as fermentable carbohydrates (FODMAPs) that can result in IBS symptoms, including abdominal pain.19,20 In addition, alcohol is also noted to have gut mucosal injury in animals21 and in chronic alcohol abusers.22 Furthermore, chronic alcohol exposure alters the microbiota reducing its variability (i.e., microbial richness),23 and this change has been found to correlate with elevated serum endotoxin levels.24 These chronic changes in response to alcohol could in part explain the greater differences we observed between persons than within a person.
Our study observed that diarrhea, stomach pain, and indigestion were strongly associated with binge drinking. Furthermore, our study reports that associations between alcohol intake and GI symptoms were strongest among the IBS-D group. Of the studies reporting on GI symptoms, several found alcohol to be associated with various GI symptoms,5,6 but not all.12 Specifically, 8% of people with IBS reported alcohol intake to be associated with loose stool/urgency.6 Another study reported that high alcohol intake, but not moderate intake, was associated with abdominal pain. These findings are in broad agreement with our study's findings regarding GI symptoms. Moreover, our study's findings are in agreement with the literature detailing the physiologic effects of alcohol on the gut. In particular, gut mucosal injury could explain the increased abdominal pain, nausea, and indigestion.
With respect to caffeine and cigarettes, both have been demonstrated to affect gut motility in normal individuals.1,25 However, their roles in IBS patients are less clear.1,26 Prior studies have reported that coffee drinking is not more common among IBS patients, but a role of caffeine in IBS symptoms is supported by studies demonstrating that reintroduction of coffee led to the return of IBS symptoms in up to a third of IBS patients.1 For cigarette smoking, results from one study indicated that half of IBS patients with predominant constipation who smoked cigarettes reported that cigarette smoking led to softer stools.26 However, any other evidence linking smoking to GI symptoms in IBS patients is lacking. With the exception of cigarettes and symptoms of constipation, our data suggest that caffeine and cigarettes play at most a modest role in GI symptoms among women with IBS.
The primary limitations of this study are as follows. First, our daily diaries do not provide a temporal sequence between alcohol, caffeine, cigarettes and GI symptoms within the day. However, we attempted to overcome this issue by investigating the role of lifestyle factors on next day's GI symptoms. Because symptoms related to alcohol are often observed the following day, this approach works well for investigations regarding alcohol. However, this approach may not be appropriate for cigarette smoking and caffeine because their effects on the gut may be of a shorter timeframe; thus, our analysis did not adequately address the temporal sequence between GI symptoms and smoking and caffeine intake. In addition, while the study participants were taught how to complete the alcohol section of the daily diary according to wine, beer, and liquor consumption, no information was collected on the type of alcohol within the daily diary. Overall, a primary strength of our study is our prospective data on daily cigarette, alcohol and caffeine intake, as well as prospective ascertainment of GI symptoms.
In summary, we observed alcohol consumption among IBS patients to be associated with GI symptoms, but this was primarily limited to those who consumed high amounts of alcohol and to women with IBS-D. Our data suggest that IBS patients, particularly women with IBS-D, who binge drink may experience an increase in GI symptoms that are not limited to their episodes of binge drinking. While our findings would require replication before conclusions are to be drawn, these findings provide early indications that the pattern of alcohol intake (i.e. binge drinking) predominantly influences GI symptoms of women with IBS.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Many IBS patients perceive that alcohol consumption triggers GI symptoms
The impact of differing patterns of alcohol intake on GI symptoms is unclear
WHAT IS NEW HERE
Binge drinking, but not light to moderate drinking impact GI symptoms among women with IBS
Alcohol's role in IBS may be limited to people with IBS-diarrhea
Binge drinking may affect GI symptoms chronically in women with IBS, while not affecting GI symptoms to same extent in control women
Acknowledgments
The authors would like to thank the study participants for their contribution to this research.
Financial support: Study funding was provided by NINR, NIH (NR04001 and NR04142), and an unrestricted educational grant from Novartis Pharmaceuticals. Kerryn W. Reding was supported by NINR grant K99NR012232. Our study design, analysis and report of our findings were independent of funding agencies.
Footnotes
Author contributions: Kerryn W. Reding took the primary role in data analysis, interpretation, and drafting the manuscript. Kevin C. Cain was involved in study design and planning, data collection, data analysis and interpretation. Monica E. Jarrett was involved in study design and planning, data collection, interpretation, and manuscript writing. Margaret D. Eugenio was involved in manuscript writing. Margaret M. Heitkemper was involved in study design and planning, data collection, data interpretation, and manuscript writing.
Guarantor of the article: Kerryn W. Reding
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. Journal of the American Dietetic Association. 2009 Jul;109(7):1204–1214. doi: 10.1016/j.jada.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Halpert A, Dalton CB, Palsson O, et al. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). The American journal of gastroenterology. 2007 Sep;102(9):1972–1982. doi: 10.1111/j.1572-0241.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Zinsmeister AR, Melton LJ., 3rd Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. American journal of epidemiology. 1995 Jul 1;142(1):76–83. doi: 10.1093/oxfordjournals.aje.a117548. [DOI] [PubMed] [Google Scholar]
- 4.Saito YA, Locke GR, 3rd, Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population-based case-control study. The American journal of gastroenterology. 2005 Dec;100(12):2743–2748. doi: 10.1111/j.1572-0241.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 5.Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Influence of alcohol consumption on IBS and dyspepsia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2006 Nov;18(11):1001–1008. doi: 10.1111/j.1365-2982.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 6.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 7.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. European journal of clinical nutrition. 2006 May;60(5):667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WG, Dotevall G, Drossman D. Irritable bowel syndrome: Guidelines for the diagnosis. Gastroenterol Int. 1989;2:92–95. al e. [Google Scholar]
- 9.Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of autonomic nervous system indices based on abdominal pain reports in women with irritable bowel syndrome. Biological research for nursing. 2000 Oct;2(2):97–106. doi: 10.1177/109980040000200203. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA: the journal of the American Medical Association. 1994 Dec 7;272(21):1672–1677. [PubMed] [Google Scholar]
- 11.Mancl LA, Leroux BG, DeRouen TA. Between-subject and within-subject statistical information in dental research. Journal of dental research. 2000 Oct;79(10):1778–1781. doi: 10.1177/00220345000790100801. [DOI] [PubMed] [Google Scholar]
- 12.Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol. 2010 May;44(3):223–228. doi: 10.1016/j.alcohol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagyanszki M, Krecsmarik M, De Winter BY, et al. Chronic alcohol consumption affects gastrointestinal motility and reduces the proportion of neuronal NOS-immunoreactive myenteric neurons in the murine jejunum. Anat Rec (Hoboken) 2010 Sep;293(9):1536–1542. doi: 10.1002/ar.21192. [DOI] [PubMed] [Google Scholar]
- 14.Bode C, Bode JC. Alcohol's role in gastrointestinal tract disorders. Alcohol health and research world. 1997;21(1):76–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Watson RR, Watzl B. Nutrition and alcohol. CRC Press; Boca Raton: 1992. [Google Scholar]
- 16.Bode JC. Alcohol and the gastrointestinal tract. Ergebnisse der inneren Medizin und Kinderheilkunde. 1980;45:1–75. doi: 10.1007/978-3-642-67632-1_1. [DOI] [PubMed] [Google Scholar]
- 17.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. American journal of physiology. Gastrointestinal and liver physiology. 2011 Nov;301(5):G919–928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer A, Schmidt T, Vidon N, Pehl C, Kaess H. Absorption of a nutrient solution in chronic alcoholics without nutrient deficiencies and liver cirrhosis. Scandinavian journal of gastroenterology. 1992 Dec;27(12):1023–1030. doi: 10.3109/00365529209028133. [DOI] [PubMed] [Google Scholar]
- 19.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. Journal of gastroenterology and hepatology. 2010 Aug;25(8):1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 20.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2011 Oct;24(5):487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 21.Ray M, Dinda PK, Beck IT. Mechanism of ethanol-induced jejunal microvascular and morphologic changes in the dog. Gastroenterology. 1989 Feb;96(2 Pt 1):345–354. doi: 10.1016/0016-5085(89)91558-8. [DOI] [PubMed] [Google Scholar]
- 22.Seitz HK, Kommerell B. Alcohol related diseases in gastroenterology. Springer-Verlag; Berlin ; New York: 1985. [Google Scholar]
- 23.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011 Nov;301(5):G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. American journal of physiology. Gastrointestinal and liver physiology. 2012 Jan 12; doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott AM, Kellow JE, Eckersley GM, Nolan JM, Jones MP. Cigarette smoking and nicotine delay postprandial mouth-cecum transit time. Digestive diseases and sciences. 1992 Oct;37(10):1544–1547. doi: 10.1007/BF01296500. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Lissner SA, Kaatz V, Brandt W, Keller J, Layer P. The perceived effect of various foods and beverages on stool consistency. European journal of gastroenterology & hepatology. 2005 Jan;17(1):109–112. doi: 10.1097/00042737-200501000-00020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.