Abstract
Irpex lacteus is a white rot basidiomycete proposed for a wide spectrum of biotechnological applications which presents an interesting, but still scarcely known, enzymatic oxidative system. Among these enzymes, the production, purification, and identification of a new dye-decolorizing peroxidase (DyP)-type enzyme, as well as its physico-chemical, spectroscopic, and catalytic properties, are described in the current work. According to its N-terminal sequence and peptide mass fingerprinting analyses, I. lacteus DyP showed high homology (>95%) with the hypothetical (not isolated or characterized) protein cpop21 from an unidentified species of the family Polyporaceae. The enzyme had a low optimal pH, was very stable to acid pH and temperature, and showed improved activity and stability at high H2O2 concentrations compared to other peroxidases. Other attractive features of I. lacteus DyP were its high catalytic efficiency oxidizing the recalcitrant anthraquinone and azo dyes assayed (kcat/Km of 1.6 × 106 s-1 M-1) and its ability to oxidize nonphenolic aromatic compounds like veratryl alcohol. In addition, the effect of this DyP during the enzymatic hydrolysis of wheat straw was checked. The results suggest that I. lacteus DyP displayed a synergistic action with cellulases during the hydrolysis of wheat straw, increasing significantly the fermentable glucose recoveries from this substrate. These data show a promising biotechnological potential for this enzyme.
INTRODUCTION
Irpex lacteus is a basidiomycete with a noteworthy biotechnological potential. This white-rot fungus has been applied in biodegradation of toxic compounds (1), dye decolorization (2), water and soil bioremediation (3), and biopretreatment of lignocellulosic substrates to improve sugar recoveries for bioethanol production (4, 5). Its efficiency in these processes has been mainly attributed to the release of a battery of ligninolytic enzymes, namely, Mn2+-oxidizing peroxidases (MnPs), lignin peroxidases (LiPs), and laccases (6). However, some reports have also described the production of another type of peroxidases, designated nonspecific (7) or Mn2+-independent (8, 9) peroxidases, which could have an important role in these processes but have not been isolated and/or characterized to date.
According to Welinder (10), heme peroxidases are classified into two superfamilies, animal and plant peroxidases, and the latter group is further divided into three categories according to origin. Class I peroxidases come from the prokaryotic lineage, and class II and III peroxidases are secreted by fungi and plants, respectively. In the fungal kingdom, MnPs, LiPs, and versatile peroxidases (VPs) are the main representatives of the group and the most studied so far (11). Nevertheless, a new family of heme peroxidases, known as dye-decolorizing peroxidase (DyP)-type enzymes (EC 1.11.1.19), has been described in recent years (12–15). These enzymes constitute an independent group of heme peroxidases and seem to offer attractive catalytic properties for biotechnological purposes (16–18).
DyPs show no sequence homology to other fungal peroxidases, presenting structural divergence from them, low sequence similarity with disparity in heme pocket residues, and singular catalytic properties, such as very low optimal pHs and high apparent affinity for substituted anthraquinones (13, 19). In addition to typical peroxidase activity, a hydrolase or oxygenase activity has been suggested for DyP (20). The physiological role of DyPs is still controversial, but it has been suggested that they are part of the catalytic system secreted by fungi for the oxidation of nonphenolic substrates, such as lignin and/or toxic aromatic products, or as a defense mechanism against oxidative stress (21). There are hundreds of putative DyP sequences in databases (22), and 222 members have been registered in PeroxiBase (http://peroxibase.toulouse.inra.fr/). However, only eight proteins have been characterized so far, and six belong to fungal DyPs (21). On the basis of sequence homologies and phylogenetic and tertiary structure analyses, a peroxidase from Termitomyces albuminosus and one hypothetical peroxidase (cpop21) from an unidentified Polyporaceae species have also been included in this group (12, 13).
In the current work, a novel DyP from I. lacteus, related to the putative cpop21 enzyme, has been isolated and characterized. In addition, the catalytic properties of the enzyme, as well as its application as a supplement for the enzymatic hydrolysis of wheat straw, are discussed.
MATERIALS AND METHODS
Microorganism, culture conditions, and enzyme production.
The basidiomycete I. lacteus, deposited in Centro de Investigaciones Biológicas (Madrid, Spain) as IJFM A792 (CCBAS 238 617/93), was maintained on 2% (wt/vol) malt extract agar at 4°C and precultured on the same medium at 28°C. After 1 week, four 1-cm2 agar plugs were excised and used to inoculate 250-ml flasks with 30 ml of a corn steep solids-based (CSS) medium at 28°C and 180 rpm (4). The growth medium contained corn steep solids (26.3 g), glucose (40 g), FeSO4 · 7H2O (0.4 g), (NH4)2SO4 (9 g), KH2PO4 (4 g), and CaCO3 (7 g). After 7 days, the cultures were homogenized (Omnimixer, Sorvall), and 2.5 ml was transferred to 250-ml flasks containing 30 ml of the same medium and incubated under identical conditions. Samples were taken periodically to follow enzyme secretion, as described below, and the remaining glucose was measured with a Glucose-TR kit (Spinreact).
Enzymatic assays.
Mn-oxidizing peroxidase (MnP) activity was measured by the formation of Mn+3-tartrate complex (ε238 = 6,500 cm-1 M-1) during the oxidation of 0.1 mM MnSO4 in 100 mM tartrate buffer at pH 5. DyP-like activity was followed by the oxidation of 2.5 mM 2,2′-azino-bis(3-ethylthiazoline-6-sulfonate) (ABTS) to its cation radical (ε418 = 36,000 cm-1 M-1) in 100 mM tartrate buffer at pH 5 (standard conditions) and the decolorization of an anthraquinone dye, 50 μM Reactive Blue 19 (RBlue19) (ε595 = 10,000 cm-1 M-1), in the same buffer at pH 4. In all cases, peroxidase activity assays were carried out in the presence of 0.1 mM H2O2. Laccase activity was also determined following ABTS and RBlue19 oxidation in the absence of H2O2.
An additional battery of substrates was used to check the effect of pH on I. lacteus DyP activity and to study its kinetic parameters. The selected substrates were 2,6-dimethoxyphenol (DMP) and veratryl alcohol (VA) as representatives of phenolic and nonphenolic compounds, respectively, and Reactive Black 5 (RBlack5) as an azo dye. Enzyme activities were followed by the increase in absorbance at 469 nm for DMP (ε469 = 27,500 cm-1 M-1) and 310 nm for VA (ε310 = 9,300 cm-1 M-1) and by the decrease of absorbance at 598 nm for RBlack5 (ε598 = 30,000 cm-1 M-1). These reactions were performed in the presence of 0.1 mM H2O2.
All measurements were carried out at room temperature. Control treatments without H2O2 and/or without enzyme were performed. One unit (1 U) of activity is defined as the amount of enzyme releasing 1 μmol of product per minute under the defined reaction conditions.
Enzyme purification.
When maximal DyP-like activity was detected, cultures were harvested and filtered to separate the mycelium. Then the culture broth was vacuum filtered through 0.22-μm-pore-size membranes (Millipore Corp.), concentrated 30-fold, and dialyzed against 10 mM sodium tartrate buffer (pH 5) under continuous stirring at 4°C in a tangential ultrafiltration system (Amicon, Millipore Corp.) using a 10-kDa-cutoff membrane. This enzymatic crude extract was used for further purification studies and enzymatic hydrolysis assays of wheat straw.
The enzyme was purified using an ÄKTA high-performance liquid chromatography (HPLC) system (GE Healthcare), using columns from the same provider, in three consecutive steps. The eluted fractions were monitored at 280 nm and 410 nm to detect total and heme-containing proteins, respectively. In anion exchange chromatography, columns were equilibrated with 10 mM sodium phosphate buffer at pH 7. The first separation step was performed on a HiTrap Q Fast Flow (FF) 5-ml cartridge at a flow rate of 1 ml min-1. After 12 ml, the retained proteins were eluted with a linear NaCl gradient from 0 to 20% in 50 ml and then from 20 to 100% in 13 ml; 100% NaCl was maintained for 11 ml. Peroxidase activity was measured by ABTS and Mn2+ oxidation under standard conditions, and the appropriate fractions were pooled, concentrated, dialyzed against 10 mM sodium phosphate buffer at pH 7, and loaded into a Mono-Q high-resolution (HR) 5/5 column at a flow rate of 0.8 ml min-1. A NaCl lineal gradient from 0 to 16% in 33 ml was applied, rising to 100% NaCl in 3 ml. Fractions with DyP-like activity were again pooled and concentrated, and the mixture of proteins was separated by size exclusion chromatography (SEC) in a Superdex-75 HR 10/30 column with 10 mM sodium phosphate buffer (pH 7) plus 150 mM NaCl at 0.3 ml min-1. The purified DyP was dialyzed against 10 mM sodium tartrate buffer at pH 5. The homogeneity of the protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% gels stained with Coomassie brilliant blue R-250 (Sigma). The purification yield was calculated from total protein quantification in a NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific) taking into account the peroxidase activity against ABTS.
Enzyme characterization.
The molecular mass of I. lacteus DyP was estimated by SDS-PAGE (12% polyacrylamide gel) by using prestained standard proteins (Bio-Rad) and confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Autoflex III, Bruker Daltonics), calibrated with protein calibration standard II from Bruker. The isoelectric point (pI) of the desalted protein was determined by isoelectrofocusing (IEF) in gels with 5% polyacrylamide and a mixture of Ampholines to obtain a pH range of 2.5 to 5 (mixing 85% from pH 2.5 to 5 and 15% from pH 3 to 10 (GE Healthcare), with 1 M H3PO4, and 1 M NaOH in anode and cathode, respectively. After IEF, the pH values were directly measured from the gel to obtain a pI calibration line. Proteins were stained with Coomassie brilliant blue R-250 and, for peroxidase activity (zymogram), with ABTS and H2O2 under standard conditions after the gels were washed for 10 min with 100 mM sodium tartrate buffer at pH 5.
To determine its glycosylation degree, the protein was deglycosylated with endoglycosidase H (Roche) in 10 mM tartrate sodium buffer (pH 5) at 37°C overnight. The molecular mass and pI of the deglycosylated DyP were examined as previously described for the native enzyme. The amount of N-linked carbohydrates was determined by the difference in the molecular masses of the native and the deglycosylated proteins obtained by MALDI-TOF and is expressed as weight percentage.
The UV-visible spectrum of the enzyme at resting state, in 10 mM sodium tartrate buffer, pH 5, was recorded in the range from 800 to 300 nm (UV 160 Spectrophotometer; Shimadzu).
Effect of pH and temperature on I. lacteus DyP activity and stability.
To study the effect of pH on enzyme activity, the substrates ABTS (2.5 mM), DMP (2.5 mM), VA (10 mM), RBlue19 (50 μM), and RBlack5 (25 μM) were prepared in Sorensen's glycine buffer (100 mM) from pH 1.5 to 2 and in Britton-Robinson buffer (100 mM) from pH 2 to 9. The percentage of oxidation or decolorization for each substrate was calculated considering the pH of maximum activity as 100%.
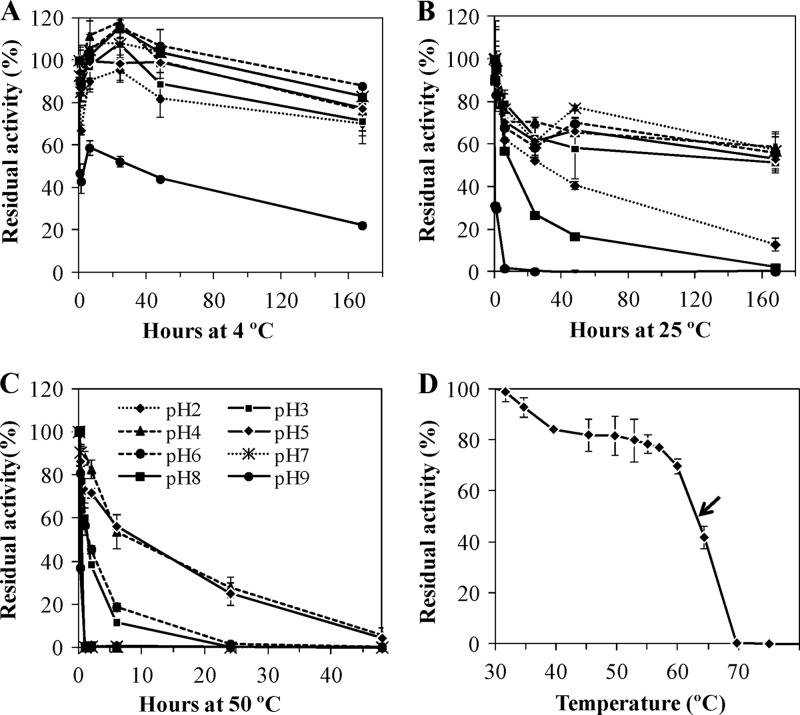
To determine the effect of pH and temperature on DyP stability, the enzyme was incubated in 100 mM Britton-Robinson buffer from pH 2 to 9 at 4, 25, and 50°C. Samples were withdrawn at 0, 0.25, 1, 6, 24, 48, and 168 h to calculate residual activity by using the standard ABTS assay at pH 3 described above. The initial activity of the enzyme was taken as 100%.
The temperature at which 50% of activity was lost in a 10-min incubation (T50 value) was also checked. To determine this thermostability index, the protein was incubated in 10 mM sodium tartrate buffer (pH 5) at various temperatures from 25 to 80°C for 10 min, cooled on ice, and rewarmed to room temperature for 5 min prior to residual activity determination by the standard assay at pH 3. The temperature at which the enzyme retained the maximum residual activity was taken as 100%.
Enzyme kinetics.
The Michaelis-Menten and catalytic constants (Km, kcat, and kcat/Km) were determined by incubating the enzyme (∼1.5 nM) with various concentrations of (i) ABTS, DMP, VA, RBlue19, and RBlack5 with a constant H2O2 concentration (0.1 mM) and (ii) H2O2 in the presence of a constant ABTS concentration (2.5 mM). The values for the kinetic parameters and their correspondent errors were calculated with Sigma Plot, version 12. The kinetics and the specificity of I. lacteus DyP toward each of these substrates were measured at the optimum pH of the enzyme: ABTS, DMP, and RBlack5 substrates were prepared in 100 mM sodium tartrate buffer at pH 3, RBlue19 was prepared in the same buffer at pH 4, and VA was prepared in 100 mM Britton-Robinson buffer at pH 2.
Enzyme activation/inactivation assays.
All of the following DyP activity measurements were performed under standard assay conditions at pH 3 with some modifications. First, enzyme inactivation by its own cosusbstrate (H2O2) at concentrations from 50 mM to 0.3 μM (2-fold serial dilutions) was tested at short reaction times (1 min) in the presence of 2.5 mM ABTS. The enzyme concentration used for this assay was 1.5 nM.
DyP inactivation was also followed over time using different [H2O2]/[enzyme] molar ratios, maintaining a constant enzyme concentration (50 nM) and using various H2O2 concentrations from 12.8 mM to 6.25 μM in 10 mM tartrate buffer, pH 5, at 25°C in the absence of reducing substrate. Samples were taken at different times, and residual activity was measured.
Additionally, standard assays at pH 3 were carried out in the presence and absence of two different concentrations (0.2 and 2 mM) of different cations (Mn2+, Co2+, Ni2+, Cu2+, Fe3+, Ca2+, Mg2+, K+, Li+, Ba2+, Zn2+, Pb2+, Hg2+, and Ag1+), EDTA, and N3Na. DyP was incubated with these compounds for 10 min prior to the activity measurement.
N-terminal sequence and peptide mass fingerprinting analyses.
The N-terminal amino acid sequence was analyzed by sequential Edman degradation in a Procise 494 protein sequencer (PerkinElmer). Homology searches were performed using the BLAST program from the National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) database.
For peptide mass fingerprinting, the purified protein was analyzed by SDS-PAGE in a 7.5% polyacrylamide gel and stained with SYPRO Ruby (Bio-Rad). The band was excised and subjected to tryptic in-gel digestion in a DigestPro MS digestor (Intavis AG). MS analyses of the tryptic peptides were performed in an Autoflex III MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) controlled by flexControl, version 3.0, software (Bruker Daltonics). Three of the tryptic peptides were chosen to carry out fragmentation and sequencing. MALDI-MS and tandem MS (MS/MS) data were combined through the BioTools, version 3.0, program (Bruker Daltonics) to search against the nonredundant protein database from the NCBI using the MASCOT, version 2.3, search engine (Matrix Science). Scores greater than 86 were considered significant (P < 0.05).
Enzymatic hydrolysis of wheat straw.
The effectiveness of different enzyme mixtures in wheat straw hydrolysis was checked by measuring the cellulose and hemicellulose digestibility yields. Prior to the enzymatic hydrolysis, wheat straw was either left untreated or subjected to biopretreatment with I. lacteus for 21 days under basal conditions, either with or without a mild alkali treatment (0.1% sodium hydroxide), as previously reported (9). Hydrolysis of the different substrates was performed in duplicate at 5% (wt/vol) in 100 mM sodium citrate buffer (pH 4.8) at 50°C and 165 rpm for 60 h using the following enzymatic mixtures: (i) commercial cellulases, such as Celluclast (15 U of global cellulase) and NS50010 (15 U of β-glucosidase), and xylanases, such as NS50013 (30 U of global xylanase) and NS50030 (30 U of β-xylosidase), all provided by Novozymes; (ii) the previous commercial cocktails plus the enzymatic crude extract of I. lacteus containing 30 U of DyP; (iii) only the enzymatic crude extract of I. lacteus (30 U of DyP); and (iv) the previous commercial cocktails plus the purified DyP (30 U of DyP which corresponded to 150 μg of protein). Global cellulase activity was determined as filter paper units, β-glucosidase against p-nitrophenyl glucopyranoside, global xylanases against birch xylan, β-xylosidase against p-nitrophenyl xylopyranoside, and DyP against RBlue19, in the presence of 0.1 mM H2O2 in sodium tartrate buffer, pH 4. Units of commercial cellulases and xylanases are measured per gram of cellulose and xylan, respectively, and DyP units are measured per gram of wheat straw. The residual activity of DyP was also checked at the end of enzymatic hydrolysis. After the stability of the enzyme against H2O2 was proved, an aliquot of 0.8 mM H2O2 was added to all samples at 0, 24, and 48 h. Controls consisted of substrates plus buffer without any enzyme supplementation and were incubated under the same conditions. Substrate characterization (percentage of cellulose and hemicellulose before and after biopretreatment), fermentable sugar release quantification, and digestibility estimations were performed as detailed by Salvachúa et al. (9).
RESULTS
Production and purification.
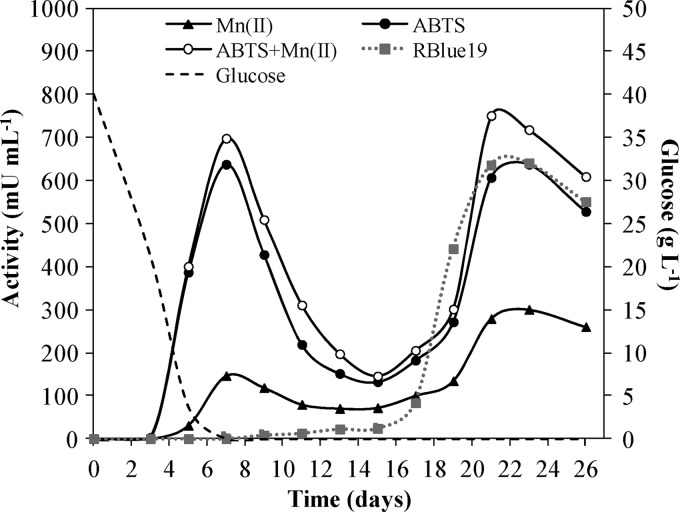
The secretion of peroxidases by I. lacteus, detected by H2O2-dependent oxidation of ABTS (to colored cation radical) and RBlue19 decolorization, started at days 4 and 12, respectively. A first activity peak against Mn2+ and ABTS, which corresponded to MnP, was detected at 7 days. This activity decreased over time, but a second MnP peak appeared at 21 days, coinciding with maximum activity against RBlue19 (Fig. 1). Laccase activity was not detected during the whole growing period.
Fig 1.
Peroxidase activities and residual glucose in I. lacteus cultures incubated in CSS medium for 26 days. The oxidation of Mn2+, ABTS, and ABTS plus 0.1 mM Mn2+ were followed at pH 5, and the oxidation of Reactive Blue 19 was followed at pH 4. Standard deviations from triplicate cultures were less than 10%.
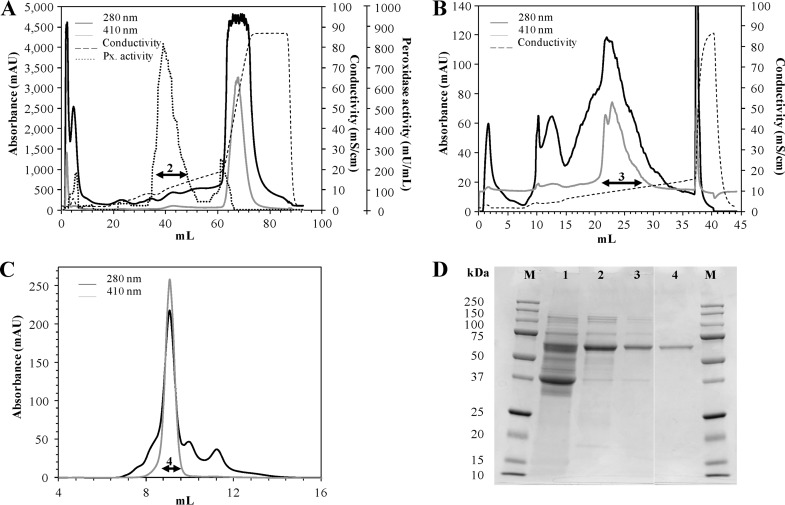
After the first purification chromatographic step (Table 1), DyP was fully separated from the other peroxidases released to the medium. The chromatogram profile showed three peaks at 410 nm (Fig. 2A), with relative ABTS activity representing 6%, 80%, and 14% of total activity, respectively. The second fraction, eluting at a NaCl concentration of 0.1 mM, was the only one without MnP activity and was selected to continue the purification process. After three chromatographic steps (Fig. 2A, B, and C), an electrophoretically homogeneous enzyme preparation was achieved (Fig. 2D), reaching a 32-fold purification factor with a yield of 18.6% (Table 1).
Table 1.
Purification of DyP from the enzymatic crude extract of I. lacteus growing in CSS liquid medium
| Purification step | Activity (U)a | Protein (mg) | Specific activity (U mg-1) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Culture filtrate | 117.0b | 9.69 | 1.2 | 100.0 | 1.0 |
| HiTrap-Q column | 90.7 | 1.39 | 6.5 | 77.5 | 5.7 |
| Mono-Q column | 45.0 | 0.22 | 13.8 | 49.6 | 12.0 |
| Superdex-75 column | 8.4 | 0.05 | 37.8 | 18.6 | 32.3 |
Activity was measured with 2.5 mM ABTS in 100 mM sodium tartrate buffer (pH 5) and 0.1 mM H2O2.
This value could be overestimated due to the presence of ABTS-oxidizing MnP in the I. lacteus culture.
Fig 2.
Purification and SDS-PAGE of DyP from the enzymatic crude extract of I. lacteus. Data are from anion exchange chromatography in HiTrap-Q FF (A) and Mono-Q (B) columns and SEC in a Superdex-75 column (C). Each collected fraction, used for the subsequent chromatographic step, is indicated with an arrow. (D) SDS-PAGE (12%) gel from the culture filtrate (lane 1) and the fractions collected after each purification step (lanes 2, 3, and 4). Lane M, molecular mass markers. AU, arbitrary units.
Characterization of I. lacteus DyP.
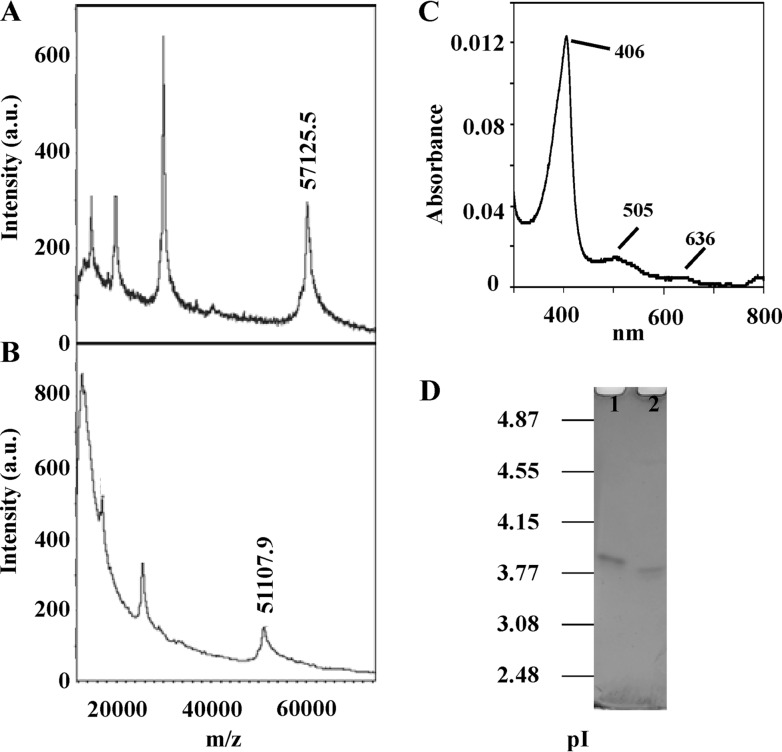
The exact molecular masses of the purified native enzyme in two forms, glycosylated and N deglycosylated, were 57,125 and 51,108 Da, respectively, as determined by MALDI-TOF (Fig. 3A and B). These data indicate that DyP contains about 10.5% of N-linked carbohydrate. Concerning pI, both forms of the enzyme appeared as single bands in IEF, with pI values of 3.85 and 3.75, respectively (Fig. 3D). DyP zymograms revealed that the deglycosylated protein was fully active (data not shown).
Fig 3.
Characterization of I. lacteus DyP. Molecular mass estimation of the purified DyP by MALDI-TOF of the glycosylated (A) and deglycosylated (B) forms. (C) Spectrum from 300 to 800 nm. (D) IEF gel from pI 2.5 to 5 of the glycosylated (lane 1) and deglycosylated (lane 2) DyPs, stained with Coomassie blue R-250.
The UV-visible spectrum of DyP at resting state showed the main Soret band at 406 nm and two charge transfer bands at 505 and 636 nm (Fig. 3C), as well as a Reinheitszahl ratio (A406/A280) of 1.59. The molar absorption coefficient of I. lacteus DyP, ε406, was 140,527 M-1 cm-1, similar to values found for other peroxidases and DyPs (23).
Effect of pH and temperature on I. lacteus DyP activity and stability.
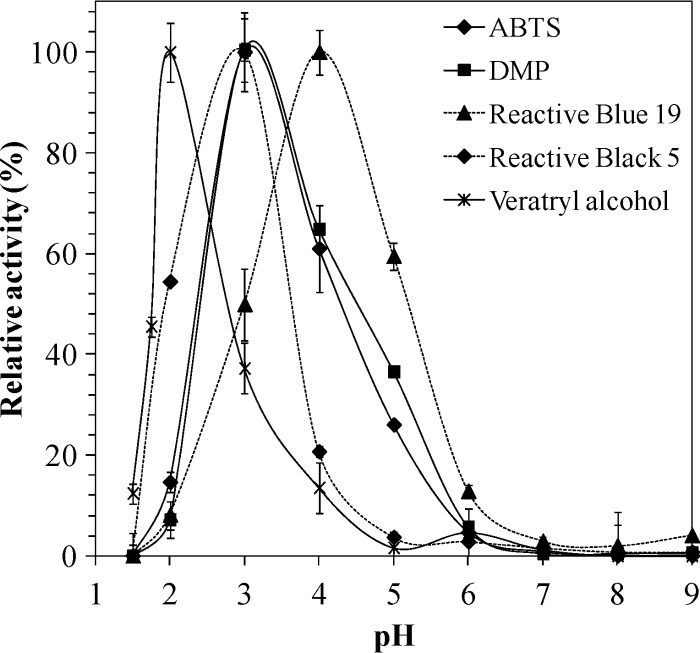
The effect of pH on DyP activity was initially examined with different substrates at pHs ranging from 1.5 to 9. As shown in Fig. 4, DyP was active in a pH range between 1.5 and 6 although relative activities and optimum pHs differed among the tested substrates. VA oxidation was optimal at pH 2, but the residual activity decreased to 40% at pHs 1.75 and 3. In contrast, the highest optimal pH was observed at pH 4 for RBlue19 decolorization. The oxidation of ABTS and DMP showed similar pH profiles, with pH 3 as the optimum value and 20% residual activity at pH 2. The optimum pH for RBlack5 oxidation was also pH 3, but in this case the enzyme retained 60% of residual activity at pH 2.
Fig 4.
Optimum pH of the purified I. lacteus DyP oxidizing different substrates.
Additionally, the stability of I. lacteus DyP was checked at three different temperatures and a pH range between 2 and 9. When the enzyme was maintained at 4°C for 1 week, the residual activity was higher than 70% at all pHs tested excluding pH 9, where the activity loss was around 80% (Fig. 5A). After a 1-week incubation at 25°C, the enzyme was also quite stable, with residual activities greater than 50% at pH values between 3 and 7 (Fig. 5B) and even retaining 15% activity at pH 2. In view of this result, some activity loss during the purification process (at pH 7 and 25°C) may occur during the first hours, but after 24 h that loss is insignificant. At 50°C, DyP displayed the highest activity at pHs 4 and 5 (Fig. 5C), with a half-life (50% of the activity) of 8 h and keeping some activity after 48 h of incubation. When the pH values were set at 3 and 6, the half-life of DyP was around 2 h. Regarding the T50 index, the calculated value was 63°C at pH 5 (Fig. 5D). The maximum activities, taken as 100%, were detected at 25 and 30°C.
Fig 5.
Thermostability of the purified I. lacteus DyP at 4°C (A), 24°C (B), and 50°C (C) at different pHs and T50 estimation at pH 5 (D).
Catalytic properties.
Diverse substrates were oxidized by DyP in the presence of H2O2, and the kinetic parameters of the enzyme were calculated for each of them, as summarized in Table 2. The highest activity was found for ABTS (kcat = 224 s-1), followed by similar values with DMP and RBlue19 (kcat = 70 s-1). The enzyme showed the lowest activities for VA and RBlack5. The Michaelis constant (Km) for dyes was lower than values for DMP and ABTS, and the catalytic efficiency (kcat/Km) was significantly greater for the anthraquinone-type dye RBlue19 (5.9 × 106 s-1 M-1); it was even higher for the synthetic substrate ABTS (7.97 × 106 s-1 M-1). The highest Km (>3,600 μM) was found toward VA. The apparent affinity for H2O2 was lower than that reported for other DyPs (17, 24).
Table 2.
Kinetic parameters of I. lacteus DyP for several substrates and pH values at which they were evaluated
| Substratea | pH | Km (μM) | kcat (s-1) | kcat/Km (s-1 M-1) |
|---|---|---|---|---|
| ABTS | 3 | 28.0 ± 2.6 | 224.0 ± 4.0 | (8.0 ± 0.7) × 106 |
| DMP | 3 | 72.6 ± 9.5 | 70.9 ± 2.1 | (9.7 ± 0.1) × 105 |
| Veratryl alcohol | 2 | 3610.0 ± 211.0 | 2.70 ± 0.1 | (8.3 ± 0.0) × 102 |
| Reactive Blue 19 | 4 | 13.5 ± 1.6 | 79.9 ± 3.2 | (5.9 ± 0.5) × 106 |
| Reactive Black 5 | 3 | 11.2 ± 0.9 | 11.9 ± 0.4 | (1.1 ± 0.05) × 106 |
| H2O2b | 3 | 79.5 ± 11.7 | 419.0 ± 18.8 | (5.3 ± 0.6) × 106 |
Unless noted otherwise, substrate oxidation was measured using 0.1 mM H2O2.
Activity was measured with 2.5 mM ABTS.
Inactivation of I. lacteus DyP.
First, inactivation of the enzyme by its own cosubstrate, H2O2, was checked at a 1-min reaction time in the presence of ABTS. The maximum relative activity was reached at final H2O2 concentrations between 0.4 and 0.8 mM, corresponding to [H2O2]/[DyP] molar ratios of 250,000 and 500,000, respectively, and then gradually dropped off (data not shown). A residual activity of 30% still remained at 12.5 mM H2O2, and the enzyme was completely inactivated in the presence of 50 mM H2O2.
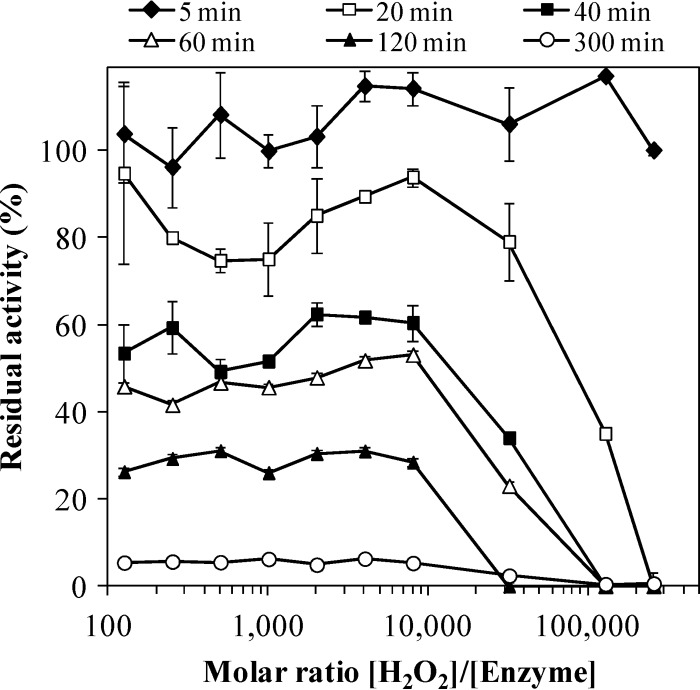
DyP inactivation by H2O2, in the absence of reducing substrate, was also assayed at different times and concentrations (Fig. 6). When a [H2O2]/[DyP] molar ratio of 250,000 was used, the enzyme was completely inactivated in 20 min of incubation. In contrast, a residual activity of 40% was observed in the treatment with a molar ratio of 128,000. DyP was inactivated progressively and similarly over time between molar ratios of 128 and 8,000 in 5 h of incubation.
Fig 6.
H2O2 stability of the purified I. lacteus DyP at different times. DyP (50 nM) was incubated at 25°C in 10 mM tartrate buffer (pH 5) containing different H2O2 concentrations (from 6.25 μM to 12.8 mM). The residual activity (measured with ABTS) was determined relative to DyP activity incubated in the absence of H2O2 (100%). Error bars are means of duplicate treatments.
The effect of two concentrations of several cations on DyP activity was also assayed, and all of them activated the enzyme (data not shown). In most cases, a residual activity higher than 60% was retained, but 80% of the enzyme's activity was lost in reactions with Fe3+ and Hg2+, and enzyme activity disappeared completely in the presence of 2 mM Pb2+, as well as 2 mM NaN3. The addition of 0.2 mM EDTA did not inhibit the enzyme, and a 2 mM concentration of the same reagent caused only slight inactivation, which suggests that there are no essential ions in the reaction mixture for the enzyme activity.
N-terminal sequencing and peptide mass fingerprinting of I. lacteus DyP.
The N-terminal region of the purified enzyme was sequenced (Table 3). Data bank homology searches (NCBI) returned the hypothetical peroxidase cpop21 from an unidentified species in the family Polyporaceae as the best hit (>95% identity), followed by the DyP from T. albuminosus (>85% identity). Lower identities and/or shorter alignments were found with the DyP from Bjerkandera adusta strain Dec 1, formerly classified as Thanatephorus cucumeris (25), and the Auricularia delicata DyP.
Table 3.
Alignment of I. lacteus DyP N-terminal sequence with other DyP sequences returned by NCBI with significant scoresa
| Microorganism | Accession no. | N-terminal sequenceb | Identity (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. lacteus | S | A | G | X | D | S | — | L | P | F | E | N | I | Q | G | D | I | L | V | G | M | ||
| Unidentified Polyporaceae speciesc | AAB58908 | S | A | G | N | D | S | — | L | P | F | E | N | I | Q | G | D | I | L | V | G | M | >95 |
| T. albuminosusd | AAM21606 | D | D | S | I | — | P | F | E | N | I | Q | A | D | I | L | V | G | M | >70 | |||
| B. adustad | BAA77283 | A | — | N | D | T | I | L | P | L | N | N | I | Q | G | D | I | L | V | G | M | >70 | |
| A. delicatad | EJD38892 | A | A | N | D | A | A | L | P | F | N | D | I | Q | G | D | I | L | A | G | M | >65 | |
A significant score is an E value of <0.05.
Conserved residues are in boldface, and missing residues are represented by dashes. The letter X indicates an unidentified residue.
The hypothetical protein sequence includes part of the signal peptide.
The amino-terminal sequence corresponds to that of the mature protein.
The combined search of the protein fingerprints and the MS/MS spectra of three of the tryptic peptides (selected for fragmentation and sequencing) rendered a maximum score of 332 for the 499-amino-acid hypothetical peroxidase cpop21 (accession numbers AAB58908 [NCBI], U77073 [GenBank], and 5097 [Peroxibase]). Table 4 shows the peptides from DyP which match cpop21.
Table 4.
Matching peptides with cpop21 after DyP trypsin digestion and PMF analysis
| Peptide sequencea | Observed m/z | Expected m/z | cpop21 position (aa)b |
|---|---|---|---|
| QTFGLDPR | 933.48 | 932.47 | 458–465 |
| CPFTAHVR | 987.49 | 986.48 | 359–366 |
| QLVPEFHK | 997.55 | 996.54 | 278–285 |
| SEPLGLDPVIGQGTR* | 1538.83 | 1537.82 | 443–457 |
| NNDFNYIHPGEDLTTDETR* | 2250.99 | 2249.99 | 340–358 |
| GTNVDGVFLIGSDDVTTTNQYR* | 2372.15 | 2371.14 | 167–188 |
Peptides chosen for fragmentation and sequencing are marked with an asterisk.
Starting and ending amino acid (aa) positions.
Enzymatic hydrolysis of wheat straw.
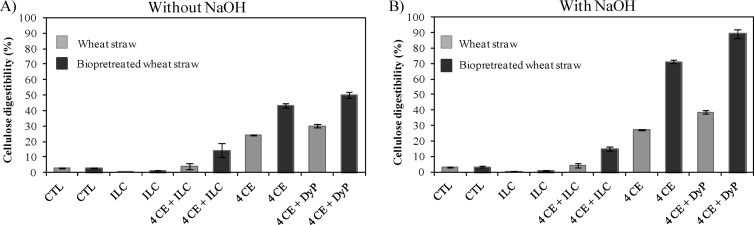
Untreated wheat straw is composed of cellulose (37%), hemicellulose (23%), and lignin (24%). After 21 days of treatment with I. lacteus, the above amounts decreased by 8%, 3%, and 9%, respectively, while a mild alkali treatment did not change sugar percentages significantly. The enzymatic hydrolysis of biopretreated wheat straw showed enhanced yields with respect to the nonbiopretreated controls (Fig. 7). In addition, the mild alkali washing also produced a considerable increase in cellulose digestibility, with a more remarkable effect in the biopretreated wheat straw. As expected, the addition of commercial cellulase and xylanase formulations strongly improved the free sugar content with respect to the control. In contrast, the hydrolysis of the lignocellulosic material using the enzymatic crude extract from I. lacteus either as the unique enzyme source or as a supplement of the commercial cocktails dropped drastically. Nevertheless, supplementation with purified DyP significantly improved cellulose digestibility. In nonbiopretreated wheat straw, with and without alkali washing, this value rose from 27 to 38% and from 24 to 30%, respectively, but the effect was more evident on biopretreated wheat straw, with a rise from 71 to 89% in NaOH-treated samples and from 43 to 50% in those not subjected to alkali pretreatment. A parallel decrease in hemicellulose digestibility was observed in some cases (data not shown). Furthermore, the residual DyP activity at the end of the treatment (60 h) was around 5 to 10%, which means that the peroxidase continued to be active.
Fig 7.
Cellulose digestibility from wheat straw without (A) and with (B) NaOH pretreatment and with or without biological pretreatment by I. lacteus for 21 days. Diverse hydrolysis treatments were applied using four enzymatic commercial cocktails (4 CE), the enzymatic crude extract of I. lacteus (ILC), and the purified peroxidase from I. lacteus (DyP). Substrates without any enzymatic supplementation were incubated under the same conditions (CTL).
DISCUSSION
I. lacteus and its extracellular oxidative enzymatic system offer a great potential for different biotechnological applications. The detection of Mn-independent peroxidase in I. lacteus growing on wheat straw has been recently described (9). With the aim of producing and purifying it, peroxidase production was followed in a liquid medium (CSS) known to produce high I. lacteus biomass in comparison with other media (9). According to the peroxidase production profile found in the present study, it can be suggested that the fungus starts producing MnPs when glucose is almost consumed. These MnPs, apparently showing both Mn-dependent and -independent activities, would belong to a recently described subfamily of short MnPs (22, 26) whose components, in addition to primary activity on Mn2+, are able to oxidize phenols and ABTS in the absence of Mn2+, as described in Pleurotus ostreatus (27) and Agrocybe praecox (28). I. lacteus reduced peroxidase production until the third week of incubation, when MnP production increased again, together with a peak of DyP, which was able to oxidize high-redox-potential substrates such as anthraquinones. DyP appeared when the carbon/nitrogen ratio in the cultures was very low, which is in accordance with the production profile reported by Cajthaml et al. (8) in I. lacteus liquid cultures spiked with polycyclic aromatic hydrocarbons (PAHs). Laccase was not detected, which suggests that both DyP and MnP are the main oxidative enzymes secreted by I. lacteus under these culture conditions. At the time of culture harvesting, the major peroxidase in the enzymatic crude extract of I. lacteus was a DyP-like peroxidase, as determined after the first chromatographic step, where 80% of the total peroxidase activity against ABTS was found. The above data suggest that the production of DyP by I. lacteus under different environmental or experimental conditions should be studied by using specific substrates to get a deeper understanding of the presence of this enzyme and to avoid imprecise enzyme identifications as Mn-independent peroxidases.
In order to confirm the I. lacteus peroxidase identification as a member of the DyP superfamily, comparison of its N-terminal sequence and peptide mass fingerprint with those from other proteins was performed. Both analyses returned, with maximal identity and score, the hypothetical cpop21 peroxidase from Polyporaceae which belongs to the DyP-type peroxidase family. The N terminus of I. lacteus DyP matched the amino acidic sequence starting at position 53 of cpop21, suggesting that an ∼50-amino-acid signal peptide exists in the latter hypothetical protein, as found in T. albuminosus DyP (29). The fourth amino acid was not identified by Edman degradation. One of the reasons to explain this fact could be the existence of an N-glycosylated asparagine residue hindering its identification. Taking into account that the sixth position is occupied by a serine residue, it suggests the existence of a consensus sequence for N-glycosylation (N-X-S/T) (30, 31) which in addition coincides with the sequence in analogous positions 56 to 58 (N-D-S) in cpop21.
Concerning the physical properties of the purified enzyme, SDS-PAGE disclosed that, similar to most fungal DyPs, I. lacteus DyP is a monomeric protein, while most bacterial DyPs form oligomeric species (32). The molecular mass was 57.1 kDa, higher than that reported for all LiPs and MnPs from I. lacteus (6) but similar to DyPs from other microorganisms (19, 21, 23). The protein contained around 10.5% of N-linked carbohydrate chains, lower than the 17% found in the native DyP from B. adusta (23). Independent of whether I. lacteus DyP is glycosylated, the protein's pI was acidic, a usual feature among fungal peroxidases (33) and DyP-like peroxidases whose pI range is between 3.7 and 4.3. The A406/A280 ratio (1.59) and absorption peaks in the UV-visible spectrum of the heme protein were similar to those found for other native DyPs (13, 23, 32).
On the basis of its substrate specificity and catalytic properties, I. lacteus DyP can be considered a high-redox-potential enzyme and can be classified into the DyP-like peroxidase family (12). As previously mentioned, this DyP did not oxidize Mn2+ and exhibited higher decolorizing activity and catalytic efficiency toward anthraquinone than azo dyes although the Kms were similar. It has been reported that hydroxyl-free anthraquinones are not usually a substrate for non-DyP-type peroxidases, and if they are, the oxidation is very low (12, 21). Furthermore, the specificity and catalytic efficiency for RBlack5 were superior to the values reported for other DyPs (21), other high-redox-potential enzymes such as VP (34), or the classical horseradish peroxidase (HRP) (32). The activity against the phenolic compound DMP reached higher values than those reported for other phenol peroxidases, such as soybean peroxidase (21), although the catalytic efficiency for this substrate was poorer than that of the dyes. I. lacteus DyP was also able to oxidize VA (23) although the Km for this substrate was quite high, in the range found for other DyPs (17) and VP (35) (although the latter enzyme has much higher kcat). The Km for H2O2 in the presence of ABTS (the substrate for which the highest activity and catalytic efficiency were obtained) was in the magnitude of values for other fungal and plant peroxidases (with Km values from 5 μM in Auricularia auricula-judae DyP to 152 μM in Coprinopsis cinerea peroxidase) (21). The catalytic efficiency for H2O2 was slightly lower than that reported in other DyPs but superior to values found in HRP and LiP from Phanerochaete chrysosporium (21). The maximum activities with the cosubstrate H2O2 were found at concentrations between 0.4 to 0.8 mM, which suggests that an increase of this cosubstrate's concentration (from 0.1 mM to 0.4 to 0.8 mM) should be used to measure enzyme activity in future work.
The optimal pHs for the enzyme activity were acidic specially for VA, which was better oxidized at pH 2, a value slightly higher than that reported for the isoforms of A. auricula-judae (21). In contrast, the optimum pH oxidizing DMP was more acid than pHs found for other DyPs but similar to the pH for VP from P. eryngii (35) and for VP and LiP from B. adusta and P. chrysosporium (21). The optimal pH for RBlue19 was one point higher than that for the other substrates, including the azo dye RBlack5. Similar pH variations between dyes were observed with the bacterial DyP from Anabaena sp. (32).
Taking into consideration its feasible biotechnological applications, the stability of I. lacteus DyP was evaluated at different pHs and temperatures. The enzyme was shown to be very stable at 4°C and 25°C in a wide range of pHs, excluding those more basic (8 and 9). In fact, at pH 2 and 25°C, the enzyme still retained 62% of activity after 6 h, similar to that found in the isoform AjII of A. auricula-judae after 4 h at 20°C and pH 2.5 (17). The ability to work efficiently under such low pH environments is a characteristic that distinguishes DyPs from most peroxidases (12). This enzyme was also shown to be active at pH 4 and pH 5 for 48 h at 50°C, which is required for its use in enzymatic hydrolysis of lignocellulosic substrates. It was more stable than AjII (17) and the DyP from Anabaena sp., (32), which had an activity loss of 90% at 50°C in 3 h. The thermal index T50 was 63°C, and the most severe drop was produced from 60°C, with 70% of the activity still remaining at that temperature. The B. adusta DyP isoenzymes lost their activity completely at 55°C, even without previous incubation (23, 31). Keeping in mind the fungal origin of I. lacteus DyP, the habitat of I. lacteus in the northern temperature zone (6), and its natural environment, wood and litter decay, the obtained data (high activity and stability at acid pHs, especially at low temperatures) fit with a potential physiological role in nature during lignocellulose degradation.
Concerning enzyme inactivation, the first compound tested was its own cosubstrate, H2O2. The enzyme showed increased activity at high H2O2 concentrations at reaction times compared to other DyPs (23, 32). That enhanced activity could be explained by the high turnover rate (kcat = 419 s-1) (Table 2) compared to the usual values in fungal peroxidases (kcat = 240 to 270 s-1) (21, 23). In general, it is very difficult to compare peroxidases in terms of their stability against H2O2 because many factors, such as pH, time, and temperature during the incubation, have a great influence (36). However, in view of these results and considering the [H2O2]/[enzyme] molar ratio and the incubation time, I. lacteus DyP seems to be even more resistant than other improved peroxidases for H2O2 stability, such as Pleurotus eryngii VP (37) and Anabaena sp. DyP (24). On the other hand, an excess of the tested ions (2 mM) produced a slight decrease in enzyme activity, and only Fe3+, Hg2+, Pb2+, and NaN3 severely affected enzyme performance. In particular, Pb2+ has been demonstrated to be an important MnP inhibitor (38). Mn2+ did not enhance DyP activity, which agrees with findings from fungal (23, 32) but not bacterial DyPs (39), where some activation has been described.
The applicability of I. lacteus DyP in second-generation ethanol production from wheat straw, a process of high biotechnological interest (9), was checked by including this enzyme in the enzymatic hydrolysis step. In the current work, it has been reasserted that (i) the type of pretreatment used to remove and/or deconstruct lignin, a heteropolymer of phenolic and nonphenolic residues and the main barrier to obtaining high fermentable sugar yields, and (ii) the enzyme mixture used to further hydrolyze cellulose and hemicellulose were essential for sugar recovery improvement from wheat straw (40). The addition of the whole enzymatic crude extract of I. lacteus negatively influenced the hydrolysis yields. This can be due to the presence, in the extracellular material secreted by this microorganism, of a heterogeneous mixture of enzymes including some proteases (data not shown), which could have hydrolyzed the commercial enzymes added. In contrast, Du et al. (41) reported that by-products from I. lacteus cultures improved the enzymatic hydrolysis of biotreated cornstalks. Nevertheless, the purified DyP increased cellulose digestibility (fermentable glucose recovery) in all cases, especially in the biologically or alkali-washed pretreated substrate. Briefly, these results suggest that DyP and cellulases exhibit a synergistic action during cellulose degradation of wheat straw through the oxidation of lignin's phenolic and nonphenolic compounds, thus making the cellulose polymers more accessible to cellulase attack. A patent describing the treatment of non-starch carbohydrates with a peroxidase of Marasmius scorodonius (42), which seems to be a DyP-type peroxidase, reported similar results. However, very few investigations have included peroxidases in this type of application, which enhances the novelty of these results.
To summarize, in the current work a novel high-redox-potential DyP has been isolated and characterized from I. lacteus. Despite its classification as a DyP-type peroxidase, this enzyme presents some advantageous features, such as high stability to pH and temperature and increased resistance to H2O2, which make it a really interesting enzyme to be applied in biotechnological processes such as enzymatic deconstruction of lignocellulose for bioethanol production or dye decolorization. Future work is focused on cloning, sequence analysis, and heterologous expression of I. lacteus DyP for further application and structure-function studies.
ACKNOWLEDGMENTS
This work has been carried out with funding from the Spanish project PRI-PIBAR-2011-1402 and EU FP7 project Peroxicats (KBBE-2010-4-265397). D.S. thanks the Spanish Ministry of Economy for a FPU fellowship.
We thank the Proteomics and Genomics and Protein Chemistry facilities at the Centro de Investigaciones Biológicas (Madrid, Spain).
Footnotes
Published ahead of print 10 May 2013
REFERENCES
- 1. Moon DS, Song HG. 2012. Degradation of alkylphenols by white rot fungus Irpex lacteus and its manganese peroxidase. Appl. Biochem. Biotechnol. 168:542–549 [DOI] [PubMed] [Google Scholar]
- 2. Kalpana D, Velmurugan N, Shim JH, Oh BT, Senthil K, Lee YS. 2012. Biodecolorization and biodegradation of reactive Levafix Blue E-RA granulate dye by the white rot fungus Irpex lacteus. J. Environ. Manage. 111:142–149 [DOI] [PubMed] [Google Scholar]
- 3. Novotny C, Erbanova P, Cajthaml T, Rothschild N, Dosoretz C, Sasek V. 2000. Irpex lacteus, a white rot fungus applicable to water and soil bioremediation. Appl. Microbiol. Biotechnol. 54:850–853 [DOI] [PubMed] [Google Scholar]
- 4. Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez AT, Martínez MJ. 2011. Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 102:7500–7506 [DOI] [PubMed] [Google Scholar]
- 5. Pinto PA, Dias AA, Fraga I, Marques G, Rodrigues MAM, Colaco J, Sampaio A, Bezerra RMF. 2012. Influence of ligninolytic enzymes on straw saccharification during fungal pretreatment. Bioresour. Technol. 111:261–267 [DOI] [PubMed] [Google Scholar]
- 6. Novotny C, Cajthaml T, Svobodova K, Susla M, Sasek V. 2009. Irpex lacteus, a white-rot fungus with biotechnological potential—review. Folia Microbiol. (Praha) 54:375–390 [DOI] [PubMed] [Google Scholar]
- 7. Shin KW. 2004. The role of enzymes produced by white-rot fungus Irpex lacteus in the decolorization of the textile industry effluent. J. Microbiol. 42:37–41 [PubMed] [Google Scholar]
- 8. Cajthaml T, Erbanova P, Kollmann A, Novotny C, Sasek V, Mougin C. 2008. Degradation of PAHs by ligninolytic enzymes of Irpex lacteus. Folia Microbiol. (Praha) 53:289–294 [DOI] [PubMed] [Google Scholar]
- 9. Salvachúa D, Prieto A, Vaquero ME, Martinez AT, Martínez MJ. 2013. Sugar recoveries from wheat straw following treatments with the fungus Irpex lacteus. Bioresour. Technol. 131:218–225 [DOI] [PubMed] [Google Scholar]
- 10. Welinder KG. 1992. Superfamily of plant, fungal and bacterial peroxidases. Curr. Opin. Struct. Biol. 2:388–393 [Google Scholar]
- 11. Martínez AT, Ruiz-Dueñas FJ, Martínez MJ, del Río JC, Gutiérrez A. 2009. Enzymatic delignification of plant cell wall: from nature to mill. Curr. Opin. Biotechnol. 20:348–357 [DOI] [PubMed] [Google Scholar]
- 12. Sugano Y, Muramatsu R, Ichiyanagi A, Sato T, Shoda M. 2007. DyP, a unique dye-decolorizing peroxidase, represents a novel heme peroxidase family. J. Biol. Chem. 282:36652–36658 [DOI] [PubMed] [Google Scholar]
- 13. Sugano Y. 2009. DyP-type peroxidases comprise a novel heme peroxidase family. Cell. Mol. Life Sci. 66:1387–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T. 2010. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 87:871–897 [DOI] [PubMed] [Google Scholar]
- 15. Ruiz-Dueñas FJ, Martínez AT. 2010. Structural and functional features of peroxidases with a potential as industrial biocatalysts, p 37–59 In Torres E, Ayala M. (ed), Biocatalysts based on heme peroxidases. Springer, Berlin, Germany [Google Scholar]
- 16. Zelena K, Hardebusch B, Huelsdau B, Berger RG, Zorn H. 2009. Generation of norisoprenoid flavors from carotenoids by fungal peroxidases. J. Agric. Food Chem. 57:9951–9955 [DOI] [PubMed] [Google Scholar]
- 17. Liers C, Bobeth C, Pecyna M, Ullrich M, Hofrichter M. 2010. DyP-like peroxidases of the jelly fungus Auricularia auricula-judae oxidizing nonphenolic lignin model compounds and high redox potential dyes. Appl. Microbiol. Biotechnol. 85:1869–1879 [DOI] [PubMed] [Google Scholar]
- 18. Zorn H, Scheibner M, Hulsdau B, Berger RG, de Boer L, Meima RB. July 2011. Novel enzymes for use in enzymatic bleaching of food products. US patent 7,981,636 B2 [Google Scholar]
- 19. Faraco V, Piscitelli A, Sannia G, Giardina P. 2007. Identification of a new member of the dye-decolorizing peroxidase family from Pleurotus ostreatus. World J. Microbiol. Biotechnol. 23:889–893 [Google Scholar]
- 20. Sugano Y, Matsushima Y, Tsuchiya K, Aoki H, Hirai M, Shoda M. 2009. Degradation pathway of an anthraquinone dye catalyzed by a unique peroxidase DyP from Thanatephorus cucumeris Dec 1. Biodegradation 20:433–440 [DOI] [PubMed] [Google Scholar]
- 21. Liers C, Pecyna MJ, Kellner H, Worrich A, Zorn H, Steffen KT, Hofrichter M, Ullrich R. 31 October 2012. Substrate oxidation by dye-decolorizing peroxidases (DyPs) from wood- and litter-degrading agaricomycetes compared to other fungal and plant heme-peroxidases. Appl. Microbiol. Biotechnol. 10.1007/s00253-012-4521-2 [DOI] [PubMed] [Google Scholar]
- 22. Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kuees U, Kumar T, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, John FS, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719 [DOI] [PubMed] [Google Scholar]
- 23. Kim SJ, Shoda M. 1999. Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl. Environ. Microbiol. 65:1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogola HJO, Hashimoto N, Miyabe S, Ashida H, Ishikawa T, Shibata H, Sawa Y. 2010. Enhancement of hydrogen peroxide stability of a novel Anabaena sp. DyP-type peroxidase by site-directed mutagenesis of methionine residues. Appl. Microbiol. Biotechnol. 87:1727–1736 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida T, Tsuge H, Konno H, Hisabori T, Sugano Y. 2011. The catalytic mechanism of dye-decolorizing peroxidase DyP may require the swinging movement of an aspartic acid residue. FEBS J. 278:2387–2394 [DOI] [PubMed] [Google Scholar]
- 26. Hilden KS, Makela MR, Hakala TK, Hatakka A, Lundell T. 2006. Expression on wood, molecular cloning and characterization of three lignin peroxidase (LiP) encoding genes of the white rot fungus Phlebia radiata. Curr. Genet. 49:97–105 [DOI] [PubMed] [Google Scholar]
- 27. Giardina P, Palmieri G, Fontanella B, Rivieccio V, Sannia G. 2000. Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch. Biochem. Biophys. 376:171–179 [DOI] [PubMed] [Google Scholar]
- 28. Steffen KT, Hofrichter M, Hatakka A. 2002. Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzyme Microb. Technol. 30:550–555 [Google Scholar]
- 29. Johjima T, Ohkuma M, Kudo T. 2003. Isolation and cDNA cloning of novel hydrogen peroxide-dependent phenol oxidase from the basidiomycete Termitomyces albuminosus. Appl. Microbiol. Biotechnol. 61:220–225 [DOI] [PubMed] [Google Scholar]
- 30. Sugano Y, Sasaki K, Shoda M. 1999. cDNA cloning and genetic analysis of a novel decolorizing enzyme, peroxidase gene dyp from Geotrichum candidum Dec 1. J. Biosci. Bioeng. 87:411–417 [DOI] [PubMed] [Google Scholar]
- 31. Shimokawa T, Shoda M, Sugano Y. 2009. Purification and characterization of two DyP isozymes from Thanatephorus cucumeris Dec 1 specifically expressed in an air-membrane surface bioreactor. J. Biosci. Bioeng. 107:113–115 [DOI] [PubMed] [Google Scholar]
- 32. Ogola HJO, Kamiike T, Hashimoto N, Ashida H, Ishikawa T, Shibata H, Sawa Y. 2009. Molecular characterization of a novel peroxidase from the cyanobacterium Anabaena sp. strain PCC 7120. Appl. Environ. Microbiol. 75:7509–7518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin KS, Kim YH, Lim JS. 2005. Purification and characterization of manganese peroxidase of the white-rot fungus Irpex lacteus. J. Microbiol. 43:503–509 [PubMed] [Google Scholar]
- 34. Banci L, Camarero S, Martínez AT, Martínez MJ, Pérez-Boada M, Pierattelli R, Ruiz-Dueñas FJ. 2003. NMR study of Mn(II) binding by the new versatile peroxidase from the white-rot fungus Pleurotus eryngii. J. Biol. Inorg. Chem. 8:751–760 [DOI] [PubMed] [Google Scholar]
- 35. Martínez MJ, Ruiz-Dueñas FJ, Guillén F, Martínez AT. 1996. Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 237:424–432 [DOI] [PubMed] [Google Scholar]
- 36. Böckle B, Martínez MJ, Guillén F, Martínez AT. 1999. Mechanism of peroxidase inactivation in liquid cultures of the ligninolytic fungus Pleurotus pulmonarius. Appl. Environ. Microbiol. 65:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. García-Ruiz E, Gonzalez-Perez D, Ruiz-Dueñas FJ, Martinez AT, Alcalde M. 2012. Directed evolution of a temperature-, peroxide- and alkaline pH-tolerant versatile peroxidase. Biochem. J. 441:487–498 [DOI] [PubMed] [Google Scholar]
- 38. Tuomela M, Steffen KT, Kerko E, Hartikainen H, Hofrichter M, Hatakka A. 2005. Influence of Pb contamination in boreal forest soil on the growth and ligninolytic activity of litter-decomposing fungi. FEMS Microbiol. Ecol. 53:179–186 [DOI] [PubMed] [Google Scholar]
- 39. Ahmad M, Roberts JN, Hardiman EM, Singh R, Eltis LD, Bugg TD. 2011. Identification of DypB from Rhodococcus jostii RHA1 as a lignin peroxidase. Biochemistry 50:5096–5107 [DOI] [PubMed] [Google Scholar]
- 40. Talebnia F, Karakashev D, Angelidaki I. 2010. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 101:4744–4753 [DOI] [PubMed] [Google Scholar]
- 41. Du W, Yu H, Song L, Zhang J, Weng C, Ma F, Zhang X. 2011. The promoting effect of byproducts from Irpex lacteus on subsequent enzymatic hydrolysis of bio-pretreated cornstalks. Biotechnol. Biofuels 4:37. 10.1186/1754-6834-4-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zorn H, Szweda R, Kumar M, Wilms J. July 2009. Method for modifying non-starch carbohydrate material using peroxidase enzyme. Patent WO2009EP58871 [Google Scholar]