Abstract
The objective of this study was to determine the effects of farm management and environmental factors on preharvest spinach contamination with generic Escherichia coli as an indicator of fecal contamination. A repeated cross-sectional study was conducted by visiting spinach farms up to four times per growing season over a period of 2 years (2010 to 2011). Spinach samples (n = 955) were collected from 12 spinach farms in Colorado and Texas as representative states of the Western and Southwestern United States, respectively. During each farm visit, farmers were surveyed about farm-related management and environmental factors using a questionnaire. Associations between the prevalence of generic E. coli in spinach and farm-related factors were assessed by using a multivariable logistic regression model including random effects for farm and farm visit. Overall, 6.6% of spinach samples were positive for generic E. coli. Significant risk factors for spinach contamination with generic E. coli were the proximity (within 10 miles) of a poultry farm, the use of pond water for irrigation, a >66-day period since the planting of spinach, farming on fields previously used for grazing, the production of hay before spinach planting, and the farm location in the Southwestern United States. Contamination with generic E. coli was significantly reduced with an irrigation lapse time of >5 days as well as by several factors related to field workers, including the use of portable toilets, training to use portable toilets, and the use of hand-washing stations. To our knowledge, this is the first report of an association between field workers' personal hygiene and produce contamination with generic E. coli at the preharvest level. Collectively, our findings support that practice of good personal hygiene and other good farm management practices may reduce produce contamination with generic E. coli at the preharvest level.
INTRODUCTION
Produce consumption, production, and safety are undergoing rapid changes. Global consumption of fruits and vegetables demonstrated an average annual increase of 4.5% from 1990 to 2004 (1). During the same period, the numbers of food-borne outbreaks and cases linked to produce have also increased (2). Increases in produce-related food-borne disease may have resulted not only from the increase in consumption of produce but also from changes in farm management and processing practices (3). Among outbreaks where a pathogen was identified, Salmonella (29%) and Escherichia coli O157:H7 (13%) were the main pathogens causing food-borne outbreaks in the United States (4). Both of these pathogens are shed through the feces of infected animals and human hosts (including asymptomatic carriers) (5). Listeria monocytogenes is another important food-borne pathogen of significant human health concern (6). It is shed through the feces of infected animals and human hosts, but it can also sustain itself in the environment as a saprophytic microorganism that thrives on decaying plant material (7). Microbial contamination of produce may occur at any point in the farm-to-fork food production chain (8). However, during the postharvest stage, it may be difficult to eliminate or counteract contamination that occurred before harvest (9). Produce is often consumed raw or after minimal processing, and therefore, pathogen contamination of produce is considered a serious human health risk. Identifying and controlling risk factors for produce contamination at the preharvest level are important steps for reducing this health risk.
The presence of E. coli in foods indicates fecal contamination and possibly the presence of pathogens carried in the intestinal tract of animals (10). This bacterium, which is commonly isolated from the intestines of warm-blooded vertebrates, is shed into the environment through feces. E. coli contamination of produce fields occurs from various sources, such as contaminated soil, fertilizer (manure/compost), wildlife, and irrigation water (11). A previous study (12) showed the usefulness of E. coli as an indicator organism for evaluating contamination with Salmonella enterica serovar Typhimurium originating from manure. Thus, to reduce the incidence of food-borne illnesses attributed to produce, it is of interest to study farm-related risk factors for E. coli produce contamination.
Previous research (13) has provided a comprehensive systematic review of the current knowledge about the effects of farm management practices, such as planting procedures, manure use, and irrigation application, on the contamination of fruits and vegetables with E. coli O157:H7, Salmonella, and L. monocytogenes For example, irrigation methods such as furrow and surface irrigation resulted in less produce contamination than spray irrigation. Additionally, the risk of E. coli contamination increased with the use of manure aged <6 months or 12 months as well as the use of cattle manure instead of another type of manure-based fertilizer (14, 15). The use of animal waste as fertilizer increased the risk of E. coli contamination of produce in organic and semiorganic farms (14); however, studies of the association between organic farming and E. coli contamination have yielded inconsistent results (15, 16). There is a need to reevaluate these and other types of inconsistencies and to assess the currently known risk factors alongside factors that have not yet been evaluated (e.g., landscape factors and workers' hygiene) in order to determine how they independently and jointly affect produce contamination. Most reported observational studies interested in the role of farm management factors in produce contamination with E. coli were conducted only in the Midwestern United States (e.g., Minnesota [14–16] and Wisconsin [14, 16, 17]), although additional states in the Western and Southwestern United States are important vegetable production areas with region-specific management and landscape factors (18). The objective of this study was to describe the distribution of generic E. coli contamination in spinach grown in Colorado and Texas as representative states of the Western and Southwestern United States and to determine the effects of farm management and environmental factors on the contamination of spinach with generic E. coli at the preharvest level.
MATERIALS AND METHODS
Study design and area.
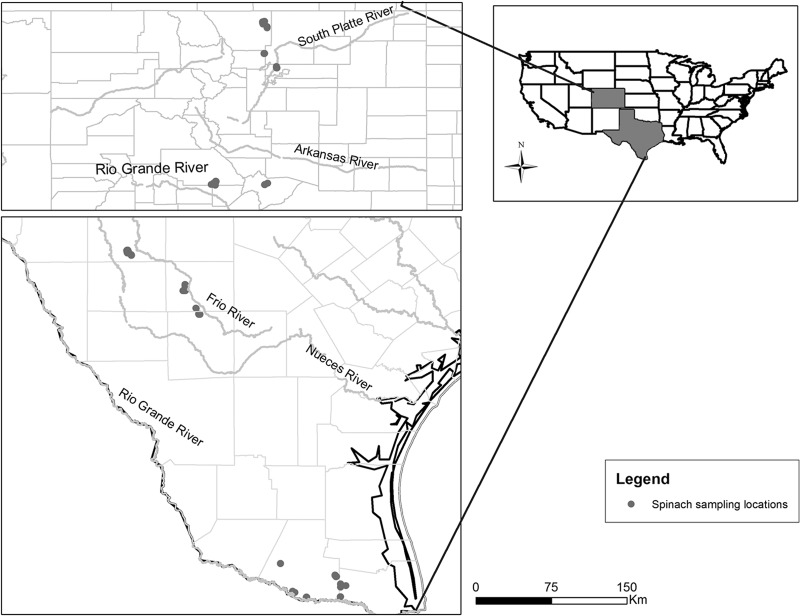
A repeated cross-sectional study over a period of 2 years (2010 to 2011) was conducted. We recruited 12 spinach farms: 4 in the Western (Colorado) and 8 in the Southwestern (Texas) United States (Fig. 1). A total of 955 spinach samples were collected over the duration of the study. Each farm was visited one to four times per growing season or up to seven times over the 2-year study period, depending on the availability of spinach fields throughout the growing seasons. At each farm visit, we chose one to six fields per farm and collected five spinach samples per field (Table 1). The number of fields sampled per farm depended on the number of available fields with spinach crop at the time of the visit. The spinach-growing season lasts from May to September in Colorado and from November to March in Texas. During the 2010 growing season, the monthly averages of mean daily temperatures around the enrolled farms ranged from 11°C to 21°C in Colorado (19) and from 13°C to 22°C in Texas (20). Likewise, the mean monthly precipitations ranged from 10 to 35 mm in Colorado and from 3 to 27 mm in Texas. During the growing season of 2011, the monthly averages of mean daily temperatures ranged from 10°C to 22°C in Colorado (21) and from 14°C to 21°C in Texas (22–25), while the mean monthly rainfalls ranged from 13 to 62 mm in Colorado and from 18 to 90 mm in Texas. In Colorado, most of the sampled spinach was grown on loam soil (70%), followed by clay loam soil (29%). In Texas, on the other hand, there was a greater diversity of soil types, with most of the sampled spinach being grown on silty clay loam soil (63%), followed by clay loam (14%), fine sandy loam soil (7%), and several other soil types (26). The meteorological and landscape factors, including temperature, precipitation, and soil types, are being investigated in more detail in a separate study.
Fig 1.
Map of sampling locations in Colorado and Texas.
Table 1.
Description of spinach sample collection
| State | Farm | Growing season | Sampling mo and yra | No. of visits | No. of fields sampled per visit | No. of collected samples | No. of samples that tested positive |
|---|---|---|---|---|---|---|---|
| Colorado | 1 | 1 | July/August/September 2010 | 3 | 3–4 | 55 | 0 |
| 2 | May/July/August 2011 | 3 | 4 | 60 | 0 | ||
| 2 | 1 | June/July/August 2010 | 3 | 4 | 60 | 4 | |
| 2 | May/June/July 2011 | 3 | 4 | 60 | 20 | ||
| 3 | 1 | June/July/August 2010 | 3 | 4 | 60 | 2 | |
| 2 | May/June/July 2011 | 3 | 4 | 60 | 0 | ||
| 4 | 1 | June/August/September 2010 | 3 | 4 | 60 | 1 | |
| 2 | June/July/August 2011 | 3 | 4 | 60 | 0 | ||
| Texas | 1 | 1 | November 2010, January/February 2011 | 3 | 4 | 60 | 5 |
| 2 | November/December 2011, January 2012 | 3 | 4 | 60 | 6 | ||
| 2 | 1 | November 2010, January/February 2011 | 3 | 4 | 60 | 2 | |
| 2 | November/December 2011, January 2012 | 3 | 4 | 60 | 2 | ||
| 3 | 1 | December 2010, January 2011 | 2 | 1 | 10 | 1 | |
| 2 | NS | ||||||
| 4 | 1 | December 2010, January/February/March 2011 | 4 | 1–4 | 45 | 3 | |
| 2 | December 2011, January/February 2012 | 3 | 2–6 | 50 | 4 | ||
| 5 | 1 | December 2010, January/February 2011 | 3 | 1–2 | 25 | 7 | |
| 2 | NS | ||||||
| 6 | 1 | January/February/March 2011 | 3 | 2 | 30 | 0 | |
| 2 | December 2011, January/February 2012 | 3 | 2 | 30 | 1 | ||
| 7 | 1 | December 2010, January 2011 | 2 | 1 | 10 | 1 | |
| 2 | NS | ||||||
| 8 | 1 | NS | 2 | 4 | 40 | 4 | |
| 2 | December 2011, January 2012 |
NS, samples not collected.
Sample collection.
Spinach samples were collected using sterile gloves. Each spinach sample consisted of at least 10 randomly selected individual plant leaves of different maturities, collected in an area within a 5-mile radius. Only random leaves were collected, without harvesting of the whole plants. Samples were placed into sterile Whirl-Pak bags (Nasco, Fort Atkinson, WI). All samples were shipped in coolers with ice packs. In year 1, the samples were shipped to the Food Safety Laboratory of the Department of Animal Sciences at Colorado State University (Fort Collins, CO). In year 2, samples were shipped to the Department of Animal and Food Sciences at Texas Tech University (Lubbock, TX). The research protocol and laboratory personnel for microbial detection were identical between the two laboratories. All samples were processed within 48 h after collection.
Microbiological analyses.
Each sample was prepared by using 25 g of spinach leaves. The spinach samples were transferred into 75 ml of phosphate-buffered saline (PBS) contained in stomacher bags. The contents of each bag were then mixed by using a laboratory blender (Smasher Lab Blender; AES-Chemunex, France) for 2 min at room temperature. A 1-ml aliquot from the sample bag followed by 1 ml of each of five 1:10 serial dilutions was then plated directly onto Petrifilm E. coli coliform count plates (3 M Microbiology, St. Paul, MN) and then incubated at 37°C ± 2°C. Petrifilm plates were counted, and blue colonies with gas bubbles, which were observed at 48 h, were considered to be E. coli colonies according to standard E. coli Petrifilm enumeration methods. The limit of detection was 4 CFU/ml of the plated dilution.
Questionnaire.
At each farm visit, we administered a comprehensive questionnaire to obtain information on the general farm-related management and environmental factors selected. The farm owner or manager was asked the questions in a face-to-face interview during each farm visit. The farmers referred to their management records to answer questions that required more detail, such as the date of manure application. The questionnaire can be found in Fig. S1 in the supplemental material. The questionnaire has two parts: parts A and B. Part A inquires about general farm information (such as farm size) that is not expected to change during the growing season. These questions were asked only at the beginning of each growing season. Part B asks about factors that may change during the growing season (e.g., history of farm intrusion by wild animals between two subsequent visits). These questions were asked at each farm visit. The questionnaire responses were coded and entered into an Excel spreadsheet. This spreadsheet was then used to create variables to be considered in the statistical analysis.
Statistical analyses.
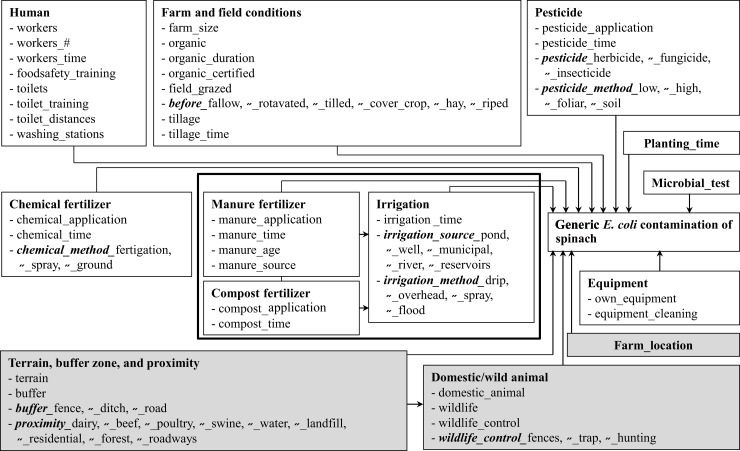
Data analyses were conducted by using R software (R Project for Statistical Computing [http://www.r-project.org/]). Except when stated otherwise, P values of <0.05 were considered statistically significant. The outcome of interest was spinach contamination with generic E. coli, evaluated as a binary variable: if any generic E. coli was detected in a spinach sample, the sample was considered contaminated; otherwise, it was considered noncontaminated (meaning that generic E. coli bacteria were either present below the limit of detection or absent all together). Table 2 lists and describes the 76 explanatory variables considered in the statistical analyses. The causal diagram in Fig. 2 shows the hypothesized associations among these variables and the outcome of interest. In the univariate and multivariable analyses, the associations between the explanatory variables (farm management and environmental) and the outcome variable (generic E. coli contamination) were evaluated by using a mixed-effect logistic regression model with farm and farm visit as random effects. Regarding the random effects, there were 12 farms (F1 to F12), each with a total of 1 to 7 visits (V1 to V7) over the course of the study. The mixed-effect models were fitted by using the “lmer” function of the R package, called “lme4” (27). At the univariate analysis level only, the significance of associations was assessed at a liberal cutoff of a P value of 0.2 to ensure that all potentially important factors and confounders reached the multivariable analysis. The validity of the linearity assumption of the developed mixed-effect logistic regression models was assessed by graphical plotting, lowess smoothing, between the continuous explanatory variables and log odds of the outcome variable (28). Natural log and quadratic transformations of continuous explanatory variables were also considered. However, because the linearity assumption was not confirmed for any of the continuous explanatory variables (including for their transformations), all continuous variables were median dichotomized before they could be assessed in the univariate and multivariable mixed-effect logistic regression models that also controlled for clustering of samples within farms and farm visits. A manual forward stepwise selection procedure was used to select an appropriate multivariable model (P < 0.05 based on the Wald Z test). Only those independent variables whose addition significantly reduced residual deviance were included in the expanded model. Significant differences in model deviance between two nested models were evaluated based on the likelihood ratio test (P < 0.05). A plot of observed proportions versus mean predicted probabilities was used to determine the goodness of fit by the “plot.logistic.fit.fnc” function of the R package, called “languageR” (29). The proportion of variation explained by clustering levels (farm and farm visit) was calculated for the final model by using a latent variable approach (28).
Table 2.
Description of explanatory variables
| Category and variable | Description and levela | Unit |
|---|---|---|
| Farm management factors | ||
| Human | ||
| workers | Farm uses temporary workers (yes/no) | |
| workers_# | No. of temporary workers used on the farm (con) | No. |
| workers_time | Time since the last workers' visit during CGS (con) | Days |
| foodsafety_training | Food safety training provided to the staff/temporary workers on the farm (yes/no) | |
| toilets | Portable toilets used in the field (yes/no) | |
| toilet_training | Training to use portable toilets to staff/temporary workers (yes/no) | |
| toilet_distances | Portable toilet distances from the work area on the field (con) | Meters |
| washing_stations | Hand-washing stations used in the field (yes/no) | |
| Farm and field conditions | ||
| farm_size | Farm size (con) | Acres |
| organic | Organic farming practices currently applied on the farm (yes/no) | |
| organic_duration | Duration of application of organic farming practices on the farm (con) | Yr |
| organic_certified | Organic farming certified by the National Organic Program (yes/no) | |
| field_grazed | Farming on field previously used for grazing (yes/no) | |
| before_fallow | Field condition before planting of the spinach during CGS, fallow (yes/no) | |
| before_rotavated | Field condition before planting of the spinach during CGS, rotavated (yes/no) | |
| before_tilled | Field condition before planting of the spinach during CGS, tilled (yes/no) | |
| before_cover_crop | Field condition before planting of the spinach during CGS, cover crop (yes/no) | |
| before_hay | Field condition before planting of the spinach during CGS, hay (yes/no) | |
| before_riped | Field condition before planting of the spinach during CGS, riped (yes/no) | |
| tillage | Tilling, rotavating, or aerating soil for CGS (yes/no) | |
| tillage_time | Time since the last tilling, rotavating, or aerating of soil for CGS (con) | Days |
| Pesticide | ||
| pesticide_application | Pesticide application (yes/no) | |
| pesticide_time | Time since the last pesticide application during CGS (con) | Days |
| pesticide_herbicide | Type of pesticide applied to the field for CGS, herbicide (yes/no) | |
| pesticide_fungicide | Type of pesticide applied to the field for CGS, fungicide (yes/no) | |
| pesticide_insecticide | Type of pesticide applied to the field for CGS, insecticide (yes/no) | |
| pesticide_method_low | Method for applying pesticide for CGS, low-vol spray (yes/no) | |
| pesticide_method_high | Method for applying pesticide for CGS, high-vol spray (yes/no) | |
| pesticide_method_foliar | Method for applying pesticide for CGS, foliar (yes/no) | |
| pesticide_method_soil | Method for applying pesticide for CGS, soil (yes/no) | |
| Chemical fertilizer | ||
| chemical_application | Chemical fertilizer spread onto the field for CGS (yes/no) | |
| chemical_time | Time since the last chemical fertilizer spreading during CGS (con) | Days |
| chemical_method_fertigation | Method for spreading chemical fertilizer onto the field for CGS, fertigation (yes/no) | |
| chemical_method_spray | Method for spreading chemical fertilizer onto the field for CGS, foliar spray (yes/no) | |
| chemical_method_ground | Method for spreading chemical fertilizer onto the field for CGS, ground application (yes/no) | |
| Manure fertilizer | ||
| manure_application | Manure spread onto the field for CGS (yes/no) | |
| manure_time | Time since the last manure spreading during CGS (con) | Days |
| manure_age | Age of manure spread onto the field for CGS (con) | Wk |
| manure_source | Source of manure spread onto the field for CGS (dairy farm/poultry farm) | |
| Compost fertilizer | ||
| compost_application | Compost spread onto the field for CGS (yes/no) | |
| compost_time | Time since the last compost spreading during CGS (con) | Days |
| Irrigation | ||
| irrigation_time | Time since the last irrigation during CGS (con) | Days |
| irrigation_source_pond | Source of irrigation water applied during CGS, pond (yes/no) | |
| irrigation_source_well | Source of irrigation water applied during CGS, well (yes/no) | |
| irrigation_source_municipal | Source of irrigation water applied during CGS, municipal (yes/no) | |
| irrigation_source_river | Source of irrigation water applied during CGS, river/stream/creek (yes/no) | |
| irrigation_source_reservoirs | Source of irrigation water applied during CGS, reservoirs (yes/no) | |
| irrigation_method_drip | Method of irrigation for CGS, drip (yes/no) | |
| irrigation_method_overhead | Method of irrigation for CGS, overhead (yes/no) | |
| irrigation_method_spray | Method of irrigation for CGS, spray (yes/no) | |
| irrigation_method_flood | Method of irrigation for CGS, flood (yes/no) | |
| Equipment | ||
| own_equipment | Use of own farm equipment for all operations (yes/no) | |
| equipment_cleaning | Cleaning of farm equipment (yes/no) | |
| Microbial_test | Routine microbial test (yes/no) | |
| Planting_time | Time since planting of spinach (con) | Days |
| Farm environmental factors | ||
| Terrain, buffer zone, and proximity | ||
| terrain | Terrain where the farm is located (flat/sloped) | |
| buffer | Buffer zone from neighbors and roads, etc. (yes/no) | |
| buffer_fence | Type of buffer zone, fence (yes/no) | |
| buffer_ditch | Type of buffer zone, ditch (yes/no) | |
| buffer_road | Type of buffer zone, road (yes/no) | |
| proximity_dairy | Proximity within 10-mile radius, dairy farm (yes/no) | |
| proximity_beef | Proximity within 10-mile radius, beef farm (yes/no) | |
| proximity_poultry | Proximity within 10-mile radius, poultry farm (yes/no) | |
| proximity_swine | Proximity within 10-mile radius, swine farm (yes/no) | |
| proximity_water | Proximity within 10-mile radius, water resources (yes/no) | |
| proximity_landfill | Proximity within 10-mile radius, landfill (yes/no) | |
| proximity_residential | Proximity within 10-mile radius, residential (yes/no) | |
| proximity_forest | Proximity within 10-mile radius, forest (yes/no) | |
| proximity_roadways | Proximity within 10-mile radius, roadways (yes/no) | |
| Domestic/wild animals | ||
| domestic_animal | Domestic animal intrusion of the field for CGS (yes/no) | |
| wildlife | Wildlife intrusion of the field for CGS (yes/no) | |
| wildlife_control | Wildlife control on the farm (yes/no) | |
| wildlife_control_fences | Wildlife control method of the farm, fences (yes/no) | |
| wildlife_control_trap | Wildlife control method of the farm, trap (yes/no) | |
| wildlife_control_hunting | Wildlife control method of the farm, hunting (yes/no) | |
| Farm_location | Farm location (Southwestern U.S./Western U.S.) |
CGS, current growing season; con, continuous variable.
Fig 2.
Causal diagram of the hypothesized farm management and environmental risk factors for generic E. coli contamination of spinach at the preharvest level. ″ means that the text above in boldface italic type applies. Gray-shaded boxes indicate environmental factors.
To evaluate the predictive performance of the final multivariable model, we calculated the model sensitivity (Se) and specificity (Sp), with the standard errors (SE) for each of these estimates, along with the model positive predictive value (PPV) and negative predictive value (NPV), based on the explanatory variables appearing in the model. The logistic regression prediction results were recorded on a continuous scale (spanning from 0 to 1) and were dichotomized for comparison with the binary (yes/no) contamination data. To find the optimal cutoff value, the model misclassification costs were estimated over the entire range of possible cutoffs while penalizing false-negative more than false-positive classifications in order to improve the Se of the model. This was achieved by setting the false-positive cost to 1 and testing the model's predictive performance when the false-negative cost was set to an integer value in the interval [1, 25]. Penalizing false negatives more than false positives also compensated for the fact that microbial culture-based tests, such as the one used here to detect spinach contamination, are expected to have better Sp than Se. Consequently, positive microbial test results can be considered true positives, while some negative results might actually be false negatives.
RESULTS
Overall, generic E. coli was isolated from 63 of 955 (6.6%) of the spinach samples. The median size of enrolled farms was 280 acres (interquartile range [IQR], 12 to 1,000 acres). In Table 3, we show summary statistics for the remaining continuous explanatory variables to aid in interpretation of the results of univariate (Table 4) and multivariable (Table 5) statistical analyses, where these variables were considered in their median-dichotomized forms. Three of the 12 enrolled farms were organic, and 2 of these were certified organic. All but one of the enrolled farms used hand-washing stations and portable toilets and trained employed workers on how to use them. That farm was also the only one that used their fields for grazing and hay production before spinach planting. Due to the simultaneous occurrence of these farm management factors, we evaluated them by using toilet use as a representative factor for the “hygiene-field status” group of factors. The hygiene-field status group had two levels: “yes” and “no.” The yes level indicated the use of portable toilets and hand-washing stations, the presence of training on the use of portable toilets, and the absence of grazing and hay production in the field before spinach planting.
Table 3.
Summary statistics for continuous variables with respect to spinach contamination with generic E. coli
| Category and variable | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 955) |
Positive (n = 63) |
Negative (n = 892) |
|||||||
| Mean | Median | IQR | Mean | Median | IQR | Mean | Median | IQR | |
| Human | |||||||||
| workers_# (no.) | 99.2 | 100 | 8–150 | 99.4 | 150 | 8–150 | 99.2 | 100 | 9–150 |
| workers_time (days) | 7.7 | 3 | 1–9 | 3 | 1.5 | 1.5–4 | 8.1 | 3 | 1–9 |
| toilet_distances (m) | 186.9 | 146.3 | 45.7–402.3 | 213.6 | 201.2 | 201.2–201.2 | 185.2 | 91.4 | 45.7–402.3 |
| Farm and field conditions | |||||||||
| organic_duration (yr) | 13.4 | 4 | 3–25 | 20.2 | 26 | 25–26 | 12.5 | 4 | 3–25 |
| tillage_time (days) | 42.5 | 17 | 15–74 | 24 | 15 | 15–15 | 45.3 | 18 | 15–74 |
| Pesticides/fertilizers/irrigation | |||||||||
| pesticide_time (days) | 24.9 | 10 | 5–41 | 24.7 | 10 | 5–41 | 29.2 | 12 | 5–50.8 |
| chemical_time (days) | 24.1 | 15 | 10–32 | 24.0 | 15 | 10–32 | 24.8 | 15 | 10–44.5 |
| manure_time (days) | 228.3 | 200 | 200–281 | 202.4 | 200 | 200–200 | 233.9 | 224 | 200–281 |
| manure_age (wk) | 12.9 | 13 | 9–13 | 14.6 | 13 | 13–13 | 12.5 | 13 | 9–13 |
| compost_time (days) | 269.1 | 275 | 237–292.5 | 328 | 328 | 328–328 | 268.6 | 269 | 237–291 |
| irrigation_time (days) | 12.9 | 5 | 2–14 | 11.4 | 3.5 | 3.5–8.5 | 13 | 5 | 2–15 |
| Planting_time (days) | 66.8 | 66 | 47–82 | 76.2 | 77 | 64–90.5 | 66.1 | 65 | 47–80 |
Table 4.
Association between generic E. coli-contaminated spinach and risk factors assessed in the univariate mixed-effect logistic regression analysis with farm and visit as random effects
| Variable (comparison level) | Frequencya | Reference level | OR (95% CI)b | P valuec |
|---|---|---|---|---|
| Farm management factor | ||||
| Human | ||||
| toilets (yes)d | 930/955 | No | 0.08 (0.01, 0.99) | 0.049 |
| Farm and field conditions | ||||
| organic_certified (yes) | 175/200 | No | 0.05 (0.00, 0.65) | 0.022 |
| before_rotavated (yes) | 490/955 | No | 0.21 (0.08, 0.54) | 0.001 |
| tillage_time (>17 days) | 85/185 | ≤17 days | 0.02 (0.00, 0.16) | <0.001 |
| Fertilizers | ||||
| manure_application (yes) | 160/955 | No | 7.9 (1.6, 39.4) | 0.011 |
| manure_time (>200 days) | 60/140 | ≤200 days | 0.08 (0.01, 0.90) | 0.041 |
| manure_age (>13 wk) | 20/150 | ≤13 wk | 156.6 (0.2, 114,716.7) | 0.133 |
| manure_source (poultry farm) | 90/150 | Dairy farm | 11.4 (1.1, 123.5) | 0.045 |
| compost_application (yes) | 140/955 | No | 0.08 (0.00, 2.02) | 0.127 |
| Irrigation | ||||
| irrigation_time (>5 days) | 365/845 | ≤5 days | 0.17 (0.05, 0.59) | 0.005 |
| irrigation_source_pond (yes) | 20/955 | No | 24.4 (2.1, 280.1) | 0.010 |
| irrigation_source_well (yes) | 635/955 | No | 0.30 (0.06, 1.51) | 0.144 |
| irrigation_source_reservoirs (yes) | 160/955 | No | 0.08 (0.01, 0.40) | 0.002 |
| Equipment | ||||
| own_equipment (yes) | 865/955 | No | 9.1 (2.4, 34.6) | 0.001 |
| Planting_time (>66 days) | 465/955 | ≤66 days | 2.6 (1.3, 5.2) | 0.008 |
| Farm environmental factors | ||||
| Terrain, buffer zone, and proximity | ||||
| terrain (sloped) | 165/955 | Flat | 8.3 (2.5, 27.3) | <0.001 |
| buffer_fence (yes) | 165/895 | No | 4.8 (1.9, 12.0) | 0.001 |
| proximity_beef (yes) | 120/955 | No | 6.0 (0.5, 79.1) | 0.174 |
| proximity_poultry (yes) | 110/955 | No | 8.7 (0.9, 88.0) | 0.067 |
| proximity_forest (yes) | 60/955 | No | 0.11 (0.03, 0.43) | 0.002 |
| proximity_roadways (yes) | 895/955 | No | 0.07 (0.02, 0.28) | <0.001 |
| Domestic/wild animals | ||||
| wildlife_control (yes) | 505/910 | No | 5.0 (1.9, 13.2) | 0.001 |
| domestic_animal (yes) | 25/935 | No | 11.8 (1.1, 122.9) | 0.039 |
| Farm_location (Southwestern U.S.) | 480/955 | Western U.S. | 4.4 (0.8, 25.5) | 0.096 |
Frequency is the number of observations with the comparison level/total number of recorded observations for the variable.
OR (95% CI), odds ratio with 95% confidence interval.
Only variables with P values of <0.2 are shown.
The estimated OR (95% CI) value applies to each factor in the “hygiene-field status” group: toilet_training (yes versus no), washing_stations (yes versus no), field_grazed (no versus yes), and before_hay (no versus yes).
Table 5.
Association of generic E. coli prevalence with risk factors based on the final multivariable mixed-effect logistic regression model with farm and visit as random effectsb
| Variable (comparison level) | Reference | OR (95% CI) | P value |
|---|---|---|---|
| toilets (yes)a | No | 0.15 (0.05, 0.45) | <0.001 |
| irrigation_time (>5 days) | ≤5 days | 0.24 (0.09, 0.67) | 0.006 |
| planting_time (>66 days) | ≤66 days | 2.7 (1.2, 6.1) | 0.018 |
| farm_location (Southwestern U.S.) | Western U.S. | 60.7 (7.1, 516.6) | <0.001 |
| irrigation_source_pond (yes) | No | 64.4 (4.9, 855.3) | 0.002 |
| proximity_poultry (yes) | No | 172.1 (21.1, 1,402.8) | <0.001 |
The estimated OR (95% CI) value applies to each factor in the “hygiene-field status” group: toilet_training (yes versus no), washing_stations (yes versus no), field_grazed (no versus yes), and before_hay (no versus yes).
Variance component values (standard deviations) were 7.2e−11 (8.5e−6) for farm and 0.53 (0.73) for farm visit. For the intercept-only model, the variance component values were 1.87 (1.37) for farm and 0.57 (0.75) for farm visit.
Based on the univariate analyses, the variables that were associated with spinach contamination at the 20% significance level were identified (Table 4). Among the farm management factors, the presence of generic E. coli on spinach samples was significantly reduced if they were collected from certified organic farms compared with the noncertified organic farms. Similarly, spinach was less likely to be contaminated if it was collected from fields that used portable toilets, fields that were rotavated before planting of the spinach crop in the season, or fields that used reservoir water for irrigation. Finally, the prevalence of contamination was lower if the time since the last manure spreading was >200 days or the time since the last irrigation was >5 days. Alternatively, the presence of generic E. coli was significantly increased by the use of manure in general, by the use of manure from dairy farms in particular, and when pond water was used for irrigation. Among the farm environmental factors, the presence of generic E. coli was significantly reduced by proximity (within 10 miles) of forest or roadways. The presence of generic E. coli was significantly increased when the farm was located on a sloped terrain and when domestic animal intrusion on the field was reported. Four additional variables were associated with the outcome at the 20% level albeit with a counterintuitive direction of association, suggesting a possible distorting effect of a confounder (28). Specifically, the probability of generic E. coli occurrence was higher when a fenced buffer zone around the farm was present, when the farm applied some means of controlling wildlife, if the farm used their own farm equipment, and when manure applied onto the field was aged longer than 13 weeks. All four variables dropped out during the multivariable analysis, indicating a lack of an association with spinach contamination after controlling for other risk factors.
The variables listed in Table 4 were tested further for inclusion in the multivariable model. The final multivariable mixed-effect model (Table 5) had 110 missing observations, all of which were for the irrigation time variable. Based on this model, the odds of spinach contamination were reduced to approximately 1 in 4 (odds ratio [OR] = 0.24) when the time since the last irrigation was longer than 5 days. Similarly, the odds of contamination were reduced to approximately 1 in 7 (OR = 0.15) when the field used portable toilets. As stated above, the use of portable toilets represents the “hygiene-field status” group of factors, meaning that the odds of spinach contamination would be equally reduced if any of the factors in this group were considered in the final model instead of the portable toilet use factor. The final model indicated that the odds of spinach contamination were increased to approximately 3 in 1 (OR = 2.7) when spinach was grown for longer than 66 days before sampling. Interestingly, the odds of contamination were considerably higher for farms located in the southwest (Texas) than in the west (Colorado) (OR = 60.7), for fields that used pond water for irrigation (OR = 64.4), and for fields in proximity (within 10 miles) of a poultry farm (OR = 172.1). While the 95% confidence intervals (CIs) for these variables did not include 1, which indicates strong evidence of an increased risk in the presence of these factors, the CIs were very wide, indicating a high level of uncertainty in the true value of their respective ORs. The proportions of variation explained at the visit and farm levels were 9.9% and 32.6%, respectively, in the intercept-only model and 13.9% and almost 0%, respectively, in the final model.
We additionally tested potential 2-way interactions between factors in the final model. Only one interaction term, between “irrigation_time” and “planting_time,” had a significant effect on the probability of spinach contamination. It indicated that if spinach was planted >66 days ago and irrigation was applied <5 days ago, the probability of spinach contamination increased by approximately 3%. The predictive performance of the model with the interaction term was comparable to the predictive performance of the simpler model without it, and therefore, the model without the interaction term was retained as the final model.
In terms of predictive performance, our final model had perfect Sp (100%; SE, 0%), while its Se was quite low (33.9%; SE, 6.2%) at the cutoff value of 0.699 that was used to dichotomize the predictions of the logistic regression model. However, because spinach contamination with generic E. coli was relatively rare, the NPV of the model was relatively high (95.3%), meaning that the probability that a negative prediction is truly negative is quite high. Since no false-positive predictions were expected, the PPV was 100%. It should be noted here that the model predictive performance was assessed on the data used for model bundling, and thus, generalization of the results to independent data should be done with caution.
DISCUSSION
The study described here undertook a comprehensive and organized approach to identify the farm management and environmental factors affecting microbial contamination of produce at the preharvest level. The results indicate that both farm management and environmental factors can affect the risk of spinach contamination with generic E. coli.
Our study identified the “hygiene-field status” group of factors to have a strong protective effect on spinach contamination. These factors were the use of portable toilets and hand-washing stations, training in the use of portable toilets, and not the use of the spinach field for grazing or hay production before spinach planting. Because these factors occurred jointly, inference based on their individual effects has to be done with caution. Within the group, the most intriguing result is the potential role that field workers' personal hygiene may play in generic E. coli contamination of produce at the preharvest level. Poor personal hygiene of workers is a well-known risk factor for the microbial contamination of produce growing in fields or during harvest, postharvest processing, and distribution (31). However, to our knowledge, no published epidemiological study has shown an association between workers' hygiene practices and produce contamination rates at the preharvest level. We found that produce contamination was significantly reduced when workers used hand-washing stations or when the farm provided portable toilets for workers and trained the workers on how to use them. As indicated by the hygiene-field status group, produce contamination was also significantly reduced if the spinach field was not used for hay production or for grazing prior to spinach planting. While the role of these factors in produce contamination is intuitive, surprisingly, only limited published information on these factors exists. One study (32) showed tomato contamination with Salmonella after planting of tomatoes in soil mixed with debris of tomato plants grown on Salmonella-inoculated soil. Grazing on or near fields used for growing of produce is considered a food safety hazard (33; B. R. Hoar, presented at the Center for Produce Safety 2010 Research Symposium, Davis, CA, 23 June 2010). Surface runoff from grazing areas onto cultivated fields has been previously recognized as a risk factor for produce contamination (34). Collectively, conclusions about the role of each individual factor from the hygiene-field status group are valuable because they are either intriguing or intuitive. However, due to their joint appearance (likely due to the small number of enrolled farms), it is impossible to determine which (if not all) of these hygiene-field status factors was truly protective or whether they all were just proxies for another unmeasured but true protective factor. With these limitations in mind, and considering the importance and novelty of our findings, we suggest that personal hygiene may be considered a potential factor for controlling microbial contamination of produce at the postharvest level. Future controlled trials should be conducted to elucidate the role of workers' personal hygiene in relation to the history of field use and in conjunction with other factors not measured in our study (such as weather).
Farms using manure had a significantly higher proportion of generic E. coli-positive samples than did farms not using it (15.6% versus 4.8%). This is consistent with results of previous studies (14, 15). In our study, 60% of farms used manure from dairy cows, and the others used manure from poultry. Studies by Islam et al., who inoculated the same concentrations of microorganisms into manure, showed inconsistent results regarding the survival rates of E. coli O157:H7 (30, 35) and Salmonella (36, 37) in vegetables grown in soil mixed with manure from cows and from poultry. In our study, spinach samples grown in soil mixed with poultry manure had a significantly higher risk of generic E. coli contamination than did those grown with cattle manure (P = 0.045) (Table 4). However, this factor was not retained in the final multivariable model. Therefore, while the use of manure, particularly poultry manure, on the farm seems to increase the probability of spinach contamination, after controlling for other risk factors, we did not find evidence that manure in general, or poultry manure specifically, significantly increased the probability of spinach contamination with generic E. coli.
The odds of spinach contamination with generic E. coli was higher in organic than in conventional farms (OR = 2.4), although this difference was not significant (P = 0.340) (data not shown). In a study by Mukherjee et al. (15), organic produce showed a significantly greater risk of E. coli contamination than conventional produce. It is possible that our study was unable to detect a significant association between the type of farming (organic versus conventional) and produce contamination due to the relatively small number of enrolled farms. However, it is also possible that the type of farming does not significantly affect the probability of produce contamination, which would support the results of another study that showed that the type of farm (organic, semiorganic, or conventional) was less likely than produce type to affect the risk of E. coli contamination (16). Interestingly, in the analysis restricted to organic farms only, the spinach from certified organic farms was less likely to be contaminated with generic E. coli than spinach from noncertified organic farms (OR = 0.05; P = 0.022). This low risk of spinach contamination with generic E. coli in the certified farm environment might be attributed to the strict implementation of national organic regulations (38).
Previous studies have shown no apparent effect of time since the last manure spreading, in the range from 90 to 120 days, on produce contamination with E. coli (14, 39). Interestingly, our univariate analysis showed a significant association between this factor and spinach contamination when a different cutoff interval (of 200 days) was used. Nevertheless, this factor was not retained in the final model. According to national organic regulations, raw animal manure should be applied at least 90 days prior to harvesting of edible produce that does not come into contact with soil or soil particles (38). Several studies have assessed the role of manure aging before spreading on produce contamination. A study by Mukherjee et al. showed the nonsignificant association between manure age (≥6 months) and E. coli prevalence in noncertified organic produce (14). However, those authors also showed that manure aged longer than 6 months in certified organic farms (14) or 1 year in organic farms (15) significantly decreased the risk of E. coli contamination. An experimental study also showed that they dramatically lowered E. coli levels by >99% after 90 days of manure storage (40). Our study did not detect any association between manure age and spinach contamination. This may be due to a true lack of association. Alternatively, it may be due to the farmers' poor recall (or record keeping) of the manure age.
At the preharvest level, irrigation is considered one of the most important modes for transmission of microorganisms from their reservoirs to produce (13). Consistent with this, our final model found that the use of pond water for irrigation was a strong predictor of spinach contamination (OR = 64.4) (Table 5). While this association was expected, care is needed in generalization of the results because only one farm in our study used for irrigation water from a pond (approximately 12,000 m2) located on the field. In the 2002 and 2005 outbreaks of salmonellosis associated with tomatoes, Salmonella enterica serovar Newport isolates from two outbreaks had the same genotype profile as isolates from pond water that was used for irrigation (41).
A time of >5 days since the last irrigation was associated with a decreased risk of generic E. coli contamination of spinach. Intuitively, this might be because irrigation near sampling with potentially contaminated water increased the risk of produce contamination. A previous study showed the persistence of E. coli O157:H7 on lettuce leaves for up to 20 days, after a single exposure to 100 ml of a solution with the pathogen at a concentration of 102 CFU/ml (42). Another study showed the persistence of E. coli O157:H7 in lettuce phyllospheres over 45 days after irrigation of seedlings with water inoculated with the pathogen (density of 107 E. coli O157:H7 bacteria · liter−1) on transplanting day and 15 days later (43). The U.S. Food and Drug Administration recommends that the quality of water directly contacting the edible parts of produce should be better than the quality of water that minimally contacts the edible parts of produce (8). Thus, when farmers irrigate leafy green or fruit vegetables with water that could potentially be contaminated with pathogens, they should irrigate the field >5 days before harvest, or they should use furrow or surface irrigation methods rather than overhead or spray irrigation.
In our study, the history of farm intrusion by wildlife was not associated with the presence of E. coli contamination of spinach. However, Orozco et al. (44) suggested that wildlife is an important vector for Salmonella transmission to tomatoes. At the univariate level, there was a significant association between the history of domestic animal intrusion and an increased risk of spinach contamination. This factor was not included into the final model due to its high correlation with the hygiene-field status factors, including field use for grazing before spinach planting. However, it is reasonable to suggest that domestic animal intrusion is one of the important risk factors for E. coli spinach contamination. The presence of wildlife might have gone unobserved, as farmers do not stay on the fields all the times. Thus, both wild and domestic animals could have contaminated spinach with E. coli in this study. A previous study (17) suggested that wildlife intrusion can be an important vehicle for E. coli contamination of produce, particularly when there is noncomposted manure piled on the farm. Interestingly, the proximity (10 miles) of a poultry farm increased the risk of spinach contamination (Table 5). This result may be just a statistical artifact caused by a high correlation between this variable and farm use of poultry manure. However, spinach could have truly become contaminated by wild birds that are known to be drawn to the poultry barns (fully enclosed housing) and surrounding habitats (45). Because E. coli can grow in soil (46, 47), animal intrusion into a produce farm cumulatively increases the risk of microbial contamination of produce. Thus, practices to prevent or repel wildlife and domestic animal intrusion should be considered as a means to prevent microbial contamination of produce.
Spinach contamination with generic E. coli increased if the time since planting of spinach was >66 days. This result is in line with previous studies that observed an increase of produce contamination in mature lettuce (48, 49) or spinach (50). Mootian et al. (49) proposed that a well-developed secondary root system of lettuce might increase microbial contamination of produce. We suspected that a longer exposure of mature spinach to E. coli resulted in more contamination in mature produce than in young produce (13). Spinach usually takes 6 or 7 weeks until its first harvest (51), and it will often be cut 2 or 3 additional times in intervals of 20 to 30 days after the first harvest. Our results suggest that the first cut of spinach crop may be considered microbiologically safer.
Our final statistical model had a perfect Sp but a very low Se (33.9%). However, when the cost of a false negative was set to 14, the Se and Sp of the final model were 71.2% and 75.4%, respectively. Therefore, our model may be practically manipulated depending on our objective (i.e., whether a better Se or Sp is of interest). Therefore, while it is subject to future model assessments on an independent data set, our statistical model may be a promising tool for the prediction of generic E. coli contamination in the field. Moreover, to our knowledge, this is the only published study that investigated the predictive performance of the developed statistical model for the considered farm management and environmental risk factors.
Generic E. coli is commonly used as an indicator of environmental fecal contamination. For example, E. coli has been recommended as a reliable indicator organism for the potential presence of S. Typhimurium in manure-fertilized soil and on vegetables grown in such soil (12). Thus, the absence of E. coli from a produce sample may be taken as a strong indication of the absence of other fecal contaminants (12). However, the presence of E. coli on a produce sample as an indication of contamination of fecal origin has to be considered with a grain of salt. E. coli has been shown to be able to maintain stable populations in temperate soil and water (47). Thus, while E. coli on produce most often originates from recent fecal contamination, it could also be from an environmentally stable population of the microorganism.
The evaluation of spinach contamination with generic E. coli described in this paper was part of a larger unpublished study involving the same spinach farms where, in addition to collection of spinach, we also collected a total of 191 drag samples of soil and 26 samples of irrigation water. The original intent of that study was to elucidate the effect of management and environmental factors on the contamination of spinach with food-borne pathogens. However, food-borne pathogens were detected at a very low frequency, which precluded their statistical evaluation, and indicators of fecal contamination had to be used instead. Briefly, in addition to testing of spinach for contamination with E. coli, all spinach, soil, and water samples were tested for contamination with L. monocytogenes, Salmonella spp., E. coli O157:H7, and Listeria spp. With spinach contamination being the main focus of the study, soil and water samples were not tested for generic E. coli contamination, and thus, no inference on the source of E. coli detected in spinach could be attempted. Regarding contamination with food-borne pathogens, no L. monocytogenes or E. coli O157:H7 was detected, and only 1 out of 955 samples was contaminated with Salmonella spp. Similarly, 5 out of 191 soil drag samples tested positive for Salmonella spp.; they were detected on two farms (with 1 and 4 positive samples out of 25 samples tested per farm). Interestingly, the Salmonella species-positive spinach sample was collected on the farm that had 20% of spinach samples positive for generic E. coli, which was the second highest farm level prevalence detected during the study. This somewhat supports previous reports (12) about the usefulness of generic E. coli as an indicator microorganism. On the same farm, one soil drag sample also tested positive for Salmonella spp., and one tested positive for Listeria spp. (for Listeria spp., this was the only positive sample detected during the course of study). These results suggest that preharvest food-borne pathogen contamination of spinach does occur albeit at a low frequency, indicating that high resources would be needed to obtain a sample size sufficiently large for evaluation of management and environmental factors affecting pathogen contamination of spinach. Therefore, studies of produce contamination using indicator organisms, such as the current study, still provide a valuable alternative.
Several studies (14–16, 52–54) have evaluated the impact of farm management practices on produce contamination. Most of those studies focused on factors related to farm management. Compared to those studies, our study comprehensively assessed a large number of farm management and environmental factors, several of which were investigated for the first time. Nevertheless, our study did have several limitations. First, our study was based on a repeated cross-sectional study design, precluding conclusions about causality for produce contamination. Second, because we studied only spinach, caution should be exercised in extrapolating these results to other vegetables or fruits. Third, soil and irrigation water were not tested for contamination with generic E. coli. Finally, our study was limited to only 12 farms, and thus, some findings may have been coincidental. Future prospective longitudinal studies should be conducted in order to validate the plausibility of the identified risk factors including a variety of farm settings (e.g., greenhouse conditions) and climate environments (e.g., Northern or Eastern United States). Likewise, intervention trials should be conducted to investigate the effects of measures such as irrigation with good-quality water, stopping irrigation up to 5 days before harvest, and improving workers' personal hygiene to validate the findings that these interventions could reduce produce contamination with E. coli in the field.
In conclusion, microbial contamination of produce is influenced by farm management and environmental factors. Specifically, microbial contamination of produce seems strongly influenced by the time since last irrigation, workers' personal hygiene, and the use of the field prior to planting. Our study may serve as a template to investigate the role of farm and environmental factors in contamination of other produce with generic E. coli and other microorganisms relevant to food safety.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Agriculture and Food Research Initiative grant 2009-04261, program code 93233, to R.I. from the U.S. Department of Agriculture National Institute of Food and Agriculture (USDA-NIFA).
Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect the views of the USDA-NIFA.
We thank Alexandra Tudor, Kelly Horgan, Anna Van Stelten, and Jake Elder for assistance with sample collection, processing, and microbiological analyses. We also thank Martin Wiedmann and Laura K. Strawn from Cornell University for generously providing a questionnaire that we adapted for use in the current study.
Footnotes
Published ahead of print 10 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00474-13.
REFERENCES
- 1. European Commission 2007. Agricultural commodity markets past developments fruits and vegetables, an analysis of consumption, production and trade based on statistics from the Food and Agriculture Organization (FAO). European Commission, Brussels, Belgium. http://ec.europa.eu/agriculture/analysis/tradepol/worldmarkets/fruitveg/072007_en.pdf Accessed 5 January 2013
- 2. Dewaal CS, Hicks G, Barlow K, Alderton L, Vegosen L. 2006. Foods associated with foodborne illness outbreaks from 1990 through 2003. Food Prot. Trends 26: 466– 473 [Google Scholar]
- 3. Beuchat LR. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microb. Infect. 4: 413– 423 [DOI] [PubMed] [Google Scholar]
- 4. Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67: 2342– 2353 [DOI] [PubMed] [Google Scholar]
- 5. Franz E, van Bruggen AH. 2008. Ecology of E. coli O157:H7 and Salmonella enterica in the primary vegetable production chain. Crit. Rev. Microbiol. 34: 143– 161 [DOI] [PubMed] [Google Scholar]
- 6. Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 75: 123– 131 [DOI] [PubMed] [Google Scholar]
- 7. Ivanek R, Grohn YT, Wiedmann M. 2006. Listeria monocytogenes in multiple habitats and host populations: review of available data for mathematical modeling. Foodborne Pathog. Dis. 3: 319– 336 [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration 1998. Guide to minimize microbial food safety hazards for fresh fruits and vegetables. US Food and Drug Administration, Washington, DC: http://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ProduceandPlanProducts/UCM169112.pdf Accessed 5 January 2013 [Google Scholar]
- 9. Materon LA. 2003. Survival of Escherichia coli O157:H7 applied to cantaloupes and the effectiveness of chlorinated water and lactic acid as disinfectants. World J. Microbiol. Biotechnol. 19: 867– 873 [Google Scholar]
- 10. Adams M, Moss M. 2000. Food microbiology, 2nd ed. The Royal Society of Chemistry, Cambridge, United Kingdom: [Google Scholar]
- 11. Beuchat LR. 2006. Vectors and conditions for preharvest contamination of fruits and vegetables with pathogens capable of causing enteric diseases. Br. Food J. 108: 38– 53 [Google Scholar]
- 12. Natvig EE, Ingham SC, Ingham BH, Cooperband LR, Roper TR. 2002. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl. Environ. Microbiol. 68: 2737– 2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park S, Szonyi B, Gautam R, Nightingale K, Anciso J, Ivanek R. 2012. Risk factors for microbial contamination in fruits and vegetables at the preharvest level: a systematic review. J. Food Prot. 75: 2055– 2081 [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee A, Speh D, Diez-Gonzalez F. 2007. Association of farm management practices with risk of Escherichia coli contamination in pre-harvest produce grown in Minnesota and Wisconsin. Int. J. Food Microbiol. 120: 296– 302 [DOI] [PubMed] [Google Scholar]
- 15. Mukherjee A, Speh D, Dyck E, Diez-Gonzalez F. 2004. Preharvest evaluation of coliforms, Escherichia coli, Salmonella, and Escherichia coli O157:H7 in organic and conventional produce grown by Minnesota farmers. J. Food Prot. 67: 894– 900 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee A, Speh D, Jones AT, Buesing KM, Diez-Gonzalez F. 2006. Longitudinal microbiological survey of fresh produce grown by farmers in the upper Midwest. J. Food Prot. 69: 1928– 1936 [DOI] [PubMed] [Google Scholar]
- 17. Ingham SC, Fanslau MA, Engel RA, Breuer JR, Breuer JE, Wright TH, Reith-Rozelle JK, Zhu J. 2005. Evaluation of fertilization-to-planting and fertilization-to-harvest intervals for safe use of noncomposted bovine manure in Wisconsin vegetable production. J. Food Prot. 68: 1134– 1142 [DOI] [PubMed] [Google Scholar]
- 18. US Department of Agriculture 2012. Vegetables 2011 summary. US Department of Agriculture, Washington, DC: http://usda01.library.cornell.edu/usda/current/VegeSumm/VegeSumm-01-26-2012.pdf Accessed 5 January 2013 [Google Scholar]
- 19. US National Oceanic and Atmospheric Administration 2011. Climatological data. Annual summary. Colorado. 2010. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-A3DFB510-90D9-448B-80DE-C3F0419777F4.pdf Accessed 23 April 2013 [Google Scholar]
- 20. US National Oceanic and Atmospheric Administration 2011. Climatological data. Annual summary. Texas. 2010. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-060FA273-ED2B-4F1F-94F4-9B28478A4D75.pdf Accessed 23 April 2013 [Google Scholar]
- 21. US National Oceanic and Atmospheric Administration 2012. Climatological data. Annual summary. Colorado. 2011. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-2F9C4C7E-2025-4EA1-82F1-FA4C88DC52BE.pdf Accessed 23 April 2013 [Google Scholar]
- 22. US National Oceanic and Atmospheric Administration 2012. Climatological data. Annual summary. Texas. 2011. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-B5DF9D91-B12A-44FA-B746-1835E87E6D21.pdf Accessed 23 April 2013 [Google Scholar]
- 23. US National Oceanic and Atmospheric Administration 2013. Climatological data. Annual summary. Texas. February 2012. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-DFDA7087-CB33-41A4-AA70-C84A01ADC14C.pdf Accessed 23 April 2013 [Google Scholar]
- 24. US National Oceanic and Atmospheric Administration 2013. Climatological data. Annual summary. Texas. January 2012. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-F072DAAF-076A-46D6-9087-CCDAC68069D2.pdf Accessed 23 April 2013 [Google Scholar]
- 25. US National Oceanic and Atmospheric Administration 2013. Climatological data. Annual summary. Texas. March 2012. US National Oceanic and Atmospheric Administration, Washington, DC: http://www1.ncdc.noaa.gov/pub/orders/IPS-C6ED4CF8-5B40-498A-83F5-BA7C0B3031F6.pdf Accessed 23 April 2013 [Google Scholar]
- 26. US Natural Resources Conservation Service 2013. Soil data mart. US Department of Agriculture, Washington, DC: http://soildatamart.nrcs.usda.gov/ Accessed 23 April 2013 [Google Scholar]
- 27. Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. http://cran.r-project.org/web/packages/lme4/lme4.pdf Accessed 5 January 2013
- 28. Dohoo IR, Martin W, Stryhn H. 2010. Veterinary epidemiologic research, 2nd ed. VER Incorporated, Prince Edward Island, Canada [Google Scholar]
- 29.Baayen RH.languageR: data sets and functions with “analyzing linguistic data: a practical introduction to statistics.”. 2012. [Accessed 5 January 2013]. http://cran.r-project.org/web/packages/languageR/languageR.pdf.
- 30. Islam M, Doyle MP, Phatak SC, Millner P, Xiuping J. 2005. Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. 22: 63– 70 [Google Scholar]
- 31. Beuchat LR. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59: 204– 216 [DOI] [PubMed] [Google Scholar]
- 32. Barak JD, Liang AS. 2008. Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants. PLoS One 3: e1657. 10.1371/journal.pone.0001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitinoja L, Gorny JR. 1999. Postharvest technology for small-scale produce marketers: economic opportunities, quality and food safety. University of California, Davis, CA: http://extension.psu.edu/food-safety/farm/resources/packing/p/at_download/file Accessed 5 January 2013 [Google Scholar]
- 34. Gelting RJ, Baloch MA, Zarate-Bermudez MA, Selman C. 2011. Irrigation water issues potentially related to the 2006 multistate E. coli O157:H7 outbreak associated with spinach. Agr. Water Manage. 98:1395– 1402 [Google Scholar]
- 35. Islam M, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J. Food Prot. 67: 1365– 1370 [DOI] [PubMed] [Google Scholar]
- 36. Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Fate of Salmonella enterica serovar Typhimurium on carrots and radishes grown in fields treated with contaminated manure composts or irrigation water. Appl. Environ. Microbiol. 70: 2497– 2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Persistence of Salmonella enterica serovar Typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog. Dis. 1: 27– 35 [DOI] [PubMed] [Google Scholar]
- 38. US Department of Agriculture 2000. National organic program; final rule. 7 CFR part 205. US Department of Agriculture, Washington, DC: http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5087165 Accessed 5 January 2013 [Google Scholar]
- 39. Ingham SC, Losinski JA, Andrews MP, Breuer JE, Breuer JR, Wood TM, Wright TH. 2004. Escherichia coli contamination of vegetables grown in soils fertilized with noncomposted bovine manure: garden-scale studies. Appl. Environ. Microbiol. 70: 6420– 6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meals DW, Braun DC. 2006. Demonstration of methods to reduce E. coli runoff from dairy manure application sites. J. Environ. Qual. 35: 1088– 1100 [DOI] [PubMed] [Google Scholar]
- 41. Greene SK, Daly ER, Talbot EA, Demma LJ, Holzbauer S, Patel NJ, Hill TA, Walderhaug MO, Hoekstra RM, Lynch MF, Painter JA. 2008. Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol. Infect. 136: 157– 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomon EB, Pang HJ, Matthews KR. 2003. Persistence of Escherichia coli O157:H7 on lettuce plants following spray irrigation with contaminated water. J. Food Prot. 66: 2198– 2202 [DOI] [PubMed] [Google Scholar]
- 43. Ibekwe AM, Watt PM, Shouse PJ, Grieve CM. 2004. Fate of Escherichia coli O157:H7 in irrigation water on soils and plants as validated by culture method and real-time PCR. Can. J. Microbiol. 50: 1007– 1014 [DOI] [PubMed] [Google Scholar]
- 44. Orozco RL, Iturriaga MH, Tamplin ML, Fratamico PM, Call JE, Luchansky JB, Escartin EF. 2008. Animal and environmental impact on the presence and distribution of Salmonella and Escherichia coli in hydroponic tomato greenhouses. J. Food Prot. 71: 676– 683 [DOI] [PubMed] [Google Scholar]
- 45. Burns TE, Ribble C, Stephen C, Kelton D, Toews L, Osterhold J, Wheeler H. 2012. Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. Can. Vet. J. 53: 158– 166 [PMC free article] [PubMed] [Google Scholar]
- 46. Byappanahalli MN, Whitman RL, Shively DA, Sadowsky MJ, Ishii S. 2006. Population structure, persistence, and seasonality of autochthonous Escherichia coli in temperate, coastal forest soil from a Great Lakes watershed. Environ. Microbiol. 8: 504– 513 [DOI] [PubMed] [Google Scholar]
- 47. Ishii S, Ksoll WB, Hicks RE, Sadowsky MJ. 2006. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 72: 612– 621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernstein N, Sela S, Neder-Lavon S. 2007. Assessment of contamination potential of lettuce by Salmonella enterica serovar Newport added to the plant growing medium. J. Food Prot. 70: 1717– 1722 [DOI] [PubMed] [Google Scholar]
- 49. Mootian G, Wu WH, Matthews KR. 2009. Transfer of Escherichia coli O157:H7 from soil, water, and manure contaminated with low numbers of the pathogen to lettuce plants. J. Food Prot. 72: 2308– 2312 [DOI] [PubMed] [Google Scholar]
- 50. Pu S, Beaulieu JC, Prinyawiwatkul W, Ge B. 2009. Effects of plant maturity and growth media bacterial inoculum level on the surface contamination and internalization of Escherichia coli O157:H7 in growing spinach leaves. J. Food Prot. 72: 2313– 2320 [DOI] [PubMed] [Google Scholar]
- 51. Robbins AR. 1974. 25 vegetables anyone can grow. Dover, New York, NY [Google Scholar]
- 52. Barker-Reid F, Harapas D, Engleitner S, Kreidl S, Holmes R, Faggian R. 2009. Persistence of Escherichia coli on injured iceberg lettuce in the field, overhead irrigated with contaminated water. J. Food Prot. 72: 458– 464 [DOI] [PubMed] [Google Scholar]
- 53. Cote C, Quessy S. 2005. Persistence of Escherichia coli and Salmonella in surface soil following application of liquid hog manure for production of pickling cucumbers. J. Food Prot. 68: 900– 905 [DOI] [PubMed] [Google Scholar]
- 54. Johannessen GS, Froseth RB, Solemdal L, Jarp J, Wasteson Y, Rorvik LM. 2004. Influence of bovine manure as fertilizer on the bacteriological quality of organic iceberg lettuce. J. Appl. Microbiol. 96: 787– 794 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.