Abstract
Here, we report a new zinc-inducible expression system for Lactococcus lactis, called Zirex, consisting of the pneumococcal repressor SczA and PczcD. PczcD tightly regulates the expression of green fluorescent protein in L. lactis. We show the applicability of Zirex together with the nisin-controlled expression system, enabling simultaneous but independent regulation of different genes.
TEXT
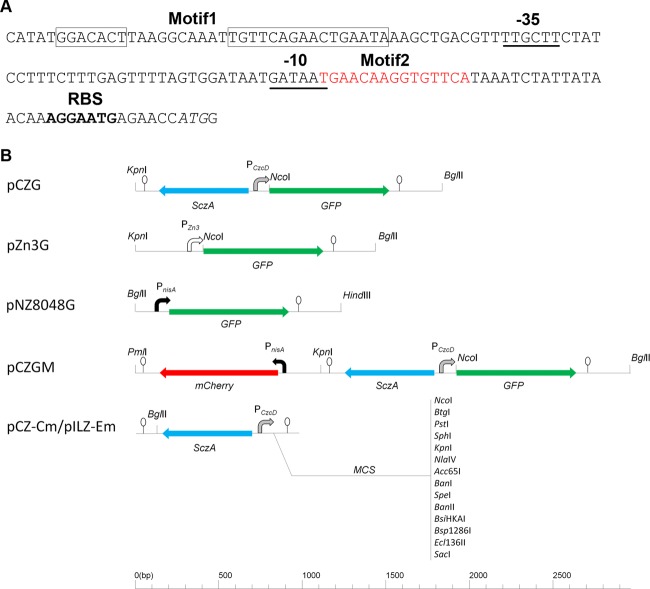
Lactococcus lactis is a Gram-positive bacterium that has been intensively engineered for the production of heterologous proteins (1, 2). In addition, it is an organism generally recognized as safe (GRAS). To date, several promoters originally from Lactococcus, regulated by inducers or environmental factors, have been documented, including the dnaJ promoter, induced by heat shock (3); the PA170 promoter, which can be upregulated at a low pH during the transition to stationary phase (4); the prtP promoter, which is regulated by the peptide concentration in the medium (5); and the PZn zitR promoter, which responds to divalent cation starvation (6). The promoter of nisin, PnisA, is the most widely used promoter for inducible protein expression in L. lactis (1, 7) and other Gram-positive bacteria (8). The expression from the PnisA promoter is regulated by the two-component regulatory system NisRK, which is triggered by nisin. For the other promoters mentioned above, there are still some drawbacks, such as relatively low induction levels or high background level at the uninduced stage, which may complicate efforts to tightly control the expression or coexpression of one or two different proteins in the same cell (9). The aims of the present work were to develop a novel tightly controlled promoter for L. lactis and to investigate if such an inducible promoter system could be coupled to the PnisA promoter to create a dual-promoter-regulated production system for different proteins. First, we searched in the genome of L. lactis MG1363 (NCBI reference sequence NC_009004.1) for proteins putatively involved in cation transport that may be regulated by the presence of cations. A putative promoter, namely, PZn3, preceding the translation of a cationic ion efflux protein (NCBI reference sequence YP_001032214.1) in L. lactis was further investigated (see below). Additionally, we explored the genome of other related Gram-positive bacteria for cation-regulated promoters. In the case of Streptococcus pneumoniae, a zinc-inducible promoter was previously described by Kloosterman et al. (10) and Eberhardt et al. (11). sczA and czcD are transcribed divergently (Fig. 1A). The promoter of czcD gene is regulated by SczA. SczA binding to the motif 2 sequence located downstream of the −10 sequence of PczcD blocks transcription of czcD in the absence of zinc. After the addition of zinc, SczA will move to motif 1 unblocking the transcription (10).
Fig 1.
(A) Nucleic acid sequence of PczcD. Motif1 (in boxes) and Motif2 (red letters) are the binding sites for the SczA regulator. The start codon of czcD is indicated in italics. The ribosome binding site (RBS) of PczcD is shown in boldface. −35 and −10 sequences are underlined (10). (B) Expression systems used in this study. Hairpins represent terminators.
Primers czcD-f and czcD-r (Table 1) were designed to amplify the regulator protein SczA and the PczcD region from the S. pneumoniae D39 (12) genome, including the restriction sites KpnI and NcoI, respectively. The gene coding for green fluorescent protein (GFP) with its own terminator was amplified from pJWV102_gfp (a kind gift from J. W. Veening) by PCR with primers gfp-f and gfp-r. A BglII site was added on the 5′ end of primer gfp-r. czcD-r and gfp-f were designed to be reverse complementary by overlapping the 5′ ends of each other, and an NcoI site was inserted in both primers. The fragment SczA-PczcD-GFP was generated by spliced overlap extension PCR with primers czcD-f and gfp-r using the mixture of SczA-PczcD and GFP-specifying amplicons as the templates (13). After digestion with KpnI and BglII, SczA-PczcD-GFP was cloned into pNZ8048 (7), digested with the same enzymes to create the plasmid pCZG (Fig. 1B). pZn3G was constructed based on pCZG, in which the gfp gene was controlled by PZn3 (Fig. 1B). Unfortunately, the low production level obtained after induction with zinc and the leakage in the noninduced state made PZn3 an unsuitable candidate for further characterization (data not shown). pNZ8048G was created by cloning gfp amplified with the primers gfp-f and gfp-r2 (Table 1) in the NcoI-HindIII sites of pNZ8048. In pNZ8048G, GFP expression is under the control of PnisA (Fig. 1B).
Table 1.
Primers used in this study
| Primer | Sequencea | Restriction site(s) |
|---|---|---|
| czcD-f | CGGGGTACCGGATCCTGCAGGCAGATATAGTTGATAATCAAGG | KpnI, SbfI |
| czcD-r | CAGCTCTTCTCCTTTTCCCATGGTTCTCATTCCTTTGTTATAATAG | NcoI |
| gfp-f | CTATTATAACAAAGGAATGAGAACCATGGGAAAAGGAGAAGAGCTG | NcoI |
| gfp-r | GGAAGATCTATTAATCGAAATACGGGCAGAC | BglII |
| gfp-r2 | CCCAAGCTTCGAAATACGGGCAGAC | HindIII |
| mCherry-f | CGGGGTACCTCCTGGTTGCAAATTTTG | KpnI |
| mCherry-r | CGTACTCACGTGCTGCAAGGCGATTAAGTTG | PmlI |
| sczA-czcD-f | ATCAAGATCTAGAAATAAGACAACTGAAGCTTTAC | BglII |
| sczA-czcD-r | AGATCCATGGTTCTCATTCCTTTGTTATAATAG | NcoI |
| pIL-f | ATCAAGATCTACAGCAAAGAATGGCGGAAACG | BglII |
| pIL-r | AATCGATAAGCTTGGCTGCAGGTC |
Restriction sites engineered in the primers are underlined.
The expression assays were carried out in L. lactis NZ9000 (9), which was transformed with pCZG (containing SczA-PczcD-GFP) according to Holo and Nes (14). All of the expression assays were conducted at 30°C in a chemically defined medium for prolonged cultivation (CDMPC) without ZnSO4 supplemented with 10 μg/ml chloramphenicol (B. Teusink, F. Santos, O. P. Kuipers, C. E. Price, J. Kok, and D. Molenaar, unpublished data). Each assay was repeated in triplicate in a 96-well microtiter plate and monitored with an Infinite 200 Pro microplate spectrophotometer (Tecan Group, Ltd., Mannedorf, Switzerland).
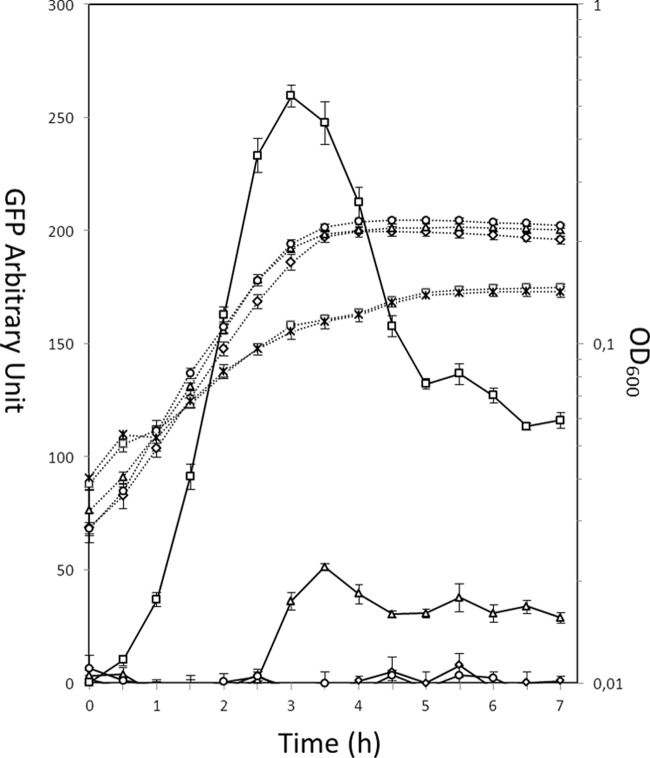
First, we investigated the optimal induction moment. For this purpose, ZnSO4 was added after 0 h, 2 h, or 4 h of growth at a final concentration of 0.5 mM. The cell growth was monitored measuring the optical density at 600 nm (OD600), and the signal of GFP was measured using an excitation wavelength of 485 nm and an emission wavelength of 535 nm (15). NZ9000(pNZ8048) was used as a negative control. We observed that the earlier we induced, the stronger the displayed signal was, with no induction observed in the stationary phase (Fig. 2). It should be noted that 0.5 mM ZnSO4 showed comparatively low toxicity when it was introduced before inoculation (0 h) of the strains with (pCZG) or without (pNZ8048) GFP. The addition after 2 h of growth does not cause a visible reduction in growth. To assess if the slower growth when the cells were induced at 0 h was caused by the Zn salt used in the study, we also studied the growth tendencies and fluorescent signals of cells induced with the same amount of Zn2+ using ZnCl2. The growth curves and the expression profiles were almost the same as those after induction with ZnSO4 (data not shown). These results indicate that different Zn2+ sources do not affect the toxicity or potency of the induction.
Fig 2.
Growth (dotted lines) and GFP expression level (solid lines) after induction with 0.5 mM zinc at different time points. NZ9000(pCZG) induced with ZnSO4 at a final concentration of 0.5 mM at 0 h (□), 2 h (△), and 4 h (○). Two control strains were run in parallel: NZ9000(pNZ8048) uninduced (◇) and induced with 0.5 mM ZnSO4 at 0 h (×). The values represent the means from three independent measurements.
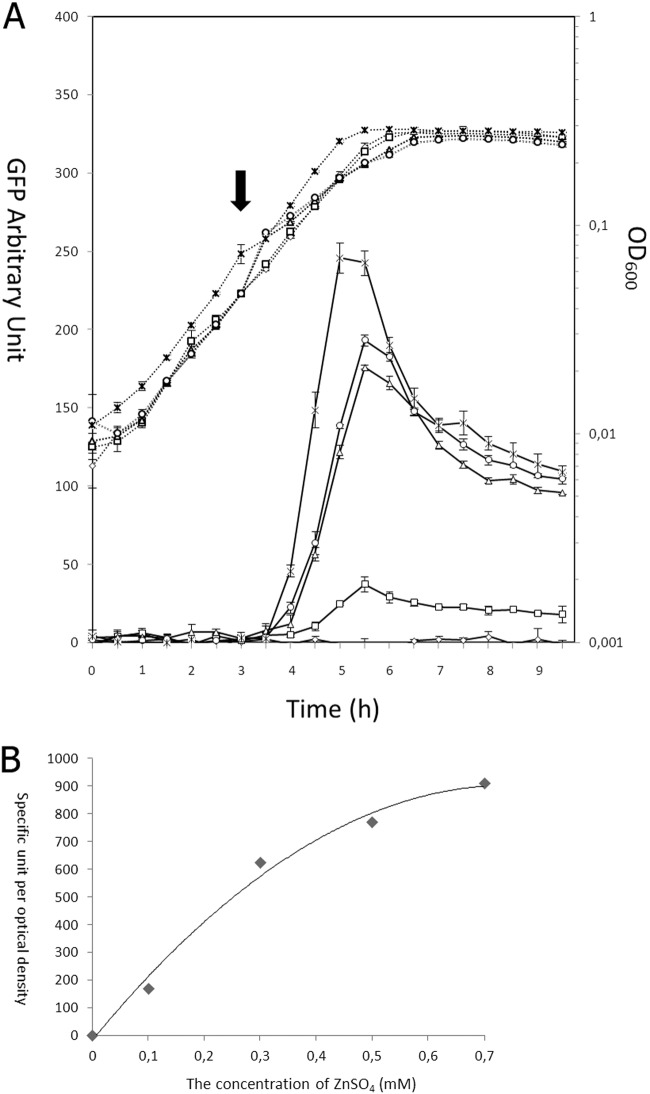
In order to assess the optimal concentration of ZnSO4 for the induction, NZ9000(pCZG) was induced at an OD600 of 0.06 (the middle of the exponential phase) with a final concentration of 0, 0.1, 0.3, 0.5, 0.7, or 1.0 mM ZnSO4. NZ9000(pNZ8048G) grown in CDMPC was induced with 5 ng/ml of nisin at an OD600 of 0.06 as a reference. The growth rate was not affected by the addition of ZnSO4, and the GFP signal produced by NZ9000(pNZ8048G) or NZ9000(pCZG) reached the highest level after 2 h or 2.5 h of induction, respectively (Fig. 3A). The highest intensity of GFP produced using the zinc-inducible system was almost 80% of that produced with the nisin-inducible system. Moreover, the GFP signal in the induced cells increased nearly proportionally with the ZnSO4 concentration in the range between 0 and 0.3 mM (Fig. 3B). Furthermore, almost no fluorescent signal was detected under uninduced conditions, which demonstrated that this pneumococcal system is also effectively repressed in the absence of zinc in L. lactis.
Fig 3.
(A) Growth (dotted lines) and GFP intensity (solid lines) after induction with different zinc concentrations. L. lactis NZ9000(pCZG) was induced at an OD600 of 0.06 with different concentrations of ZnSO4: 0 (◇), 0.1 mM (□), 0.7 mM (△), or 1.0 mM (○). A control, NZ9000(pNZ8048G) (×), induced with 5 ng/ml of nisin was also used to compare the production levels of PczcD and PnisA. The arrow indicates the time point for induction. These values represent the means from three independent measurements. (B) Dose-response curve of GFP expression of L. lactis NZ9000(pCZG). Fluorescent signal is shown as specific units per OD600. The fluorescent signal changed less sensitively at the concentration of ZnSO4 above 0.3 mM. The standard errors are less than 15% for each value.
pCZGM was constructed to observe the effect of the induction with nisin and zinc at the same time. In pCZGM, PnisA controls the expression of mCherry, whereas PczcD controls the expression of GFP. To construct this vector, a fragment encompassing from PnisA to the terminator of mCherry was amplified from pHK35C (a generous gift from H. Karsens) using the primers mCherry-f and mCherry-r, containing at their 5′ end a KpnI site and a PmlI site, respectively. After digestion with KpnI and PmlI, the fragment was inserted into pCZG cut with the same enzymes, resulting in pCZGM (Fig. 1B). The signal of mCherry was measured using an excitation wavelength of 590 nm and an emission wavelength of 620 nm, and GFP was measured as mentioned above. Cultures were induced with 0.7 mM ZnSO4 at an OD600 of 0.06 and with 5 ng/ml nisin 1 h later. Uninduced controls lacking either nisin or zinc were run in parallel. In Table 2, we can observe the expression level of GFP or mCherry achieved after 2.5 h of the induction with ZnSO4 or nisin. These data show that simultaneous overexpressions of mCherry and GFP in this system cause around 23% and 11% reduction of the two fluorescent signals, respectively.
Table 2.
Simultaneous production of GFP and mCherry
| ZnSO4 as inducer (0.7 mM)a | Nisin as inducer (5 ng/ml 1 h later)a | Fluorescent intensity (AU)b |
|
|---|---|---|---|
| GFP (2.5 h) | mCherry (2.5 h) | ||
| + | − | 181.00 ± 8.01 | 0.67 ± 4.19 |
| + | + | 138.00 ± 2.49 | 544.33 ± 17.68 |
| − | + | 5.33 ± 3.00 | 612.00 ± 39.04 |
+, inducer present; −, inducer absent.
AU, arbitrary units.
Based on the results described above, we created pCZ-Cm for general use as a chloramphenicol-resistant expression vector for L. lactis. In this vector, the multiple-cloning site (MCS) of pNZ8048 was fused behind PczcD. For this purpose, the region SczA-PczcD was amplified from pCZG with the primers sczA-czcD-f and sczA-czcD-r (Table 1). After digestion with BglII and NcoI, the fragment SczA-PczcD was inserted into pNZ8048 digested with the same restriction enzymes, rendering pCZ-Cm (Fig. 1B). An additional expression vector, termed pILZ-Em, containing this zinc-inducible expression system with the same MCS and erythromycin resistance was also constructed from the plasmid pIL253 (Fig. 1B) (16). A BglII site was inserted into pIL253 by round PCR with the primers pIL-f and pIL-r (Table 1) in order to insert the BglII-SacI region from pCZ-Cm.
In order to assess the usefulness of this double inducible system, the structural gene of nisin, nisA, was cloned into plasmid pCZ-Cm under the control of PczcD and transformed into NZ9800 (17). In this strain, the enzymes responsible for the maturation and modification of nisin are controlled by PnisA. Comparison of the production of nisin in CDM medium (18, 19) with a constant concentration of nisin and various amounts of ZnSO4 was performed (Fig. 4). We measured the production of nisin using an activity test against L. lactis NZ9000 (20). The activity assay clearly shows that nisin can be successfully expressed in the system in a tightly regulated fashion when the gene nisA is controlled by PczcD and the modification enzymes are regulated by nisin (Fig. 4).
Fig 4.
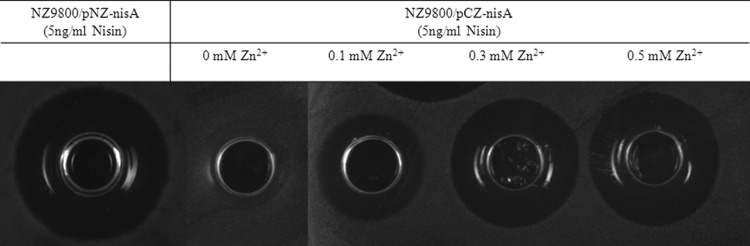
Expression of nisin measured in terms of activity. In the control strain, NZ9800(pNZ-nisA) (20), nisA and the modification enzymes that process nisin are controlled by PnisA. In NZ9800(pCZ-nisA), nisA was controlled by PczcD. A constant concentration of nisin (5 ng/ml) was used to induce both strains. In NZ9800(pCZ-nisA), no activity was detected in the absence of Zn2+, whereas increasing amounts of Zn2+ led to the production of nisin proportionally to the concentration used.
In our study, we introduced the streptococcal promoter PczcD together with the gene coding for its regulatory protein, SczA, in L. lactis, yielding an effective zinc-regulated expression system, called Zirex. Our results clearly show that this system can effectively control the overexpression of proteins in response to modest and nontoxic zinc additions to the medium in L. lactis. The very low basal expression without inducer suggests that SczA is also expressed in L. lactis and tightly represses the system in the absence of zinc. Notably, L. lactis showed high tolerance to zinc in the millimolar range when induced in the exponential phase (6). Previously, a zinc-repressed expression system (PZn zitR promoter) was reported. It was based on the L. lactis zit operon, which encodes an emergency Zn2+ uptake ABC transporter (6). The presence of Zn2+ can repress the expression of the emergency Zn2+ uptake ABC transporter, which partly explains the high tolerance of L. lactis to Zn2+. The initiation of the PZn zitR promoter is caused by the addition of a chelating agent, which reduces the available zinc in the medium, therefore activating the transcription of the emergency uptake system (6). A drawback of this system is that the induction based on the depletion of Zn2+, which is achieved with the addition of EDTA, can hamper the overexpression of proteins or enzymes that require cations. The zinc-inducible system presented here constitutes, to our knowledge, the first zinc-inducible promoter developed for L. lactis. It can be extremely useful for the overproduction of enzymes such as lanthipeptide cyclases, which require Zn2+ to be active, or other metalloenzymes. This advantage makes the expression system presented in this paper a suitable candidate for the production of lanthipeptides (21). So far the nisin-inducible expression (NICE) system is the most widely used and potent protein expression system in L. lactis. Compared to the nisin-inducible system, the common drawbacks of other regulated expression systems found in L. lactis are their low expression level and/or high leakage (9). The zinc-inducible system presented here achieves a high expression level comparable to that of nisin (ca. 80%), which is higher than the expression level obtained with the PZn zitR promoter (20% of that achieved with nisin) (6). Moreover, we demonstrate that it is possible to combine both inducible promoters for the expression of different proteins at different times during cell growth. This can be a useful tool for the overexpression of proteins or the creation of controlled gene regulatory circuits in L. lactis and expands the toolbox available for this bacterium. Moreover, the plasmid described here could be directly applicable for use in other Gram-positive hosts, as is the case for the NICE system, although this has to be further investigated to assess the specific characteristics.
ACKNOWLEDGMENTS
We thank Tomas G. Kloosterman for the genomic DNA sample of S. pneumoniae D39 and helpful discussions.
D. Mu was supported by funding from the China Scholarship Council (no. 2010605032). M. Montalbán-López was supported by the NWO-ESF EuroSynbio program on a project called SynMod. Y. Masuda was funded by a grant from SNN (Province of Groningen) on project T2006, LanthioPEP-RUG.
Footnotes
Published ahead of print 10 May 2013
REFERENCES
- 1. de Ruyter PG, Kuipers OP, De Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dieye Y, Usai S, Clier F, Gruss A, Piard J. 2001. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 183:4157–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Asseldonk M, Simons A, Visser H, De Vos WM, Simons G. 1993. Cloning, nucleotide sequence, and regulatory analysis of the Lactococcus lactis dnaJ gene. J. Bacteriol. 175:1637–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madsen SM, Arnau J, Vrang A, Givskov M, Israelsen H. 1999. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis. Mol. Microbiol. 32:75–87 [DOI] [PubMed] [Google Scholar]
- 5. Marugg JD, Meijer W, van Kranenburg R, Laverman P, Bruinenberg PG, De Vos WM. 1995. Medium-dependent regulation of proteinase gene expression in Lactococcus lactis: control of transcription initiation by specific dipeptides. J. Bacteriol. 177:2982–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llull D, Poquet I. 2004. New expression system tightly controlled by zinc availability in Lactococcus lactis. Appl. Environ. Microbiol. 70:5398–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15–21 [Google Scholar]
- 8. Renye J, Jr, Somkuti G. 2010. Nisin-induced expression of pediocin in dairy lactic acid bacteria. J. Appl. Microbiol. 108:2142–2151 [DOI] [PubMed] [Google Scholar]
- 9. Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM. 1997. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 15:135–140 [DOI] [PubMed] [Google Scholar]
- 10. Kloosterman TG, Der Kooi-Pol V, Magdalena M, Bijlsma JJ, Kuipers OP. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 65:1049–1063 [DOI] [PubMed] [Google Scholar]
- 11. Eberhardt A, Wu LJ, Errington J, Vollmer W, Veening J. 2009. Cellular localization of choline-utilization proteins in Streptococcus pneumoniae using novel fluorescent reporter systems. Mol. Microbiol. 74:395–408 [DOI] [PubMed] [Google Scholar]
- 12. Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higuchi R, Krummel B, Saiki R. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holo H, Nes I. 1995. Transformation of Lactococcus by electroporation. Methods Mol. Biol. 47:195–199 [DOI] [PubMed] [Google Scholar]
- 15. Solopova A, Bachmann H, Teusink B, Kok J, Neves AR, Kuipers OP. 2012. A specific mutation in the promoter region of the silent cel cluster accounts for the appearance of lactose-utilizing Lactococcus lactis MG1363. Appl. Environ. Microbiol. 78:5612–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon D, Chopin A. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559–566 [DOI] [PubMed] [Google Scholar]
- 17. Kuipers OP, Beerthuyzen MM, Siezen RJ, De Vos WM. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281–291 [DOI] [PubMed] [Google Scholar]
- 18. Otto R, Brink B, Veldkamp H, Konings WN. 1983. The relation between growth rate and electrochemical proton gradient of Streptococcus cremoris. FEMS Microbiol. Lett. 16:69–74 [Google Scholar]
- 19. Poolman B, Konings WN. 1988. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J. Bacteriol. 170:700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Heel AJ, Mu D, Montalbán-López M, Hendriks D, Kuipers OP. 1 February 2013. Designing and producing modified, new-to-nature, peptides with antimicrobial activity by use of a combination of various lantibiotic modification enzymes. ACS Synth. Biol. 10.1021/sb3001084 [DOI] [PubMed] [Google Scholar]
- 21. Li B, Yu JPJ, Brunzelle JS, Moll GN, Van der Donk WA, Nair SK. 2006. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science 311:1464–1467 [DOI] [PubMed] [Google Scholar]