Abstract
A number of operons encoding the nutrient germinant receptors (GRs) in dormant spores of Bacillus megaterium and Bacillus subtilis species have small open reading frames (ORFs) of unknown function within or immediately adjacent to the operons. Inactivation of the genes in these ORFs, encoding proteins now termed D proteins, either significantly increased or decreased spore germination via the associated GR but had no effects on germination via non-GR-dependent germinants. These effects on GR-dependent germination were complemented by ectopic expression of the appropriate D gene (gene encoding D protein). However, substitution of noncognate D genes in two GR operons resulted in inhibition of germination via the GR manipulated, although ectopic overexpression of a D gene had no effect on overall GR-dependent germination. The various D genes studied were expressed in the forespore during sporulation in parallel with the associated GR operon, and transcription of a B. subtilis D gene was controlled by RNA polymerase sigma factor σG. These results indicate that proteins encoded by small ORFs within or adjacent to operons encoding GRs play major roles in modulating GR function in spores of Bacillus species. In B. subtilis, deletion of a D gene (B. subtilis gerKD [gerKDbs]) adjacent to the gerK operon encoding the GerK GR or ectopic expression or overexpression of gerKDbs had no major effect on the levels of GR subunits or of two other germination proteins.
INTRODUCTION
Spores of various Bacillus species are metabolically dormant and extremely resistant to a variety of harsh treatments (1). As a consequence, such spores can survive for years in the absence of exogenous nutrients. However, these spores constantly sense their environment, and if nutrients become available, spores can rapidly return to life in the process of germination followed by outgrowth (2, 3). Nutrients generally trigger spore germination through their interactions with proteins called germinant receptors (GRs) located in the inner membranes of spores, and Bacillus spores most often have multiple GRs, each with a different specificity for a nutrient germinant or nutrient germinant mixture.
GRs are thought to be composed of three protein subunits, termed A, B, and C, with the A and B subunits likely being integral membrane proteins and the C subunit likely being a lipid-anchored peripheral membrane protein. By far the best-studied Bacillus species is Bacillus subtilis, and spores of this species contain five operons, each encoding the three subunits of similar GRs. These operons are expressed most likely in parallel late in sporulation in the developing spore and under the control of the forespore-specific RNA polymerase sigma factor σG (2–6). The functions of two of these GRs, those encoded by the yndEFG and yfkQRST operons are not known, as deletions of either one or both of these operons have no effect on spore germination, even in the absence of the other three GRs (7). The GerA GR responds specifically to l-alanine or l-valine alone, while the GerB and GerK GRs are required together for germination with a mixture of l-asparagine (or l-alanine) plus d-glucose, d-fructose, and K+ ions called AGFK (3, 8). In B. subtilis, all three GR subunits are required for a particular GR's function, and GRs are localized together in a cluster termed the germinosome in the inner membranes of spores, with this clustering essential for rapid spore germination (9).
Bacillus megaterium belongs to a deeply rooted phylogenetic lineage within the Bacillus genus, being quite distinct from members of the Bacillus subtilis and Bacillus cereus families. Its genome contains six GR operons, the best studied of which is the plasmid-borne gerU operon, which encodes a GR that can trigger spore germination in response to glucose, proline, leucine, or certain inorganic salts (10, 11). The gerU operon is unusual in that products of three different genes that encode GR B-subunit proteins have been shown to interact with the products of the gerUA and gerUC genes to produce GRs with overlapping germinant recognition patterns, demonstrating at the same time a rarely observed interchangeability between GR subunits. Of the five chromosomal GR operons, four have been characterized as being functional via mutagenesis analyses (36). However, whereas the GerU receptor can trigger germination in response to single germinant compounds, the chromosomally encoded GRs all appear to require combinations of germinants—typically glucose plus inorganic salts and certain amino acids—to promote efficient germinative responses.
Recently, the presence of a fourth protein that might be a component of at least some GRs was suggested in a bioinformatic analysis of genes encoding GRs in both Bacillus and Clostridium species (2). This putative GR component, termed a D protein, is a 60- to 85-amino-acid (aa) protein encoded by a gene either within or adjacent to a GR operon. In the current work, we have examined the expression, location, and function of the putative GR D proteins in both Bacillus megaterium and B. subtilis and present strong evidence that these new proteins can modulate rates of GR-dependent spore germination.
MATERIALS AND METHODS
Bacillus strains.
The Bacillus strains used in this work are listed in Table 1. B. subtilis strains are derivatives of strain 168 and are isogenic derivatives of strain PS832. The B. subtilis strain with a gerB-lacZ fusion in the PS832 background was generated by transformation of strain PS832 to MLSr (resistance to erythromycin plus lincomycin) with chromosomal DNA from strain AM1247 (12), giving strain PS3709. A B. subtilis strain with much of the B. subtilis gerKD (hereafter termed gerKDbs) coding sequence replaced by a tetracycline resistance (Tcr) cassette was constructed as follows. The Tcr cassette was PCR amplified from plasmid pFE149 (7), and the upstream region of the gerKDbs gene (−500 to +19 relative to the gerKDbs translation start site [+1]) and the downstream region of the gerKDbs gene (+222 to +721) were PCR amplified from chromosomal DNA of strain PS832. The latter two PCR products plus the amplified Tcr cassette were used for a three-way overlap PCR, and the product was purified and ligated to pGEM-T Easy vector (Promega Corp., Madison, WI) to generate plasmid pXY1223 in Escherichia coli. The tet gene's promoter is oriented in the direction opposite that of the B. subtilis gerK (hereafter termed gerKbs) operon to minimize the effects of transcription of the tet gene on transcription of the gerKbs operon. Plasmid pXY1223 was used to transform B. subtilis strain PS832 to Tcr by a double-crossover event, giving strain PS4256; the expected chromosome structure in the gerKDbs region of this strain was confirmed by PCR.
Table 1.
Bacillus strains used in this study
| Bacillus strain | Relevant genotype or phenotype or descriptiona | Reference or source |
|---|---|---|
| B. subtilis strains | ||
| AM1247b | gerB-lacZ; MLSr | 12 |
| PS533 | Wild-type; Kmr | 34 |
| PS767 | gerA-lacZ; MLSr | 4 |
| PS832 | Wild-type prototroph; laboratory strain | |
| PS3709 | gerB-lacZ; MLSr | This work |
| PS4256 | ΔgerKDbs; Tcr | This work |
| PS4290 | gerKDbs-lacZ; Cmr | This work |
| PS4293c | gerKDbs-lacZ ΔsigE; Cmr MLSr | This work |
| PS4294c | gerKDbs-lacZ ΔsigF; Cmr | This work |
| PS4295c | gerKDbs-lacZ ΔsigKbs; Cmr MLSr | This work |
| PS4296c | gerKDbs-lacZ ΔsigG; Cmr Kmr | This work |
| PS4313 | ΔgerKDbs amyE::gerKDbs; Cmr Tcr | This work |
| PS4314 | amyE::PsspB-gerKDbs; Cmr | This work |
| PS4319 | gerA-lacZ amyE::PsspB-gerKDbs; Cmr MLSr | This work |
| PS4320 | gerB-lacZ amyE::PsspB-gerKDbs; Cmr MLSr | This work |
| PY79c | Wild-type | R. Losick |
| SC137c | ΔsigE; MLSr | 35 |
| SC1159c | ΔsigF | 35 |
| SC64c | ΔsigK; MLSr | 35 |
| RL831c | ΔsigG; Kmr | 35 |
| B. megaterium strains | ||
| QM B1551 | Wild-type | P. S. Vary |
| PV361 | Plasmidless derivative of QM B1551; ΔgerU | P. S. Vary |
| GC614d | GR null (ΔgerU ΔgerKbm ΔgerK2 ΔgerA ΔgerA2); Kmr MLSr Cmr Spr | 36 |
| GC615e | GR null pHT-gerUD gerU*; Kmr MLSr Cmr Spr Tcr | This work |
| GC630e | GR null pHT-ΔgerUD gerU*; Kmr MLSr Cmr Spr Tcr | This work |
| GC631e | GR null pHT-gerUDM10stop gerU*; Kmr MLSr Cmr Spr Tcr | This work |
| GC632e | GR null pHT-gerUDM10stop gerU*, with gerUD located between gerUC and gerVB; Kmr MLSr Cmr Spr Tcr | This work |
| GC633e | GR null pHT-gerUD gerU*, with gerKDbm located between gerUC and gerVB; Kmr MLSr Cmr Spr Tcr | This work |
| GC634e | GR null pHT-gerUDM10stop gerU*, with gerKDbm located between gerUC and gerVB; Kmr MLSr Cmr Spr Tcr | This work |
| GC635e | GR null pHT-gerKbm; Kmr MLSr Cmr Spr Tcr | This work |
| GC636e | GR null pHT-gerKbm ΔgerKDbm; Kmr MLSr Cmr Spr Tcr | This work |
| GC637e | GR null pHT-gerKbm, with gerUD in place of gerKDbm; Kmr MLSr Cmr Spr Tcr | This work |
| GC638f | gerUA-lacZ; Kmr | 36 |
| GC639f | gerUD-lacZ; Kmr | This work |
| GC640f | gerKAbm-lacZ; Kmr | 36 |
| GC641f | gerKDbm-lacZ; Kmr | This work |
Abbreviations for antibiotic resistance: Kmr, kanamycin resistance (5 and 10 μg/ml for B. megaterium and B. subtilis, respectively); Spr, spectinomycin resistance (100 μg/ml); Cmr, chloramphenicol resistance (5 μg/ml); MLSr, resistance to erythromycin (1 μg/ml) plus lincomycin (25 μg/ml); Tcr, tetracycline resistance (12.5 μg/ml).
This strain has the SC64 background.
These strains have the PY79 genetic background.
This strain is isogenic with PV361.
These strains have the GC614 background and were transformed to Tcr with plasmid pHT315t carrying the described receptor genes. gerU*, GR operon comprising gerUA, gerUC, and gerVB.
QM B1551 genetic background.
A B. subtilis strain with a transcriptional fusion of the putative promoter region of gerKDbs to the E. coli lacZ gene was constructed as follows. The region between bp −447 to −1 relative to the putative gerKDbs translation start site (+1) was PCR amplified from B. subtilis PS832 DNA using primers containing EcoRI and BamHI sites (all primer sequences are available on request). The purified PCR product was digested with EcoRI and BamHI and then ligated to plasmid pDG268 (13) that was digested with the same restriction enzymes. The resulting plasmid, pPS4289, was isolated in E. coli and used to transform various B. subtilis strains to a chloramphenicol-resistant (Cmr) amylase-negative phenotype by a double-crossover event at the amyE locus.
A B. subtilis strain expressing gerKDbs at amyE in a ΔgerKDbs background with the gerKDbs gene expressed from its own likely promoter (see Results) was constructed as follows. A DNA fragment from −500 to +350 bp relative to the gerKDbs translation start site (defined as +1), and encompassing the likely gerKDbs promoter (see Results) and its coding sequence, was amplified from strain PS832 chromosomal DNA with primers containing an upstream EcoRI site and a downstream BamHI site. The PCR product was purified, digested with EcoRI and BamHI, and ligated to similarly cut plasmid pDG364 (14). The recombinant plasmid was isolated in E. coli and used to transform strain PS4256 (ΔgerKDbs) to a Cmr amylase-negative phenotype, giving strain PS4313. The double-crossover event leading to disruption of amyE by the gerKDbs gene plus the Cmr cassette in strain PS4313 was confirmed by PCR.
To construct a B. subtilis strain in which gerKDbs expression was under the control of the strong forespore-specific PsspB promoter (4), the region from −500 to −1 bp relative to the translation start site of the B. subtilis sspB gene (this region has PsspB as well as a strong ribosome binding site [RBS]) was amplified from PS832 DNA using primers with an EcoRI site in the upstream primer and a region of overlap with the gerKDbs translation start site in the downstream primer. The upstream primer for gerKDbs amplification was complementary to the downstream primer for PsspB, and the downstream gerKDbs primer would amplify the complete gerKDbs coding region plus 112 downstream bp that should include the likely gerKDbs transcription terminator and a 3′ BamHI site. For overlap PCR, we amplified a product that has a PsspB promoter plus RBS just upstream of the gerKDbs coding region and between the EcoRI and BamHI sites, using the amplified PsspB promoter and gerKDbs fragments as the template. The overlap PCR product of the expected size was purified, digested with EcoRI and BamHI, and ligated to similarly cut plasmid pDG364 and the recombinant plasmid was isolated in E. coli. This plasmid was used to transform B. subtilis strain PS832 to a Cmr amylase-negative phenotype by a double-crossover event, giving strain PS4314, and the expected genomic structure in the amyE region of this strain was confirmed by PCR. Chromosomal DNA from strain PS4314 was also used to transform strains PS767 and PS3709 to a Cmr MLSr amylase-negative phenotype, and the resultant strains were termed PS4319 and PS4320, respectively.
B. megaterium strain GC614, which is isogenic with the plasmidless QM B1551 derivative PV361 and lacks all functional GRs due to insertion-deletions in the A-cistrons of four respective GR loci and excision of the gerU-containing plasmid pBM700 (36), was the host strain for plasmid-based complementation analyses. A plasmid containing the entire B. megaterium gerK operon (hereafter referred to as gerKbm) plus upstream promoter sequence was prepared by ligating a 4.7-kb PCR fragment, amplified from QM B1551 genomic DNA using primers with BamHI sites at the 5′ ends (all primer sequences are available on request), with plasmid pHT315t (modified from the plasmid in reference [15]) digested with the same enzyme. The resulting plasmid, pHT-gerKbm, was purified from E. coli and used to transform B. megaterium GC614 to Tcr, giving strain GC635. The same plasmid served as the template for an inverse PCR using primers designed to remove the entire gerKDbm open reading frame (ORF), which upon blunt-end religation would leave a 20-bp region between the stop codon of gerKCbm and the predicted translational start site of gerKBbm; the latter is preceded by an appropriately positioned RBS. The resultant plasmid, pHT-(gerKbm ΔgerKDbm), was isolated from E. coli, verified by DNA sequencing, and used to transform strain GC614 to Tcr, giving strain GC636.
Construction of a pHT315-based plasmid containing the gerU* (gerUA, gerUC, and gerVB) receptor operon has been described previously (16). The cloned locus contains approximately 400 bp of sequence upstream of the predicted gerUA translational start site, within which the putative gerUD ORF is located. This entire region was amplified by PCR using primers with BamHI sites and ligated with plasmid pHT315t digested with the same enzyme to prepare plasmid pHT-(gerUD gerU*), which was compatible with complementation experiments in the multiantibiotic-resistant GC614 genetic background. A plasmid in which the entire gerUD ORF was deleted, but leaving the predicted gerUA promoter intact, was prepared by PCR amplification of the region comprising −132 bp relative to the gerUA translational start site to ∼200 bp downstream of the predicted gerVB stop codon. This DNA fragment, which had flanking BamHI restriction sites, was digested and ligated with pHT315t digested with the same enzyme to prepare plasmid pHT-ΔgerUD gerU* which was used to transform strain GC614 to Tcr, giving strain GC630. Site-directed mutagenesis (SDM), conducted with a QuikChange Lightning SDM kit (Agilent Technologies, Wokingham, United Kingdom), was used to prepare plasmid pHT-gerUDM10stop gerU*, in which the methionine encoded by predicted codon 10 of the gerUD ORF, was changed to a stop codon. This plasmid, designed to result in the expression of a severely truncated GerUD protein while minimizing potential disruption to the gerUA promoter, was used to transform strain GC614 to Tcr, giving strain GC631.
The Gibson Assembly technique (New England BioLabs, Hitchin, United Kingdom) was used to prepare plasmids in which either gerUD or gerKDbm were located between the gerUC and gerVB ORFs within the gerU* operon. First, a PCR fragment spanning 444 bp with respect to the gerUA translational start site to position 2826, which included ORFs for gerUDM10stop, gerUA, and gerUC, was prepared using plasmid pHT-gerUDM10stop gerU* as the template DNA. A second PCR fragment comprising the gerVB ORF plus 104 bp upstream of the gerVB translational start site and 200 bp downstream of the stop codon was prepared from the same plasmid. Finally, PCR fragments spanning the gerUD and gerKDbm ORFs were prepared from genomic DNA. The appropriate fragments, including BamHI-linearized pHT315t vector backbone were subsequently purified and assembled using Gibson Assembly master mix (New England BioLabs, Hitchin, United Kingdom), and the reaction mixtures were used to transform E. coli. Purified and sequence-validated pHT-(gerUDM10stop gerUA gerUC gerUD gerVB) was used to transform strain GC614 to Tcr, giving strain GC632. Similarly, plasmid pHT-(gerUDM10stop gerUA gerUC gerKDbm gerVB) was used to construct strain GC634. A similar approach was used to prepare a plasmid in which the gerKDbm ORF was located between gerUC and gerVB in a gerU* operon with the intact gerUD gene, serving as the basis of strain GC633. The Gibson Assembly technique was also used to create a pHT315-based plasmid encoding a modified gerKbm operon, in which the gerKDbm ORF was replaced with the gerUD ORF. Essentially, PCR fragments encoding (i) gerKAbm and gerKCbm ORFs plus upstream promoter sequence, (ii) the gerUD ORF, and (iii) the gerKBbm ORF plus 200 bp of downstream sequence were amplified from B. megaterium QM B1551 genomic DNA. The PCR fragments, plus BamHI-linearized pHT315t vector, were purified and then assembled with Gibson Assembly master mix. Plasmid pHT-(gerKAbm gerKCbm gerUD gerKBbm) was isolated from E. coli, verified by PCR and sequencing, and used to transform B. megaterium GC614 to Tcr, giving strain GC637.
B. megaterium strains with transcriptional fusions between the E. coli lacZ gene and either the gerU or gerKbm A and D genes (genes encoding the A and D proteins) were prepared essentially as described previously (17). PCR was used to amplify ∼500-bp DNA fragments starting at the predicted translational start sites for gerUA, gerUD, gerKAbm, and gerKDbm using gene-specific primers with 5′ extensions to create attB-flanked PCR products compatible with Gateway cloning (Life Technologies Ltd., Paisley, United Kingdom) into pDONRtet (17) entry plasmids. Entry plasmids were isolated from E. coli and used in a series of LR reactions to create appropriate receptor gene-pNFd13-derived (17) destination plasmids. pNFd13-derived plasmids were then introduced into B. megaterium QM B1551 via polyethylene glycol (PEG)-mediated protoplast transformation. Colonies that had undergone homologous recombination, integrating the pNFd13-derived plasmid at the cloned locus and placing lacZ under the control of the promoter of the designated receptor gene, were isolated after incubation on solid medium at 42°C. The correct construction of the various strains was confirmed by PCR.
Spore preparation and purification.
Spores of B. subtilis strains were prepared at 37°C on 2× Schaeffer's-glucose plates as described previously (18, 19). After incubation for 2 or 3 days, the plates were left an additional 1 to 3 days at 23°C, then scraped from plates, and washed with water by repeated centrifugation with intermittent sonication treatment. All B. subtilis spore preparations used in this work were free (>95%) from growing or sporulating cells and germinated spores as determined by phase-contrast microscopy.
B. megaterium spores were prepared by inoculating 200 ml of supplemented nutrient broth (SNB) (20) with 0.5 ml of a mid-log-phase SNB culture and incubated in 2-liter baffled flasks at 22°C (unless otherwise indicated) for 72 h. Spores were harvested and purified by repeated rounds of centrifugation (4,300 × g for 7 min at 4°C) and washing in sterile ice-cold deionized water, removing the upper layer of cellular debris with each cycle, until the spore pellet was observed by phase-contrast microscopy to comprise >99% dormant spores. Purified spores were stored on ice at an optical density at 600 nm (OD600) of ∼50.
Spore germination.
B. subtilis spores were germinated following heat shock (30 min at 75°C) and cooling on ice. Spores at an OD600 of 0.5 were germinated for 2.5 h at 37°C in 200 μl of 25 mM K-HEPES buffer (pH 7.4) with nutrient germinants added at various concentrations and with duplicate samples measured at all germinant concentrations. B. subtilis spore germination was monitored by measuring the release of the spores' large depot of dipicolinic acid (DPA) by inclusion of 50 μM TbCl3 in all germination mixtures and measuring Tb-DPA fluorometrically in a multiwell plate reader as described previously (21). For all germination experiments, rates of germination were determined in arbitrary units (AU) by determining the maximum rates of increases in Tb-DPA fluorescence and correcting the values for slight differences in the DPA contents in different spore preparations; these corrections were always ≤10%. In a few experiments, spores were germinated as described above but without Tb3+ present from the initiation of germination. Instead, at various times after germination was initiated, aliquots of the germinating culture were centrifuged, the supernatant fluid was made 50 μM in TbCl3 and Tb-DPA fluorescence was measured as described previously (22). Spore germination was also routinely monitored at the end of germination incubations by phase-contrast microscopy. The total amount of DPA present in spores was assessed by Tb-DPA fluorescence after DPA had been released from spores by boiling (21).
In addition to nutrient germinants that trigger spore germination via GRs, there are also several agents that trigger spore germination without either GR involvement or a heat shock requirement, including the cationic surfactant dodecylamine and the 1:1 chelate of Ca2+ and DPA (CaDPA) (1, 3). B. subtilis spores germinated in dodecylamine at 45°C in 25 mM K-HEPES buffer (pH 7.4) plus 50 μM TbCl3 with 1.2 mM dodecylamine and spores at an OD600 of 0.5, and germination was assessed by measuring the percentage of maximum Tb-DPA fluorescence obtained as described above. Germination of B. subtilis spores with CaDPA was at 23°C and in 60 mM CaDPA made to pH 7.5 with Tris base and with spores at an OD600 of 2. Spore germination with CaDPA was monitored by examining ∼100 individual spores at various times of germination by phase-contrast microscopy, since germinated spores become phase dark.
For B. megaterium spore germination, concentrated spore suspensions (OD600 of ∼50) were heat shocked (60°C for 10 min for gerU-associated experiments; 75°C for 30 min for gerKbm-associated experiments) and cooled on ice immediately prior to conducting germination assays. The physiological basis for apparent GR-specific heat shock regimens in B. megaterium has yet to be determined. Germination of B. megaterium spores was monitored by recording the decrease in optical density of 300-μl aliquots of spores suspended at an initial OD600 of 0.4 to 0.5 in 5 mM Tris-HCl (pH 7.5), supplemented with typically 5% (wt/vol) beef extract (for gerKbm-associated experiments) or 10 mM glucose or proline (for gerU-associated experiments), using a Perkin-Elmer EnVision-Xcite multilabel plate reader fitted with a 600-nm photometric filter. Germination assays were conducted typically for 90 min at 37°C, with 10 s of orbital shaking performed prior to OD600 measurements taken every minute. Experiments were conducted in triplicate, with at least two spore preparations for each strain.
Kinetic values associated with germination of B. megaterium spores were obtained by incubating heat-shocked spores at 37°C in 5 mM Tris-HCl (pH 7.5) containing various concentrations (0.1 mM to 1,000 mM) of glucose or proline. Germination data were plotted in SigmaPlot 11.0 (Systat Software Inc.), and the slope of the linear portion of curves was used to determine apparent Km and Vmax values using the program's ligand-binding macro. Plots of germinant concentration versus percent spore germination were analyzed with the same program to determine approximate concentrations of germinant required to stimulate the germination of 50% of the spore population in 60 min [K0.5 germ].
Analytical methods.
For analysis of the levels of germination proteins of B. subtilis spores, spores of various strains were prepared, purified, decoated, ruptured with lysozyme, and subjected to brief sonication treatment, giving a spore lysate; in some cases, the spore inner membrane fraction was isolated by differential centrifugation of the spore lysate (all methods described previously [23–26]). The levels of germination proteins were determined by Western blotting analyses on equal amounts of both spore lysate and inner membrane protein as determined by analysis of samples run on gels by SDS-polyacrylamide gel electrophoresis (PAGE), and staining the gels with Coomassie blue as described previously (23–27). The antisera used have been shown to be specific for various germination proteins, including the various GR subunits, as well as the GerD protein essential for rapid GR-dependent spore germination and the SpoVAD protein essential for DPA movement into and out of spores (2, 3).
For measurement of gerKDbs-lacZ expression during sporulation, B. subtilis strains were sporulated at 37°C by resuspension in Sterlini-Mandelstam medium (28). At various times after resuspension (defined as time zero of sporulation), duplicate aliquots were centrifuged and washed with water, and pellets were frozen for subsequent analysis. β-Galactosidase activity in these samples was determined after lysozyme permeabilization, assaying equal aliquots of cell suspension using methylumbelliferyl-β-d-galactoside (MUG) as the substrate in 1.2 ml, with incubation at 30°C and measuring methylumbelliferone production fluorometrically after 40 min as described previously (4, 12). Note that this assay does not measure β-galactosidase in spores that have become lysozyme resistant, due to the assembly of the mature coat structure on the outer surfaces of the spores. DPA accumulation in the samples from the resuspension cultures was monitored by first boiling cells pelleted from 1 ml of culture in 1 ml of water, followed by centrifugation and measurement of DPA in supernatant fluids by its fluorescence with Tb as described previously (21).
In a few cases, equal amounts (4 × 109) of dormant B. subtilis spores of either B. subtilis PS533 (wild type) or strains carrying gerA- or gerB-lacZ fusions with or without gerKDbs overexpression were prepared on plates. The spores were purified, decoated, and permeabilized with lysozyme, and 8 × 108 spores were assayed for β-galactosidase as described above.
With dormant B. megaterium spores, β-galactosidase activity was assayed in lysates from spores with lacZ transcriptional fusions to GR A and D genes. Typically, equal amounts of spores (1 ml at an OD600 of 10) were suspended in 0.6 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol [pH 7.0]) and then lysed by two rounds of shaking (instrument setting 6 for 30 s) in a FastPrep FP120 cell disrupter (Fisher Scientific, Loughborough, United Kingdom), with chilling on ice for 5 min between cycles. Spore lysates were recovered from the FastPrep tubes and centrifuged for 1 min at 15,000 × g, and 0.2 ml of 40 μg/ml MUG was added to the supernatant fluid. Reaction mixtures were incubated at 30°C for 40 min and then terminated by the addition of 0.4 ml of 1 M Na2CO3. Fluorescence associated with methylumbelliferone production was recorded in triplicate using a Tecan Infinite 200 series plate reader, using excitation and emission filters set at 365 nm and 450 nm, respectively.
For reverse transcriptase PCR (RT-PCR) to determine the time of expression of various GR A and D genes during B. megaterium sporulation, wild-type B. megaterium cells were cultured at 30°C in 200 ml SNB, and duplicate samples, adjusted to an OD600 of 10, were collected on an hourly basis following entry into stationary phase (designated time zero in sporulation), with this point determined by the plateauing of OD600 values recorded every 30 min during the growth phase of the culture. Samples were immediately centrifuged (15,000 × g for 1 min) and washed with RNAprotect bacterial reagent (Qiagen Ltd.), and the cell pellets were stored at −80°C until further analysis. RNA was subsequently extracted and purified from thawed cell pellets using an RNeasy minikit (Qiagen Ltd.), and then stored at −80°C. Approximately 1 μg of RNA from each sample was converted to cDNA using a QuantiTect reverse transcription kit (Qiagen Ltd., Manchester, United Kingdom), using random hexamers (Life Technologies Ltd., Paisley, United Kingdom) as primers for cDNA synthesis. Finally, cDNA samples for each time point served as templates for PCRs employing NovaTaq DNA polymerase (Merck Chemicals Ltd., Nottingham, United Kingdom), and gene-specific primers were designed to amplify ≤300-bp fragments of the genes of interest.
RESULTS
Identification of genes encoding putative D proteins in B. megaterium and B. subtilis.
Bioinformatic analysis of the B. megaterium QM B1551 genome revealed a number of ORFs encoding putative GR D proteins. The most conspicuous of these ORFs (BMQ_1272) is the third of four genes that comprise the gerKbm GR operon (Fig. 1) and is predicted to encode a 78-aa GerKDbm protein with two transmembrane (TM) domains. The GerKbm receptor has been characterized recently as responsible for triggering efficient germination in several rich undefined media, in particular solubilized beef extract, of B. megaterium spores that lack the GerU GR (36).
Fig 1.
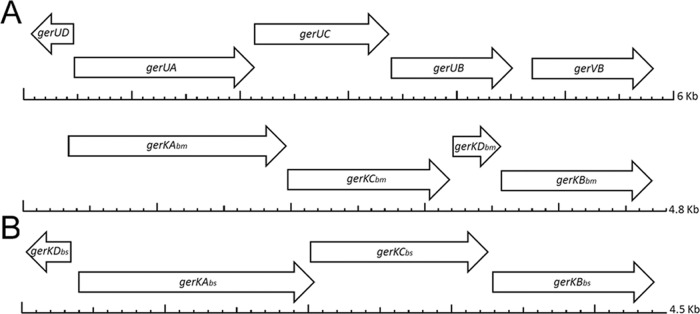
Locations of putative D genes in or adjacent to operons that encode GRs in B. megaterium (A) and B. subtilis (B). Scales below the genes are in kilobase pairs.
Several other putative GR D genes are evident on the B. megaterium chromosome, typically in the region of other GR-associated loci. These genes include BMQ_2235, predicted to encode a 78-aa protein with two TM domains located between two ORFs (BMQ_2236 and BMQ_2234) predicted to encode GR B-subunit proteins, which unusually do not appear to be organized in an operon with genes encoding associated A- and C-subunit proteins. Similarly, BMQ_3107, predicted to encode a 78-aa integral membrane protein, is immediately upstream of an apparently orphan GR B-subunit gene (BMQ_3108).
The best-studied B. megaterium GR, GerU, responsible for mediating spore germination with several single germinants, including either glucose or proline (11), also has a potential D protein encoded nearby. In this case, the putative D gene (BMQ_pBM70069) is located a short distance upstream of (133 bp) and on the opposite strand to the start codon of the first gene (gerUA) of the GR operon (Fig. 1). The provisionally entitled GerUD protein is predicted to comprise 76 aa and have two TM domains, similar to other putative D proteins identified in this work.
Three small genes encoding potential GR D proteins were also identified in B. subtilis as follows: (i) in the yfkQRST operon as the yfkS gene; (ii) as the ynzB gene immediately upstream of the yndEFG operon; and (iii) as the yczF gene (now termed gerKDbs; Fig. 1) immediately upstream of the gerKbs operon; note that the arrangement of gerKDbs and the gerKbs operon in B. subtilis is similar to that of gerUD and the gerU operon in B. megaterium (Fig. 1). All three of the putative B. subtilis D genes have a good RBS prior to the likely ATG translation initiation codon. YfkS and GerKDbs are also predicted to contain two TM segments by multiple prediction programs (data not shown). The expression of yfkS parallels that of yfkQ, yfkR, and yfkT as expected (29), given the presence of all four genes in an operon. In addition, gerKDbs transcription largely parallels that of gerKA, the first gene in the immediately adjacent gerKbs operon, although ynzB transcription does not parallel that of the yndDFG operon (29). These data together are consistent with at least the YfkS and GerKDbs proteins playing some role in the function of the YfkQRST and GerKbs GRs, respectively.
Since at least some of the D proteins described above might play roles in GR function, with B. subtilis we focused on analysis of the role and regulation of expression of GerKDbs in the function of the GerKbs GR, since the role of this GR in spore germination is known, in contrast to that of the GR encoded by the yfkQRST operon (7). With B. megaterium we examined the function and expression of GerKDbm and GerUD, since the functions of the GerKbm and GerU GRs in spore germination have been determined (10, 11).
Effects of deletion, complementation, and overexpression of gerKDbs on B. subtilis spore germination.
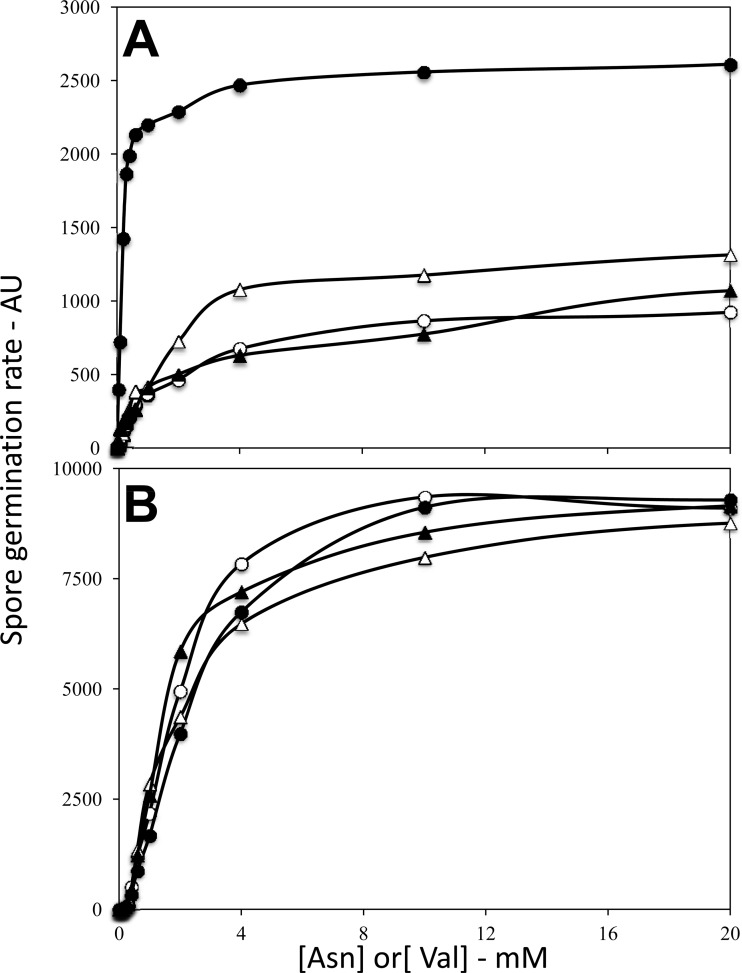
To assess the function of the B. subtilis gerKDbs gene immediately upstream of the gerKbs operon, this small gene was largely deleted, and its coding sequence was replaced by a Tcr cassette. The resulting ΔgerKDbs strain sporulated normally (data not shown). However, B. subtilis spore germination with the AGFK mixture, which requires both the GerB and GerKbs GRs, was significantly faster with ΔgerKDbs spores than with wild-type spores at multiple concentrations of l-asparagine and constant high GFK concentrations (Fig. 2A). In addition, the l-asparagine concentration needed for 50% maximal germination with AGFK was significantly higher for wild-type spores than for ΔgerKDbs spores (Fig. 2A). In contrast, germination of the ΔgerKDbs spores with l-valine alone via the GerA GR was essentially identical to that of wild-type spores at multiple l-valine concentrations (Fig. 2B). The similar germination of ΔgerKDbs and wild-type spores with l-valine and the faster germination of ΔgerKDbs spores with AGFK were seen with three independent spore preparations.
Fig 2.
Rates of germination of wild-type and ΔgerKDbs B. subtilis spores with AGFK (A) and l-valine (B). Spores of B. subtilis strains PS533 (wild type) and PS4256 (ΔgerKDbs) were germinated with various concentrations of l-valine (A) or l-asparagine with d-glucose and d-fructose constant at 10 mM, and K+ constant at ∼15 mM (B), and rates of spore germination were assessed by Tb-DPA fluorescence and given in arbitrary units (AU) as described in Materials and Methods. Symbols: ○, wild-type (PS533) spores; ●, ΔgerKDbs (PS4256) spores; △, ΔgerKDbs amyE::gerKDbs (PS4313) spores; ▲, amyE::PsspB-gerKDbs (PS4314) spores.
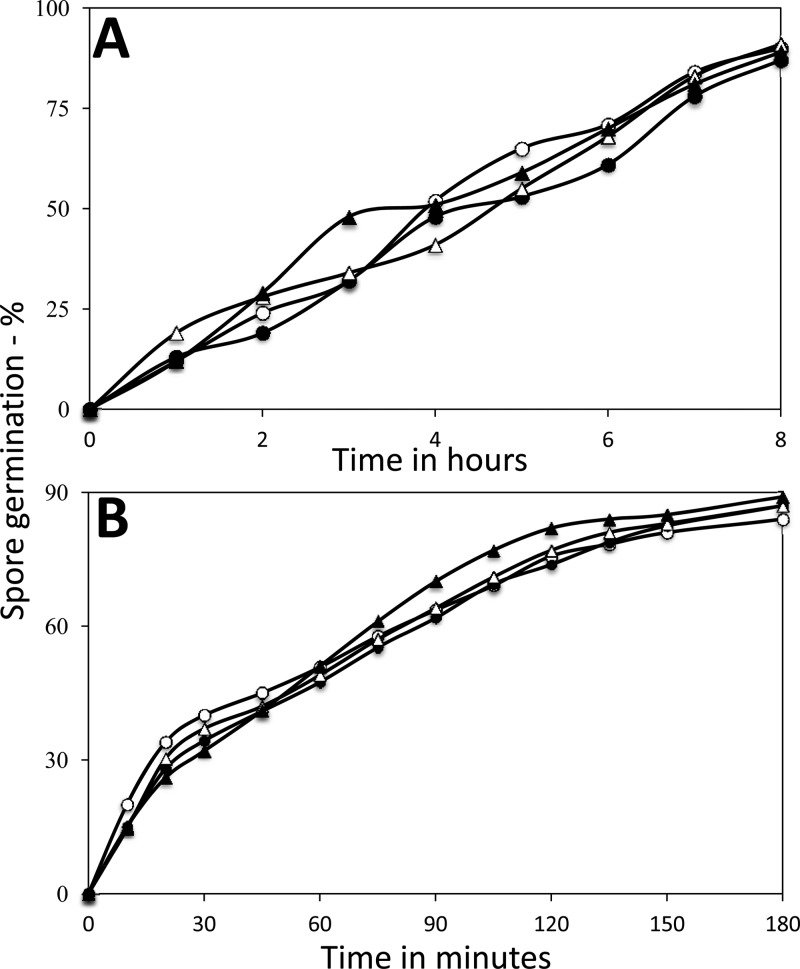
While AGFK germination was significantly affected by deletion of gerKDbs, AGFK triggers germination via GRs and there are other germinants that trigger germination in GR-independent processes (1, 3). Two germinants that do not require GRs for triggering germination are CaDPA and dodecylamine, with CaDPA directly activating hydrolysis of the peptidoglycan cortex of the spores, and dodecylamine most likely directly activating CaDPA release from the spore core (1, 3). In contrast to the significant effect of the gerKDbs deletion on AGFK germination, germination of ΔgerKDbs spores with either CaDPA or dodecylamine was essentially identical to that of wild-type spores (Fig. 3A and B).
Fig 3.
Germination of spores of various B. subtilis strains with CaDPA (A) and dodecylamine (B). Spores of B. subtilis strains PS533 (wild type), PS4256 (ΔgerKDbs), PS4313 (ΔgerKDbs amyE::gerKDbs), and PS4314 (amyE::PsspB-gerKDbs) germinated in the presence of CaDPA (A) or dodecylamine (B), and spore germination with these two agents was measured as described in Materials and Methods. The dormant spores of these four strains had essentially the same amounts of DPA. Symbols: ○, PS533 spores; ●, PS4256 spores; △, PS4313 spores; ▲, PS4314 spores.
Since the gerKDbs gene is located just upstream of the B. subtilis gerKbs operon, it was possible that the gerKDbs deletion could alter transcription of the gerKbs operon, even though the tet gene inserted in gerKDbs was oriented such that its transcription would be away from the gerKbs operon. To show that the effects of the gerKDbs deletion were indeed due to effects of the loss of the GerKDbs protein, we examined the effects of ectopic expression of gerKDbs on the germination of spores lacking the normal gerKDbs gene (Fig. 2A and B). Strikingly, expression of gerKDbs from its own promoter at the amyE locus in the ΔgerKDbs strain resulted in spores that exhibited significantly lower rates of AGFK germination, and this rate was comparable to that of wild-type spores, as was the dependence of the spore germination rate on l-asparagine concentration (Fig. 2A); this was also the case when l-valine germination was examined (Fig. 2B). In addition, when gerKDbs was overexpressed at amyE under PsspB control in an otherwise wild-type strain, the rates of both AGFK and l-valine germination of the spores were again very similar to those of wild-type spores (Fig. 2A and B). One trivial explanation for the effects on AGFK germination of gerKDbs expression at amyE in the ΔgerKDbs strain is that ectopic gerKDbs expression somehow interferes with sporulation such that spore coat synthesis or assembly is defective and since Tb3+ can strongly inhibit germination of coat-defective spores (29), perhaps this inhibition is more effective for germination of ΔgerKbs spores with AGFK than with l-valine. However, this was not the case, as the slower germination of spores of strains expressing or overexpressing gerKDbs at amyE was seen even if TbCl3 was added only at multiple times after the initiation of germination to measure DPA release (data not shown).
In contrast to the slower AGFK germination of B. subtilis spores ectopically expressing gerKDbs in the ΔgerKDbs background, these spores exhibited essentially identical germination to wild-type spores with the GR-independent germinants CaDPA and dodecylamine (Fig. 3A and B). This was also the case with spores overexpressing GerKDbs in an otherwise wild-type background (Fig. 3A and B). Since spores with coat defects often exhibit major alterations in their germination with CaDPA and dodecylamine (2, 3), the normal germination of ΔgerKDbs spores ectopically expressing gerKDbs with these agents is further evidence that these spores do not have a coat defect as indicated above.
Effects of deletion and complementation of GR D genes on B. megaterium spore germination.
In order to assess the roles of the putative GerKDbm and GerUD proteins on B. megaterium spore germination, a number of mutant constructs were generated in B. megaterium strain GC414, which lacks all five functional spore GRs (36). When complemented with intact gerU* (gerUA gerUC and gerVB plus the upstream gerUD gene) or the gerKbm operon genes plus native regulatory sequences on plasmids that are maintained at a low copy number (16), GC414-derived spores germinated efficiently (>95%) in response to appropriate germinant compounds. In both cases, germination rates were comparable to those of spores of appropriate parental strains (data not shown), indicating that any effects on germination resulting from differential expression of plasmid-borne GR operons was minimal. Spores of strain GC635, for example, which are complemented with the intact gerKbm operon, germinated almost completely in 60 min in 5% (wt/vol) beef extract (Table 2). Spores of strain GC636, which have plasmid-borne gerKbm ΔgerKDbm, also germinated fully and at an increased rate (Table 2).
Table 2.
Germination of B. megaterium GR null spores complemented with gerKbm and gerKbm ΔgerKDbm operonsa
| Strain | Relevant phenotype or genotype | Germination rate (%) with the following beef extract concn (wt/vol)b: |
||||
|---|---|---|---|---|---|---|
| 0.1% | 0.5% | 1% | 5% | 10% | ||
| GC614 | GR null | <5 | <5 | <5 | <5 | <5 |
| GC635 | GR null pHT-gerKbm | 12 | 53 | 73 | 98 | 83 |
| GC636 | GR null pHT-gerKbm ΔgerKDbm | 99 | 98 | 94 | 97 | 89 |
| GC637 | GR null pHT-gerKbm, with gerUD in place of gerKDbm | 11 | 41 | 67 | 83 | 64 |
Heat-shocked spores were germinated at 37°C for 60 min in various concentrations of beef extract in 5 mM Tris-HCl (pH 7.5), and germination was measured as described in Materials and Methods.
Rates of spore germination are given relative to the OD600 loss (55%) for spores of strain PV361 in 5% (wt/vol) beef extract, which was set at 100. This value is equivalent to 100% germination in 60 min. Values are the means of three independent experiments conducted with the same spore preparations, where standard deviations were <10% of the mean. Similar results were obtained with additional spore preparations.
Differences in germination efficiency between spores complemented with gerKbm or gerKbm ΔgerKDbm became even more pronounced as the beef extract concentration in the germination buffer was reduced (Table 2). Spores complemented with gerKbm minus the gerKDbm gene (strain GC636), for example, exhibited full germination in 0.1% (wt/vol) beef extract, whereas only ∼12% of spores complemented with the intact gerKbm operon (strain GC635) germinated under the same conditions (Table 2). Indeed, a 10-fold-higher concentration of beef extract was required to trigger 50% germination of pHT-gerKbm spores (0.44%) versus spores complemented with pHT-gerKbm ΔgerKDbm (0.049%). These data indicate that GerKDbm has a negative effect on the function of the B. megaterium GerKbm GR, similar to the effect of the loss of GerKDbs in B. subtilis. However, spores of strain GC637, in which gerKDbm was replaced within the gerKbm operon by gerUD, did not show the same apparent increase in sensitivity to germinants, with a K0.5 germ value of 0.67% (wt/vol) beef extract revealing a slight but significant (P ≤ 0.05) reduction in sensitivity to beef extract compared to spores complemented with the native gerK operon (K0.5 germ of 0.44% [wt/vol] beef extract) (where K0.5 germ is the value required to trigger germination of 50% of the spore population).
Having examined the role of gerKDbm, we then turned our attention to the role of the gerUD gene that encodes a putative D subunit associated with the GerU GR. Two different mutants were engineered to examine the role of gerUD; first, a strain in which the entire predicted gerUD ORF was deleted from the pHT-gerU* plasmid, leaving 132 bp upstream of the gerUA start codon. Although the latter region has been confirmed by 5′ RACE (rapid amplification of cDNA ends) experiments to contain the transcription start site of gerUA (S. Gupta and G. Christie, unpublished data) and putative SigG −35 and −10 consensus sequences remain intact upstream of gerUA on the modified plasmid (see below), it is possible that deletion of the gerUD gene may have compromised the integrity of the gerU promoter. Hence, a second plasmid was prepared in which a codon predicted to encode the 10th residue (methionine) of GerUD was modified by SDM to a stop codon (TAA), which should result in a severely truncated version of the protein being expressed while minimizing the likelihood of interfering with the gerU promoter. Preliminary experiments indicated that spores of strains complemented with either construct displayed essentially identical germination phenotypes (data not shown), and therefore the gerUDM10stop variant was employed in subsequent analyses.
In contrast to observations concerning the impact of GerKDbm on GerKbm GR-mediated germination, kinetic analyses conducted with spores exposed to different concentrations of either proline or glucose, two compounds that trigger spore germination via GerU, revealed that the absence of gerUD had a deleterious effect on spore germination (Table 3). Indeed, for both glucose and proline, the concentration of germinant required to stimulate 50% germination was increased ∼10-fold in spores lacking the gerUD gene (strain GC631) compared to spores complemented with the entire gerU or gerUD locus. This was also reflected in results from kinetic analyses, as similar Vmax values were obtained for spore germination triggered by either proline or glucose via GerU with and without the GerUD protein, but the apparent affinity of the ΔgerUD spores for either germinant was decreased (Table 3).
Table 3.
Kinetic parameters of germination of B. megaterium GC614 (GR null) spores complemented with GerU containing various combinations of GR D proteinsa
| Strain | Relevant genotype | Kinetic parameter of germination in spores incubated with: |
|||||
|---|---|---|---|---|---|---|---|
| Glucose |
Proline |
||||||
| Kmb | Vmaxc | K0.5 germb,d | Km | Vmax | K0.5 germ | ||
| GC615 | pHT-gerUD gerU*e | 1.7 | 0.05 | 0.24 | 1.7 | 0.05 | 0.24 |
| GC631 | pHT-gerUDM10stop gerU* | 7.4 | 0.04 | 2.49 | 5.6 | 0.04 | 2.49 |
| GC632 | pHT-gerUDM10stop gerU*, with gerUD between gerUC and gerVB | 1.6 | 0.08 | 0.22 | 1.5 | 0.07 | 0.24 |
| GC633 | pHT-gerUD gerU*, with gerKDbm between gerUC and gerVB | 23.4 | 0.02 | 47 | 16.5 | 0.02 | 41 |
| GC634 | pHT-gerUDM10stop gerU*, with gerKDbm between gerUC and gerVB | 131 | 0.01 | 276 | 174 | 0.01 | 305 |
Spores were germinated in 5 mM Tris-HCl, pH 7.5, for 90 min with concentrations of glucose and proline ranging from 0.1 to 1,000 mM. Germination was monitored, and kinetic parameters were calculated as described in Materials and Methods. The values for each strain are from three independent experiments conducted with the same spore preparation; similar values were obtained with at least one other spore preparation for each strain. Standard deviations from mean values are less than 10%.
Values given in millimolar.
Values represent the decrease in OD600 units/min, where starting values are normalized to 1 OD600 unit.
Concentrations of glucose and proline required to stimulate 50% spore germination [K0.5 germ] were calculated after incubation for 90 min with the respective germinants. Values presented for each strain are from three independent experiments conducted with the same spore preparation; similar values were obtained with other spore preparations. Standard deviations from mean values are less than 10%.
gerU* comprises gerUA, gerUC, and gerVB and appropriate regulatory sequences.
Intriguingly, kinetic values for the germination of spores of strain GC632 in which the gerUD gene has been relocated from its native, divergently oriented position upstream of gerUA to a position within the gerU operon (encoded on the same strand between gerUC and gerVB) were similar to the benchmark pHT-gerU gerUD (strain GC615) spores (Table 3). These data demonstrate that the influence of the gerUD gene is not necessarily a trans effect, since the positive influence it appears to impart on GerU function was achieved when it is presumably cotranscribed as part of a GR operon. However, data in Table 3 also reveal that gerKDbm cannot be substituted for gerUD within a similarly organized gerU operon without adversely affecting GR function. This effect was most pronounced in the absence of functional GerUD, as evidenced by spores of strain GC634, which required ≥1,000-fold-higher germinant concentrations to stimulate 50% spore germination. The presence of intact gerUD partially alleviated the adverse effects associated with the presence of gerKDbm within the gerU operon, although significantly increased germinant concentrations (>150-fold) were still required to stimulate 50% germination of spores of strain GC633 compared to GC615 spores (Table 3).
Effects of loss of gerKDbs or its ectopic expression or overexpression on the levels of germination proteins in B. subtilis spores.
The results noted above indicated that alterations in levels of the various GR D proteins exert either positive or negative effects on GR function. Previous work has shown that rates of spore germination with particular nutrient germinants are often elevated by increases in levels of GRs that recognize the particular germinants (8, 23, 25, 26, 30). Consequently, it seemed possible that effects of alterations in D-protein levels on rates of spore germination might be mediated through effects on GR levels. Unfortunately, specific antisera are not available against B. megaterium GR subunits, but antisera against a number of B. subtilis GR subunits as well as several other B. subtilis germination proteins are available. To test whether the ΔgerKDbs mutation had resulted in alterations in GR subunit levels, we determined levels of such proteins as well as GerD (31) and SpoVAD in either spore lysates or inner membrane fractions, as these proteins are located in the inner membrane (1–3, 9, 23–26). Total spore lysates as well as isolated inner membrane fractions were used, because the lysates give much higher yields of inner membrane proteins, as spore fractionation by differential centrifugation recovers only ∼10% of the inner membrane and inner membrane proteins in the final inner membrane pellet fraction (K.-A.V. Stewart and P. Setlow, unpublished results). Strikingly, the levels of SpoVAD, GerD, and the GerAA, GerAC, GerBC, and GerKA GR subunits showed less than a 25% difference in wild-type and ΔgerKDbs spores when either spore lysates or inner membrane fractions were analyzed (Fig. 4 and 5 and Table 4).
Fig 4.
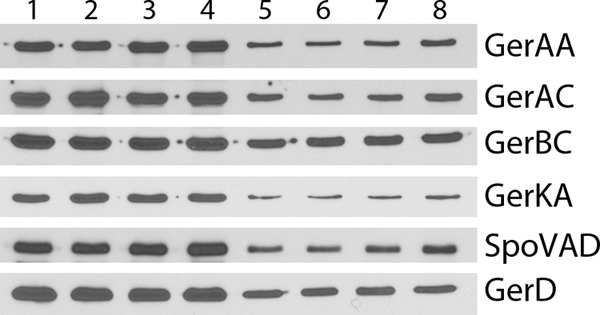
Western blot analysis of germination proteins in total lysates of spores of various B. subtilis strains. Total spore lysates from spores of B. subtilis strains PS533 (wild type) (lanes 1 and 5), PS4256 (ΔgerKDbs) (lanes 2 and 6), PS4213 (amyE::gerKDbs ΔgerKDbs) (lanes 3 and 7), and PS4314 (amyE::PsspB-gerKDbs) (lanes 4 and 8) were isolated, and samples with identical amounts of protein from ∼7 × 106 spores (lanes 1 to 4) or 1.8 × 106 spores (lanes 5 to 8) (with amounts of protein adjusted appropriately based on preliminary SDS-PAGE and Coomassie blue staining) were run on SDS-polyacrylamide gels and subjected to Western blot analysis with antisera against various germination proteins as described in Materials and Methods. The strips probed for the various antigens were from several different blots.
Fig 5.
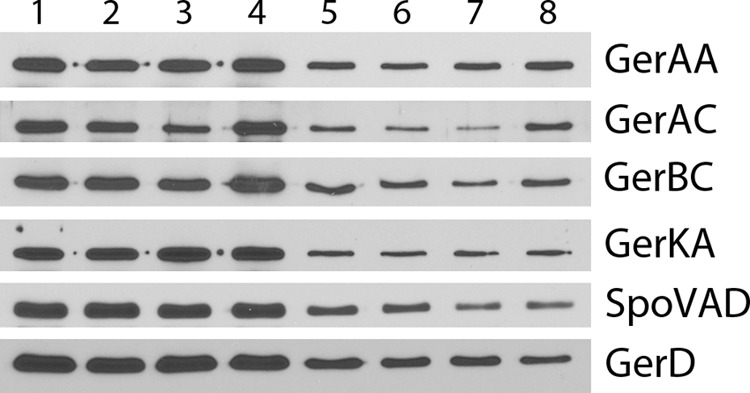
Western blot analysis of germination proteins in the isolated inner membrane fraction from spores of various B. subtilis strains. The inner membrane fraction was isolated from the total spore lysates analyzed in Fig. 4 from spores of B. subtilis strains PS533 (wild type) (lanes 1 and 5), PS4256 (ΔgerKDbs) (lanes 2 and 6), PS4213 (amyE::gerKDbs ΔgerKDbs) (lanes 3 and 7), and PS4314 (amyE::PsspB-gerKDbs) (lanes 4 and 8). Samples with identical amounts of inner membrane protein from ∼2.3 × 108 spores (lanes 1 to 4) or 5.6 × 107 spores (lanes 5 to 8) (with amounts of protein adjusted appropriately based on preliminary SDS-PAGE and Coomassie blue staining) were run on SDS-polyacrylamide gels and subjected to Western blot analysis with antisera against various germination proteins as described in Materials and Methods. The strips probed for the various antigens were from several different blots.
Table 4.
Levels of germination proteins in total spores and the inner membrane fraction of spores of various B. subtilis strainsa
| Protein | Level in the following strain relative to that in wild-type sporesb |
|||
|---|---|---|---|---|
| Wild-type (PS533) | ΔgerKDbs (PS4256) | ΔgerKDbs amyE::gerKDbs (PS4313) | amyE::PsspB-gerKDbs (PS4314) | |
| GerAA | 1.0 (1.0) | 0.8 (0.8) | 1.0 (0.9) | 1.0 (1.0) |
| GerAC | 1.0 (1.0) | 0.9 (0.8) | 1.0 (0.6) | 1.1 (1.2) |
| GerBC | 1.0 (1.0) | 0.9 (0.9) | 0.8 (0.8) | 1.0 (0.9) |
| GerKA | 1.0 (1.0) | 1.2 (0.9) | 1.1 (1.0) | 1.2 (1.0) |
| GerD | 1.0 (1.0) | 0.9 (1.0) | 0.9 (0.8) | 1.0 (0.8) |
| SpoVAD | 1.0 (1.0) | 0.9 (0.9) | 1.0 (1.0) | 1.2 (0.8) |
Levels of each germination protein in wild-type spores from analysis of total spores or inner membrane fractions were set at 1.0. The first relative values (not in parentheses) are from total spores (Fig. 4), and the second values (values in parentheses) are from the inner membrane fraction (Fig. 5). Values are ±25%.
The slowed GR-dependent germination of ΔgerKDbs B. subtilis spores expressing gerKDbs from its own promoter was striking and suggested that this slow germination might be reflected in lower levels of the GerKbs GR, but perhaps not other GR proteins. However, ectopic expression of gerKDbs from its own promoter or ectopic overexpression of gerKDbs from PsspB also had no major effect on the levels of GR subunits, GerD, or SpoVAD when either total spore lysates or isolated inner membrane fractions were analyzed (Fig. 4 and 5 and Table 4). The lack of effect of ectopic overexpression of gerKBbs on the levels of various B. subtilis germination proteins was further assessed by examination of the levels of β-galactosidase expressed from either a gerA-lacZ fusion or a gerB-lacZ fusion in spores with and without ectopic gerKDbs overexpression (Table 5). As expected (4, 12), there was significant β-galactosidase in the spores carrying gerA-lacZ or gerB-lacZ fusions, and the level of this activity was much higher than in wild-type spores with no lacZ fusion. However, ectopic overexpression of gerKDbs had essentially no effect on the levels of β-galactosidase in spores from either the gerA-lacZ or gerB-lacZ fusions.
Table 5.
Levels of β-galactosidase from gerA-lacZ or gerB-lacZ fusions in spores with or without gerKDbs overexpressiona
| Strain | Relevant genotype | β-Galactosidase activity (RFU)b |
|---|---|---|
| PS533 | Wild-type | 335 |
| PS767 | gerA-lacZ | 10,700 |
| PS4319 | gerA-lacZ amyE::PsspB-gerKDbs | 9,700 |
| PS3709 | gerB-lacZ | 6,135 |
| PS4320 | gerB-lacZ amyE::PsspB-gerKDbs | 5,250 |
Spores of various strains were isolated, decoated, and permeabilized, and 8 × 108 spores were assayed for β-galactosidase as described in Materials and Methods.
RFU, relative fluorescence units.
Expression of genes encoding putative GR D proteins during sporulation.
Analysis of the regions upstream of gerKDbs and gerUD revealed sequences with good homology to the consensus sequences recognized by RNA polymerase with the forespore-specific sigma factor σG that transcribes the operons encoding the GRs, including GerKbs, as well as the yndEFG and yfkQRST operons that appear likely to encode GRs of unknown function (Fig. 6) (7). However, while transcription of gerKDbs has been observed during B. subtilis sporulation and correlates well with gerKbs operon transcription (29, 32), the transcription of the gerKDbs gene alone has not been well studied, and expression of gerKDbm or gerUD has also not been well studied. Consequently, we prepared transcriptional lacZ fusions to these D genes in order to examine their expression during sporulation.
Fig 6.

Potential transcription and translation signals upstream of the gerKDbs and gerUD coding sequences. The perfect B. subtilis mRNA ribosome binding site (rbs) (28) and consensus −10 and −35 sequences recognized by B. subtilis RNA polymerase with σG and the rbs are shown above the gerKDbs sequence. The X in the −35 consensus sequence denotes a position that is any nucleotide, the Y in the −10 sequence denotes residues that are most often A or C, and the Z in the −10 sequence denotes a position that is most often A or T. The translation-initiating ATG codons are underlined. In the sequences shown, the values in parentheses between the −35 and −10 sequences, the −10 sequence and the rbs, and the rbs and the ATG codon are the consensus spacings between these elements (3, 27). The dashes indicate positions that are any nucleotide.
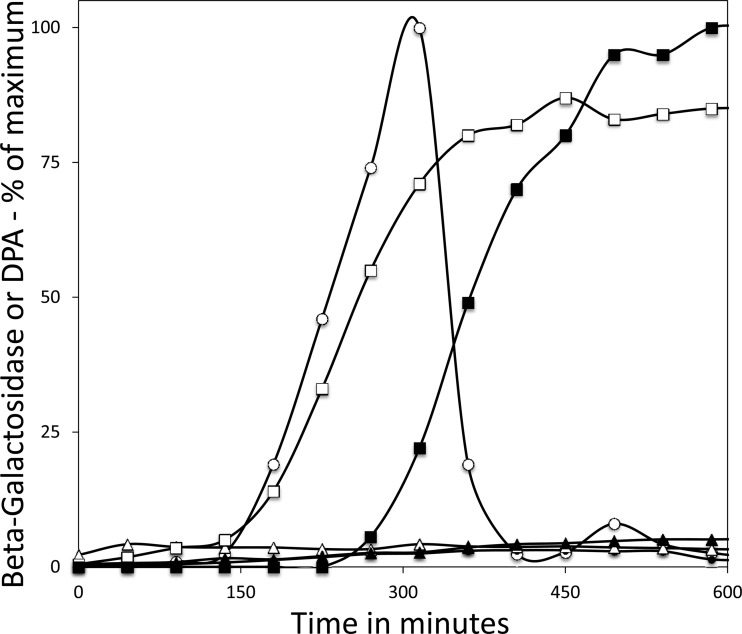
With gerKDbs-lacZ in an otherwise wild-type B. subtilis strain, significant β-galactosidase activity appeared ∼2.5 h after the start of sporulation, although the β-galactosidase activity in this culture fell rapidly and almost to background levels after 6 h (Fig. 7). Note also that DPA accumulated ∼2 h later than gerKDbs-lacZ accumulation. The latter timing as well as the fall in gerKDbs-driven lacZ expression late in sporulation is consistent with gerKDbs expression being confined to the developing forespore, as the forespore becomes refractory to lysozyme permeabilization as sporulation proceeds (33). If this is indeed the case, then high levels of β-galactosidase should be present in gerKDbs-lacZ spores. This was the case, as dormant gerKbs-lacZ spores had β-galactosidase activity similar to the maximum level accumulated during sporulation in liquid resuspension medium (data not shown).
Fig 7.
Levels of β-galactosidase and DPA during sporulation of strains with a gerKDbs-lacZ fusion and with or without sporulation-specific RNA polymerase σ factors. Various B. subtilis strains carrying gerKDbs-lacZ were sporulated by resuspension, and equal aliquots of cultures were assayed for β-galactosidase (○, ●, △, ▲, □) or DPA (■) as described in Materials and Methods. The strains analyzed were PS4290 (has all sigma factors) (○, ■), PS4293 (no σE) (●), PS4294 (no σF) (△), PS4295 (no σG) (▲), and PS4296 (no σK) (□). Strain PS533 that lacks a lacZ fusion was also sporulated by resuspension in parallel, and the levels of β-galactosidase from this strain were subtracted from those of other samples taken at the same time. The maximum value of these subtracted values was <10% of the maximum value obtained with the wild-type strain carrying gerKDbs-lacZ.
To further assess the regulation of gerKDbs expression during B. subtilis sporulation, the gerKDbs-lacZ fusion was introduced into strains lacking an RNA polymerase σ factor (σE, σF, σG, or σK) needed for sporulation-specific transcription in either the mother cell or forespore compartment of the sporulating cell, and β-galactosidase was assayed during resuspension sporulation of these strains (Fig. 7). These assays showed that the absence of σE, σF, or σG eliminated β-galactosidase accumulation from gerKDbs-lacZ, while the absence of σK did not. However, in the absence of σK, the gerKDbs-driven β-galactosidase level did not decrease late in sporulation, consistent with this enzyme being accumulated only in the forespore, which does not become lysozyme resistant in a σK deletion. Together, these data indicate that gerKDbs is transcribed by RNA polymerase that contains σG, the σ factor that also directs the transcription of the operons encoding GRs, including gerKbs, as well as a number of other forespore-specific genes (1, 4–6, 12, 32).
To analyze expression of the B. megaterium gerKDbm and gerUD genes, lacZ translational fusions were generated in the wild-type background, fusing lacZ to the 3′ ends of the gerKDbm (creating a gerKbm null mutant in this case as a result of disrupting the gerK operon) and gerUD genes and then assaying extracts from disrupted spores for β-galactosidase activity. These data (Table 6) indicate that gerKDbm is expressed at a near identical level to gerKAbm, which is as expected, since both genes are located in the same operon and other GR operons have been shown to be transcribed as single mRNA molecules (4, 12). RT-PCR analyses conducted with cDNA prepared from sporulating wild-type cultures reinforced this observation, with transcription of both gerKAbm and gerKDbm being detected at the same time in sporulation (4 h after entry into sporulation on) in two apparent waves of expression (Fig. 8). The gerUD gene also appears to be expressed during sporulation, apparently at a higher level than gerU receptor genes encoded on the opposite strand according to analyses with lacZ fusion spores, although both gerUD and gerUA appear to be expressed at a lower level than gerKbm genes (Table 6). RT-PCR analyses supported the lacZ fusion data, indicating that despite their divergent genetic organization, gerUD and gerUA (and presumably the entire gerU operon) are expressed at the same time during sporulation (3 h after entry into sporulation on, although only as a single wave of expression) (Fig. 8), which is consistent with GerUD being associated with GerU receptor function.
Table 6.
Levels of β-galactosidase in B. megaterium spores with gerU-lacZ and gerKbm-lacZ fusionsa
| Strain | Relevant genotype | β-Galactosidase activity (RFU)b |
|---|---|---|
| QM B1551 | Wild-type | 500 |
| GC638 | gerUA-lacZ | 16,300 |
| GC639 | gerUD-lacZ | 29,200 |
| GC640 | gerKAbm-lacZ | 43,000 |
| GC641 | gerKDbm-lacZ | 42,500 |
Spores of various strains (∼109) were isolated, disrupted, and assayed for β-galactosidase activity as described in Materials and Methods. β-Galactosidase values are the average of triplicate measurements conducted with two different spore preparations. Standard deviations for all values are <10%.
RFU, relative fluorescence units.
Fig 8.
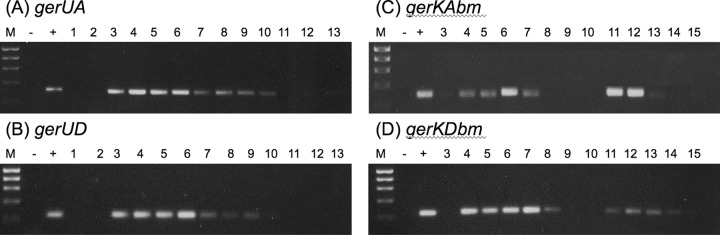
RT-PCR analysis of expression of B. megaterium GR A- and D-protein genes during sporulation. RT-PCR was conducted using gene-specific primers designed to amplify 230- to 300-bp fragments of gerUA (A), gerUD (B), gerKAbm (C), and gerKDbm (D) from RNA isolated from sporulating cultures as described in Materials and Methods. The numbers above the lanes refer to the time (in hours) after entry into sporulation. Positive-control reactions (+) (i.e., genomic DNA) and negative-control reactions (−) (i.e., no template DNA/RNA) reactions are indicated above the lanes. Lanes M, molecular weight markers (M).
DISCUSSION
The identification of the potential GR D genes by bioinformatic analysis was somewhat surprising, not the least because putative D genes were not found associated with the B. subtilis gerA and gerB operons encoding two of the major GRs of this organism. However, the location of a number of likely D genes immediately upstream of or within operons encoding GRs in both B. megaterium and B. subtilis suggests that these small genes may play roles in at least some functions of GRs. This suggestion is strongly supported by the findings for the B. subtilis gerKDbs gene and the B. megaterium gerKDbm and gerUD genes presented in this work. Thus, these three genes were expressed in parallel with their associated GR operon and only in the developing spore, and gerKDbs and the gerKbs operon are under the control of the same RNA polymerase σ factor, σG. Noteworthy also from an expression perspective was the observation that the gerKbm operon, including gerKDbm, is transcribed in two apparent waves of expression. Similar bimodal expression of GRs, and indeed other σG-dependent genes, has been observed previously in B. subtilis, although the biological relevance of this phenomenon has yet to be revealed (32). More importantly, deletion and subsequent ectopic expression of the deleted D genes had significant effects on GR-dependent spore germination. Notably, the effects of alteration of D gene expression were only on GR-dependent germination, suggesting that the effects of these proteins on spore germination are not directly on either cortex peptidoglycan hydrolysis or the CaDPA release process.
The major question arising from the results noted above is how the D proteins exert their effects. The fact that the putative GR D proteins with documented effects on spore germination appear to be composed primarily of two membrane-spanning segments suggests that these proteins are located in the spore's inner membrane, where the GRs themselves are located. Thus, the D proteins could influence the organization of their GRs in the cluster of germination proteins, including GRs and GerD termed the germinosome, and there is evidence indicating that germinosome assembly is important for optimum GR function (9). In addition, the dramatic inhibition of the B. megaterium GerU function by substitution of gerKDbm for gerUD in the gerU operon suggests that the various D proteins are specific for their cognate GRs. A less pronounced but significant reduction in apparent receptor efficiency was also observed when gerUD was substituted for gerKDbm in the B. megaterium gerKbm operon, lending further support to this idea. D proteins can certainly affect the maximum germination rates via specific GRs, and this was achieved at least in B. subtilis spores with no significant alteration in the levels of subunits of the relevant GRs. In addition, the D proteins can markedly alter the affinity of their associated GRs for their cognate nutrient germinants, as exemplified by kinetic analysis of the effects of GerUD on the function of the B. megaterium GerU GR and GerKDbs on the function of the B. subtilis GerK GR. Thus, a D protein may somehow modulate either germinant access to GRs or alter GR conformation such that germinant binding affinities are either decreased or increased. Similarly, substitution of gerKDbm in the gerU operon resulted in a marked decrease in GerU function, both in terms of spores' apparent affinity for germinants and maximal rates of germination achieved. Hence, at least in this case, a noncognate D protein could not be tolerated as part of or associated with the GR complex, although the structural and/or physiological basis for this is not clear. Indeed, at this time, we do not understand how D proteins act to modulate spore germination rates.
In addition to the major question noted above on what exactly D proteins do, a second major question is whether D proteins are associated with all GRs. In particular, are there D proteins associated with all GRs in B. megaterium and B. subtilis? In B. megaterium and B. subtilis, several operons encoding GRs have no obvious D genes in or adjacent to the operons. One possibility is that these GRs have no associated D proteins. However, since D proteins can clearly exert effects in trans, perhaps GRs encoded by operons without obvious nearby D genes utilize D proteins encoded far away, and there are potential D genes not associated with GR operons in Bacillus genomes. Clearly, there is much yet to be learned about these unexpected components of the spore germination apparatus.
ACKNOWLEDGMENTS
This work was supported in part by a U.S. Department of Defense Multi-University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911NF-09-1-0286. S.G. is the recipient of a RA Fisher bursary award from Gonville and Caius College, University of Cambridge.
Footnotes
Published ahead of print 26 April 2013
REFERENCES
- 1. Setlow P, Johnson EA. 2013. Spores and their significance, p 45–79 In Doyle MP, Buchanan RL. (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC [Google Scholar]
- 2. Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 3. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 4. Feavers IM, Foulkes J, Setlow B, Sun D, Nicholson W, Setlow P, Moir A. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol. Microbiol. 4:275–282 [DOI] [PubMed] [Google Scholar]
- 5. Steil L, Serrano M, Henriques AO, Volker U. 2005. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology (Reading, Engl.) 151:399–420 [DOI] [PubMed] [Google Scholar]
- 6. Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, Sato T, Setlow P, Losick R, Eichenberger P. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16–37 [DOI] [PubMed] [Google Scholar]
- 7. Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol. Microbiol. 81:1061–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christie G, Gotzke H, Lowe CR. 2010. Identification of a receptor subunit and putative ligand-binding residues involved in the Bacillus megaterium QM B1551 spore germination response to glucose. J. Bacteriol. 192:4317–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christie G, Lowe CR. 2007. Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J. Bacteriol. 189:4375–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corfe BM, Moir A, Popham D, Setlow P. 1994. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology (Reading, Engl.) 140:3079–3083 [DOI] [PubMed] [Google Scholar]
- 13. Antoniewski C, Savelli B, Stragier P. 1990. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 172:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cutting SM. 1990. Genetic analysis, p 27–74 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 15. Arantes O, Lereclus D. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115–119 [DOI] [PubMed] [Google Scholar]
- 16. Christie G, Lazarevska M, Lowe CR. 2008. Functional consequences of amino acid substitutions to GerVB, a component of the Bacillus megaterium spore germinant receptor. J. Bacteriol. 190:2014–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher N, Hanna P. 2005. Characterization of Bacillus anthracis germinant receptors in vitro. J. Bacteriol. 187:8055–8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 19. Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. English JD, Vary PS. 1986. Isolation of recombination-defective and UV-sensitive mutants of Bacillus megaterium. J. Bacteriol. 165:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi X, Bond C, Sarker MR, Setlow P. 2011. Efficient inhibition of germination of coat-deficient bacterial spores by multivalent metal cations, including terbium (Tb3+). Appl. Environ. Microbiol. 77:5536–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghosh S, Scotland M, Setlow P. 2012. Levels of germination proteins in dormant and superdormant spores of Bacillus subtilis. J. Bacteriol. 194:2221–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pelczar PL, Setlow P. 2008. Localization of the germination protein GerD to the inner membrane in Bacillus subtilis spores. J. Bacteriol. 190:5635–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramirez-Peralta A, Stewart KAV, Thomas SK, Setlow B, Chen Z, Li YQ, Setlow P. 2012. Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J. Bacteriol. 194:3417–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramirez-Peralta A, Zhang P, Li YQ, Setlow P. 2012. Effects of sporulation conditions on the germination and germination protein levels of Bacillus subtilis spores. Appl. Environ. Microbiol. 78:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart KAV, Yi X, Ghosh S, Setlow P. 2012. Germination protein levels and rates of germination of spores of Bacillus subtilis with overexpressed or deleted genes encoding germination proteins. J. Bacteriol. 194:3156–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterlini JM, Mandelstam J. 1969. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem. J. 113:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicolas P, Mader U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Hartig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rugheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stulke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Volker U, Bessieres P, Noirot P. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–1106 [DOI] [PubMed] [Google Scholar]
- 30. Cabrera-Martinez RM, Tovar-Rojo F, Vepachedu VR, Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelczar PL, Igarashi T, Setlow B, Setlow P. 2007. Role of GerD in germination of Bacillus subtilis spores. J. Bacteriol. 189:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Igarashi T, Setlow P. 2006. Transcription of the Bacillus subtilis gerK operon, which encodes a spore germinant receptor, and comparison with that of operons encoding other germinant receptors. J. Bacteriol. 188:4131–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mason JM, Hackett RH, Setlow P. 1988. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J. Bacteriol. 170:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bagyan I, Setlow B, Setlow P. 1998. New small, acid-soluble proteins unique to spores of Bacillus subtilis: identification of the coding genes and regulation and function of two of these genes. J. Bacteriol. 180:6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta S, Üstok FI, Johnson CL, Bailey DMD, Lowe CR, Christie G. 2013. Investigating the functional hierarchy of Bacillus megaterium PV361 spore germinant receptors. J. Bacteriol. 195:3045–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]