Abstract
A subset of rifampin resistance (rpoB) mutations result in the overproduction of antibiotics in various actinomycetes, including Streptomyces, Saccharopolyspora, and Amycolatopsis, with H437Y and H437R rpoB mutations effective most frequently. Moreover, the rpoB mutations markedly activate (up to 70-fold at the transcriptional level) the cryptic/silent secondary metabolite biosynthetic gene clusters of these actinomycetes, which are not activated under general stressful conditions, with the exception of treatment with rare earth elements. Analysis of the metabolite profile demonstrated that the rpoB mutants produced many metabolites, which were not detected in the wild-type strains. This approach utilizing rifampin resistance mutations is characterized by its feasibility and potential scalability to high-throughput studies and would be useful to activate and to enhance the yields of metabolites for discovery and biochemical characterization.
INTRODUCTION
Actinomycetes produce a variety of natural products that are of major importance in the pharmaceutical industry. More than 50% of all anti-infective and anticancer compounds developed over the past 25 years have been natural products or derivatives thereof (1). Discovery of novel antibiotics and strain improvement for overproduction are important in applied microbiology research, especially in the production of clinically important antibiotics as well as antibiotics important in veterinary medicine and agriculture. There is accumulating evidence that the ability of actinomycetes to produce antibiotics and other bioactive secondary metabolites has been underestimated due to the presence of cryptic gene clusters. That is, genome sequencing projects have revealed many biosynthetic gene clusters for the production of unknown secondary metabolites. For example, Streptomyces coelicolor, Streptomyces avermitilis, Streptomyces griseus, and Saccharopolyspora erythraea are each known to produce three to five secondary metabolites but actually possess >20 clusters that encode known or predicted biosynthetic pathways for secondary metabolites (2–5). Exploitation of such genetic potential in actinomycetes may lead to the isolation of new biologically active compounds (6–8). We recently described a new method to increase antibiotic production in bacteria by modulating ribosomal components (ribosomal proteins or rRNA), i.e., by introducing mutations conferring drug resistance, as many antibiotics target the ribosome (9–11). This new approach, called “ribosome engineering” (12, 13), has several advantages, including the ability to screen for drug resistance mutations by simple selection on drug-containing plates, even if the mutation frequency is extremely low (e.g., <10−10), and the ability to select for mutations without prior genetic information. Hence, this method requires no induced mutagenesis. Interestingly, the introduction of several drug resistance mutations has a cumulative effect on antibiotic production (14–16).
In addition to enhancement of antibiotic production, we have demonstrated that certain rpoB mutations which arose in the RNA polymerase (RNAP) β-subunit (and certain rpsL mutations which arose in ribosomal protein S12) are effective for activating the “silent” secondary metabolite biosynthetic genes, eventually leading to discovery of novel antibiotics (17–19). Importantly, Derewacz et al. (20) recently reported that drug resistance mutations (e.g., streptomycin resistance or rifampin resistance) affect broad changes in the metabolic phenotype of Nocardiopsis sp. in addition to secondary metabolism of this organism. Although earlier work was conducted mainly with S. coelicolor A3(2), we have now demonstrated that rpoB mutations are widely effective in enhancing the production of antibiotics by various actinomycetes and that rpoB mutations activate the secondary metabolite biosynthetic gene clusters, which are “silent” or poorly expressed under ordinary culture conditions. Analysis of the metabolite profile demonstrates that the rpoB mutants produced many metabolites, which were not detected in the wild-type strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The wild-type strains S. coelicolor A3(2), Streptomyces antibioticus strain 3720, Streptomyces parvulus ATCC 12434, S. griseus IFO13189, Streptomyces lavendulae MA406A1, Amycolatopsis orientalis NBRC12806, and S. erythraea NRRL2338 were used. Spontaneous rifampin-resistant mutants were obtained as colonies that grew within 5 to 10 days after spores were spread on GYM agar (21) containing various concentrations of rifampin. Mutations in the rpoB gene were determined by DNA sequencing using the primers listed in Table S1 in the supplemental material. Strains were grown in GYM medium (21), SPY medium (21), SYM medium (21), MPY medium (22), R4 medium (23), R3/1 medium (24), or vancomycin production medium (25) at 25°C or 30°C under rotary shaking (220 rpm) as described in the text. All measurements for antibiotic productivity were performed in triplicate or more flasks, and the antibiotic titers were expressed as means ± standard deviations (SD).
Assays for antibiotics.
Actinorhodin produced in liquid culture was determined by the method of Kieser et al. (26). The level of actinorhodin production on the plates was assessed directly by determination of the intensity of the blue color. Actinomycin was determined photometrically as described previously (11). Streptomycin, formycin, and erythromycin were determined by bioassay (agar diffusion method) using test organisms as described previously (11, 21, 22). Vancomycin was determined by bioassay using Staphylococcus aureus 209P as a test organism.
Determination of MICs.
MICs were determined by spotting spore solutions (∼106) onto rifampin-containing GYM plates, followed by incubation at 30°C for the indicated times. The minimum drug concentration able to fully inhibit growth was defined as the MIC.
Transcriptional analysis by real-time qPCR.
Total RNAs were extracted and purified from cells grown for the indicated times, using Isogen reagent (Nippon Gene) according to the manufacturer's protocol. Real-time quantitative PCR (qPCR) was performed as described previously (27). Each transcription assay was normalized relative to the corresponding transcriptional level of hrdB (for S. griseus and S. coelicolor) or sigB (for S. erythraea), a gene encoding the principal sigma factor. The primers used for real-time qPCR are listed in Table S1 in the supplemental material.
Conjugation procedure.
Conjugation-based gene transfer between S. coelicolor strains was performed as described by Kieser et al. (26), using the actII-open reading frame 4 (ORF4) disruptant (with hygromycin resistance). For transfer of the disrupted actII-ORF4 gene between the disruptant and the rpoB mutant KO-1130, conjugants were selected for rifampin resistance and hygromycin resistance. Mutations were confirmed by DNA sequencing.
Analysis of metabolites by UPLC/MS.
A double volume of acetonitrile was added to the culture broth and mixed well. The mixture was then centrifuged at 15,000 × g for 1 min, and the supernatant was analyzed directly by ultraperformance liquid chromatography-mass spectrometry (UPLC/MS). The analytical conditions were as follows: device, Waters Acquity UPLC H-class; column, Waters Acquity UPLC ethylene bridged hybrid (BEH) C18 (2.1 by 150 mm; particle size, 1.7 μm); column temperature, 40°C; gradient elution, solvent A (acetonitrile), solvent B (MeOH), solvent C (0.1% CH3COONH4 in deionized water), solvent D (deionized water); gradient profile, 0 to 1 min, 20% A, 20% to 60% B, 20% C, 40% to 0% D; 1 to 6 min, 20% A, 60% B, 20% C, 0% D; 6 to 6.5 min, 20% A, 60% to 20% B, 20% C, 0% to 40% D; 6.5 to 9.0 min, 20% A, 20% B, 20% D, 40% D; flow rate, 0.25 ml/min; detection, m/z between 200 and 800 (for S. coelicolor) or between 200 and 500 (for S. griseus and S. erythraea) using a Waters SQ detector mass spectrometer; ionization mode, electrospray ionization (ESI) positive, capillary voltage, 3.3 kV; source temperature, 120°C; desolvation temperature, 350°C; desolvation gas flow, 600 liters/h; cone gas flow, 50 liters/h; cone voltage, 10 V for S. griseus, 60 V for S. coelicolor, and 40 V for S. erythraea metabolite analyses.
RESULTS
Isolation and characterization of rifampin-resistant mutants.
To investigate the effects of rpoB mutations in various actinomycetes, we isolated a number of spontaneous rifampin-resistant (rif) mutants. When spores of strains were spread and incubated on GYM agar containing various concentrations of rifampin, rif mutants developed after 5 to 10 days at a frequency of 10−7 to 10−8. The sequence of the rpoB gene, which encodes the RNAP β-subunit, was determined by DNA sequencing analysis, and the majority of the mutants tested had a mutation in the so-called “rif cluster” of the rpoB gene (we analyzed 21 to 84 rif mutants for each actinomycete) (Table 1). Strikingly, the introduction of certain rpoB mutations effectively increased antibiotic production by S. griseus (streptomycin producer), S. coelicolor (actinorhodin producer), S. antibioticus (actinomycin producer), S. lavendulae (formycin producer), S. erythraea (erythromycin producer), and A. orientalis (vancomycin producer). These antibiotics are characterized structurally as polyketides, polyethers, glycopeptides, macrolides, polypeptides, nucleotides, and aminoglycosides. Only S. parvulus (actinomycin producer) showed essentially no increase in antibiotic production in any media examined, including 2× SYM medium, although actinomycin production by S. antibioticus was markedly enhanced by introducing certain rpoB mutations (Table 1). The rpoB mutations that yielded antibiotic overproduction are summarized in Table 2. All rpoB mutations detected in this study were located within positions 1264C to 1327G, representing amino acid residues Leu422 to Ala443. Importantly, rpoB H437Y (altering His at position 437 to Tyr) and H437R (altering His at position 437 to Arg) mutations were most often effective in a wide variety of actinomycetes. It is also notable that alteration of Ser at position 433 or 442 was also often effective, although position 442 is variable among species (e.g., Asn in S. coelicolor).
Table 1.
Characterization of rifampin resistance mutations of Streptomyces griseus IFO13189, Streptomyces coelicolor A3(2), Saccharopolyspora erythraea NRRL2338, Streptomyces antibioticus 3720, Streptomyces parvulus ATCC 12434, Streptomyces lavendulae MA406A1, and Amycolatopsis orientalis NBRC12806
| Strain | Rifampin concn (μg/ml) used for selection | Mutation in rpoBa | Amino acid substitution | Frequency of mutants with same mutation | Resistance to rifampin (μg/ml)b | Concn of antibiotic produced |
|---|---|---|---|---|---|---|
| S. griseus | Streptomycin (μg/ml)c | |||||
| Wild type | NAd | NA | NA | NA | 0.5 | 48 ± 14 |
| KO-1167 | 5 | 1270C→A | Gln424→Lys | 1/30 | >200 | 178 ± 27 |
| KO-1168 | 5 | 1275C-1277T→Δ | Phe425,Met426→Leu | 1/30 | >200 | 9 ± 1 |
| KO-1169 | 5 | 1276A-1278G→Δ | Met426→Δ | 1/30 | >200 | 0 |
| KO-1170 | 5 | 1280A→T | Asp427→Val | 2/30 | 10 | 23 ± 5 |
| KO-1171 | 5 | 1309C→G | His437→Asp | 8/30 | 50 | 34 ± 7 |
| KO-1172 | 5 | 1309C→T | His437→Tyr | 8/30 | 200 | 42 ± 9 |
| KO-1173 | 5 | 1310A→T | His437→Leu | 1/30 | 10 | 23 ± 7 |
| KO-1174 | 5 | 1310A→G | His437→Arg | 1/30 | >200 | 96 ± 4 |
| KO-1175 | 5 | 1325C→T | Ser442→Leu | 1/30 | 20 | 143 ± 16 |
| S. coelicolor | Actinorhodin (OD640)e | |||||
| Wild type | NA | NA | NA | NA | 20 | 0.59 ± 0.05 (+) |
| KO-1123 | 100 | 1267T→A | Ser423→Thr | 2/32 | 300 | 20.7 ± 1.9 (++) |
| KO-1124 | 100 | 1272G→T | Gln424→His | 1/32 | 300 | 0.88 ± 0.23 (+) |
| KO-1125 | 100 | 1276A→G | Met426→Val | 2/32 | 300 | 1.46 ± 0.23 (++) |
| KO-1126 | 100 | 1280A→C | Asp427→Ala | 1/32 | 300 | 19.6 ± 1.5 (++) |
| KO-1127 | 100 | 1280A→G | Asp427→Gly | 1/32 | >500 | 0.56 ± 0.15 (+) |
| KO-1128 | 100 | 1280A→T | Asp427→Val | 5/32 | >500 | 1.4 ± 0.26 (++) |
| KO-1129 | 100 | 1281C→A | Asp427→Glu | 1/32 | >500 | 2.1 ± 0.47 (++) |
| KO-1130 | 100 | 1298C→T | Ser433→Leu | 3/32 | 500 | 28.7 ± 1.3 (+++) |
| KO-1131 | 100 | 1309C→T | His437→Tyr | 3/32 | >500 | 1.3 ± 0.20 (++) |
| KO-1132 | 100 | 1310A→T | His437→Leu | 1/32 | >500 | 1.9 ± 0.09 (+++) |
| KO-1133 | 100 | 1319G→A | Arg440→His | 3/32 | >500 | 0.31 ± 0.01 (+) |
| KO-1134 | 100 | 1324A→T | Asn442→Tyr | 1/32 | >500 | 0.55 ± 0.08 (++) |
| KO-1135 | 100 | 1327G→C | Ala443→Pro | 1/32 | 200 | 0.37 ± 0.14 (+) |
| S. erythraea | Erythromycin (μg/ml)f | |||||
| Wild type | NA | NA | NA | NA | 0.5 | 42 ± 7 |
| KO-1194 | 10 | 1309C→G | His437→Asp | 1/35 | >300 | 88 ± 6 |
| KO-1195 | 10 | 1309C→T | His437→Tyr | 2/35 | >300 | 90 ± 12 |
| KO-1196 | 10 | 1310A→G | His437→Arg | 1/35 | >300 | 163 ± 34 |
| KO-1197 | 10 | 1318C→T | Arg440→Trp | 11/35 | 200 | 7 ± 1 |
| KO-1198 | 10 | 1318C→G | Arg440→Gly | 1/35 | 200 | 41 ± 4 |
| KO-1199 | 10 | 1319G→T | Arg440→Leu | 2/35 | 80 | 33 ± 5 |
| KO-1200 | 10 | 1319G→A | Arg440→Gln | 4/35 | 30 | 20 ± 5 |
| KO-1201 | 10 | 1325C→A | Ser442→Tyr | 1/35 | >300 | 68 ± 18 |
| KO-1202 | 10 | 1325C→T | Ser442→Phe | 9/35 | 300 | 79 ± 11 |
| S. antibioticus | Actinomycin (μg/ml)g | |||||
| Wild type | NA | NA | NA | NA | 1 | 11 ± 2 |
| KO-1212 | 10 | 1264C-1275C→Δ | Leu422-Phe425→Δ | 1/21 | 300 | 2 ± 1 |
| KO-1160 | 10 | 1267T-1278G→Δ | Ser423-Met426→Δ | 1/21 | >300 | 53 ± 9 |
| KO-1161 | 10 | 1270C→G | Gln424→Glu | 1/21 | 50 | 33 ± 7 |
| KO-1162 | 10 | 1309C→G | His437→Asp | 2/21 | 300 | 9 ± 2 |
| KO-1163 | 10 | 1309C→T | His437→Tyr | 5/21 | >300 | 79 ± 9 |
| KO-1164 | 10 | 1310A→G | His437→Arg | 3/21 | >300 | 86 ± 16 |
| KO-1165 | 10 | 1319G→A | Arg440→His | 1/21 | 50 | 23 ± 5 |
| KO-1166 | 10 | 1325C→T | Ser442→Leu | 1/21 | 50 | 19 ± 2 |
| S. parvulus | Actinomycin (μg/ml)h | |||||
| Wild type | NA | NA | NA | NA | 20 | 6 ± 1 |
| KO-1183 | 100 | 1261C-1269C→Δ | Gln421-Ser423→Δ | 1/23 | >300 | 6 ± 1 |
| KO-1184 | 100 | 1279G→A | Asp427→Asn | 2/23 | >300 | 6 ± 2 |
| KO-1185 | 100 | 1279G→T | Asp427→Tyr | 3/23 | >300 | 6 ± 1 |
| KO-1186 | 100 | 1280A→G | Asp427→Gly | 2/23 | >300 | 7 ± 3 |
| KO-1187 | 100 | 1280A→T | Asp427→Val | 1/23 | >300 | 10 ± 1 |
| KO-1188 | 100 | 1281C-1283T→Δ | Asp427,Gln428→Glu | 1/23 | >300 | 6 ± 1 |
| KO-1189 | 100 | 1284G-1289A→Δ | Gln428-Asn430→His | 1/23 | >300 | 4 ± 1 |
| KO-1190 | 100 | 1298C→T | Ser433→Leu | 2/23 | >300 | 4 ± 3 |
| KO-1191 | 100 | 1309C→A | His437→Asn | 1/23 | >300 | 7 ± 1 |
| KO-1192 | 100 | 1309C→T | His437→Tyr | 4/23 | >300 | 4 ± 1 |
| KO-1193 | 100 | 1310A→T | His437→Leu | 1/23 | >300 | 6 ± 2 |
| S. lavendulae | Formycin (μg/ml)i | |||||
| Wild type | NA | NA | NA | NA | 1 | 16 ± 1 |
| KO-1176 | 10 | 1265T→C | Leu422→Pro | 1/23 | 20 | 40 ± 7 |
| KO-1177 | 10 | 1280A→G | Asp427→Gly | 6/23 | 20 | 0 |
| KO-1178 | 10 | 1298C→T | Ser433→Leu | 1/23 | 30 | 0 |
| KO-1179 | 10 | 1309C→T | His437→Tyr | 4/23 | 200 | 0 |
| KO-1180 | 10 | 1309C→G | His437→Asp | 1/23 | 200 | 8 ± 1 |
| KO-1181 | 10 | 1310A→G | His437→Arg | 1/23 | 300 | 0 |
| KO-1182 | 10 | 1319G→A | Arg440→His | 1/23 | 50 | 55 ± 14 |
| A. orientalis | Vancomycin (μg/ml)j | |||||
| Wild type | NA | NA | NA | NA | 20 | 90 ± 7 |
| KO-1153 | 30 | 1271A→T | Gln424→Leu | 1/84 | >300 | 19 ± 1 |
| KO-1154 | 30 | 1309C→T | His437→Tyr | 22/84 | >300 | 172 ± 7 |
| KO-1155 | 30 | 1310A→G | His437→Arg | 29/84 | >300 | 80 ± 5 |
| KO-1156 | 30 | 1319G→T | Arg440→Leu | 1/84 | >300 | 0 |
| KO-1157 | 30 | 1319G→A | Arg440→His | 7/84 | >300 | 0 |
| KO-1158 | 30 | 1325C→A | Ser442→Tyr | 1/84 | >300 | 270 ± 17 |
| KO-1159 | 30 | 1325C→T | Ser442→Phe | 23/84 | >300 | 101 ± 5 |
Numbered from the start codon of the open reading frame of S. coelicolor.
Determined after 5 days of incubation on GYM medium.
Strains were grown in SPY medium at 25°C for 3 days. All measurements were performed in triplicate.
NA, not applicable (parent strain).
Results for actinorhodin were determined after 5 days of incubation in GYM liquid medium or 7 days of incubation on GYM agar plate. Shown are mean values of optical density at 640 nm (OD640) of triplicate flasks (in liquid culture). +, poor production; ++, moderate; +++, abundant (in solid culture).
Strains were grown in R3/1 medium at 30°C for 6 days. All measurements were performed in triplicate.
Strains were grown in SYM medium at 30°C for 5 days. All measurements were performed in triplicate.
Strains were grown in 2× SYM medium at 30°C for 6 days. All measurements were performed in triplicate.
Strains were grown in MPY medium at 25°C for 2 days. All measurements were performed in triplicate.
Strains were grown in vancomycin production medium at 30°C for 6 days. All measurements were performed in triplicate.
Table 2.
Summary of rpoB mutations effective for antibiotic overproduction
| Microorganism | Antibiotic | Effective rpoB mutation(s)a | Source or reference |
|---|---|---|---|
| S. lividans | Actinorhodin | S433P, S433L, H437R, H437Y, R440C, R599C | 19, 40 |
| S. mauvecolor | Piperidamycin | H437D, H437L, H437R, H437Y | 18 |
| S. albus | Salinomycin | S442F | 14 |
| S. incarnatus | Sinefungin | D427G | 49 |
| S. coelicolor | Actinorhodin | S423T, D427A, S433L, S433P, H437L, H437R, H437Y | 42; this study |
| S. griseus | Streptomycin | Q424K, H437R, S442L | This study |
| S. antibioticus | Actinomycin | Q424E, H437R, H437Y | This study |
| S. parvulus | Actinomycin | D427V | This study |
| S. lavendulae | Formycin | L422P, R440H | This study |
| A. orientalis | Vancomycin | H437Y, S442Y | This study |
| S. erythraea | Erythromycin | H437D, H437R, H437Y, S442F, S442Y | This study |
Mutations are numbered from the start codon of the open reading frame of S. coelicolor. Boldface characters show mutations that were often detected as antibiotic overproduction mutations.
Effects of rpoB mutations on streptomycin biosynthetic genes in S. griseus.
We reported previously that rsmG mutations conferring a low level of resistance to streptomycin cause antibiotic overproduction in S. coelicolor and S. griseus and that enhanced expression of the metK gene encoding S-adenosylmethionine synthetase is responsible for the enhanced production of actinorhodin and streptomycin (10, 23). In fact, addition of S-adenosylmethionine causes overproduction of streptomycin in S. griseus, accompanied by enhanced expression of adpA (encoding a transcriptional regulator) and strR (encoding the streptomycin biosynthesis operon regulator). As the Q424K mutation in rpoB was most effective in enhancing streptomycin production (Table 1), we analyzed transcription of metK, adpA, and strR, together with several genes (strB1, strF, and strD) involved in streptomycin biosynthesis. As expected, expression of strR was markedly enhanced in the rpoB Q424K mutant KO-1167 compared to the wild-type strain at both early (9-h) and late (24-h) growth phases, accompanied by enhanced expression of biosynthetic genes (strB1, strD, and strF), underlying the enhanced production of streptomycin in KO-1167 (Fig. 1). In contrast, expression of metK was marginally increased only in the early growth phase, and expression of adpA was rather impaired. Thus, unlike the case for rsmG mutant, enhanced expression of strR appears to be solely responsible for the observed streptomycin overproduction in the rpoB Q424K mutant, although AdpA acts as a central transcriptional regulator in the A-factor regulatory cascade in the S. griseus wild-type strain (28).
Fig 1.
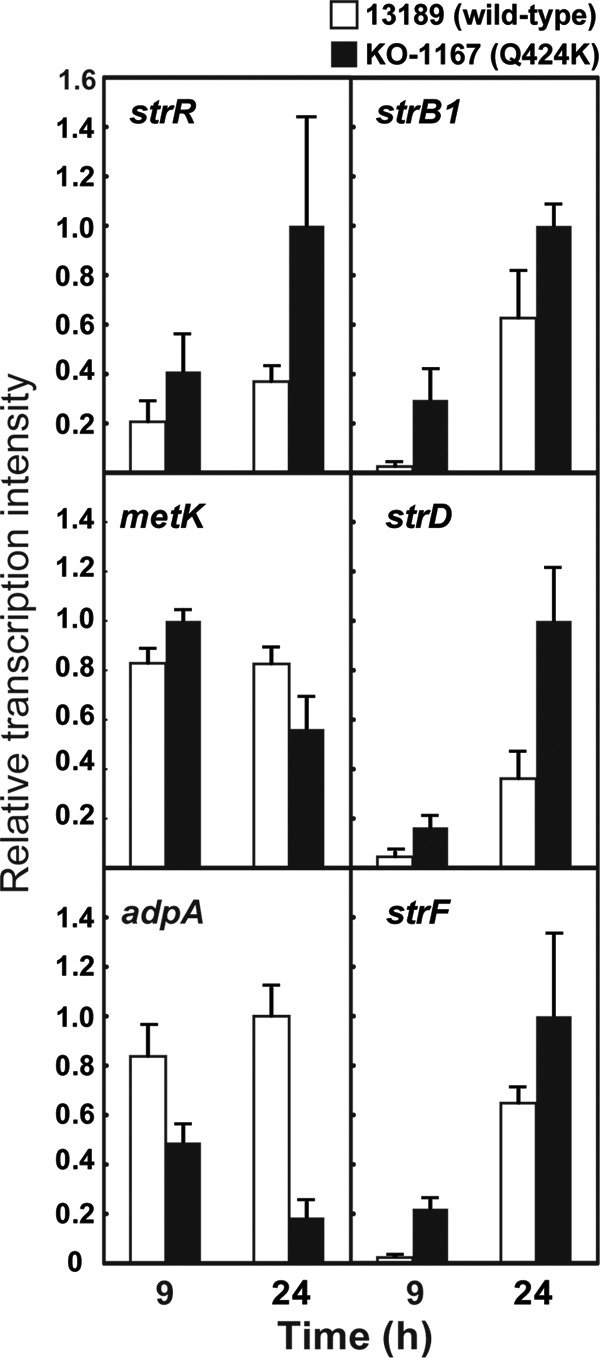
Transcriptional analysis of strR, metK, adpA, strB1, strD, and strF by real-time qPCR in S. griseus. The RNAs were extracted from cells of wild-type (13189) and KO-1167 (rpoB Q424K) mutant strains grown at 25°C to the early (9 h) or late growth phase (24 h) in SPY medium. Total RNA preparation and real-time qPCR were performed as described in Materials and Methods. The maximum expression levels were taken as unity (= 1). The error bars indicate the standard deviations of the means of three or more samples.
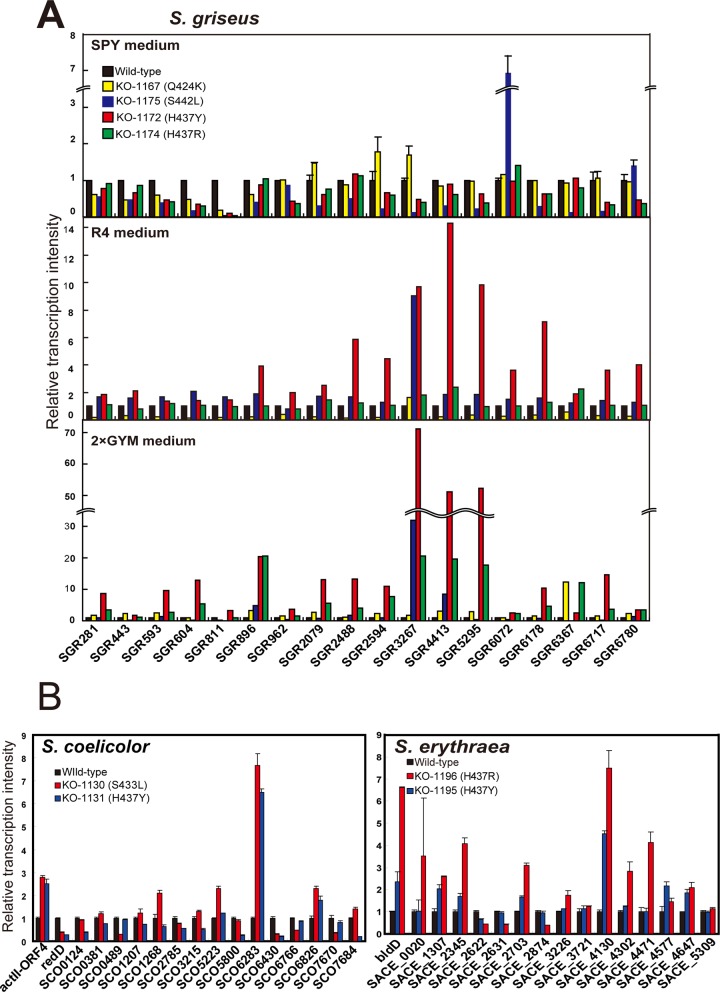
Similarly, the biosyntheses of actinorhodin (in S. coelicolor) and erythromycin (in S. erythraea) are controlled by transcription regulatory proteins ActII-ORF4 and BldD, respectively (29, 30). Notably, rpoB mutants (KO-1130 and KO-1131 of S. coelicolor and KO-1195 and KO-1196 of S. erythraea) with enhanced production of actinorhodin and erythromycin displayed enhanced expression of actII-ORF4 and bldD, respectively, accounting for the increased antibiotic production in these rpoB mutants (Fig. 2B). In accordance with the upregulation of bldD, expression of eryCII (a gene for erythromycin biosynthesis) increased 2- to 5-fold in the rpoB H437Y mutant of S. erythraea (data not shown). On the other hand, expression of another transcription regulatory gene, redD (controlling the biosynthesis operon for undecylprodigiosin in S. coelicolor), was not upregulated but rather downregulated, showing differential effects of each rpoB mutation on the expression of each transcription regulatory gene (Fig. 2B).
Fig 2.
Transcriptional analysis of the genes involved in the secondary metabolite biosynthetic gene clusters in the rpoB mutant strains of S. griseus, S. coelicolor, and S. erythraea. Total RNA preparation and real-time qPCR were performed as described in Materials and Methods. The maximum expression levels detected in each culture were compared, taking the maximum expression levels of the wild-type strain as unity (= 1). (A) The RNAs were extracted from S. griseus cells grown to the late growth phase (24 h in SPY medium at 25°C; 24, 36, 48, and 60 h in R4 medium at 30°C; 12, 24, and 36 h in 2× GYM medium at 30°C). (B) The RNAs were extracted from cells grown at 30°C to the late growth phase (24, 36, and 48 h in GYM medium for S. coelicolor; 12, 24, 36, and 48 h in 2× GYM medium for S. erythraea).
rpoB mutations widely activate transcription of cryptic secondary metabolite biosynthetic gene clusters.
A recent study in our laboratory indicated that certain mutations in rpoB or rpsL genes can activate silent or weakly expressed genes of actinomycetes or Bacillus subtilis, leading to the discovery of novel antibacterial agents (17, 18). Later, we found that rsmG mutations, which confer a low level of resistance to streptomycin, can activate not only streptomycin production but also the expression of other secondary metabolite biosynthetic genes in S. griseus (31). Therefore, we turned our attention to the effects of rpoB mutations on the expression of cryptic secondary metabolite biosynthetic gene clusters in various actinomycetes to assess the broad applicability of the rpoB mutation method. First, a total of 18 genes belonging to 18 secondary metabolite biosynthetic gene clusters of S. griseus (Table 3) were subjected to transcriptional analysis by real-time quantitative PCR (qPCR), comparing the wild-type and rpoB mutant (Q424K, S442L, H437Y, and H437R) strains. The RNAs were extracted from cells grown to late growth phase, and the maximum expression levels detected in each culture were compared (Fig. 2A). The profiles of changes in expression of each gene are shown in Fig. S1 and S2 in the supplemental material. Strikingly, of the rpoB mutants examined, strain KO-1172 with the H437Y mutation grown in 2× GYM medium showed remarkable activation of cryptic genes at the transcriptional level. This was especially pronounced for SGR3267, SGR4413, and SGR5295, which were activated by 50- to 70-fold. Likewise, the H437Y mutation was effective when cells were grown in R4 medium (activated up to 14-fold), while the efficacy of the H437Y mutation was no longer detected when cells were grown in SPY medium (a medium developed for streptomycin production). Although the H437Y mutation was effective in both 2× GYM medium and R4 medium, the H437R mutation exerted its effect only in R4 medium. It is notable that although the Q424K and S442L mutations were quite effective at enhancing streptomycin production (Table 1), these mutations were not effective in activating the cryptic genes, except for SGR3267, SGR6072, and SGR6367, which were activated by 7- to 30-fold (Fig. 2A). These results indicated that the abilities of rpoB mutations to activate the cryptic genes are medium dependent, with each rpoB mutation exerting differential effects on the activation of each cryptic gene cluster.
Table 3.
Secondary metabolite biosynthetic genes of S. griseus IFO13189, S. coelicolor A3(2), and S. erythraea NRRL2338 analyzed in this study
| Organism and genea | Product(s) | Secondary metabolite biosynthetic gene cluster product(s)b |
|---|---|---|
| S. griseus | ||
| metK | S-Adenosylmethionine synthetase | —c |
| adpA | Transcriptional regulator | — |
| strR | Streptomycin biosynthesis operon regulator | Streptomycin |
| strB1 | Scyllo-inosamine-4-phosphate amidinotransferase | Streptomycin |
| strD | Putative glucose-1-phosphate thymidylyltransferase | Streptomycin |
| strF | StrF protein | Streptomycin |
| SGR281 | Hypothetical protein | PKS-NRPS hybrid (SGR278–SGR283) |
| SGR443 | Putative ABC transporter ATPase and permease component | NRPS for siderophore (SGR443–SGR455) |
| SGR593 | Hypothetical protein | NRPS (SGR574–SGR593) |
| SGR604 | Putative enediyne biosynthesis protein | Enediyne PKS (SGR604–SGR611) |
| SGR811 | Putative oxidoreductase | PKS-NRPS hybrid (SGR810–SGR815) |
| SGR896 | Putative O-methyltransferase | NRPS (SGR895–SGR901) |
| SGR962 | Putative squalene-hopene cyclase | Hopanoid (SGR962–SGR966) |
| SGR2079 | Putative terpene cyclase | Terpene (SGR2079) |
| SGR2488 | Putative dehydrogenase | Type I PKS, NRPS (SGR2482–SGR2489) |
| SGR2594 | Putative integral membrane ion antiporter | NRPS (SGR2586–SGR2598) |
| SGR3267 | Putative cytochrome P450 | Type II PKS, NRPS (SGR3239–SGR3288) |
| SGR4413 | Putative lantibiotic biosynthesis protein | Lantibiotic (SGR4408–SGR4421) |
| SGR5295 | 5-Aminolevulinate synthase | Unknown (SGR5285–SGR5295) |
| SGR6072 | Putative ketosteroid isomerase | Type I PKS (SGR6071–SGR6083) |
| SGR6178 | Putative thioesterase | Type I PKS (SGR6177–SGR6183) |
| SGR6367 | Putative oxidoreductase | Type I PKS (SGR6360–SGR6387) |
| SGR6717 | Putative ABC transporter ATPase and permease component | NRPS for siderophore (SGR6709–SGR6717) |
| SGR6780 | Putative malonyl-coenzyme A:ACP transacylase | Type I PKS, PKS-NRPS hybrid (SGR6776–SGR6786) |
| S. coelicolor | ||
| SCO0124 | Hypothetical protein | Eicosapentaenoic acid (type I iterative PKS; SCO0124–SCO0129) |
| SCO0381 | Putative glycosyl transferase | Unknown (deoxysugar; SCO0381–SCO0401) |
| SCO0489 | Conserved hypothetical protein | Coelichelin (NRPS; SCO0489–SCO0499) |
| SCO1207 | Putative cytochrome P450 | Tetrahydroxynaphthalene (chalcone synthase; SCO1206–SCO1208) |
| SCO1268 | Putative acyltransferase | Unknown (type II fatty acid synthase; SCO1265–SCO1273) |
| SCO2785 | Conserved hypothetical protein | Desferrioxamines (siderophore synthetase; SCO2782–SCO2785) |
| SCO3215 | Hypothetical protein | CDAd (NRPS; SCO3210–SCO3249) |
| SCO5085 (actII-ORF4) | Actinorhodin cluster activator protein | Actinorhodin (type II PKS; SCO5071–SCO5092) |
| SCO5223 | Putative cytochrome P450 | Unknown (sesquiterpene synthase; SCO5222–SCO5223) |
| SCO5800 | Conserved hypothetical protein | Unknown (siderophore synthetase; SCO5799–SCO5801) |
| SCO5877 (redD) | Transcriptional regulator RedD | Prodiginines (NRPS, type I modular PKS; SCO5877–SCO5898) |
| SCO6283 | Conserved hypothetical protein | Unknown (type I modular PKS; SCO6273–SCO6288) |
| SCO6430 | Hypothetical protein | Unknown (NRPS; SCO6429–SCO6438) |
| SCO6766 | Conserved hypothetical protein | Hopanoids (squalene-hopene cyclase; SCO6759–SCO6771) |
| SCO6826 | Conserved hypothetical protein | Unknown (type I modular PKS; SCO6826–SCO6827) |
| SCO7670 | Conserved hypothetical protein | Unknown (chalcone synthase; SCO7669–SCO7671) |
| SCO7684 | Conserved hypothetical protein | Coelibactin (NRPS; SCO7681–SCO7691) |
| S. erythraea | ||
| bldD | Putative transcriptional regulator | — |
| SACE_0020 | Hypothetical protein | Pfa (polyketides; SACE_0018–SACE_0028) |
| SACE_1307 | Hypothetical protein | Nrps1 (nonribosomal peptides; SACE_1304– SACE_1310) |
| SACE_2345 | Putative hydrolase | Pks1 (polyketides; SACE_2342–SACE_2347) |
| SACE_2622 | Cytochrome P450 | Nrps2-Pks (nonribosomal peptides; SACE_1304–SACE_1310) |
| SACE_2631 | Cytochrome P450-like enzyme | Pks2 (polyketides; SACE_2628–SACE_2631) |
| SACE_2703 | Hypothetical protein | Nrps3 (nonribosomal peptides; SACE_2692–SACE_2703) |
| SACE_2874 | Phospho-2-dehydro-3-deoxyheptonate aldolase | Pks3 (polyketides; SACE_2864–SACE_2879) |
| SACE_3226 | Hypothetical protein | Nrps6 (nonribosomal peptides; SACE_3223–SACE_3229) |
| SACE_3721 | Methyltransferase | Tpc2 (terpens; SACE_3721–SACE_3723) |
| SACE_4130 | Hypothetical protein | Pke (polyketides; SACE_4128–SACE_4145) |
| SACE_4302 | Hypothetical protein | Pks4 (polyketides; SACE_4302–SACE_4307) |
| SACE_4471 | Hypothetical protein | Pks5 (polyketides; SACE_4471–SACE_4478) |
| SACE_4577 | Hypothetical protein | Pks6 (polyketides; SACE_4567–SACE_4578) |
| SACE_4647 | UbiA prenyltransferase | Tpc4 (terpens; SACE_4645–SACE_4654) |
| SACE_5309 | Putative cytochrome P450 | Pks7 (polyketides; SACE_5306–SACE_5309) |
Next, we analyzed cryptic gene activation in S. coelicolor and S. erythraea. A total of 15 genes belonging to 15 secondary metabolite biosynthetic gene clusters (Table 3) were subjected to transcription analysis using cells grown to the late growth phase. As expected, the H437Y and H437R mutations, which were effective in enhancing erythromycin production (Table 1), were widely effective in enhancing the activity of the cryptic genes of S. erythraea; 6 of the 15 genes examined showed a 3-fold or more increase in transcription (Fig. 2B). Similarly, the S433L and H437Y mutations, which were effective in enhancing actinorhodin production (Table 1), were effective, although not remarkable, in enhancing the activity of the cryptic genes of S. coelicolor. The profiles of changes in expression of each gene are shown in Fig. S3 (for S. coelicolor) and S4 (for S. erythraea) in the supplemental material.
The S. griseus cryptic genes SGR3267 and SGR5295 cannot be activated under general stress conditions.
It is widely accepted that bacterial secondary metabolism usually starts when cells encounter adverse environmental conditions, as represented by nutrient limitation or the presence of stress stimuli. As the S. griseus cryptic genes SGR3267 and SGR5295 were markedly activated by introducing certain rpoB mutations, we were interested in whether these cryptic genes can be activated under certain stressful conditions. Therefore, we conducted transcriptional analysis of SGR3267 and SGR5295 using cells that had been subjected to 30 different stress conditions, followed by a further 3 h of incubation (Table 4). These stress stimuli included pH changes, temperature shifts, and addition of chemicals, such as heavy metals, antibiotics, and flavonoids. As heavy metals and antibiotics are potent growth inhibitors, these chemicals were added at sublethal concentrations (i.e., one-third of the MIC). Despite the wide variety of stress conditions, these genes were not activated under the stress conditions examined. Treatment with rifampin was also ineffective. These results can be taken as evidence that the genes SGR3267 and SGR5295 are “silent” under laboratory fermentation conditions and, in turn, emphasize the efficacy of rpoB mutations that resulted in 50- to 70-fold activation.
Table 4.
Transcriptional analysis of the S. griseus genes SGR3267 and SGR5295 under various stressful conditionsa
| Treatment | Relative expression level ofb: |
|
|---|---|---|
| SGR3267 | SGR5295 | |
| Control (no treatment) | 1 | 1 |
| H2O2 (0.1%) | 0.6 | 0.6 |
| Oxygen limitation (no shaking) | 1.1 | 0.6 |
| Acidified (pH 5) | 1.1 | 1.4 |
| Alkalinized (pH 10) | 0.6 | 0.7 |
| High temp (37°C) | 1.2 | 1.1 |
| Low temp (16°C) | 0.9 | 1.8 |
| Ethanol (2%) | 0.7 | 0.6 |
| Sorbitol (10%) | 0.3 | 0.5 |
| NaCl (10%) | 0.2 | 0.3 |
| 2-Deoxy-d-glucose (2%) | 0.6 | 0.7 |
| Serine hydroxamate (2 mM) | 0.6 | 0.7 |
| EDTA (10 mM) | 0.5 | 0.4 |
| CuSO4 (1 mM) | 1.5 | 1.2 |
| ZnSO4 (3 mM) | 1.8 | 1.5 |
| CoCl2 (0.3 mM) | 0.9 | 1.9 |
| MnCl2 (10 mM) | 1.0 | 1.7 |
| Ga2(SO4) (1 mM) | 0.7 | 1.4 |
| DMSOc (0.2%) | 0.7 | 0.5 |
| Decoynine (0.3 mM) | 0.7 | 1.1 |
| Rifampin (0.5 μg/ml) | 0.9 | 0.8 |
| Rifampin (0.17 μg/ml) | 0.8 | 0.7 |
| Gentamicin (0.3 μg/ml) | 1.1 | 0.6 |
| Penicillin (10 μg/ml) | 0.8 | 0.5 |
| Vancomycin (0.3 μg/ml) | 0.7 | 0.5 |
| Caffeine (50 mM) | 1.6 | 1.1 |
| Quercetin (3 mM) | 1.6 | 0.6 |
| Kaempferol (2.3 mM) | 0.4 | 0.5 |
| Apigenin (1.23 mM) | 0.4 | 0.6 |
| Luteolin (2.3 mM) | 0.8 | 0.8 |
| Scandium chloride (0.5 mM) | 3.0 | 2.8 |
| Lanthanum chloride (1 mM) | 4.1 | 4.2 |
S. griseus IFO13189 (wild-type strain) was grown in 2× GYM medium at 30°C for 12 h. Then the cells were subjected to each stress treatment (addition of chemicals, pH changes, or temperature shift), followed by a further 3 h of incubation. RNAs were extracted as described in Materials and Methods. The results with the rare earth elements are shown in boldface.
The expression levels are shown taking the expression level of the control (no treatment) as unity (= 1).
DMSO, dimethyl sulfoxide.
We reported previously that rare earth elements, such as scandium (Sc) and lanthanum (La), not only enhance antibiotic production but also activate expression of the cryptic secondary metabolite biosynthetic gene clusters in S. coelicolor (27). Strikingly, scandium and lanthanum exerted their apparent effects on transcription of SGR3267 and SGR5295 of S. griseus when added to the medium at low concentrations. Transcription levels of these genes were upregulated by 4-fold with treatment of the cells with a sublethal concentration (one-third of the MIC) of lanthanum (Table 4), thus highlighting rare earth elements as distinguished stress stimuli.
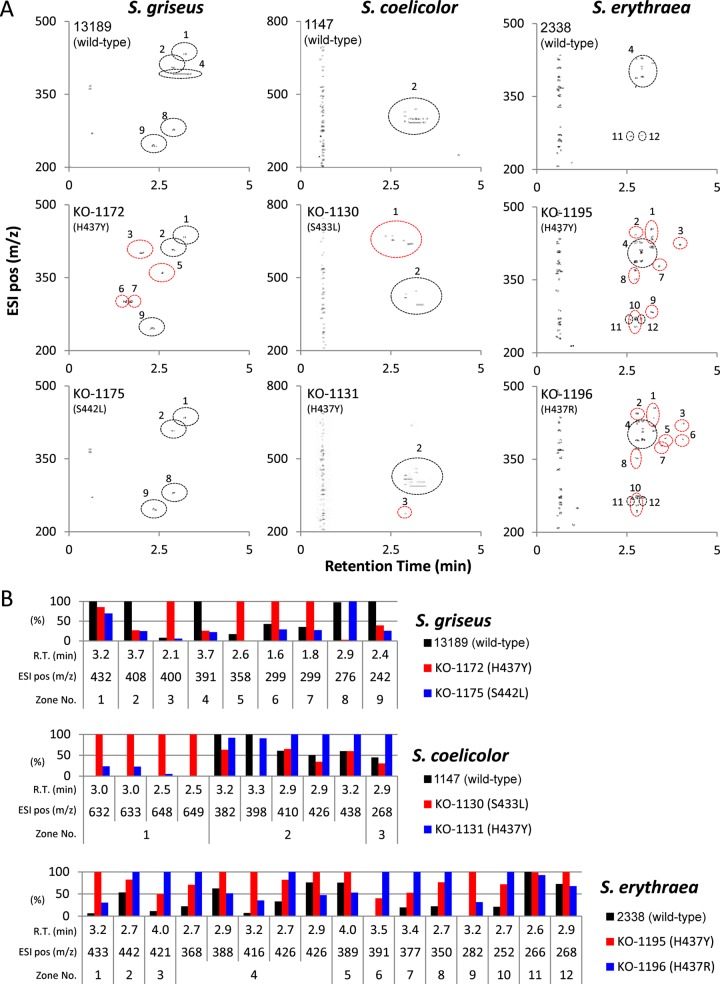
The rpoB mutants produce many metabolites not detected in the wild-type strain. (i) Metabolite analysis with 2D-UPLC/MS.
As the rpoB mutations markedly upregulated the expression of cryptic genes at the transcriptional level, we compared metabolic profiles between the wild-type and rpoB mutant strains. Strains of S. griseus, S. coelicolor, and S. erythraea were grown to the late growth phase, and then the metabolites produced were analyzed by two-dimensional (2D)-UPLC/MS. Strikingly, the rpoB mutants produced many metabolites that were not detected in the wild-type strains. This was especially pronounced in S. griseus KO-1172 (H437Y) and S. erythraea KO-1195 (H437Y) and KO-1196 (H437R), as indicated by red circles in Fig. 3A. For quantitative description, we calculated each peak area from selected ion chromatograms and compared the peak areas among the wild-type and rpoB mutant strains (Fig. 3B). In S. griseus, the levels of production of metabolites designated zones no. 3 (m/z 400) and no. 5 (m/z 358) were markedly activated, and zones no. 6 (m/z 299) and no. 7 (m/z 299) showed extensive activation. In S. coelicolor, the production levels of metabolites designated zone no. 1 (m/z 631, 633, 647, and 649) were remarkably activated, and that of zone no. 3 (m/z 268) was extensively activated. The metabolites with m/z of 631, 633, and 649 were assigned as γ-actinorhodin, γ′-actinorhodin, and ε-actinorhodin, respectively (26, 32, 33), while the metabolite with m/z 647 was considered a novel actinorhodin-related compound, because disruption of actII-ORF4 in KO-1130 resulted in almost complete abolition of these four metabolites simultaneously (see Fig. S5A in the supplemental material). The structures of these known actinorhodin-related compounds, together with the possible structure of the unknown actinorhodin, are shown in Fig. S5B.
Fig 3.
Analysis of the metabolite profile derived from the rpoB mutants. The strains were grown for 4 days in 2× GYM medium (for S. griseus) or GYM medium (for S. coelicolor) as described in the legend to Fig. 2. S. erythraea strains were grown for 2 days in 2× GYM medium. The metabolite analysis was performed as described in Materials and Methods. (A) Metabolite profiles of wild-type and rpoB mutant strains as analyzed by 2D-UPLC/MS. The results are shown as 2D plots (positive [pos] ions [m/z] versus retention time [min]). The spots detected in the wild type are marked by black circles, while those not detected in the wild type are marked by red circles. (B) Comparison of peak area of metabolites produced by wild-type and rpoB mutant strains. The differences in peak areas were calculated from selected ion chromatograms. The largest peak area was designated 100%. R.T., retention time.
Quantitative comparison of metabolites in S. erythraea strains was characterized by marked activation of the production of many metabolites (Fig. 3B), as expected from the results of 2D-UPLC/MS (Fig. 3A).
(ii) Differential effects of rpoB mutations on metabolite profile.
Although the results shown in Fig. 3B suggest differential effects of rpoB mutations on metabolite profile, this was confirmed by analyzing the metabolite profiles of nine S. griseus rpoB mutants. As shown in Fig. 4, it is apparent that the best rpoB mutation to enhance the yield of each metabolite varies from metabolite to metabolite, although the H437Y mutation was most often the best. For example, the H437D mutation was effective for productivity enhancement of the metabolites designated zone no. 3 but ineffective for zones no. 6 and 7. Similarly, the Q424K mutation was effective for zone no. 1 but ineffective for other metabolites.
Fig 4.
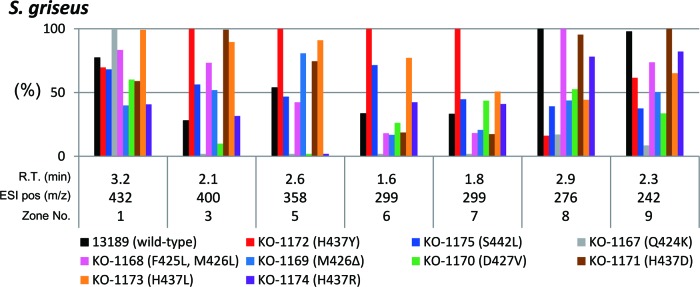
Differential effects of rpoB mutations on the metabolite profile in S. griseus. Nine rpoB mutants were grown as described in the legend to Fig. 3, and the metabolites were analyzed by 2D-UPLC/MS. The peak areas calculated from selected ion chromatograms are shown, taking the largest peak area as unity (100%).
DISCUSSION
Genome sequencing of Streptomyces, fungi, and myxobacteria showed that, although each strain contains genes encoding the enzymes necessary to synthesize a plethora of potential secondary metabolites, only a fraction are expressed during fermentation. There has been increasing interest in the activation of these cryptic pathways. In this study, we demonstrated that rpoB mutations are widely effective not only in enhancing antibiotic production but also in activating the silent genes at both the transcriptional and metabolite levels in various actinomycetes. Therefore, this approach may solve the early stage discovery problems of (i) inducing some level of expression of cryptic biosynthetic gene clusters (“waking” the sleeping genes) and (ii) rapidly increasing product yields to obtain enough material to characterize chemically and biologically (early stage yield enhancement) (34). It is notable that the activation of silent gene clusters by rpoB mutations at the transcriptional level was medium dependent, with each rpoB mutation exerting differential effects on the activation of each silent gene cluster. Conceivably, the expression of each silent gene cluster is controlled by multiple factors, which are affected, qualitatively and quantitatively, under various culture conditions. These findings suggest that strains containing different rpoB mutations (e.g., H437Y and H437R) should be grown in different media to access the full spectrum of silent gene activation. It is also notable that the rpoB mutations were effective in enhancing actinomycin production in S. antibioticus but not S. parvulus, indicating the strain dependency of the rpoB mutation effect. Similarly, erythromycin resistance (ery) mutations were effective in enhancing actinomycin production in S. parvulus but not S. antibioticus, although the reason for this difference remains unknown (35).
The genetic regulation of streptomycin biosynthesis has been studied in detail in relation to A-factor (28). Briefly, ArpA (A-factor receptor protein) negatively regulates the expression of adpA in the absence of A-factor. The resulting transcriptional activator, AdpA, synthesized in the presence of A-factor, positively regulates the expression of strR, leading to synthesis of StrR. This StrR protein switches on expression of the streptomycin biosynthesis operon as a pathway-specific regulator protein (36). The present study clearly demonstrated a detrimental effect of the rpoB Q424K mutation on adpA expression (Fig. 1). Nevertheless, the expression of strR was markedly upregulated, resulting in enhanced expression of biosynthetic genes (strB1, strD, and strF) and eventual streptomycin overproduction. Mutant RNAP with the Q424K mutation was apparently more favorable than the native RNAP in transcribing strR. These results, in turn, showed that modulation of downregulated genes (as represented by pathway-specific regulatory genes strR in streptomycin production and actII-ORF4 in actinorhodin production) is more essential in subjecting the rpoB mutations to antibiotic overproduction. S. erythraea has been widely studied as a model system for antibiotic production (37, 38). Interestingly, BldD, a key developmental regulator in actinomycetes (30), regulates the synthesis of erythromycin (39). In accordance with this previous finding, the rpoB H437R mutation caused a marked (6.5-fold) enhancement of bldD expression, eventually leading to upregulation of a biosynthetic gene (eryCII) and erythromycin overproduction (Fig. 2B, Table 1). As discussed by Carata et al. (24), the increased erythromycin production may also be due, in part, to the effects of rpoB H437R on the expression of genes encoding key enzymes involved in carbon and nitrogen metabolism, which may result in activation of erythromycin feeder pathways. The recent study of Derewacz et al. (20) working with Nocardiopsis sp. supports this notion. In turn, although we did not analyze in the present study the rpoB mutants with reduced ability to produce antibiotics, it is possible that mutant RNAPs with such rpoB mutations may have a reduced affinity to the promoter region of transcription regulatory genes for secondary metabolite biosynthetic genes or may perturb the expression of key genes for primary metabolism, which may result in deactivation of antibiotic feeder pathways.
The introduction of the rpoB mutation S487L into a B. subtilis strain resulted in cells that overproduced an amino sugar antibiotic, 3,3′-neotrehalosadiamine (NTD), the production of which is dormant in the wild-type strain (17). Unlike the wild-type RNAP, it is possible that the mutant RNAP efficiently recognized the σA-dependent promoters, resulting in marked activation of the NTD biosynthesis pathway. On the other hand, assessment of Streptomyces mauvecolor 631689 demonstrated that two rpoB mutants (H437D or H437L) produced a family of novel antibiotics, the piperidamycins. In this case, the activation of silent genes was attributed, at least in part, to the increased affinity of mutant RNAP for the silent gene promoters (18). The observed upregulation of cryptic genes by rpoB mutations in actinomycetes (Fig. 2) could be accounted for, at least in part, in this way. In contrast, the downregulation observed in several cryptic genes (Fig. 2) may be accounted for by the decreased affinity of mutant RNAP for the promoters.
The amino acid alteration at position H437 (corresponding to H406, H482 and H526 in Thermus thermophilus, B. subtilis, and Escherichia coli, respectively) was often effective in activating the cryptic pathways (Table 2). The mutation at position H437 has been shown to circumvent the detrimental effects of the relA and afsB mutations (in S. coelicolor) and the relC mutation (in S. lividans) on actinorhodin production, perhaps by mimicking the ppGpp-bound form of RNAP (40–43). Taken together, ppGpp likely participates significantly even in the activation of cryptic gene clusters as well as highly expressed, well-known, secondary metabolite gene clusters. Precocious overexpression of cryptic gene clusters and cell lysis observed at late growth phase of S. griseus with the rpoB H437Y mutation (see Fig. S2 in the supplemental material) can be explained by its unique spectrum of effects in modulating the transcription of each gene. In fact, the rpoB S444F mutation (corresponding to S442F in this study) of S. erythraea markedly alters the transcriptional profile of this organism (24). The defective effects of the rpoB H437Y mutation on sporulation and competence were also reported in B. subtilis (44).
In addition to marked activation of cryptic genes, it should be emphasized that the rpoB mutations actually rendered cells active in producing a number of metabolites, which were not detected in the wild-type strain (Fig. 3A). These results demonstrated that introducing the rpoB mutations not only enhanced expression of the cryptic genes at the transcriptional level but could be effective in discovering novel secondary metabolites. It is also notable that differential effects of rpoB mutation were observed at both the gene transcription level (Fig. 2A) and the metabolite level (Fig. 4).The discovery of a new actinorhodin-related compound in the S. coelicolor rpoB mutant encourages us to utilize the rpoB mutation approach for drug discovery. The proposed structure of the new actinorhodin-related compound based on its molecular size most closely resembles ε-actinorhodin (see Fig. S5B in the supplemental material), and these two actinorhodin-related compounds are major metabolites in the rpoB mutant KO-1130 (see Fig. S5A), possibly reflecting a close relationship in biosynthesis.
Flavonoids, exuded by plant cells, are abundant in soil, especially in the rhizosphere. Certain flavonoids possess antibacterial activity and, interestingly, induce the expression of certain bacterial genes (45). The flavonoids examined, however, had no significant effects on the expression of S. griseus cryptic genes (Table 4). Similarly, the expression was not significantly affected by heavy metals, such as Cu, Zn, Co, Mn, and Ga. Thus, the effect of rare earth elements on activation should be highlighted. The rare earth elements have recently been shown to be involved in the overproduction of antibiotics or enzymes (46, 47). The effects of scandium were exerted at the level of transcription of pathway-specific regulatory genes, as demonstrated by marked upregulation of actII-ORF4 in S. coelicolor (47). Notably, rare earth elements were effective not only in activating actII-ORF4 but also in activating silent or poorly expressed secondary metabolite biosynthetic genes (27). As rare earth elements are distributed ubiquitously in soil, it is possible that microorganisms have acquired the ability to respond to low levels of these elements over the course of their long evolutionary history, possibly as a means of adapting their physiology to prevailing conditions.
In addition to our approach, other methods have been developed to activate silent biosynthetic pathways in Streptomyces, including manipulation of nucleoid structure; the addition of N-acetylglucosamine to the medium or deletion of the dasR gene, which encodes an N-acetylglucosamine-responsive regulatory protein; the constitutive overexpression of a pathway-specific LAL regulatory gene; metabolic remodeling; and cell-to-cell interaction (reviewed by Ochi and Hosaka [13]). These are all characterized by applicability to a wide range of actinomycetes and potential scalability to high-throughput studies. Myxobacterial genomes have been shown to encode many genes involved in the synthesis of secondary metabolites (e.g., 8.6% of the Myxococcus xanthus genome), suggesting the possibility of discovering clinically relevant natural products (48). Hence, activation or enhancement of cryptic genes of this microbial group by introducing the rpoB mutation or by other approaches is of particular interest. Apart from the technology, it is important to envisage why cryptic genes are silent under laboratory fermentation conditions. Understanding the mechanism(s) underlying the silencing of cryptic genes would facilitate the full utilization of the microbial gene clusters for secondary metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to K.O. from the Ministry of Education, Culture, Sports, and Technology of the Japanese Government (Effective Promotion of Joint Research of Special Coordination Funds) and from the National Agriculture and Food Research Organization (Programme for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry).
We are grateful to Susumu Okamoto for the generous gift of the actII-ORF4 disruptant strain of S. coelicolor and Shigeo Tojo for supporting gene expression analysis. Y. Tanaka performed rpoB mutant preparation, antibiotic production, and gene expression analysis by real-time qPCR. K.H., Y.K., K.M., and R.K. conducted metabolite analysis of rpoB mutants using 2D-UPLC/MS. K.O. designed the research work, conducted genetic experiments, and wrote the article.
Footnotes
Published ahead of print 19 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00147-13.
REFERENCES
- 1. Newman DJ, Cragg GM. 2007. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70:461–477 [DOI] [PubMed] [Google Scholar]
- 2. Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147 [DOI] [PubMed] [Google Scholar]
- 3. Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526–531 [DOI] [PubMed] [Google Scholar]
- 4. Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, Yamashita A, Hattori M, Horinouchi S. 2008. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J. Bacteriol. 190:4050–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliynyk M, Samborskyy M, Lester JB, Mironenko T, Scott N, Dickens S, Haydock SF, Leadlay PF. 2007. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 25:447–453 [DOI] [PubMed] [Google Scholar]
- 6. Clardy J, Fischbach MA, Walsh CT. 2006. New antibiotics from bacterial natural products. Nat. Biotechnol. 24:1541–1550 [DOI] [PubMed] [Google Scholar]
- 7. Craney A, Ozimok C, Pimentel-Elardo SM, Capretta A, Nodwell JR. 2012. Chemical perturbation of secondary metabolism demonstrates important links to primary metabolism. Chem. Biol. 19:1020–1027 [DOI] [PubMed] [Google Scholar]
- 8. Ochi K, Okamoto S. 2012. A magic bullet for antibiotic discovery. Chem. Biol. 19:932–934 [DOI] [PubMed] [Google Scholar]
- 9. Hosaka T, Xu J, Ochi K. 2006. Increased expression of ribosome recycling factor is responsible for the enhanced protein synthesis during the late growth phase in an antibiotic-overproducing Streptomyces coelicolor ribosomal rpsL mutant. Mol. Microbiol. 61:883–897 [DOI] [PubMed] [Google Scholar]
- 10. Nishimura K, Hosaka T, Tokuyama S, Okamoto S, Ochi K. 2007. Mutations in rsmG, encoding a 16S rRNA methyltransferase, result in low-level streptomycin resistance and antibiotic overproduction in Streptomyces coelicolor A3(2). J. Bacteriol. 189:3876–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka Y, Komatsu M, Okamoto S, Tokuyama S, Kaji A, Ikeda H, Ochi K. 2009. Antibiotic overproduction by rpsL and rsmG mutants of various actinomycetes. Appl. Environ. Microbiol. 75:4919–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ochi K. 2007. From microbial differentiation to ribosome engineering. Biosci. Biotechnol. Biochem. 71:1373–1386 [DOI] [PubMed] [Google Scholar]
- 13. Ochi K, Hosaka T. 2013. New strategies for drug discovery: activation of silent or weakly expressed microbial gene clusters. Appl. Microbiol. Biotechnol. 97:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamehiro N, Hosaka T, Xu J, Hu H, Otake N, Ochi K. 2003. Innovative approach for improvement of an antibiotic-overproducing industrial strain of Streptomyces albus. Appl. Environ. Microbiol. 69:6412–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu H, Ochi K. 2001. Novel approach for improving the productivity of antibiotic-producing strains by inducing combined resistant mutations. Appl. Environ. Microbiol. 67:1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Hosaka T, Ochi K. 2008. Dramatic activation of antibiotic production in Streptomyces coelicolor by cumulative drug resistance mutations. Appl. Environ. Microbiol. 74:2834–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inaoka T, Takahashi K, Yada H, Yoshida M, Ochi K. 2004. RNA polymerase mutation activates the production of a dormant antibiotic 3,3′-neotrehalosadiamine via an autoinduction mechanism in Bacillus subtilis. J. Biol. Chem. 279:3885–3892 [DOI] [PubMed] [Google Scholar]
- 18. Hosaka T, Ohnishi-Kameyama M, Muramatsu H, Murakami K, Tsurumi Y, Kodani S, Yoshida M, Fujie A, Ochi K. 2009. Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat. Biotechnol. 27:462–464 [DOI] [PubMed] [Google Scholar]
- 19. Hu H, Zhang Q, Ochi K. 2002. Activation of antibiotic biosynthesis by specified mutations in the rpoB gene (encoding the RNA polymerase β subunit) of Streptomyces lividans. J. Bacteriol. 184:3984–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Derewacz DK, Goodwin CR, McNees CR, McLean JA, Bachmann BO. 2013. Antimicrobial drug resistance affects broad changes in metabolomic phenotype in addition to secondary metabolism. Proc. Natl. Acad. Sci. U. S. A. 110:2336–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ochi K. 1987. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A factor. J. Bacteriol. 169:3608–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ochi K. 1974. Biosynthesis of formycin: role of certain amino acids in formycin biosynthesis. J. Antibiot. (Tokyo) 27:902–916 [DOI] [PubMed] [Google Scholar]
- 23. Okamoto S, Lezhava A, Hosaka T, Okamoto-Hosoya Y, Ochi K. 2003. Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2). J. Bacteriol. 185:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carata E, Peano C, Tredici SM, Ferrari F, Tala A, Corti G, Bicciato S, De Bellis G, Alifano P. 2009. Phenotypes and gene expression profiles of Saccharopolyspora erythraea rifampicin-resistant (rif) mutants affected in erythromycin production. Microb. Cell Fact. 8:18–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIntyre JJ, Bull AT, Bunch AW. 1996. Vancomycin production in batch and continuous culture. Biotechnol. Bioeng. 49:412–420 [DOI] [PubMed] [Google Scholar]
- 26. Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. John Innes Foundation Norwich, United Kingdom [Google Scholar]
- 27. Tanaka Y, Hosaka T, Ochi K. 2010. Rare earth elements activate the secondary metabolite-biosynthetic gene clusters in Streptomyces coelicolor A3(2). J. Antibiot. (Tokyo) 63:477–481 [DOI] [PubMed] [Google Scholar]
- 28. Ohnishi Y, Yamazaki H, Kato JY, Tomono A, Horinouchi S. 2005. AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci. Biotechnol. Biochem. 69:431–439 [DOI] [PubMed] [Google Scholar]
- 29. Gramajo HC, Takano E, Bibb MJ. 1993. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol. Microbiol. 7:837–845 [DOI] [PubMed] [Google Scholar]
- 30. Elliot MA, Bibb MJ, Buttner MJ, Leskiw BK. 2001. BldD is a direct regulator of key developmental genes in Streptomyces coelicolor A3(2). Mol. Microbiol. 40:257–269 [DOI] [PubMed] [Google Scholar]
- 31. Tanaka Y, Tokuyama S, Ochi K. 2009. Activation of secondary metabolite-biosynthetic gene clusters by generating rsmG mutations in Streptomyces griseus. J. Antibiot. (Tokyo) 62:669–673 [DOI] [PubMed] [Google Scholar]
- 32. Bystrykh LV, Fernandez-Moreno MA, Herrema JK, Malpartida F, Hopwood DA, Dijkhuizen L. 1996. Production of actinorhodin-related “blue pigments” by Streptomyces coelicolor A3(2). J. Bacteriol. 178:2238–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okamoto S, Taguchi T, Ochi K, Ichinose K. 2009. Biosynthesis of actinorhodin and related antibiotics: discovery of alternative routes for quinone formation encoded in the act gene cluster. Chem. Biol. 16:226–236 [DOI] [PubMed] [Google Scholar]
- 34. Baltz RH. 2011. Strain improvement in actinomycetes in the postgenomic era. J. Ind. Microbiol. Biotechnol. 38:657–666 [DOI] [PubMed] [Google Scholar]
- 35. Imai Y, Fujiwara T, Ochi K, Hosaka T. 2012. Development of the ability to produce secondary metabolites in Streptomyces through the acquisition of erythromycin resistance. J. Antibiot. (Tokyo) 65:323–326 [DOI] [PubMed] [Google Scholar]
- 36. Retzlaff L, Distler J. 1995. The regulator of streptomycin gene expression, StrR, of Streptomyces griseus is a DNA binding activator protein with multiple recognition sites. Mol. Microbiol. 18:151–162 [DOI] [PubMed] [Google Scholar]
- 37. Katz L, Donadio S. 1995. Macrolides. Biotechnology 28:385–420 [DOI] [PubMed] [Google Scholar]
- 38. Leadlay PF. 1997. Combinatorial approaches to polyketide biosynthesis. Curr. Opin. Chem. Biol. 1:162–168 [DOI] [PubMed] [Google Scholar]
- 39. Chng C, Lum AM, Vroom JA, Kao CM. 2008. A key developmental regulator controls the synthesis of the antibiotic erythromycin in Saccharopolyspora erythraea. Proc. Natl. Acad. Sci. U. S. A. 105:11346–11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai C, Xu J, Tozawa Y, Okamoto-Hosoya Y, Yao X, Ochi K. 2002. Genetic and physiological characterization of rpoB mutations that activate antibiotic production in Streptomyces lividans. Microbiology 148:3365–3373 [DOI] [PubMed] [Google Scholar]
- 41. Tala A, Wang G, Zemanova M, Okamoto S, Ochi K, Alifano P. 2009. Activation of dormant bacterial genes by Nonomuraea sp. strain ATCC 39727 mutant-type RNA polymerase. J. Bacteriol. 191:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang G, Tanaka Y, Ochi K. 2010. The G243D mutation (afsB mutation) in the principal sigma factor sigmaHrdB alters intracellular ppGpp level and antibiotic production in Streptomyces coelicolor A3(2). Microbiology 156:2384–2392 [DOI] [PubMed] [Google Scholar]
- 43. Xu J, Tozawa Y, Lai C, Hayashi H, Ochi K. 2002. A rifampicin resistance mutation in the rpoB gene confers ppGpp-independent antibiotic production in Streptomyces coelicolor A3 (2). Mol. Genet. Genomics 268:179–189 [DOI] [PubMed] [Google Scholar]
- 44. Maughan H, Galeano B, Nicholson WL. 2004. Novel rpoB mutations conferring rifampin resistance on Bacillus subtilis: global effects on growth, competence, sporulation, and germination. J. Bacteriol. 186:2481–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirooka K, Danjo Y, Hanano Y, Kunikane S, Matsuoka H, Tojo S, Fujita Y. 2009. Regulation of the Bacillus subtilis divergent yetL and yetM genes by a transcriptional repressor, YetL, in response to flavonoids. J. Bacteriol. 191:3685–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Inaoka T, Ochi K. 2011. Scandium stimulates the production of amylase and bacilysin in Bacillus subtilis. Appl. Environ. Microbiol. 77:8181–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawai K, Wang G, Okamoto S, Ochi K. 2007. The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol. Lett. 274:311–315 [DOI] [PubMed] [Google Scholar]
- 48. Wenzel SC, Muller R. 2009. The biosynthetic potential of myxobacteria and their impact in drug discovery. Curr. Opin. Drug Discov. Devel. 12:220–230 [PubMed] [Google Scholar]
- 49. Fukuda K, Tamura T, Ito H, Yamamoto S, Ochi K, Inagaki K. 2010. Production improvement of antifungal, antitrypanosomal nucleoside sinefungin by rpoB mutation and optimization of resting cell system of Streptomyces incarnatus NRRL 8089. J. Biosci. Bioeng. 109:459–465 [DOI] [PubMed] [Google Scholar]
- 50. Challis GL, Hopwood DA. 2003. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. U. S. A. 100:14555–14561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.