Abstract
An extracellular acid phosphatase was detected in the growth media of Leishmania donovani promastigotes. The enzyme was released at all stages of the growth cycle and in amounts which accounted for 90% of the total amount of this enzyme in the culture. The exoenzyme exhibited a pH optimum of 4.5 to 5.0 and was active with a variety of organic phosphates. The enzymatic activity was excluded from Sephacryl S-300 and was retained by ultrafilters with nominal molecular weight cutoffs of up to 300,000. The results of comparative studies indicated that the extracellular enzyme was distinct from a surface membrane-bound acid phosphatase of L. donovani promastigotes which has been previously described.
Full text
PDF

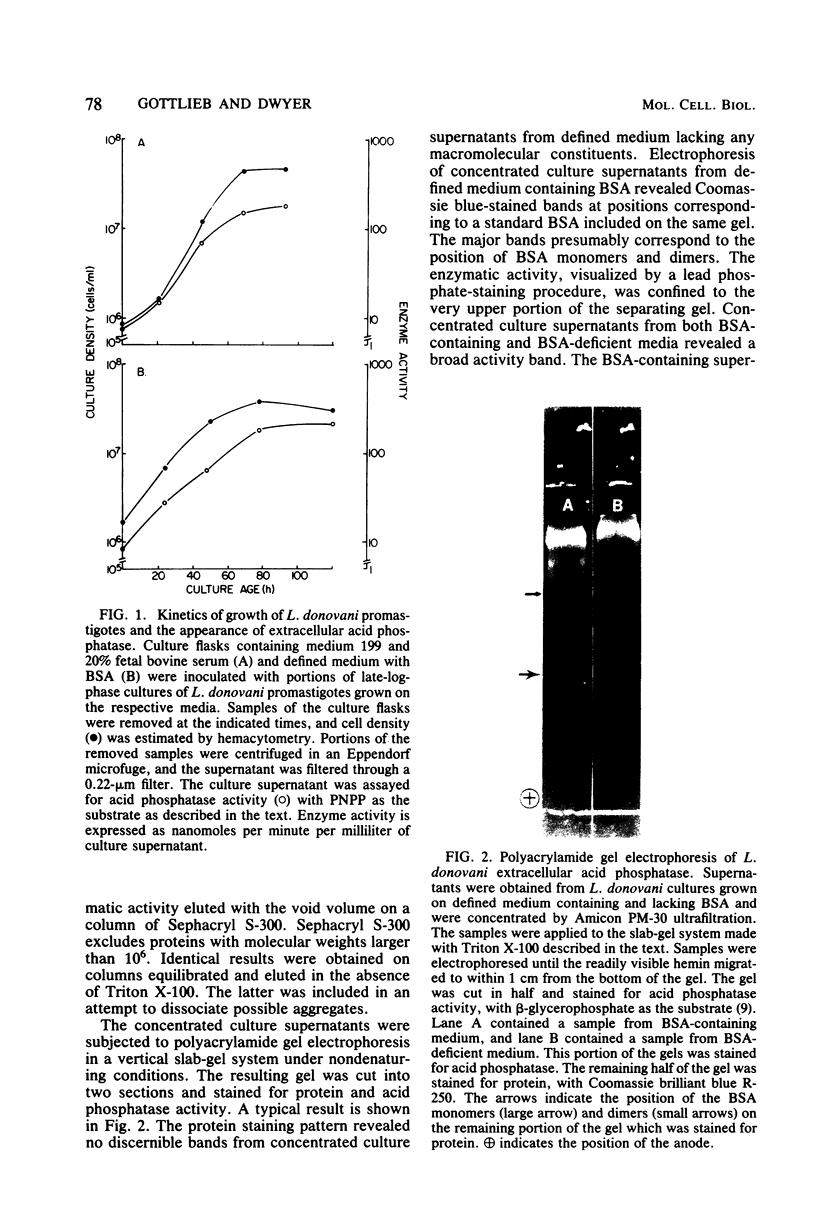
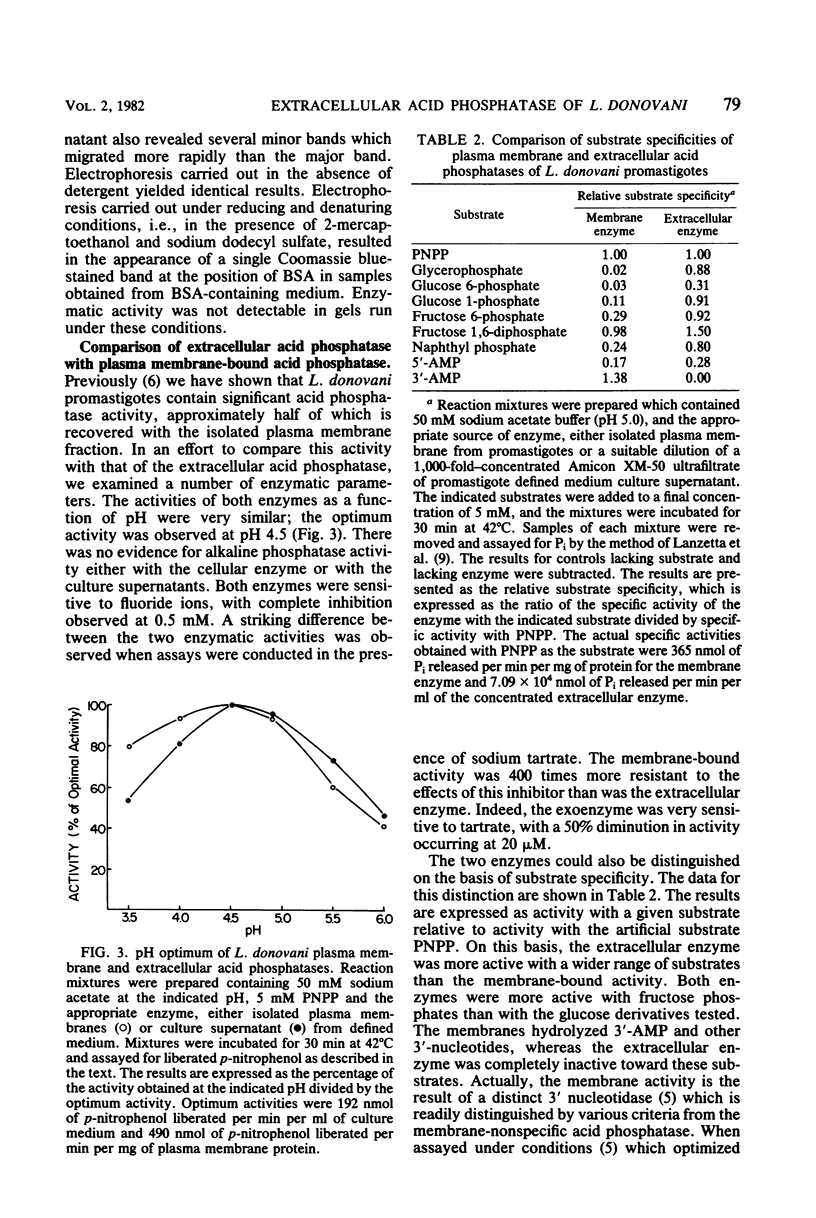


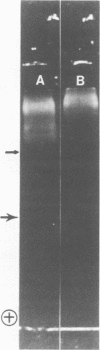
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- Decker J. E., Janovy J., Jr Leishmania donovani and L. mexicana: production of the excretion factor. Comp Biochem Physiol B. 1974 Nov 15;49(3):513–523. doi: 10.1016/0305-0491(74)90187-4. [DOI] [PubMed] [Google Scholar]
- El-On J., Schnur L. F., Greenblatt C. L. Leishmania donovani: physicochemical, immunological, and biological characterization of excreted factor from promastigotes. Exp Parasitol. 1979 Apr;47(2):254–269. doi: 10.1016/0014-4894(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Leishmania donovani: surface membrane acid phosphatase activity of promastigotes. Exp Parasitol. 1981 Aug;52(1):117–128. doi: 10.1016/0014-4894(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Injeyan H. S., Meerovitch E. The subcellular distribution and properties of Crithidia sp. hydrolases with particular reference to pyrophosphate and orthophosphate monoester phosphohydrolases. Can J Biochem. 1976 Apr;54(4):365–381. doi: 10.1139/o76-053. [DOI] [PubMed] [Google Scholar]
- Müller M. Secretion of acid hydrolases and its intracellular source in Tetrahymena pyriformis. J Cell Biol. 1972 Feb;52(2):478–487. doi: 10.1083/jcb.52.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Dhawale S. W., Aaronson S. Extracellular phosphatases of Chlamydomonas reinhardi and their regulation. J Bacteriol. 1977 Apr;130(1):205–211. doi: 10.1128/jb.130.1.205-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein T. L., Blum J. J. Lysosomal physiology in Tetrahymena. I. Effect of glucose, acetate, pyruvate, and carmine on intracellular content and extracellular release of three acid hydrolases. J Cell Biol. 1973 Jun;57(3):630–641. doi: 10.1083/jcb.57.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Semprevivo L. H. Exometabolites of Leishmania donovani promastigotes. I. Isolation and initial characterization. Proc Soc Exp Biol Med. 1978 Oct;159(1):105–110. doi: 10.3181/00379727-159-40293. [DOI] [PubMed] [Google Scholar]
- Slutzky G. M., El-On J., Greenblatt C. L. Leishmanial excreted factor: protein-bound and free forms from promastigote cultures of Leishmania tropica and Leishmania donovani. Infect Immun. 1979 Dec;26(3):916–924. doi: 10.1128/iai.26.3.916-924.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutzky G. M., Greenblatt C. L. Isolation of a carbohydrate-rich immunologically active factor from cultures of Leishmania tropica. FEBS Lett. 1977 Aug 15;80(2):401–404. doi: 10.1016/0014-5793(77)80485-7. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Opperdoes F. R., Bontemps J. Subcellular fractionation of Trypanosoma brucei bloodstream forms with special reference to hydrolases. Eur J Biochem. 1980 Mar;105(1):163–175. doi: 10.1111/j.1432-1033.1980.tb04486.x. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977 Aug;24(3):437–441. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc Soc Exp Biol Med. 1956 Apr;91(4):569–571. doi: 10.3181/00379727-91-22330. [DOI] [PubMed] [Google Scholar]