Abstract
The cultivation of genetically engineered Bacillus thuringiensis toxin-expressing (Bt) maize continues to increase worldwide, yet the effects of Bt crops on arbuscular mycorrhizal fungi (AMF) in soil are poorly understood. In this field experiment, we investigated the impact of seven different genotypes of Bt maize and five corresponding non-Bt parental cultivars on AMF and evaluated plant growth responses at three different physiological time points. Plants were harvested 60 days (active growth), 90 days (tasseling and starting to produce ears), and 130 days (maturity) after sowing, and data on plant growth responses and percent AMF colonization of roots at each harvest were collected. Spore abundance and diversity were also evaluated at the beginning and end of the field season to determine whether the cultivation of Bt maize had a negative effect on AMF propagules in the soil. Plant growth and AMF colonization did not differ between Bt and non-Bt maize at any harvest period, but AMF colonization was positively correlated with leaf chlorophyll content at the 130-day harvest. Cultivation of Bt maize had no effect on spore abundance and diversity in Bt versus non-Bt plots over one field season. Plot had the most significant effect on total spore counts, indicating spatial heterogeneity in the field. Although previous greenhouse studies demonstrated that AMF colonization was lower in some Bt maize lines, our field study did not yield the same results, suggesting that the cultivation of Bt maize may not have an impact on AMF in the soil ecosystem under field conditions.
INTRODUCTION
Genetically modified (GM) crops were commercially introduced in 1996 and now represent the majority of maize, cotton, and soybean grown in the United States (1). In 2012, 88% of the maize cultivated in the United States was genetically engineered to express herbicide tolerance, insect resistance, or some combination of stacked traits (1). Genetically modified crops also continue to be adopted by an increasing number of farmers worldwide (2). One of the most broadly cultivated GM crops is maize that has been genetically engineered to express one or more insecticidal toxins derived from the soil bacterium Bacillus thuringiensis (i.e., Bt corn). There are at least 60 different B. thuringiensis crystalline (Cry) proteins that have been identified that are targeted to certain insect groups (reviewed in references 3 and 4). The B. thuringiensis insecticidal toxins (Bt toxins) incorporated into crop plants help to protect against damage by agricultural pests, such as the European corn borer (Ostrinia nubilalis) and corn root worm (Diabrotica virgifera). When an insect ingests Bt plant material, Bt proteins bind to specific receptors in the gut, killing the insect larvae (5; reviewed in reference 6). Bt toxins can enter soil through pollen deposition or incorporation of Bt crop residue through plowing or through root exudates (reviewed in references 3 and 7). Genetic alterations within Bt plants may have nontarget effects on soil organisms associated with plant roots, such as arbuscular mycorrhizal fungi (AMF). Despite the widespread cultivation of Bt crops, few studies have examined the interactions between Bt maize and symbiotic fungi in the soil ecosystem (reviewed in references 3 and 7).
Arbuscular mycorrhizal fungi form symbiotic relationships with plant roots and have been shown to improve plant growth, enhance nutrient and water uptake, help protect against plant pathogens, and contribute to soil structure and function (8). Arbuscular mycorrhizal fungi are obligate symbionts and thus require a plant host for nutrition and reproduction. Plants supply carbon to the fungi, and fungi provide the plant with nutrients such as nitrogen and phosphorus and can improve drought tolerance (8). Recent studies have suggested that some types of Bt crops may have a negative impact on AMF (9–12), although the mechanism is not yet known. Although there is no evidence for a direct effect of B. thuringiensis proteins on soil fungi, AMF may be uniquely sensitive to genetic changes within a plant because of their reliance on a host plant. In particular, AMF may be sensitive to alterations in root exudates (13, 14), differences in root architecture or physiology (e.g., see references 15 and 16), or changes in root enzymes (17–19) that may influence carbon dynamics in the rhizosphere (20–22).
Recent greenhouse studies demonstrated that AMF associations were reduced in multiple lines of Bt maize (9–12) and that differences in AMF colonization between Bt and non-Bt maize can vary as a result of experimental and environmental conditions, such as spore density and fertilizer level (10). Under low-fertilizer conditions, AMF associations with Bt maize were significantly lower than those with the non-Bt parental (P) maize (10, 11). When residual effects of the cultivation of Bt maize were tested with a subsequently planted crop (Glycine max; soybean), there was no difference in AMF colonization of G. max grown to maturity in Bt or non-Bt preconditioned soil (11). However, lower AMF colonization was reported in Medicago sativa (alfalfa) grown in pots that had previously been cultivated in Bt maize and had Bt plant material incorporated into the soil (9). Other studies have reported no effect of Bt crop cultivation on AMF in greenhouse and microcosm studies (Bt maize [23–25]) or in field experiments (Bt cotton [26]). Because these studies were conducted with different types of Bt crops and vary substantially in nutrient levels, spore density, growing conditions, plant age at harvest, and plant genotype, experimental results to date are difficult to compare. To date, there have been no studies that have evaluated the effects of the cultivation of Bt maize on AMF in the field. Given that several greenhouse studies from independent research labs have reported a negative effect of Bt maize on AMF, it is important to examine these symbiotic relationships under more-natural field conditions.
In this field study, we evaluated AMF colonization and growth response for seven different lines of genetically modified Bt maize and five corresponding non-Bt parental isolines. Soil samples were collected from each plot at the beginning and end of the field season to determine whether spore abundance or diversity was reduced in the Bt plots after one growing season. Maize plants were harvested at three different physiological time points (60, 90, and 130 days after sowing) to examine temporal differences in AMF colonization in each line of Bt and non-Bt maize and to evaluate potential differences in yield at the end of the season. Because we used the same Bt and non-Bt maize genotypes as in previous studies, we hypothesized that results from this field experiment would support findings of our greenhouse studies (10, 11) and demonstrate that AMF colonization is lower for the Bt maize lines than for their non-Bt parental controls under field conditions. While we acknowledge that there are differences in soil properties and likely differences in AMF communities between our greenhouse and field studies, previous greenhouse studies, conducted in independent laboratories with different soils and different sources of AMF inocula (e.g., field soil and pure spores of Glomus mosseae), demonstrated an altered relationship between Bt maize and AMF (9–12), providing evidence that AMF colonization can be reduced in Bt maize under at least some environmental conditions. We also predicted that if AMF colonization levels were lower in the Bt maize lines, AMF spore abundance and diversity would also be lower in the Bt plots at the end of the field season. Finally, we hypothesized that plants with higher levels of AMF colonization in roots would have a greater shoot biomass and higher leaf chlorophyll (Chl) content, consistent with a beneficial gain from the symbiosis.
MATERIALS AND METHODS
Study site.
This field experiment was conducted from May to November 2009 near Corvallis, OR, USA, which is located in the Willamette Valley of western Oregon. The climate in this region is relatively mild throughout the year and is characterized by cool, wet winters and warm, dry summers. The mean annual high temperature is 17.4°C, and the mean annual low temperature is 5.6°C; the mean annual precipitation is 111 cm/year (27). The soil in this region is classified as Chehalis series fine-silty, mixed superactive, mesic Cumulic Ultic Haploxerolls (28). The soil at the field site has a clay loam texture (22% sand, 50% silt, and 27% clay), pH 5.7 to 6.1, medium levels of nitrogen (13 to 20 ppm NO3-N) and potassium (333 to 438 ppm), and high levels of available phosphorus (27 to 32 ppm weak Bray) (A& L Western Agricultural Laboratories, Portland, OR, USA). The field site was previously a cow pasture with mixed grasses and forbs.
Maize cultivars.
Seven different lines of Bt maize (Zea mays) and five corresponding non-Bt parental base hybrids were obtained from three seed companies (Syngenta Seeds Inc., Boise, ID, Monsanto Company, St. Louis, MO, and an additional representative seed industry seed supplier). The Bt maize lines (B1 to B4 and B6 to B8) used in this study differed in type (sweet corn or field corn), the Bt protein expressed (Cry1Ab, Cry34/35Ab1, Cry1F plus Cry34/35Ab1, Cry1F, or Cry3Bb1), and background genetics (P1 to P5), representing a cross-section of the broad range of Bt maize lines commercially available (7). The non-Bt maize seeds obtained from Monsanto Co. were described as non-Bt near-isoline control hybrids, and the non-Bt maize seeds obtained from Syngenta and the other seed industry supplier were described as near-isogenic parental base hybrids or parental (P) isolines.
Construction of plots.
The field site measured 35 m by 10 m and had 24 plots, arranged in three sets of eight plots. Plots were 3 m long by 2 m wide, with a 1-m buffer between plots and a 2-m buffer around the perimeter of the field site. On 26 May 2009, seeds of seven different Bt lines (B1 to B4 and B6 to B8) and five corresponding non-Bt parental isolines (P1 to P5) (Table 1) were sown in replicate plots (each plot contained a single genotype), with 35 to 50 seeds per row, depending on the previously determined germination rate of each cultivar. Each plot contained three rows, with 61-cm spacing between rows. Two replicate plots of each genotype were distributed randomly throughout the field site, representing 12 different Bt and non-Bt maize genotypes. After germination, plants were thinned to a maximum of 35 plants per row, and each plant was given a unique identification number. No fertilizer was added to the field plots during this experiment, and weeds were controlled by hand pulling. Plants were irrigated with overhead sprinklers as necessary to ensure that plants were not drought stressed.
Table 1.
Evaluation of seven different Bt and five non-Bt parental maize genotypes for AMF colonization in a field experimentd,e,f
| Bt maize hybrid | Company; plant ID and descriptiong | Cry protein | Target(s) of efficacy | Maize type | Parental isoline (P) |
|---|---|---|---|---|---|
| B1 | Syngenta; attribute, Bt 11: BC0805 | Cry1Ab | European corn borer, corn ear worm, fall armyworm | Triple sweet hybrid sweet corn | P1a |
| B2 | NAb | Cry34/35Ab1 | Western corn rootworm, Northern corn rootworm, and Mexican corn rootworm; glufosinate tolerance, glyphosate tolerance | Field corn | P2 |
| B3 | NA | Cry34/35Ab1 | Western corn rootworm, Northern corn rootworm, and Mexican corn rootworm; glufosinate tolerance | Field corn | P3 |
| B4 | NA | Cry1F Cry34/35Ab1 | Western bean cutworm, European corn borer, black cutworm, and fall armyworm; glufosinate tolerance; Western corn rootworm, Northern corn rootworm; glyphosate tolerance | Field corn | P4 |
| B6 | NA | Cry1F | Western bean cutworm, European corn borer, black cutworm, and fall armyworm; glyphosate tolerance, glufosinate tolerance | Field corn | P3 |
| B7 | Monsanto; DKC51-41, Mon 863, NK603c | Cry3Bb1 | Corn rootworm; glyphosate tolerance (RR2) | Field corn | P5, DKC51-45 (RR2) |
| B8 | Monsanto; DKC50-20, Mon810, Nk603c | Cry1Ab | European corn borer; glyphosate tolerance (RR2) | Field corn | P5, DKC51-45 (RR2) |
The Bt 11 transgene was backcrossed into one of the parents of Providence (P1) to create the variety BC0805. This Bt 11 cultivar was transformed using plasmid pZ01502 (containing the cry1Ab, pat, and amp genes) to express the Cry1Ab protein of B. thuringiensis.
NA, not available. Our seed agreement prohibits us from disclosing information about this seed industry representative, the genetics of the Bt and parental isolines, or other information related to the seeds provided for this study.
Nk603 is the gene for Round Up Ready 2 (RR2) glyphosate tolerance.
Information on plant ID, Cry protein, protection, and maize type was obtained from the seed suppliers and the U.S. Environmental Protection Agency Current and Previously Registered Section PIP Registrations (64).
The Bt maize hybrids were assigned numbers B1 to B8 (B5 was not included in this experiment), and their corresponding non-Bt parental base hybrids were assigned numbers P1 to P5. Note that P3 was the parental line for B3 and B6, and P5 was the parental line for B7 and B8. The Bt maize cultivars that express the same proteins differ in the background genetics of their parental line.
This table was modified from reference 11 with permission from the publisher.
ID, identifier.
Test of AMF spore composition.
Five soil samples were collected from the 0-to-15-cm fraction of soil along the center of each plot and pooled to determine the initial spore abundance and diversity in each plot prior to planting. Spores were extracted (29) and enumerated using the methods of McKenney and Lindsey (30). Briefly, 10 g of soil was agitated in a 5% Alconox solution to break up soil particles and wet sieved using 20-cm-diameter 500-, 250-, and 38-μm-mesh sieves (29). Spores collected from the 38- and 250-μm fraction were combined and centrifuged in a sucrose gradient (31). Quantification was carried out on Millipore membrane filters (47-mm diameter, 0.45-μm pore size, with 3.1-mm square grids; Millipore Corporation, Billerica, MA, USA) after vacuum filtration (30). Spores were counted on filter paper using a stereomicroscope (Leica MZ16) and assigned to five different morphological categories based on color and size (large black, large brown, medium brown, medium red, and small brown). At the end of the growing season, after plants had senesced, five soil samples were collected from the 0-to-15-cm fraction along the center of each plot and processed as before to determine whether the plots that had been cultivated in Bt maize had a negative effect on AMF spore abundance or diversity after one growing season. Spores per gram soil were calculated based on soil dry weight (a separate 10-g sample dried at 60°C for at least 48 h and weighed).
Assessment of maize plant growth.
Plants were harvested at 60, 90, and 130 days after sowing when plants were in an active growth stage, tasseling, and at maturity, respectively. Plant height and leaf number were recorded 45 days after sowing and at each harvest to determine whether plants with higher levels of AMF colonization exhibited any growth benefits as a result of the symbiosis. Plant height was recorded from the base of each plant to the top of the tallest outstretched leaf; the leaf number was recorded as the total number of live and dead leaves on each plant (only live-leaf numbers were used in the analysis); and leaf chlorophyll content was taken from the 5th live leaf from the bottom of the plant using a chlorophyll meter (Spad-502 leaf Chl meter; Minolta, Osaka, Japan). At each harvest, roots were subsampled for AMF assessment, and then roots and shoots were dried at 60°C to a constant weight. Once plants reached the reproductive stage (90 and 130 days after sowing), data were also collected on ear number per plant and weight of corn ears (dried in paper bags at 60°C to a constant weight). Five plants were harvested from each plot at 60 days, 10 plants were harvested from each plot at 90 days, and 5 plants were harvested from each plot at 130 days after sowing, for a total of 480 plants sampled over the course of the growing season. Based on preliminary studies, we anticipated the highest levels of AMF colonization at 90 days and reduced the sampling load to 5 plants per plot at the 60- and 130-day harvests.
Mycorrhizal fungus colonization assessment.
Roots were rinsed in tap water, and subsamples of at least 50 cm were collected from each plant. Root samples were stained with a trypan blue solution to visualize fungal structures (32) and scored for mycorrhizal fungus colonization using the slide-intersect method (33). To ensure that the researcher was not aware of which root type (Bt or non-Bt) was being analyzed at the time of data collection, histocassettes were mixed haphazardly during processing and slides were labeled using a sequential number system that was not associated with the Bt or P treatment.
Data analysis.
Differences in initial spore abundance and diversity between plots (α = 0.05) were analyzed using univariate analysis of variance (ANOVA) using the Proc GLM procedure of the SAS software program (version 9.2). The Shannon-Weaver diversity index (H) was calculated as H = −∑ pi ln(pi), where pi is the relative abundance of each spore group. To test for differences in initial spore abundance and diversity between plots cultivated in Bt and P maize, “plant type” (Bt or non-Bt) was treated as a fixed effect in the model; response variables were the spore categories (medium brown, large brown, large black, small brown, medium red, total spore number, and number of fungal taxa in one gram of dry soil). To test for differences in initial spore abundance and diversity between plots cultivated with each genotype of Bt or non-Bt maize, “cultivar” was treated as a fixed effect in the model with the same response variables as before. However, because there were only two replicate plots of each cultivar (due to limitations in field space and personnel), the primary emphasis for this data analysis is based on plant type (Bt versus P). To test for differences in initial and final spore abundance as affected by variation in the field plots, “plot” was treated as a fixed effect in the model, with total spores as the response variable. Because of unequal variance between initial and final soil samples, a Welch t test was used to test for overall differences in initial (May 2009) versus final (October 2009) spore counts in each plot.
Differences in arbuscular mycorrhizal fungal colonization (hyphae, arbuscules, vesicles, and total percent AMF colonization) and plant growth responses between Bt and P maize (α = 0.05) were analyzed using the Proc Mixed procedure of SAS (version 9.2). To test for differences in AMF colonization between Bt and P maize, Bt was treated as a fixed effect, and parental, Bt * parental, and plot * row were treated as random effects. To test for differences in plant growth responses at 60 days (root and shoot biomass), 90 days (root biomass, shoot biomass, and ear number per plant), and 130 days (root biomass, shoot biomass, ear number per plant, and ear dry weight), Bt, initial plant size (plant height × leaf no.), leaf chlorophyll content, and AMF colonization levels were treated as fixed effects, and parental, Bt * parental, and plot * row were treated as random effects. To test for differences in leaf chlorophyll content at each harvest period, Bt, initial size, and AMF colonization levels were treated as fixed effects, and random effects were as previously described.
For each analysis, data were examined for normal distribution using Shapiro-Wilks tests and for equal variance using equal-variance tests. Data were transformed as necessary to meet the assumptions of each model. Data analysis was performed using R software (version 2.14.1) and SAS (version 9.2).
RESULTS
Effect of Bt maize on spore abundance and diversity.
There was no difference in initial spore abundance between Bt and non-Bt designated plots at the beginning of the growing season (F1,23 = 0.26; P = 0.62) (Fig. 1). The mean initial spore counts in 1 g of dry soil collected from Bt and P plots were 15.42 and 16.05, respectively. The mean numbers of fungal taxa in initial samples, as determined by spore morphology, in Bt and P plots were 4.00 and 3.90, respectively. The numbers of fungal taxa were not different between Bt and non-Bt plots at the beginning of the field season (F1,23 = 0.10; P = 0.75). There was no difference in the Shannon index of diversity (H) between spores extracted from Bt and non-Bt plots at the beginning of the field season (0.98 and 0.87, respectively; F1,23 = 3.09; P = 0.09).
Fig 1.
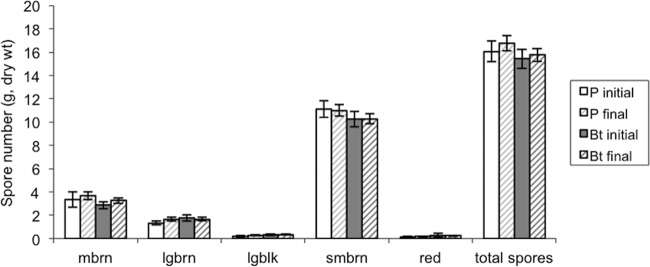
Spores per gram of dry soil in soil samples collected from each Bt and P plot in May 2009 (initial) and October 2009 (final). Five initial soil samples were collected from each plot and pooled for the spore extraction to determine initial spore abundance and diversity per plot before seeding. Spores were categorized into five morphological groups (medium brown [mbrn], large brown [lgbrn], large black [lgblk], small brown [smbrn], and red), and total spores per gram dry soil were calculated. Five final soil samples were collected from each plot at the end of the field season, and spores were extracted from five soil samples per plot to determine whether Bt maize had a negative effect on spore abundance and diversity after one growing season. Open bars represent the means (± SE) of spore counts from initial soil samples collected from P plots, and solid bars represent the means (± SE) of initial spore counts from Bt plots; bars with hatched lines represent final spore counts collected from P plots (light gray lines) and Bt plots (dark gray lines). n = 10 for P initial; n = 14 for Bt initial; n = 50 for P final; n = 70 for Bt final.
At the end of the field season, after plants had senesced, there was no difference in AMF spore abundance between Bt and non-Bt plots (F1,118 = 1.41; P = 0.24) (Fig. 1). The mean spore counts in 1 g of dry soil collected from Bt and P plots at the end of the season were 15.75 and 16.75, respectively. The mean numbers of fungal taxa in final soil samples as determined by spore morphology in Bt and P plots were 3.80 and 3.50, respectively, and did not differ between Bt and P plots (F1,118 = 3.66; P = 0.06). There was no difference in final spore diversity (H) between Bt and non-Bt plots at the end of the field season (H = 0.99 and H = 0.95, respectively; F1,118 = 1.79; P = 0.18). There was also no difference between spore abundances in field plots between the beginning and end of the field season. Overall, total spore counts varied most by plot at the end of the field season (F1,23 = 2.82; P = 0.0002), but this was not related to Bt or P cultivation. Because there was no effect of plant type (Bt or P) on spore abundance or diversity, spores were not identified to the species level.
Effect of Bt maize on AMF colonization.
There was no difference in colonization by AMF hyphae, arbuscules, vesicles, or total percentages of AMF colonization between Bt and non-Bt maize at the 60-day harvest, when plants were actively growing, at the 90-day harvest, when plants were tasseling and starting to produce ears, or at the 130-day harvest, when plants were mature (Table 2; Fig. 2). Mean AMF colonization levels were 29.69% in Bt maize and 28.94% in non-Bt maize at the 60-day harvest, 32.6% in Bt maize and 28.8% in non-Bt maize at the 90-day harvest, and 44.9% in Bt maize and 42.7% in non-Bt maize at the 130-day harvest.
Table 2.
Proc Mixed results (F values) of effects of plant type (Bt or non-Bt maize) on colonization of roots by AMF hyphae, arbuscules, and/or vesicles and total percent AMF colonization (presence/absence of any fungal structure per 100 intersects) at 60-day, 90-day, and 130-day harvests
| AMF response | df | F value | P value |
|---|---|---|---|
| 60-day harvest | |||
| Hyphae | 1,4 | 0.14 | 0.73 |
| Arbuscules | 1,4 | 0.13 | 0.73 |
| Vesicles | 1,4 | 1.14 | 0.34 |
| Total AMF | 1,4 | 0.20 | 0.68 |
| 90-day harvest | |||
| Hyphae | 1,4 | 2.03 | 0.23 |
| Arbuscules | 1,4 | 2.34 | 0.20 |
| Vesicles | 1,4 | 0.23 | 0.66 |
| Total AMF | 1,4 | 2.11 | 0.22 |
| 130-day harvest | |||
| Hyphae | 1,4 | 0.15 | 0.72 |
| Arbuscules | 1,4 | 0.06 | 0.81 |
| Vesicles | 1,4 | 0.49 | 0.52 |
| Total AMF | 1,4 | 0.15 | 0.72 |
Fig 2.
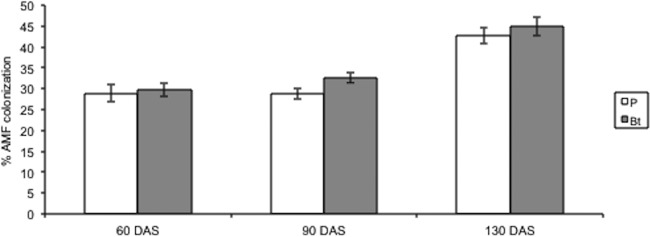
Percent AMF colonization of non-Bt parental (P) and Bt maize roots 60, 90, and 130 day after sowing (DAS). Open bars represent the means (± SE) for non-Bt parental maize lines, and solid bars represent the means (± SE) for Bt maize lines. Five plants were harvested from each plot 60 DAS, 10 plants were harvested from each plot 90 DAS, and 5 plants were harvested from each plot 130 DAS, for a total of 480 root samples over the course of this experiment.
Effect of AMF colonization and cultivar on maize growth.
At the 60-day harvest, when plants were actively growing, there was no effect of AMF colonization on root biomass, shoot biomass, or chlorophyll content of leaves (Table 3). Initial size was positively correlated with root biomass (Pearson correlation coefficient = 0.74 and P < 0.0001; Proc mixed F1,51 = 56.52 and P < 0.0001), shoot biomass (Pearson correlation coefficient = 0.83 and P < 0.0001; Proc mixed F1,51 = 124.18 and P < 0.0001), and leaf chlorophyll content (Pearson correlation coefficient = 0.55 and P < 0.0001; Proc mixed F1,52 = 49.46 and P < 0.0001). Chlorophyll content in leaves at the time of harvest was positively correlated with root biomass (Pearson correlation coefficient = 0.68 and P < 0.0001; Proc mixed F1,51 = 34.58 and P < 0.0001) and shoot biomass (Pearson correlation coefficient = 0.71 and P < 0.0001; Proc mixed F1,51 = 47.87 and P < 0.0001). There was no difference in root biomass, shoot biomass, or chlorophyll content between the Bt and non-Bt maize cultivars at the 60-day harvest (Table 4). Mean root biomass was 3.19 g in Bt maize and 3.62 g in non-Bt maize, mean shoot biomass was 28.85 g in Bt maize and 28.57 g in non-Bt maize, and mean leaf chlorophyll content was 47.87 in Bt maize and 46.76 in non-Bt maize at the 60-day harvest.
Table 3.
Proc Mixed results (F values) of effects of percentages of AMF colonization in roots (presence of AMF hyphae, arbuscules, and/or vesicles per 100 intersects) on maize growth (root dry weight, shoot dry weight, leaf chlorophyll content, ear number, and ear dry weight) at 60-day, 90-day, and 130-day harvests
| Growth response | df | F value | P valuea |
|---|---|---|---|
| 60-day harvest | |||
| Root biomass | 1,51 | 0.20 | 0.66 |
| Shoot biomass | 1,51 | 0.21 | 0.65 |
| Leaf Chl content | 1,52 | 1.05 | 0.31 |
| 90-day harvest | |||
| Root biomass | 1,167 | 0.03 | 0.87 |
| Shoot biomass | 1,168 | 0.59 | 0.44 |
| Leaf Chl content | 1,169 | 1.81 | 0.18 |
| Ear no. | 1,168 | 0.17 | 0.68 |
| 130-day harvest | |||
| Root biomass | 1,89 | 0.31 | 0.58 |
| Shoot biomass | 1,89 | 0.01 | 0.94 |
| Leaf Chl content | 1,90 | 4.61 | 0.03* |
| Ear no. | 1,89 | 0.11 | 0.74 |
| Ear wt | 1,88 | 1.50 | 0.22 |
*, P ≤ 0.05.
Table 4.
Proc Mixed results (F values) of effects of plant type (Bt or non-Bt maize) on plant growth (root dry weight, shoot dry weight, leaf chlorophyll content, ear number, and ear dry weight) at 60-day, 90-day, and 130-day harvests
| Growth response | df | F value | P value |
|---|---|---|---|
| 60-day harvest | |||
| Root biomass | 1,4 | 0.22 | 0.66 |
| Shoot biomass | 1,4 | 0.00 | 0.96 |
| Leaf Chl content | 1,4 | 0.66 | 0.46 |
| 90-day harvest | |||
| Root biomass | 1,4 | 0.88 | 0.40 |
| Shoot biomass | 1,4 | 0.58 | 0.49 |
| Leaf Chl content | 1,4 | 1.82 | 0.25 |
| Ear no. | 1,4 | 1.44 | 0.30 |
| 130-day harvest | |||
| Root biomass | 1,4 | 0.01 | 0.92 |
| Shoot biomass | 1,4 | 0.08 | 0.79 |
| Leaf Chl content | 1,4 | 1.22 | 0.33 |
| Ear no. | 1,4 | 1.03 | 0.34 |
| Ear wt | 1,4 | 0.46 | 0.53 |
At the 90-day harvest, when maize plants were tasseling and starting to produce ears, there was no effect of the percentage of AMF colonization on root biomass, shoot biomass, chlorophyll content of leaves, or ear number (Table 3). Initial size was positively correlated with root biomass (Pearson correlation coefficient = 0.54 and P < 0.0001; Proc mixed F1,167 = 37.92 and P < 0.0001), shoot biomass (Pearson correlation coefficient = 0.63 and P < 0.0001; Proc mixed F1,168 = 99.57 and P < 0.0001), leaf chlorophyll content (Pearson correlation coefficient = 0.34 and P < 0.0001; Proc mixed F1,169 = 45.37 and P < 0.0001), and ear number per plant (Pearson correlation coefficient = 0.45 and P < 0.0001; Proc mixed F1,168 = 22.68 and P < 0.0001). Chlorophyll content was positively correlated with root biomass (Pearson correlation coefficient = 0.58 and P < 0.0001; Proc mixed F1,167 = 102.44 and P < 0.0001), shoot biomass (Pearson correlation coefficient = 0.61 and P < 0.0001; Proc mixed F1,168 = 93.04 and P < 0.0001), and ear number per plant (Pearson correlation coefficient = 0.46 and P < 0.0001; Proc mixed F1,168 = 48.81 and P < 0.0001). There was no difference in root biomass, shoot biomass, leaf chlorophyll content, or ear number between the Bt and non-Bt maize cultivars at the 90-day harvest (Table 4). Mean root biomass was 7.59 g in Bt maize and 6.95 g in non-Bt maize, mean shoot biomass was 93.88 g in Bt maize and 89.97 g in non-Bt maize, mean leaf chlorophyll content was 46.35 in Bt maize and 48.02 in non-Bt maize, and mean ear number was 1.41 in Bt maize and 1.29 in non-Bt maize at the 90-day harvest.
At the 130-day harvest, when maize plants had reached maturity, there was no effect of the percentage of AMF colonization on root biomass, shoot biomass, ear number, or ear weight (Table 3); however, AMF colonization was positively correlated with chlorophyll content (Pearson correlation coefficient = 0.22; P = 0.02) (Table 3; Fig. 3). Initial size was positively correlated with root biomass (Pearson correlation coefficient = 0.62 and P < 0.0001; Proc mixed F1,89 = 51.73 and P < 0.0001), shoot biomass (Pearson correlation coefficient = 0.68 and P < 0.0001; Proc mixed F1,89 = 90.73 and P < 0.0001), leaf chlorophyll content (Pearson correlation coefficient = 0.26 and P = 0.005; Proc mixed F1,90 = 17.05 and P < 0.0001), ear number (Pearson correlation coefficient = 0.38 and P < 0.0001; Proc mixed F1,89 = 10.62 and P = 0.002), and ear weight (Pearson correlation coefficient = 0.67 and P < 0.0001; Proc mixed F1,88 = 84.28 and P < 0.0001). Chlorophyll content was positively correlated with root biomass (Pearson correlation coefficient = 0.35 and P < 0.0001; Proc mixed F1,89 = 16.31 and P = 0.0001), shoot biomass (Pearson correlation coefficient = 0.44 and P < 0.0001; Proc mixed F1,89 = 28.08 and P < 0.0001), ear number (Pearson correlation coefficient = 0.48 and P < 0.0001; Proc mixed F1,89 = 24.76 and P < 0.0001), and ear weight (Pearson correlation coefficient = 0.57 and P < 0.0001; Proc mixed F1,88 = 48.47 and P < 0.0001). There was no difference in root biomass, shoot biomass, chlorophyll content, ear number, or ear weight between Bt and non-Bt maize at 130 days (Table 4). Mean root biomass was 8.01 g in Bt maize and 7.37 g in non-Bt maize, mean shoot biomass (shoots plus ears) was 185.92 g in Bt maize and 164.16 g in non-Bt maize, mean leaf chlorophyll content was 40.58 in Bt maize and 42.54 in non-Bt maize, mean ear number was 1.47 in Bt maize and 1.38 in non-Bt maize, and mean ear weight was 116.04 g in Bt maize and 103.76 g in non-Bt maize at the 130-day harvest.
Fig 3.
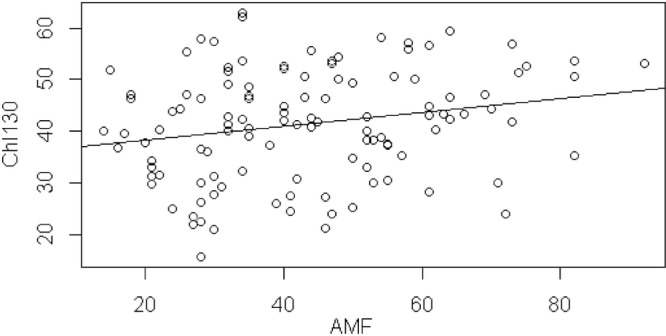
Leaf chlorophyll content was positively correlated with percentage AMF colonization of roots at the 130-day harvest. Pearson correlation coefficient = 0.22 and P = 0.02; Proc mixed F1,90 = 4.61 and P = 0.03. Leaf chlorophyll content was assessed for 5 plants per plot 130 days after sowing for a total of 120 leaf chlorophyll measurements.
Arbuscular mycorrhizal fungal colonization was highest in the 130-day samples (Fig. 2), and total plant biomass increased with each harvest (Fig. 4). Variation in plot had the most significant effect on AMF colonization and growth responses throughout the experiment as assessed using Proc GLM in SAS (at 60 days, root biomass F1,23 = 1.63 and P = 0.05; at 90 days, AMF F1,23 = 4.65 and P < 0.001, root biomass F1,23 = 2.23 and P = 0.002, and leaf chlorophyll content F1,23 = 2.38 and P = 0.0006; at 130 days, AMF F1,23 = 4.92 and P < 0.001, root biomass F1,23 = 2.16 and P = 0.005, shoot biomass F1,23 = 2.36 and P = 0.002, leaf chlorophyll content F1,23 = 3.44 and P < 0.0001, ear number F1,23 = 1.86 and P = 0.02, and total ear weight F1,23 = 2.28 and P = 0.003).
Fig 4.
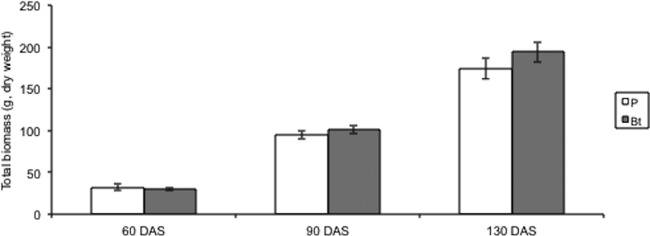
Total biomass (roots plus shoots plus ears) of non-Bt parental (P) and Bt maize 60, 90, and 130 days after sowing (DAS). Open bars represent the means (± SE) for non-Bt parental maize lines, and solid bars represent the means (± SE) for Bt maize lines. From each plot, five plants were harvested 60 DAS, 10 plants were harvested 90 DAS, and 5 plants were harvested 130 DAS, for a total of 480 plants over the course of the experiment.
DISCUSSION
In this field study, there were no differences observed in AMF colonization between Bt and non-Bt maize 60 days, 90 days, or 130 days after sowing. Based on previous greenhouse studies, we predicted that field-grown Bt maize would display a lower level of AMF colonization than non-Bt maize at each harvest period, but this hypothesis was not supported. This is surprising, because the same Bt maize genotypes that had previously exhibited lower AMF colonization in greenhouse studies (11) were also utilized here. Further, we detected no difference in plant biomass, leaf chlorophyll content, ear number, or ear weight between Bt and non-Bt maize at any harvest date. However, AMF was positively correlated with leaf chlorophyll content at the 130-day harvest, when plants were fully mature. We found no difference in spore counts between soil collected from Bt versus P plots at the beginning or end of the field season, and our counts were similar to spore densities reported in other maize field studies (34–36). While our spore diversity was low compared to those in many natural systems, it is typical of the low mycorrhizal diversity reported for other agricultural and monocropping systems (37, 38). Although there was no difference in AMF spore abundances and diversities in field plots at the beginning of the field season, there was a significant effect of plot on total spore counts at the end of the field season, indicating spatial heterogeneity of AMF propagules in these field plots. However, these differences in spore counts between plots were not a result of maize genotype (Bt versus P).
The symbiosis between maize and AMF can vary strongly depending on experimental or environmental conditions (10), and more generally the plant-AMF relationship can fluctuate along a parasitism-mutualism continuum (39). Arbuscular mycorrhizal fungi are considered parasitic when the net cost of the symbiosis exceeds net benefits for the plant and are mutualistic when both partners benefit from the relationship, although there have been some recent discussions of the use of these terms (40, 41). Our field experiment showed increasing levels of AMF colonization in both Bt and non-Bt parental maize roots over time, with the highest levels of AMF colonization detected at the 130-day harvest, when plants were mature. These results support the findings of Grigera et al., who documented an increase in carbon allocation to AMF during the reproductive period of maize (42) and demonstrated that AMF were most abundant at the end of the maize growing season as assessed by fatty acid methyl ester (FAME) biomarkers (43). We also found a positive correlation between percent AMF colonization of roots and chlorophyll content of live leaves at the 130-day harvest, suggesting that the higher levels of AMF colonization led to higher nitrogen levels in maize at maturity (e.g., see references 44, 45, and 46). While variation in soil nitrogen availability might have influenced mycorrhizal colonization levels (and thus affected leaf chlorophyll content), plots were randomly assigned to Bt and non-Bt cultivars prior to planting, and data were combined in a single analysis where differences among plots were controlled for statistically. Thus, we were able to assess the overall relationship between percent AMF colonization of maize roots and leaf chlorophyll content, minimizing any plot-specific nitrogen effects. The conditions of the field site may also help to explain the increase in AMF colonization in the maize roots at the end of the growing season. The study site was historically covered with mixed pasture grasses and forbs that were likely in symbiosis with AMF. When our study commenced, these plants were removed and the ground was turned under. Since weeds were hand pulled throughout the study, the cultivated corn was the only host plant for the AMF in our field plots.
Variation in soil conditions may also be a key factor influencing the relationship between AMF and Bt and non-Bt maize (47). When nitrogen and phosphorus are readily available in soil, plants often have lower levels of AMF colonization in roots because the carbon cost of supporting fungal symbionts is higher than the benefits received (e.g., see references 10 and 48). In previous greenhouse studies, we found that AMF colonization was lower in multiple lines of Bt maize grown in 50% field soil collected from Vancouver, WA, USA (11) and that Bt and non-Bt maize grown without fertilizer or in low-fertilizer treatments (0.23 g liter−1) recruited more AMF than maize grown in high-fertilizer (1.87 g liter−1) treatments (10). However, in the current Corvallis field study, we observed no differences in AMF colonization between many of the same lines of Bt and non-Bt maize used in the greenhouse study. Although we did not fertilize our field plots, the maize plants did not exhibit any obvious signs of nutrient stress and grew with vigor, indicating that the soils were not nutrient limited. The Corvallis field soils differ in nutrient availability and likely contain a different community of AMF than the Vancouver soils, potentially explaining the contradictory results we have observed. Future investigation of the differences in soil nutrient availability and spore composition in AMF colonization of Bt and non-Bt maize will help to elucidate the interplays between these plant-fungus partners. The significant plot effects observed in the growth responses and AMF colonization levels in our maize plants suggest spatial heterogeneity of nutrient availability and/or spore density in the soil; however, these were not related to maize genotype. Increasing the plot number of each cultivar in future field studies would likely help to minimize the impact of spatial heterogeneity in similar studies.
Interestingly, we detected no differences in spore abundance between field plots at the beginning and end of the growing season. There are several potential reasons for this. The field site was plowed prior to soil collection and planting in the spring, so perhaps the spores that were in the soil at that time were not actively colonizing the weeds/pasture plants at the time of plot preparation (i.e., spore bank). We collected final soil samples at the end of the field season after plants had senesced because we expected spore production to be the highest in the fall after plants had produced seed. It is possible, however, that we missed the sporulation event (perhaps it was in the late summer) and spores recolonized any remaining maize roots or weeds that grew after the 130-day harvest. It is also possible that in this system, roots and vesicles were serving as propagules instead of spores. Indeed, for one study that took place in vineyards in the Willamette Valley of western Oregon, a similar number of AMF species in roots and soil (based on amplification of AMF DNA in root samples and spore morphology) was reported; however, roots and soil had a different AMF community, indicating that the spores in the soil may not necessarily reflect the AMF taxa actively colonizing plant roots (49). This lends support to the idea that there may be a spore bank in our field soil that may not represent the AMF taxa colonizing the maize plants in our study, but this remains to be tested.
Future investigations evaluating the impact of Bt maize and other genetically modified agronomic species on AMF in the soil ecosystem will be beneficial to both the scientific and agricultural community. Although crop plants that are irrigated and fertilized may not benefit significantly from symbiosis with AMF (reviewed in reference 50), arbuscular mycorrhizal fungi are important for nutrient acquisition and drought tolerance in many sustainable agricultural and/or low input systems (reviewed in references 51, 52, and 53) and are important considerations in crop rotation (54, 55) and for native plant establishment in grassland restorations of former agricultural fields (e.g., see references 56 and 57). Arbuscular mycorrhizal fungi can also be affected by tillage (e.g., see references 58, 59, and 60), plant type (e.g., see references 54 and 61), and management practices (e.g., see references 62 and 63). Although results from our field experiment indicate no difference in spore abundance and diversity in the soil and no differences in AMF colonization levels between Bt and non-Bt maize over one growing season, the diversity of AMF colonizing the various maize genotypes remains unknown. Future studies should aim to resolve the causal factors contributing to the widespread variation between AMF and Bt maize which has been observed to date and would benefit from determining whether there is any variation in taxonomic and/or functional diversity of AMF colonizing Bt maize and non-Bt parental isolines under field conditions.
ACKNOWLEDGMENTS
This work was supported by the Charles A. and Anne Morrow Lindbergh Foundation, the U.S. Environmental Protection Agency under the Science to Achieve Results Graduate Fellowship Program, Sigma Delta Epsilon Graduate Women in Science, the National Science Foundation (DEB-1011525), and Sigma Xi.
We thank M. R. LaPlante, C. R. Guidry, B. A. Pace and other members of the Cruzan laboratory group for research assistance. The manuscript benefited from valuable contributions from K. Tidwell and A. Raffel, statistical analysis with J. D. Bever, and feedback from Monsanto Co. and an additional seed industry seed supplier. Maize seed for this research was provided by Syngenta Seeds Inc. (Boise, ID, USA), Monsanto Company (St. Louis, MO, USA), and an additional seed industry seed supplier.
The funding agencies have not officially endorsed this publication, and the views expressed herein may not reflect the views of the funding agencies.
Footnotes
Published ahead of print 26 April 2013
REFERENCES
- 1. USDA 2012. Adoption of genetically engineered crops in the U.S.: corn varieties. USDA, Washington, DC: http://www.ers.usda.gov/Data/BiotechCrops/ExtentofAdoptionTable1.htm Accessed 15 November 2012 [Google Scholar]
- 2. James C. 2012. Global status of commercialized biotech/GM crops: 2011. ISAAA brief no. 43. International Service for the Acquisition of Agri-Biotech Applications. http://www.isaaa.org/resources/publications/briefs/43/default.asp Accessed 15 November 2012
- 3. Icoz I, Stotzky G. 2008. Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol. Biochem. 40:559–586 [Google Scholar]
- 4. Sanchis V. 2011. From microbial sprays to insect-resistant transgenic plants: history of the biospesticide Bacillus thuringiensis. A review. Agron. Sustain. Dev. 31:217–231 [Google Scholar]
- 5. Federici BA. 1993. Insecticidal bacterial proteins identify the midgut epithelium as a source of novel target sites for insect control. Arch. Insect Biochem. Physiol. 22:357–371 [Google Scholar]
- 6. Bravo A, Gill SS, Soberon M. 2007. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheeke TE. 2012. Effects of the cultivation of genetically modified Bt crops on nontarget soil organisms, p 153–227 In Cheeke TE, Coleman DC, Wall DH. (ed), Microbial ecology in sustainable agroecosystems. CRC Press, Boca Raton, FL [Google Scholar]
- 8. Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd ed Academic Press, San Diego, CA [Google Scholar]
- 9. Castaldini M, Turrini A, Sbrana C, Benedetti A, Marchionni M, Mocali S, Fabiani A, Landi S, Santomassimo F, Pietrangeli B, Nuti MP, Miclaus N, Giovannetti M. 2005. Impact of Bt corn on rhizospheric and soil eubacterial communities and on beneficial mycorrhizal symbiosis in experimental microcosms. Appl. Environ. Microbiol. 71:6719–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheeke TE, Pace BA, Rosenstiel TN, Cruzan MB. 2011. The influence of fertilizer level and spore density on arbuscular mycorrhizal colonization of transgenic Bt 11 maize (Zea mays) in experimental microcosms. FEMS Microbiol. Ecol. 75:304–312 [DOI] [PubMed] [Google Scholar]
- 11. Cheeke TE, Rosenstiel TN, Cruzan MB. 2012. Evidence of reduced arbuscular mycorrhizal fungal colonization in multiple lines of Bt maize. Am. J. Bot. 99:700–707 [DOI] [PubMed] [Google Scholar]
- 12. Turrini A, Sbrana C, Nuti MP, Pietrangeli BM, Giovannetti M. 2004. Development of a model system to assess the impact of genetically modified corn and aubergine plants on arbuscular mycorrhizal fungi. Plant Soil 266:69–75 [Google Scholar]
- 13. Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM. 2008. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 74:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bucking H, Abubaker J, Govindarajulu M, Tala M, Pfeffer PE, Nagahashi G, Lammers P, Shachar-Hill Y. 2008. Root exudates stimulate the uptake and metabolism of organic carbon in germinating spores of Glomus intraradices. New Phytol. 180:684–695 [DOI] [PubMed] [Google Scholar]
- 15. Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW. 2005. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 168:293–303 [DOI] [PubMed] [Google Scholar]
- 16. Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF. 2000. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 40:358–364 [Google Scholar]
- 17. Garcia-Rodriguez S, Azcon-Aguilar C, Ferrol N. 2007. Transcriptional regulation of host enzymes involved in the cleavage of sucrose during arbuscular mycorrhizal symbiosis. Physiol. Plant. 129:737–746 [Google Scholar]
- 18. Schaarschmidt S, Kopka J, Ludwig-Muller J, Hause B. 2007. Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J. 51:390–405 [DOI] [PubMed] [Google Scholar]
- 19. Schaarschmidt S, Roitsch T, Hause B. 2006. Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J. Exp. Bot. 57:4015–4023 [DOI] [PubMed] [Google Scholar]
- 20. Bucking H, Shachar-Hill Y. 2005. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 165:899–912 [DOI] [PubMed] [Google Scholar]
- 21. Doidy J, van Tuinen D, Lamotte O, Corneillat M, Alcaraz G, Wipf D. 2012. The Medicago truncatula sucrose transporter family: characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Mol. Plant 5:1346–1358 [DOI] [PubMed] [Google Scholar]
- 22. Blee KA, Anderson AJ. 1998. Regulation of arbuscule formation by carbon in the plant. Plant J. 16:523–530 [Google Scholar]
- 23. de Vaufleury A, Kramarz PE, Binet P, Cortet J, Caul S, Andersen MN, Plumey E, Coeurdassier M, Krogh PH. 2007. Exposure and effects assessments of Bt-maize on non-target organisms (gastropods, microarthropods, mycorrhizal fungi) in microcosms. Pedobiologia 51:185–194 [Google Scholar]
- 24. Tan FX, Wang JW, Chen ZN, Feng YJ, Chi GL, Rehman SU. 2011. Assessment of the arbuscular mycorrhizal fungal community in roots and rhizosphere soils of Bt corn and their non-Bt isolines. Soil Biol. Biochem. 43:2473–2479 [Google Scholar]
- 25. Verbruggen E, Kuramae EE, Hillekens R, de Hollander M, Kiers ET, Roling WFM, Kowalchuk GA, van der Heijden MGA. 2012. Testing potential effects of maize expressing the Bacillus thuringiensis Cry1Ab endotoxin (Bt maize) on mycorrhizal fungal communities via DNA- and RNA-based pyrosequencing and molecular fingerprinting. Appl. Environ. Microbiol. 78:7384–7392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knox OGG, Nehl DB, Mor T, Roberts GN, Gupta V. 2008. Genetically modified cotton has no effect on arbuscular mycorrhizal colonisation of roots. Field Crops Res. 109:57–60 [Google Scholar]
- 27. National Oceanic and Atmospheric Administration 2012. National Climatic Data Center. http://ncdc.noaa.gov/cdo-web Accessed 15 November 2012
- 28. USDA Natural Resources Conservation Service 2012. Soil Survey Geographic (SSURGO) database for Corvallis, Oregon, vol 2012 USDA, Washington, DC: http://websoilsurvey.nrcs.usda.gov/app/WebSoilSurvey.aspx Accessed 15 November 2012 [Google Scholar]
- 29. Gerdemann J, Nicolson TH. 1963. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 46:235–244 [Google Scholar]
- 30. McKenney MC, Lindsey DL. 1987. Improved method for quantifying endomycorrhizal fungi spores from soil. Mycologia 79:779–782 [Google Scholar]
- 31. Walker C, Mize CW, McNabb H., Jr 1982. Populations of endogonaceous fungi at two locations in central Iowa. Can. J. Bot. 60:2518–2529 [Google Scholar]
- 32. Phillips JM, Hayman DS. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55:158–160 [Google Scholar]
- 33. McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. 1990. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol. 115:495–501 [DOI] [PubMed] [Google Scholar]
- 34. Isobe K, Sugimura H, Maeshima T, Ishii R. 2008. Distribution of arbuscular mycorrhizal fungi in upland field soil of Japan. 2. Spore density of arbuscular mycorrhizal fungi and infection ratio in soybean and maize fields. Plant Prod. Sci. 11:171–177 [Google Scholar]
- 35. Kabir Z, O'Halloran IP, Widden P, Hamel C. 1998. Vertical distribution of arbuscular mycorrhizal fungi under corn (Zea mays L.) in no-till and conventional tillage systems. Mycorrhiza 8:53–55 [Google Scholar]
- 36. Oehl F, Sieverding E, Ineichen K, Ris EA, Boller T, Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165:273–283 [DOI] [PubMed] [Google Scholar]
- 37. Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW. 1998. Ploughing up the wood-wide web? Nature 394:431. [DOI] [PubMed] [Google Scholar]
- 38. Oehl F, Sieverding E, Ineichen K, Mader P, Boller T, Wiemken A. 2003. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 69:2816–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson NC, Graham JH, Smith FA. 1997. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 135:575–586 [Google Scholar]
- 40. Johnson NC, Graham JH. 2013. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 363:411–419 [Google Scholar]
- 41. Smith FA, Smith SE. 2013. How useful is the mutualism-parasitism continuum of arbuscular mycorrhizal functioning? Plant Soil 363:7–18 [Google Scholar]
- 42. Grigera MS, Drijber RA, Shores-Morrow RH, Wienhold BJ. 2007. Distribution of the arbuscular mycorrhizal biomarker C16: 1cis11 among neutral, glyco and phospholipids extracted from soil during the reproductive growth of corn. Soil Biol. Biochem. 39:1589–1596 [Google Scholar]
- 43. Grigera MS, Drijber RA, Wienhold BJ. 2007. Increased abundance of arbuscular mycorrhizal fungi in soil coincides with the reproductive stages of maize. Soil Biol. Biochem. 39:1401–1409 [Google Scholar]
- 44. Blackmer TM, Schepers JS. 1995. Use of a chlorophyll meter to monitor nitrogen status and schedule fertigation for corn. J. Prod. Agric. 8:56–60 [Google Scholar]
- 45. Wood CW, Reeves DW, Duffield RR, Edmisten KL. 1992. Field chlorophyll measurements for evaluation of corn nitrogen status. J. Plant Nutr. 15:487–500 [Google Scholar]
- 46. Bullock DG, Anderson DS. 1998. Evaluation of the Minolta SPAD-502 chlorophyll meter for nitrogen management in corn. J. Plant Nutr. 21:741–755 [Google Scholar]
- 47. Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol. 162:9–24 [Google Scholar]
- 48. Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908 [Google Scholar]
- 49. Schreiner RP, Mihara KL. 2009. The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitis vinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia 101:599–611 [DOI] [PubMed] [Google Scholar]
- 50. Ryan MH, Graham JH. 2002. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 244:263–271 [Google Scholar]
- 51. Harrier LA, Watson CA. 2003. The role of arbuscular mycorrhizal fungi in sustainable cropping systems. Adv. Agronomy 79:185–225 [Google Scholar]
- 52. Hooker JE, Black KE. 1995. Arbuscular mycorrhizal fungi as components of sustainable soil-plant systems. Crit. Rev. Biotechnol. 15:201–212 [Google Scholar]
- 53. Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM. 2003. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 37:1–16 [Google Scholar]
- 54. Gavito ME, Miller MH. 1998. Changes in mycorrhiza development in maize induced by crop management practices. Plant Soil 198:185–192 [Google Scholar]
- 55. Johnson NC, Pfleger FL, Crookston RK, Simmons SR, Copeland PJ. 1991. Vesicular arbuscular mycorrhizas respond to corn and soybean cropping history. New Phytol. 117:657–663 [Google Scholar]
- 56. McCain KNS, Wilson GWT, Blair JM. 2011. Mycorrhizal suppression alters plant productivity and forb establishment in a grass-dominated prairie restoration. Plant Ecol. 212:1675–1685 [Google Scholar]
- 57. Middleton EL, Bever JD. 2012. Inoculation with a native soil community advances succession in a grassland restoration. Restor. Ecol. 20:218–226 [Google Scholar]
- 58. Alguacil MM, Lumini E, Roldan A, Salinas-Garcia JR, Bonfante P, Bianciotto V. 2008. The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops. Ecol. Appl. 18:527–536 [DOI] [PubMed] [Google Scholar]
- 59. Douds DD, Galvez L, Janke RR, Wagoner P. 1995. Effect of tillage and farming system upon populations and distribution of vesicular-arbuscular mycorrhizal fungi. Agric. Ecosys. Environ. 52:111–118 [Google Scholar]
- 60. Galvez L, Douds DD, Drinkwater LE, Wagoner P. 2001. Effect of tillage and farming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize. Plant Soil 228:299–308 [Google Scholar]
- 61. Lekberg Y, Koide RT, Twomlow SJ. 2008. Effect of agricultural management practices on arbuscular mycorrhizal fungal abundance in low-input cropping systems of southern Africa: a case study from Zimbabwe. Biol. Fertil. Soils 44:917–923 [Google Scholar]
- 62. Johnson NC. 1993. Can fertilization of soil select less mutualistic mycorrhizae. Ecol. Appl. 3:749–757 [DOI] [PubMed] [Google Scholar]
- 63. Martinez TN, Johnson NC. 2010. Agricultural management influences propagule densities and functioning of arbuscular mycorrhizas in low- and high-input agroecosystems in arid environments. Appl. Soil Ecol. 46:300–306 [Google Scholar]
- 64. US Environmental Protection Agency 2011. Pesticides: regulating biopesticides, plant incorporated protectants. Current & previously registered section 3 PIP registrations. US Environmental Protection Agency, Washington, DC: http://www.epa.gov/pesticides/biopesticides/pips/pip_list.htm [Google Scholar]


