Abstract
We examined nitrate-dependent Fe2+ oxidation mediated by anaerobic ammonium oxidation (anammox) bacteria. Enrichment cultures of “Candidatus Brocadia sinica” anaerobically oxidized Fe2+ and reduced NO3− to nitrogen gas at rates of 3.7 ± 0.2 and 1.3 ± 0.1 (mean ± standard deviation [SD]) nmol mg protein−1 min−1, respectively (37°C and pH 7.3). This nitrate reduction rate is an order of magnitude lower than the anammox activity of “Ca. Brocadia sinica” (10 to 75 nmol NH4+ mg protein−1 min−1). A 15N tracer experiment demonstrated that coupling of nitrate-dependent Fe2+ oxidation and the anammox reaction was responsible for producing nitrogen gas from NO3− by “Ca. Brocadia sinica.” The activities of nitrate-dependent Fe2+ oxidation were dependent on temperature and pH, and the highest activities were seen at temperatures of 30 to 45°C and pHs ranging from 5.9 to 9.8. The mean half-saturation constant for NO3− ± SD of “Ca. Brocadia sinica” was determined to be 51 ± 21 μM. Nitrate-dependent Fe2+ oxidation was further demonstrated by another anammox bacterium, “Candidatus Scalindua sp.,” whose rates of Fe2+ oxidation and NO3− reduction were 4.7 ± 0.59 and 1.45 ± 0.05 nmol mg protein−1 min−1, respectively (20°C and pH 7.3). Co-occurrence of nitrate-dependent Fe2+ oxidation and the anammox reaction decreased the molar ratios of consumed NO2− to consumed NH4+ (ΔNO2−/ΔNH4+) and produced NO3− to consumed NH4+ (ΔNO3−/ΔNH4+). These reactions are preferable to the application of anammox processes for wastewater treatment.
INTRODUCTION
Iron is a prevalent redox-active metal element present in the Earth and occurs in two oxidation states (Fe2+ and Fe3+) in nature. The state of iron depends on various physicochemical parameters, particularly pH, temperature, and redox potential (1, 2). Some microorganisms drive the oxidation of Fe2+ to gain energy for growth, with molecular oxygen or NO3− as the electron acceptor (3). There are four types of biological Fe2+ oxidation, (i) aerobic Fe2+ oxidation under acidophilic conditions; (ii) neutrophilic Fe2+ oxidation under microaerobic conditions; (iii) neutrophilic, light-dependent Fe2+ oxidation under anoxic conditions; and (iv) neutrophilic, nitrate-dependent Fe2+ oxidation under anoxic conditions (4, 5, 6, 7). Because the redox potential of Fe3+/Fe2+ is +0.77 V at pH 2, molecular oxygen is used for Fe2+ oxidation under acidophilic conditions. Nitrate can also be used for Fe2+ oxidation under neutrophilic conditions since the redox potentials of iron composites are sufficiently low to reduce nitrate (+0.42 V for NO3−/NO2−), e.g., −0.236 V for Fe(OH)3/Fe2+, +0.2 V for Fe(OH)3 + HCO3−/FeCO3, and from −0.1 to +0.1 V for ferrihydrite/Fe2+ at pH 7 (5, 8). Nitrate-dependent Fe2+ oxidation involves the oxidation of Fe2+ coupled with denitrification or dissimilatory nitrate reduction to ammonium (DNRA); therefore, it influences both N and Fe cycles in the environment. Nitrate-dependent Fe2+ oxidation has been observed in various environments, including freshwater springs (9), paddy soils (10), blackish-water and marine sediments (11), freshwater sediments (12, 13, 14), groundwater (15, 16), and activated sludge (17).
Anammox bacteria are obligate anaerobic and chemoautotrophic bacteria affiliated with a monophyletic group in the bacterial order Brocadiales in the phylum Planctomycetes (18). Anammox bacteria anaerobically oxidize ammonium with nitrite to produce nitrogen gas (N2) through a hydrazine intermediate; the stoichiometry is shown in the following equation (19, 20): NH4+ + 1.32NO2− + 0.066HCO3− + 0.13H+ → 1.02N2 + 0.26NO3− + 2.03H2O + 0.066CH2O0.5N0.15.
In addition to the anammox reaction, these bacteria are capable of performing dissimilatory nitrate (21, 22), Fe3+, and Mn(IV) (23, 24) reduction by using organic matter as an electron donor. Interestingly, the ability to oxidize Fe2+ with NO3− as an electron acceptor has also been described for an anammox bacterium, “Candidatus Kuenina stuttgartiensis.” When an enrichment culture of “Ca. Kuenina stuttgartiensis” was anaerobically incubated with added Fe2+ and NO3−, Fe2+ was oxidized at a rate of 4.8 nmol mg protein−1 min−1 (23). However, the details of nitrate-dependent Fe2+ oxidation by anammox bacteria, including the stoichiometry of the reaction, affinity for NO3−, and impact on the anammox reaction, are poorly understood.
Our objectives were (i) to examine nitrate-dependent Fe2+ oxidation by anammox bacteria other than “Ca. Kuenina stuttgartiensis,” (ii) to characterize the physiological traits of nitrate-dependent Fe2+ oxidation, including temperature and pH dependence, and the mean half-saturation constant (Ks) for NO3−, and (iii) to examine the influence of nitrate-dependent Fe2+ oxidation on the stoichiometry of the anammox reaction. Experiments were carried out primarily by using an anammox bacterium, “Candidatus Brocadia sinica,” that was enriched from activated sludge from a municipal wastewater treatment plant in Japan (25, 26). Activity of nitrate-dependent Fe2+ oxidation was also examined in another anammox bacterium, “Candidatus Scalindua sp.,” that was originally obtained from marine sediment in Hiroshima Bay, Japan (27, 28).
MATERIALS AND METHODS
Bacteria strains.
Planktonic “Ca. Brocadia sinica” cells were obtained from a membrane bioreactor (MBR) as previously described (29). A hollow-fiber membrane unit composed of 300 polyethylene tubes (pore size, 0.1 μm; tube diameter, 1 mm; length, 70 mm) was installed in a 3-liter jar fermentor (Eyela, Tokyo, Japan) to sustain the “Ca. Brocadia sinica” cells in the MBR. This MBR has been operated at 37 ± 1°C for more than 1 year with a continuous supply of inorganic nutrient medium containing NH4+ and NO2− (each at 40 to 60 mM). Inorganic nutrient medium contained the following (mM): MgSO4 · 7H2O (0.24), CaCl2 (0.47), KH2PO4 (0.18), KHCO3 (1.0), yeast extract at 1.0 mg liter−1 (Becton, Dickinson Co., Franklin Lakes, NJ), and 0.5 ml of trace element solutions I and II (30). Fluorescence in situ hybridization (FISH) analysis with the oligonucleotide probe AMX820 (31) showed that anammox bacteria accounted for more than 94% of the total biomass. The dominance of “Ca. Brocadia sinica” in a monospecies culture was confirmed by determining the 16S rRNA gene sequence of cells in the enrichment culture. To obtain a highly enriched “Ca. Brocadia sinica” culture, Percoll separation was performed as described previously (32). A red-colored band in the bottom layer of a centrifugation tube was recovered with a plastic syringe and washed twice with inorganic nutrient medium. “Ca. Brocadia sinica” accounted for more than 99.9% of the total biomass in this highly enriched culture.
The small floccular biomass (<100 μm) of “Ca. Scalindua sp.” was obtained from another MBR. An enrichment culture of “Ca. Scalindua sp.” previously described by Kindaichi et al. (27) was inoculated into the MBR, and it was operated for more than half of a year at 20°C with a continuous supply of inorganic nutrient medium containing artificial sea salt (2.5% [wt/vol]; Marine Tech, Tokyo, Japan). Anammox bacteria accounted for more than 90% of the total biomass in the culture, as determined by FISH analysis with the oligonucleotide probe AMX820. Partial 16S rRNA gene nucleotide sequences (Escherichia coli 16S rRNA position, bp 225 to 645) retrieved from the culture showed 99% sequence similarity to the clone husup-a7 (accession number AB573103) (27).
Activity tests.
Standard anaerobic techniques (26, 33) were used with an anaerobic chamber (Coy Laboratory Products, Grass Lake Charter Township, MI). Biomass taken from the MBRs was centrifuged (10,000 × g, 10 min, 20°C), and the biomass pellets were washed twice and resuspended at a biomass concentration of 0.1 to 1.0 mg protein ml−1 in an inorganic nutrient medium (pH 7.3 to 7.5) supplemented with 10 to 15 mM Fe2+ (in the form of FeSO4) and 2.5 mM NO3−. Inorganic nutrient medium was prepared by purging with N2 gas (99.999%) for 30 min, and this medium was left for 1 week in an anaerobic chamber. Prior to activity testing, KHCO3 and FeSO4 · 7H2O were weighed in the anaerobic chamber and dissolved in medium.
Biomass suspension (1 to 10 ml) was dispensed into 5- or 25-ml serum glass vials sealed with butyl rubber stoppers and aluminum caps. The headspace was replaced with He gas (>99.99995%), and the vials were incubated at 37°C in the dark for 10 to 98 h. To determine the extent of chemical Fe2+ oxidation or NO3− reduction, negative-control vials without biomass were incubated in the same manner.
Fe2+ oxidation and NO3− reduction activities were measured under different pH and temperature conditions. The influence of pH was examined over a pH range of 2.7 to 11.2. The pH was adjusted by adding 2 N H2SO4 or 1 N NaOH. The influence of temperature was examined over a range of 4 to 75°C.
The value of Ks for NO3− was determined under NO3−-limiting conditions. The initial concentrations of Fe2+ and NO3− were set at 10 and 0.7 mM, respectively. The value of Ks was evaluated on the basis of the Hanes-Wool plot.
The stoichiometry of nitrate-dependent Fe2+ oxidation by “Ca. Brocadia sinica” was determined by incubating the biomass with 2.5 mM 15N-labeled NO3− (Cambridge Isotope Laboratories, Andover, MA) and 15 mM Fe2+. As controls, vials (i) with autoclaved biomass, (ii) without added Fe2+, and (iii) without added NO3− were incubated in parallel. Additionally, “Ca. Brocadia sinica” cells were incubated with 500 mg liter−1 penicillin G (Sigma-Aldrich, St. Louis, MO) and 200 mg liter−1 chloramphenicol (Wako Pure Chemicals, Osaka, Japan).
Incorporation of CO2.
Incorporation of CO2 was examined by incubating the biomass with NaH14CO3 (PerkinElmer, Waltham, MA) at a final concentration of 20 μCi mg volatile suspended solids−1 as previously described (34). Biomass was collected after 4 days of incubation, washed three times with phosphate-buffered saline (PBS), and mixed with scintillation cocktail Ultima Gold XR (PerkinElmer). Radioactivity was measured with an LSC-6100 liquid scintillation counter (Hitachi-Aloka Medicals, Tokyo, Japan). Additionally, cold runs were performed in parallel to determine the rates of NO3− reduction and Fe2+ oxidation. Autoclaved biomass was incubated in the same manner to examine nonbiological CO2 incorporation.
Co-occurrence of Fe2+ oxidation and anammox.
Concurrent Fe2+ oxidation and anammox were examined by incubating “Ca. Brocadia sinica” cells with 15N-labeled NO3−, unlabeled NH4+ (each 2.5 mM), and Fe2+ (10 mM). The influence of nitrate-dependent ferrous iron oxidation on the stoichiometry of the anammox reaction was further examined by incubating the biomass with NH4+, NO2− (each at 2 mM), and Fe2+ (1 mM). Controls included vials (i) without Fe2+ and (ii) with 1 mM Fe3+ instead of Fe2+, which were incubated in parallel.
Chemical analysis.
To determine Fe2+, NH4+, NO2−, and NO3− concentrations, 1 ml of biomass suspension was mixed with 50 μl of 4 M HCl and incubated for 30 min at ambient temperature. A portion (∼10 μl) of this mixture was for Fe2+ concentration measurement. The remaining biomass was centrifuged at 18,200 × g for 10 min, and NH4+, NO2−, and NO3− were measured in the supernatant. These procedures were conducted in an anaerobic chamber to prevent chemical oxidation of Fe2+. Headspace gas was collected from the vials with a gas-tight glass syringe (VICI AG International, Schenkon, Switzerland) and immediately analyzed by gas chromatography-mass spectrometry (GC-MS) as described below.
Concentrations of NH4+ and NO3− were determined with an IC-2010 ion chromatograph equipped with a TSKgel SuperIC-Anion HS or a TSKgel SuperIC-Cation HS column (Tosoh, Tokyo, Japan) for anion or cation analysis, respectively. When the value of Ks for NO3− was determined, the concentration of NO3− was measured via the colorimetric brucine method (35). For colorimetric determination of NO3−, removal of Fe2+ and Fe3+ from the bulk solution was essential because they interfere with brucine color development. Liquid samples were passed over a TOYOPAK IC-SP cation-exchange column (Tosoh, Tokyo, Japan). This filtration does not decrease or increase the NO3− concentration. Filtered samples were mixed with 80% (vol/vol) sulfuric acid and brucine sulfanilic acid solution (10 mM brucine and 5.8 mM sulfanilic acid) and heated in boiling water for 20 min. After cooling, the absorbance at 405 nm was determined with an ARBO MX microplate reader (PerkinElmer).
The NO2− concentration was determined by the naphthylethylenediamine method (36). The sample was mixed with 4.9 mM naphthylethylenediamine solution, and the absorbance at 540 nm was measured.
The Fe2+ concentration was determined by the 1,10-phenanthroline method (36). Briefly, the sample was mixed with 5.5 mM 1,10-phenanthroline solution and 1 M acetate buffer (pH 4.6) in an anaerobic chamber and then the absorbance at 405 nm was measured.
15N-labeled gaseous compound concentrations (29N2, 30N2, 31NO, and 46N2O) were determined by GC-MS analysis as previously described (37, 38). Fifty microliters of headspace gas was injected at a split ratio of 1:50 into a GCMS-QP2010 SE gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a CP-Pora BOND Q fused silica capillary column (Agilent Technologies, Santa Clara, CA). A standard curve for 29N2 and 30N2 gas quantification was prepared with 30N2 gas (>98% purity) (Cambridge Isotope Laboratories).
Biomass concentration.
The culture fluid was centrifuged at 18,200 × g for 10 min, and the biomass pellets were resuspended in 10% (wt/vol) sodium dodecyl sulfate solution. After boiling for 10 min, the supernatant was obtained after centrifugation at 18,200 × g for 10 min, followed by determination of the protein concentration. The protein concentration was determined with a DC-protein assay kit (Bio-Rad, Hercules, CA) by following to the manufacturer's instructions. Bovine serum albumin (Wako Pure Chemicals) was used as the protein standard.
Microscopy.
Epifluorescence microscopy was performed after staining the biomass with 1:1,000 SYBR gold (Life Technologies, Carlsbad, CA) for 10 min. Specimens on the glass slide (Matsunami Glass, Osaka, Japan) were examined with an AxioVert 200 microscope equipped with an Axio Cam MRC charge-coupled device camera (Carl Zeiss, Jena, Germany).
Scanning electron microscopy (SEM) was performed as previously described (39). Rusty precipitates were washed two times with PBS and fixed with 2.5% glutaraldehyde in PBS overnight at 4°C. The sample was washed three times with PBS for 10 min. The fixed samples were dehydrated in a sequential acetone series (50, 70, 80, 90, and 95% for 15 min each and 100% three times for 15 min) and substituted with isoamyl acetate. Samples were dried with a critical-point drier with liquid CO2 and coated with platinum and palladium for 2 min. The coated samples were examined by SEM with a model S-4000 microscope (Hitachi).
RESULTS
Nitrate-dependent Fe2+ oxidation by anammox bacteria.
Nitrate-dependent Fe2+ oxidation was demonstrated by incubating “Ca. Brocadia sinica” cells with Fe2+ and 15NO3−. As shown in Fig. 1, a simultaneous decrease in Fe2+ and NO3− concentrations was observed until NO3− was depleted (after 50 h of incubation). The 30N2 gas accumulated in the headspace after 30 h of incubation, and nearly all of the 15N-labeled nitrogen supplemented as 15NO3− was recovered as 30N2 gas at 98 h. Accumulation of NH4+ and NO2− in the liquid phase and the production of 29N2, 31NO, and 46N2O gas in the headspace were not detected throughout incubation. These results clearly indicate that “Ca. Brocadia sinica” oxidized Fe2+ coupled with the reduction of NO3− to N2.
Fig 1.
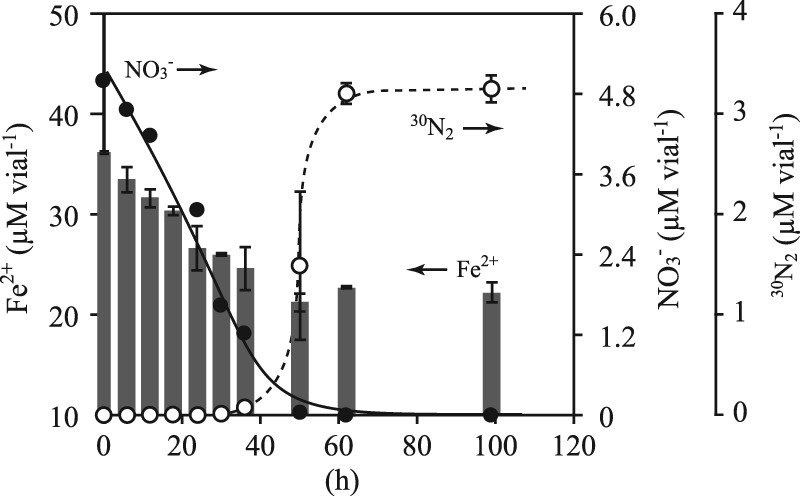
Oxidation of Fe2+ (gray bars, left y axis) with reduction of 15NO3− (closed circles, right y axis) and production of 30N2 gas (empty circles, right y axis). “Ca. Brocadia sinica” cells (2.5-ml sample in a 5-ml glass vial) were incubated at 37°C with 15NO3− (2.5 mM) and Fe2+ (15 mM). Error bars indicate the range of SDs derived from three replicated vials.
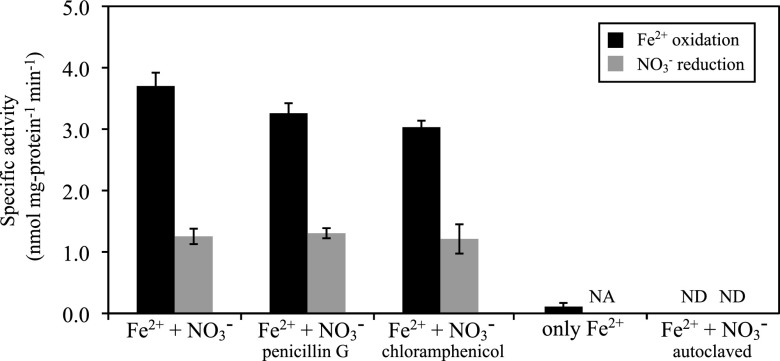
The contribution of “Ca. Brocadia sinica” to nitrate-dependent Fe2+ oxidation was further verified by using a highly enriched culture (>99.9% of the total biomass). This highly enriched “Ca. Brocadia sinica” culture also oxidized Fe2+ and reduced NO3− at rates of 3.7 ± 0.2 and 1.3 ± 0.1 nmol mg protein−1 min−1 (mean ± SD), respectively (Fig. 2). Therefore, the calculated molar ratio of reduced NO3− (ΔNO3−) to oxidized Fe2+ (ΔFe2+) was 0.351. Fe2+ oxidation and NO3− reduction activities were not strongly affected by the addition of penicillin G or chloramphenicol (Fig. 2). In contrast, Fe2+ oxidation was greatly (>95%) reduced when the cells were incubated without NO3−. Additionally, no activity of Fe2+ oxidation or NO3− reduction was observed in vials containing autoclaved biomass.
Fig 2.
Specific nitrate-dependent Fe2+ oxidation activities of a highly enriched culture of “Ca. Brocadia sinica” supplemented with (i) Fe2+ and NO3−; (ii) Fe2+, NO3−, and penicillin G; or (iii) Fe2+, NO3−, and chloramphenicol. Penicillin G and chloramphenicol inhibit the activities of most bacteria but not anammox bacteria. Specific activities were also examined during incubation with added Fe2+ only. Additionally, the autoclaved biomass was incubated with Fe2+ and NO3− in the same manner as the control. Error bars indicate the range of SDs derived from three replicated vials. NA, not applicable; ND, not detected.
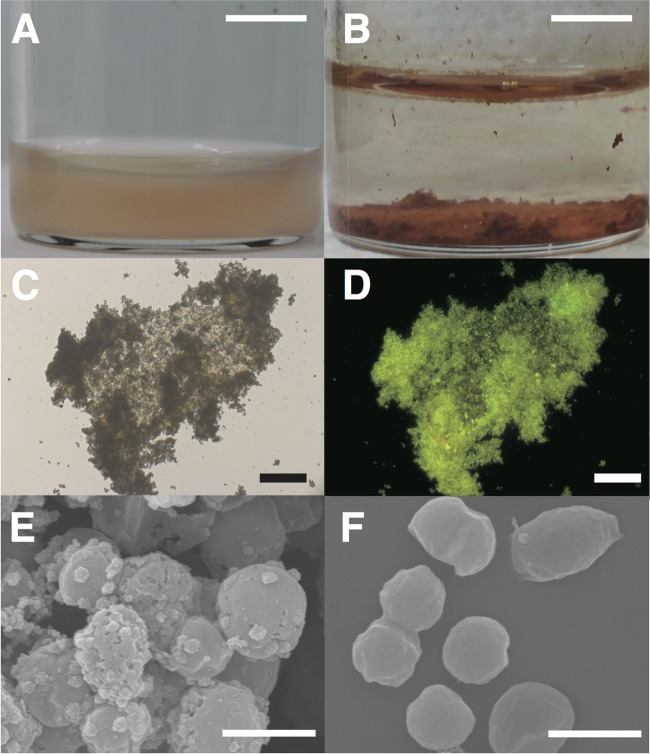
“Ca. Brocadia sinica” cells were planktonic and colored red (Fig. 3A); however, they formed rusty precipitates (diameters of 20 to 800 μm) (Fig. 3B) within 1 day of incubation. Epifluorescence microscopy after staining with SYBR gold revealed that rusty precipitates were aggregates of microbial cells (Fig. 3C and D). SEM revealed that the cells in aggregates were covered with small amorphous particles (Fig. 3E), which were likely oxidized iron-containing minerals since no amorphous particles were observed prior to incubation (Fig. 3F).
Fig 3.
Microscopy of rusty precipitates developed during nitrate-dependent Fe2+ oxidation by “Ca. Brocadia sinica.” Cell suspensions of “Ca. Brocadia sinica” were anaerobically incubated with NO3− and Fe2+ at 37°C (A). Rusty precipitates exhibiting a reddish color appeared at the bottoms of the vials after 1 day of incubation (B). Precipitates were stained with SYBR gold and subsequently examined by epifluorescence microscopy. Precipitates gave a signal for SYBR gold. Panels C and D are bright-field and fluorescence microscopy images, respectively, of SYBR gold. The precipitates were further examined by SEM (E). Cells were covered with small amorphous particles and were attached via the particles. As a control, “Ca. Brocadia sinica” cells were examined prior to incubation (F). Amorphous particles were not observed in image F. Scale bars are 6 cm (A and B), 100 μm (C and D), and 1 μm (E and F).
Fe2+ oxidation and NO3− reduction activities were also determined in the enrichment culture of “Ca. Scalindua sp.,” with the cells exhibiting Fe2+ oxidation and NO3− reduction activities at rates of 4.7 ± 0.59 and 1.45 ± 0.05 nmol mg protein−1 min−1, respectively. The molar ratio of ΔNO3− to ΔFe2+ was 0.309 for “Ca. Scalindua sp.,” which is similar to that for “Ca. Brocadia sinica” (i.e., 0.351).
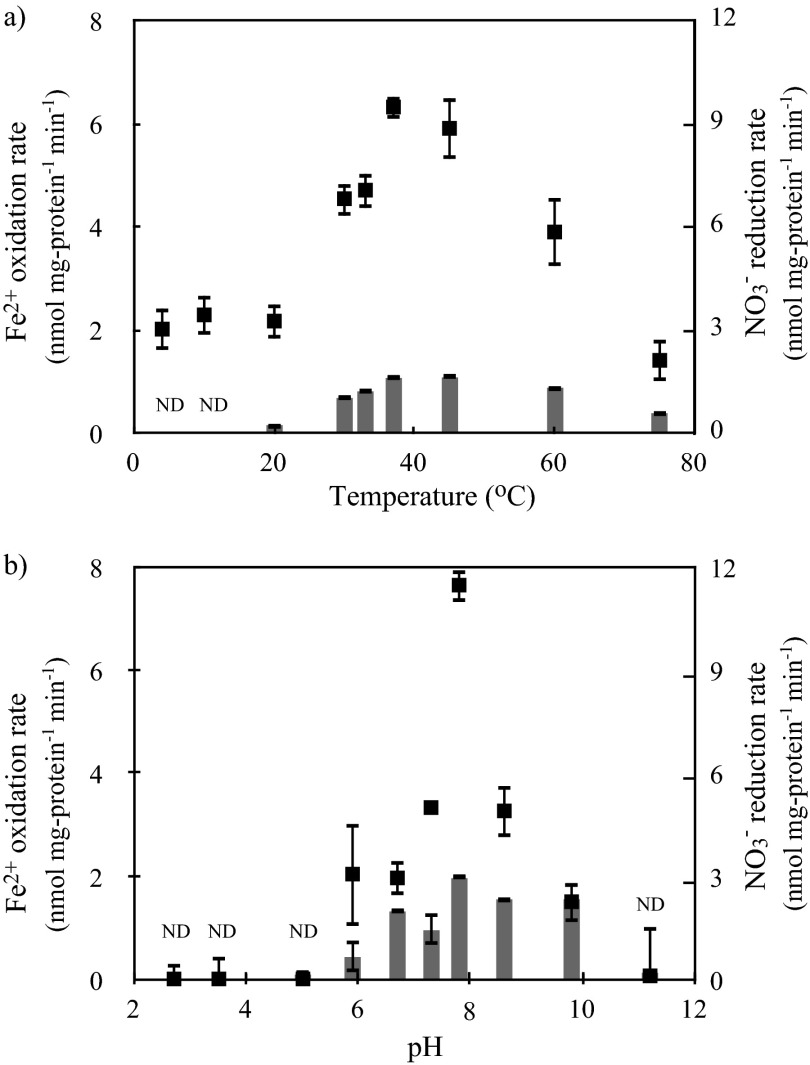
Temperature and pH dependence and Ks for NO3−.
Higher activities of Fe2+ oxidation and NO3− reduction were seen in the range of 30 to 45°C (Fig. 4a), which corresponds to the physiological anammox temperature of “Ca. Brocadia sinica” (25 to 45°C) (26). The pH range was determined to be 5.9 to 9.8 (Fig. 4b). Fe2+ oxidation and NO3− reduction activities were not observed below pH 5.0, indicating that “Ca. Brocadia sinica” is a neutrophilic Fe2+-oxidizing bacterium. The molar ratio of ΔNO3− to ΔFe2+ increased from 0.26 at 20°C to 0.42 at 76°C. These molar ratios ranged from 0.39 to 1.55 over a pH range of 5.9 to 9.8. The mean (± SD) Ks value for NO3− was determined to be 51 ± 21 μM on the basis of three independent batch experiments (see Fig. S1 in the supplemental material).
Fig 4.
Temperature (a) and pH (b) dependence of Fe2+ oxidation (filled squares) and NO3− reduction (gray bars) activities. “Ca. Brocadia sinica” cells were (a) incubated at pH 7.8 over a temperature range of 4 to 75°C and (b) incubated at 37°C over a pH range of 2.7 to 11.2. Error bars indicate the range of SDs derived from three replicated vials. ND, not detected for the NO3− reduction rate.
Autotrophic growth by nitrate-dependent Fe2+ oxidation.
Incorporation of 14C-labeled CO2 was below the detection limit (<0.12 mM), although “Ca. Brocadia sinica” consumed 2.76 mM Fe2+ and 0.868 mM NO3− over 4 days of incubation. This indicates that the molar ratio of oxidized Fe2+ to incorporated CO2 was greater than 23, which agrees with the high molar ratio obtained for the aerobic Fe2+ oxidizer Acidothiobacillus ferrooxidans (i.e., 71) (40).
Co-occurrence of Fe2+ oxidation and anammox.
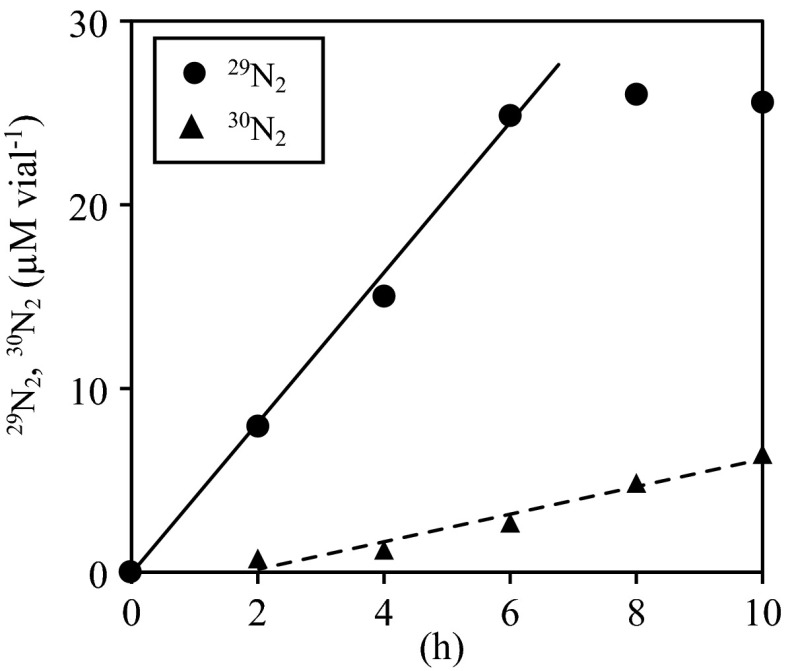
When “Ca. Brocadia sinica” cells were incubated with Fe2+, 15N-labeled NO3−, and unlabeled NH4+, 29N2 gas was produced immediately after incubation started (Fig. 5). In addition to the production of 29N2 gas, accumulation of 30N2 gas was observed in the headspace, but its production was lower than that of 29N2 gas.
Fig 5.
Accumulation of 29N2 (circles) and 30N2 gas (triangles). “Ca. Brocadia sinica” cells (10-ml samples in 25-ml glass vials) were incubated at 37°C with 15NO3−, 14NH4+ (each at 2.5 mM), and Fe2+ (10 mM).
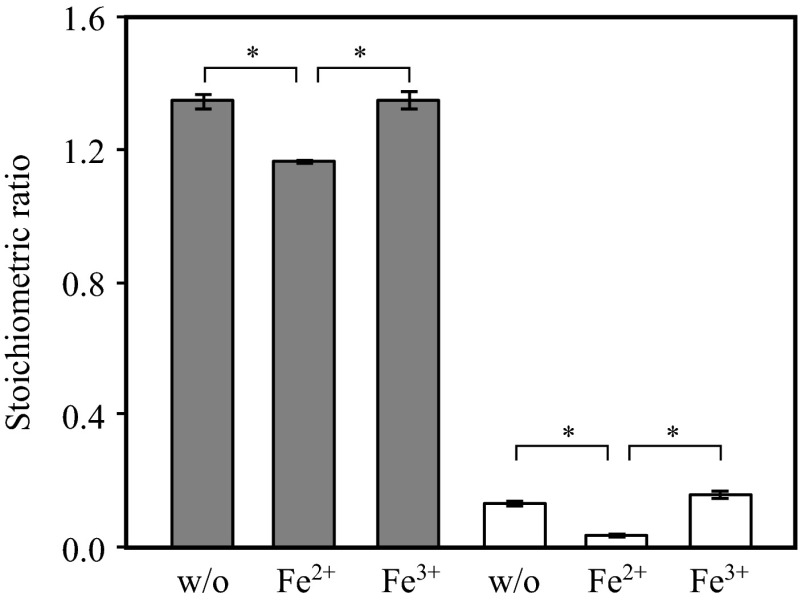
The presence of Fe2+ influenced the stoichiometry of the anammox reaction. When “Ca. Brocadia sinica” was cultivated in the presence of Fe2+, NH4+, and NO2−, the molar ratios of ΔNO2−/ΔNH4+ and ΔNO3−/ΔNH4+ were significantly lower than those found when Fe2+ was absent and when Fe3+ rather than Fe2+ was present (Fig. 6). The presence of Fe2+ and Fe3+ (up to 1 mM) did not result in an inhibitory effect on the activity of anaerobic ammonium oxidation.
Fig 6.
Stoichiometric ΔNO2−/NH4+ (gray bars) and ΔNO3−/NH4+ (white bars) ratios in “Ca. Brocadia sinica” cultures incubated in the presence of (i) NH4+, NO2−, and NO3− (labeled as w/o); (ii) NH4+, NO2−, NO3−, and Fe2+ (labeled as Fe2+); and (iii) NH4+, NO2−, NO3−, and Fe3+ (labeled as Fe3+). Error bars indicate the range of SDs derived from three replicated vials. P values were calculated with Student's t test. *, P < 0.01.
DISCUSSION
Nitrate-dependent Fe2+ oxidation has been observed in various bacterial and archaeal species (3, 41). We showed that anammox bacteria “Ca. Brocadia sinica” and “Ca. Scalindua sp.” are capable of carrying out nitrate-dependent iron oxidation. Their contributions to nitrate-dependent Fe2+ oxidation, particularly that of “Ca. Brocadia sinica,” were carefully evaluated by using highly enriched cultures and supplementation with antibiotics (penicillin G and chloramphenicol) that are not active against anammox bacteria but inhibit the activity of most heterotrophs (42, 43). Moreover, control experiments without the addition of biomass were performed to examine the extent of abiotic Fe2+ oxidation [e.g., Fe2+ oxidation by Mn(III) and Mn(IV) oxide, copper, green rust, NO2−, and N2O] (44, 45, 46, 47) and NO3− reduction. Therefore, the activities of Fe2+ oxidation coupled with NO3− reduction described in this report represent microbial activities.
Several metabolic pathways, including nitrate reduction to nitrite (NO3− → NO2−), denitrification (NO3− → NO2− → NO → N2O → N2), and DNRA (NO3− → NO2− → NH4+), can be coupled with Fe2+ oxidation (47, 48, 49). We confirmed the production of 29N2 gas from 15N-labeled NO3− and unlabeled NH4+ (Fig. 5), indicating that NO3− was reduced to NO2− and the 15N-labeled NO2− produced was subsequently utilized for the anammox reaction. This is because (i) 29N2 gas is produced solely in the anammox reaction (50) and (ii) NO3− is not a direct substrate of the anammox reaction (19, 20). Moreover, accumulation of 30N2 gas shows that 15N-labeled NO2− was further reduced to NH4+ and then consumed in the anammox reaction. DNRA is the likely pathway for NO3− reduction to NH4+ via NO2−, as previously demonstrated by anammox bacteria other than “Ca. Brocadia sinica” (21, 22, 51). Indeed, we found that “Ca. Brocadia sinica” carried a gene essential for DNRA (nrfA-encoding ammonia-forming nitrite reductase) and expressed this protein at moderate levels. On the other hand, “Ca. Brocadia sinica” does not contain a gene for denitrification (nosZ, which encodes nitrous oxide reductase) (M. Oshiki et al., unpublished data). However, the contribution of NrfA to DNRA by “Ca. Brocadia sinica” remains unknown and requires additional analysis. Notably, the production of 30N2 gas lagged behind NO3− reduction (Fig. 1) and the production of 29N2 gas (Fig. 5), indicating that reduction of the intermediate produced from NO3− was the rate-limiting step in the anammox reaction. This observation agrees with a previous study stating that NO2− reduction to NH4+ is the rate-limiting step in the DNRA reaction of “Ca. Kuenina stuttgartiensis” (22).
The two mechanisms postulated for microbial Fe2+ oxidation are cell surface and intracellular Fe2+ oxidation (52). In the former case, electrons derived from Fe2+ oxidation must be transported into cells and finally passed to nitrate reductase to reduce nitrite. In the latter case, membrane proteins are essential for Fe2+ uptake and further for Fe3+ export out of cells after Fe2+ oxidation (53). Since anammox bacterial cells contain three separate compartments (paryphoplasm, riboplasm, and anammoxosome) (54), Fe2+ or electrons must pass through these compartments. The biochemistry of nitrite-dependent Fe2+ oxidation by anammox bacteria is of great interest and requires further investigation.
The molar ratios of ΔNO3− to ΔFe2+ (0.351 in “Ca. Brocadia sinica” and 0.309 in “Ca. Scalindua sp.” at 37°C and pH 7.3 to 7.5) were higher than the theoretical ratio of Fe2+ oxidation coupled to NO3− reduction to the equimolar NO2− and NH4+ (i.e., 0.20). Moreover, the theoretical ratio further decreases when electrons derived from Fe2+ oxidation are utilized in the subsequent anammox reaction; i.e., 1 and 3 mol of electrons are required for reduction of nitrate to nitric oxide and synthesis of hydrazine from equimolar levels of NO and NH4+ (19, 20). High molar ratios of ΔNO3− to ΔFe2+ were previously observed in cultures of freshwater sediment microorganisms (55, 56). These studies examined the microbial activities of Fe2+ oxidation coupled with DNRA, and the observed molar ratios of ΔNO3− to ΔFe2+, respectively, were 0.191 and 0.25. Those values were nearly twice as high as the excepted molar ratio of 0.125. A possible explanation is that Fe3+ may have been reduced by endogenous respiration and/or the oxidation of dead cells and extracellular polymeric substances as electron donors. The metabolic capability of Fe3+ reduction by anammox bacteria has been demonstrated in “Ca. Kuenina stuttgartiensis” and “Candidatus Scalindua profunda” with exogenous formate as an electron donor (23, 24). An alternative explanation is that the production of unidentified N species occurred during NO3− reduction (47). For instance, the occurrence of incomplete denitrification under unfavorable pH conditions has been previously demonstrated in a denitrifying culture (57) and in soil samples (58).
The Fe2+ oxidation activities of three phylogenetically distinct anammox bacteria, “Ca. Kuenina stuttgartiensis” (23), “Ca. Brocadia sinica,” and “Ca. Scalindua sp.,” are in the range of 3.7 to 8.7 nmol Fe2+ mg protein−1 min−1. These values correspond to 3.8 to 8.9 mmol Fe2+ mmol C−1 h−1 when a conversion factor of 0.0585 mol C g protein−1 (26) is used, indicating that the three anammox bacteria show Fe2+ oxidation activity lower than that of acidophilic Fe2+-oxidizing bacteria (6.8 to 20 mmol Fe2+ mmol C−1 h−1) (59, 60). “Ca. Brocadia sinica” and “Ca. Scalindua sp.” showed NO3− reduction activities that were an order of magnitude lower than their anammox activities, which are in the range of 10 to 75 nmol NH4+ mg protein−1 min−1 (26, 28). On the basis of the Ks value for NO3− of “Ca. Brocadia sinica,” 51 ± 21 μM, the nitrate-dependent Fe2+ oxidation reaction by “Ca. Brocadia sinica” can be anticipated in circumneutral environments where NO3− and Fe2+ are available at several micromolar concentrations, such as in anoxic groundwater sediments (15, 61).
The molar ratios of ΔNO3−/ΔNH4+ and ΔNO2−/ΔNH4+ in the anammox reaction decreased significantly upon the addition of Fe2+ to the culture medium (Fig. 6). NO3− produced during the anammox reaction was utilized for DNRA (NO3− → NO2− → NH4+) coupled with Fe2+ oxidation; the intermediate of DNRA, NO2−, can also be utilized for the anammox reaction. Thus, the molar ratios of ΔNO3−/ΔNH4+ and ΔNO2−/ΔNH4+ were lower than those without Fe2+ addition. In contrast, there are two other possible explanations for decreased molar ratios of ΔNO3−/ΔNH4+ and ΔNO2−/ΔNH4+. The reducing power of CO2 fixation may be obtained not only from NO2− oxidation but also from Fe2+ oxidation, resulting in lower production of NO3−, and maintenance energy is increased in the presence of Fe2+, leading to a decreased growth yield of anammox bacteria. These hypotheses must be examined in future studies. Decreased ΔNO3−/ΔNH4+ and ΔNO2−/ΔNH4+ ratios are preferable in the application of anammox for wastewater treatment because they require lower levels of NO2− in the influents and there is a low discharge of NO3− (62, 63). We recommend the further study of nitrogen removal performance in anammox processes wherein ferrous iron is supplemented with wastewater.
Supplementary Material
ACKNOWLEDGMENTS
This research was financially supported by a grant-in-aid from the New Energy and Industrial Technology Development Organization, Japan; the Steel Foundation for Environmental Protection Technology, Japan; and the Japan Science and Technology Agency, CREST. M.O. was supported by a grant-in-aid for JSPS Fellows from the Japan Society for the Promotion of Science.
We sincerely appreciate T. Kindaichi (Hiroshima University) for kindly providing the biomass of “Ca. Scalindua sp.”
Footnotes
Published ahead of print 26 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00743-13.
REFERENCES
- 1. Stumm W, Lee GF. 1961. Oxygenation of ferrous iron. Ind. Eng. Chem. 53:143–146 [Google Scholar]
- 2. Sung W, Morgan JJ. 1980. Kinetics and product of ferrous iron oxygenation in aqueous systems. Environ. Sci. Technol. 14:561–568 [Google Scholar]
- 3. Emerson D, Fleming EJ, McBeth JM. 2010. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu. Rev. Microbiol. 64:561–583 [DOI] [PubMed] [Google Scholar]
- 4. Blake R, Shute EA, Waskovsky J, Harrison AP. 1992. Respiratory components in acidophilic bacteria that respire on iron. Geomicrobiol. J. 10:173–192 [Google Scholar]
- 5. Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. 1993. Ferrous iron oxidation by an oxygenic phototrophic bacteria. Nature 362:834–836 [Google Scholar]
- 6. Neubauer SC, Emerson D, Megonigal JP. 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl. Environ. Microbiol. 68:3988–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sobolev D, Roden EE. 2002. Evidence for rapid microscale bacterial redox cycling of iron in circumneutral environments. Antonie Van Leeuwenhoek 81:587–597 [DOI] [PubMed] [Google Scholar]
- 8. Straub KL, Benz M, Schink B. 2001. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 34:181–186 [DOI] [PubMed] [Google Scholar]
- 9. Hegler F, Lösekann-Behrens T, Hanselmann K, Behrens S, Kappler A. 2012. Influence of seasonal and geochemical changes on iron geomicrobiology of an iron-carbonate mineral water spring. Appl. Environ. Microbiol. 78:7185–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ratering S, Schnell S. 2001. Nitrate-dependent iron(II) oxidation in paddy soil. Environ. Microbiol. 3:100–109 [DOI] [PubMed] [Google Scholar]
- 11. Benz M, Brune A, Schink B. 1998. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 169:159–165 [DOI] [PubMed] [Google Scholar]
- 12. Straub KL, Buchholz-Cleven BEE. 1998. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl. Environ. Microbiol. 64:4846–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hauck S, Benz M, Brune A, Schink B. 2001. Ferrous iron oxidation by denitrifying bacteria in profundal sediments of a deep lake (Lake Constance). FEMS Microbiol. Ecol. 37:127–134 [Google Scholar]
- 14. Melton ED, Schmidt C, Kappler A. 2012. Microbial iron(II) oxidation in littoral freshwater lake sediment: the potential for competition between phototrophic vs. nitrate-reducing iron(II)-oxidizers. Front. Microbiol. 3:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jørgensen CJ, Jacobsen Elberling OS, Aamand BJ. 2009. Microbial oxidation of pyrite coupled to nitrate reduction in anoxic groundwater sediment. Environ. Sci. Technol. 43:4851–4857 [DOI] [PubMed] [Google Scholar]
- 16. Haaijer SCM, Crienen G, Jetten MSM, Op den Camp HJM. 2012. Anoxic iron cycling bacteria from an iron sulfide- and nitrate-rich freshwater environment. Front. Microbiol. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielsen JL, Nielsen PH. 1998. Microbial nitrate-dependent oxidation of ferrous iron in activated sludge. Environ. Sci. Technol. 32:3556–3561 [Google Scholar]
- 18. Strous M, Fuerst J, Kramer E, Logemann S, Muyzer G, van de Pas-Schoonen K, Webb R, Kuenen J, Jetten M. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446–449 [DOI] [PubMed] [Google Scholar]
- 19. Kartal B, Maalcke WJ, de Almeida NM, Cirpus I, Gloerich J, Geerts W, Op den Camp HJM, Harhangi HR, Janssen-Megens EM, Francoijs KJ, Stunnenberg HG, Keltjens JT, Jetten MSM. 2011. Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130 [DOI] [PubMed] [Google Scholar]
- 20. Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJM, Jetten MSM, Keltjens JT. 2013. How to make a living from anaerobic ammonium oxidation. FEMS Microbiol. Rev. 37:428–461 [DOI] [PubMed] [Google Scholar]
- 21. Güven D, Dapena A, Kartal B, Schmid MC, Maas B, van de Pas-Schoonen K, Sozen S, Mendez R, Op den Camp HJM, Jetten MSM, Strous M, Schmidt I. 2005. Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl. Environ. Microbiol. 71:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kartal B, Kuypers MMM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM, Strous M. 2007. Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ. Microbiol. 9:635–642 [DOI] [PubMed] [Google Scholar]
- 23. Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, Taylor M, Horn M, Daims H, Bartol-Mavel D, Wincker P, Barbe V, Fonknechten N, Vallenet D, Segurens B, Schenowitz-Truong C, Medigue C, Collingro A, Snel B, Dutilh B, Op den Camp H, van der Drift C, Cirpus I, van de Pas-Schoonen K, Harhangi H, van Niftrik L, Schmid M, Keltjens J, van de Vossenberg J, Kartal B, Meier H, Frishman D, Huynen M, Mewes H, Weissenbach J, Jetten M, Wagner M, Le Paslier D. 2006. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794 [DOI] [PubMed] [Google Scholar]
- 24. van de Vossenberg J, Rattray JE, Geerts W, Kartal B, van Niftrik L, van Donselaar EG, Sinninghe Damsté JS, Strous M, Jetten MSM. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Environ. Microbiol. 10:3120–3129 [DOI] [PubMed] [Google Scholar]
- 25. Tsushima I, Kindaichi T, Okabe S. 2007. Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Res. 41:785–794 [DOI] [PubMed] [Google Scholar]
- 26. Oshiki M, Shimokawa M, Fujii N, Satoh H, Okabe S. 2011. Physiological characteristics of the anaerobic ammonium-oxidizing bacterium ‘Candidatus Brocadia sinica.’ Microbiology 157:1706–1713 [DOI] [PubMed] [Google Scholar]
- 27. Kindaichi T, Awata T, Suzuki Y, Tanabe K, Hatamoto M, Ozaki N, Ohashi A. 2011. Enrichment using an up-flow column reactor and community structure of marine anammox bacteria from coastal sediment. Microbes Environ. 26:67–73 [DOI] [PubMed] [Google Scholar]
- 28. Awata T, Oshiki M, Kindaichi T, Ozaki N, Ohashi A, Okabe S. 12 April 2013, posting date Physiological characterization of an anaerobic ammonium-oxidizing bacterium belonging to the “Candidatus Scalindua” group. Appl. Environ. Microbiol. (Epub ahead of print.) 10.1128/AEM.00056-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oshiki M, Satoh H, Okabe S. 2011. Long-term operation of anaerobic ammonium oxidation (anammox) process in a membrane bioreactor equipped with hollow fibred membranes, p 23 Abstr. 4th IWA-ASPIRE Conference & Exhibition International Water Association, Tokyo, Japan [Google Scholar]
- 30. van de Graaf AA, de Bruijn P, Robertson LA, Jetten MM, Gijs Kuenen J. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187–2196 [Google Scholar]
- 31. Schmid M, Schmitz-Esser S, Jetten M, Wagner M. 2001. 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium-oxidizing bacteria: implications for phylogeny and in situ detection. Environ. Microbiol. 3:450–459 [DOI] [PubMed] [Google Scholar]
- 32. Kartal B, Geerts W, Jetten MSM. 2011. Cultivation, detection and ecophysiology of anaerobic ammonium-oxidizing bacteria. Methods Enzymol. 486:89–108 [DOI] [PubMed] [Google Scholar]
- 33. Ito T, Nielsen JL, Okabe S, Watanabe Y, Nielsen PH. 2002. Phylogenetic identification and substrate uptake patterns of sulfate-reducing bacteria inhabiting an oxic-anoxic sewer biofilm determined by combining microautoradiography and fluorescent in situ hybridization. Appl. Environ. Microbiol. 68:356–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okabe S, Kindaichi T, Ito T. 2005. Fate of 14C-Labeled microbial products derived from nitrifying bacteria in autotrophic nitrifying biofilms. Appl. Environ. Microbiol. 71:3987–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenkins D, Medsker LL. 1964. Brucine method for determination of nitrate in ocean, estuarine, and fresh waters. Anal. Chem. 36:610–612 [Google Scholar]
- 36. Rice EW, Baird RB, Eaton AD, Clesceri LS. (ed). 2012. Standard methods for the examination of water and wastewater, 22nd ed, p 376–380, 4115,, 4120–4121 American Public Health Association, Washington, DC [Google Scholar]
- 37. Waki M, Yasuda T, Suzuki K, Sakai T, Suzuki N, Suzuki R, Matsuba K, Yokoyama H, Ogino A, Tanaka Y, Ueda S, Takeuchi M, Yamagishi T, Suwa Y. 2010. Rate determination and distribution of anammox activity in activated sludge treating swine wastewater. Bioresour. Technol. 101:2685–2690 [DOI] [PubMed] [Google Scholar]
- 38. Yoshinaga I, Amano T, Yamagishi T, Okada K, Ueda S, Sako Y, Suwa Y. 2011. Distribution and diversity of anaerobic ammonium oxidation (anammox) bacteria in the sediment of a eutrophic freshwater lake, Lake Kitaura, Japan. Microbes Environ. 26:189–197 [DOI] [PubMed] [Google Scholar]
- 39. May T, Ito A, Okabe S. 2009. Induction of multidrug resistance mechanism in Escherichia coli biofilms by interplay between tetracycline and ampicillin resistance genes. Antimicrob. Agents Chemother. 53:4628–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelly DP. 1978. Bionergetics of chemolithotrophic bacteria, p 363–386 In Bull AT, Meadow PM. (ed), Companion to microbiology; selected topics for further discussion. Longman, London, United Kingdom [Google Scholar]
- 41. Bonnefoy V, Holmes DS. 2012. Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments. Environ. Microbiol. 14:1597–1611 [DOI] [PubMed] [Google Scholar]
- 42. Dapena-Mora A, Fernandez I, Campos JL, Mosquera-Corral A, Mendez R, Jetten MSM. 2007. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzyme Microb. Technol. 40:859–865 [Google Scholar]
- 43. Okabe S, Oshiki M, Takahashi Y, Satoh H. 2011. N2O emission from a partial nitrification anammox process and identification of a key biological process of N2O emission from anammox granules. Water Res. 45:6461–6470 [DOI] [PubMed] [Google Scholar]
- 44. Buresh RJ, Moraghan JT. 1976. Chemical reduction of nitrate by ferrous iron. J. Environ. Qual. 5:320–325 [Google Scholar]
- 45. Myers CR, Nealson KN. 1988. Microbial reduction of manganese oxides: interaction with iron and sulfur. Geochim. Cosmochim. Acta 52:2727–2732 [Google Scholar]
- 46. Hansen HCB, Koch CB, Nancke-Krogh H, Borggaard OK, Sørensen J. 1996. Abiotic nitrate reduction to ammonium: key role of green rust. Environ. Sci. Technol. 30:2053–2056 [Google Scholar]
- 47. Straub KL, Benz M, Schink B, Widdel AF. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clément J-C, Shrestha J, Ehrenfeld JG, Jaffé PR. 2005. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol. Biochem. 37:2323–2328 [Google Scholar]
- 49. Carlson HK, Clark IC, Melnyk RA, Coates JD. 2012. Toward a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: balancing electron uptake and detoxification. Front. Microbiol. 3:57. 10.3389/fmicb.2012.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thamdrup B, Dalsgaard T. 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van de Vossenberg J, Woebken D, Maalcke WJ, Wessels HJ, Dutilh BE, Kartal B, Janssen-Megens EM, Roeselers G, Yan J, Speth D, Gloerich J, Geerts W, van der Biezen E, Pluk W, Francoijs KJ, Russ L, Lam P, Malfatti SA, Tringe SG, Haaijer SC, Op den Camp HJ, Stunnenberg HG, Amann R, Kuypers MM, Jetten MS. 2013. The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda’ illustrates the versatility of this globally important nitrogen cycle bacterium. Environ. Microbiol. 15:1275–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schädler S, Burkhardt C, Hegler F, Straub KL, Miot J, Benzerara K, Kappler A. 2009. Formation of cell-iron-mineral aggregates by phototrophic and nitrate-reducing anaerobic Fe(II)-oxidizing bacteria. Geomicrobiol. J. 26:93–103 [Google Scholar]
- 53. Miot J, Benzerara K, Morin G, Kappler A, Bernard S, Obst M, Férard C, Skouri-Panet F, Guigner J-M, Posth N, Galvez M, Brown GE, Jr, Guyot F. 2009. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 73:696–711 [Google Scholar]
- 54. van Niftrik L, Jetten MS. 2012. Anaerobic ammonium-oxidizing bacteria: unique microorganisms with exceptional properties. Microbiol. Mol. Biol. Rev. 76:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber KA, Urrutia MM, Churchill PF, Kukkadapu RK, Roden EE. 2006. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 8:100–113 [DOI] [PubMed] [Google Scholar]
- 56. Coby AJ, Picardal F, Shelobolina E, Xu H, Roden EE. 2011. Repeated anaerobic microbial redox cycling of iron. Appl. Environ. Microbiol. 77:6036–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thomsen JK, Geest T, Cox RP. 1994. Mass spectrometric studies of the effect of pH on the accumulation of intermediates in denitrification by Paracoccus denitrificans. Appl. Environ. Microbiol. 60:536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dörsch P, Braker G, Bakken LR. 2012. Community-specific pH response of denitrification: experiments with cells extracted from organic soils. FEMS Microbiol. Ecol. 79:530–541 [DOI] [PubMed] [Google Scholar]
- 59. Breed AW, Dempers CJ, Searby GE, Gardner MN, Rawlings DE, Hansford GS. 1999. The effect of temperature on the continuous ferrous-iron oxidation kinetics of a predominantly Leptospirillum ferrooxidans culture. Biotechnol. Bioeng. 65:44–53 [DOI] [PubMed] [Google Scholar]
- 60. Candy RM, Blight KR, Ralph DE. 2009. Specific iron oxidation and cell growth rates of bacteria in batch culture. Hydrometallurgy 98:148–155 [Google Scholar]
- 61. Smolders AJP, Lucassen ECHET, Bobbink R, Roelofs JGM, Lamers LPM. 2010. How nitrate leaching from agricultural lands provokes phosphate eutrophication in groundwater fed wetlands: the sulphur bridge. Biogeochemistry 98:1–7 [Google Scholar]
- 62. Kartal B, Rattray J, van Niftrik LA, van de Vossenberg J, Schmid MC, Webb RI, Schouten S, Fuerst JA, Damsté JS, Jetten MS, Strous M. 2007. Candidatus “Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 30:39–49 [DOI] [PubMed] [Google Scholar]
- 63. Winkler MK, Yang J, Kleerebezem R, Plaza E, Trela J, Hultman B, van Loosdrecht MC. 2012. Nitrate reduction by organotrophic anammox bacteria in a nitritation/anammox granular sludge and a moving bed biofilm reactor. Bioresour. Technol. 114:217–223 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.