Abstract
During hematogenously disseminated infection, blood-borne Candida albicans invades the endothelial cell lining of the vasculature to invade the deep tissues. Although the C. albicans Als3 invasin is critical for invasion and damage of endothelial cells in vitro, a C. albicans als3Δ/Δ mutant has normal virulence in the mouse model of disseminated infection. We hypothesized that the contribution of Als3 to virulence is obscured by the presence of additional C. albicans invasins. To elucidate the in vivo function of Als3, we heterologously expressed C. albicans ALS3 in Candida glabrata, a yeast that lacks a close ALS3 ortholog and has low virulence in mice. We found that following intravenous inoculation into mice, the ALS3-expressing strain preferentially trafficked to the brain, where it induced significantly elevated levels of myeloperoxidase, tumor necrosis factor, monocyte chemoattractant protein 1, and gamma interferon. Also, the ALS3-expressing strain had enhanced adherence to and invasion of human brain microvascular endothelial cells in vitro, demonstrating a potential mechanism for ALS3-mediated neurotropism. In addition, upon initiation of infection, the ALS3-expressing strain had increased trafficking to the cortex of the kidneys. With prolonged infection, this strain persisted in the kidneys at significantly higher levels than the control strain but did not induce an elevated inflammatory response. Finally, the ALS3-expressing strain had increased resistance to neutrophil killing in vitro. These results indicate that during disseminated infection, Als3 mediates initial trafficking to the brain and renal cortex and contributes to fungal persistence in the kidneys.
INTRODUCTION
The invasion of blood-borne Candida albicans cells across the endothelial cell lining of the vasculature is a key event in the initiation of hematogenously disseminated candidiasis (1). One of the mechanisms by which C. albicans can invade endothelial cells is by inducing its own endocytosis (2). Previously, we found that C. albicans hyphae express the invasin Als3, which binds to N-cadherin on endothelial cells and induces endocytosis (3, 4). Mutants of C. albicans that lack Als3 have significantly reduced capacity to adhere to, invade, and damage endothelial cells in vitro (3, 5, 6). In addition, latex beads that are coated with recombinant Als3 and clones of Saccharomyces cerevisiae that heterologously express C. albicans Als3 are avidly endocytosed by endothelial cells in vitro (3, 7). Moreover, both ALS3 mRNA and Als3 protein are highly expressed by C. albicans in the kidneys of mice with disseminated disease (8, 9), and healthy individuals and patients with candidemia have high-titer antibodies against Als3 (10). Finally, vaccination of mice with recombinant Als3 protects them against disseminated candidiasis (11). Collectively, these data suggest that Als3 is likely to play an important role in the pathogenesis of hematogenously disseminated candidiasis.
Based on this information, one would predict that a C. albicans mutant lacking Als3 would have markedly attenuated virulence in the mouse model of disseminated candidiasis. Surprisingly, it was recently reported that an als3Δ/Δ mutant has wild-type virulence in this model (12). Although there are multiple potential reasons for this result, one possible explanation is that because ALS3 is a member of a multigene family, other members of the ALS gene family can compensate for its absence. Because the ALS gene family contains 8 members (13), it would be technically difficult to construct a mutant in which all ALS genes other than ALS3 were deleted in order to investigate the pathogenic function of Als3 in isolation. Therefore, to evaluate the function of Als3 in vivo, we used an alternative approach in which we expressed C. albicans ALS3 in Candida glabrata, a yeast with no close ALS3 ortholog and low virulence in mice. We found that following intravenous inoculation into mice, an ALS3-expressing strain of C. glabrata had increased trafficking to the brain, where it induced a strong inflammatory response. In addition, this strain preferentially traveled to the cortex of the kidneys, where it caused a persistent infection with minimal inflammation. These data suggest that Als3 mediates trafficking to the brain and the renal cortex in vivo.
MATERIALS AND METHODS
Candida strains and culture conditions.
The C. albicans wild-type strain, DAY185, and the als3Δ/Δ null and als3Δ/Δ+ALS3 complemented strains were constructed as described previously (14, 15). C. glabrata BG14 (ura3Δ::Tn903Neor) was used as the parent strain in all experiments (16). This strain was transformed with either the ALS3 expression plasmid pGRB2.2-ALS3 to create strain Cg-Als3 or the backbone vector pGRB2.2 (17) to create strain Cg-control. To construct pGRB2.2-Als3, the ALS3 protein coding sequence was amplified by PCR using primers ATATAAAACATCTAGATGCTACAACAATATACATTGTTACTC and GGGTTGTGTTCTCGATTAAATAAACAAGGATAATAATGTGATCAAACC and then cloned into the XbaI and XhoI sites of pGRB2.2 using an In-Fusion 2.0 Dry-Down PCR cloning kit per the manufacturer's instructions (Clontech Laboratories). For use in the experiments, all strains were grown in liquid synthetic complete medium with or without uridine (MP Biomedicals) overnight in a shaking incubator at 30°C. The in vitro growth rate experiments were performed in the same medium.
Flow cytometry.
Flow cytometry was used to analyze the surface expression of Als3 on C. glabrata using our previously described method (3). Briefly, after fixing of the organisms in 3% paraformaldehyde and blocking with 1% goat serum, the cells were incubated with a rabbit polyclonal antiserum raised against the recombinant N-terminal region of Als3 (3). Next, the cells were rinsed extensively and incubated with a goat anti-rabbit secondary antibody conjugated with Alexa Fluor 488 (Invitrogen). The fluorescence intensity of the cells was measured using a FACSCaliber flow cytometer (Becton, Dickinson). Fluorescence data for 10,000 cells of each strain were collected.
Endothelial cells.
Human umbilical vein endothelial cells (HUVECs) and human brain microvascular endothelial cells (HBMECs) were grown as described previously (18–21).
Adherence and endocytosis.
The capacity of the two strains of C. glabrata to adhere to and induce their own endocytosis by HUVECs and HBMECs was determined using our previously described differential fluorescence assay (3, 20). Briefly, the endothelial cells were grown to confluence on fibronectin-coated coverslips in 24-well tissue culture plates. They were infected with 105 cells of each C. glabrata strain suspended in RPMI 1640 medium (Irvine Scientific) to achieve a multiplicity of infection of 1:1. After 90 min for human umbilical vein endothelial cells and 180 min for human brain microvascular endothelial cells, the wells were rinsed with Hanks balanced salt solution (Irvine Scientific) and fixed with 3% paraformaldehyde. The adherent but nonendocytosed organisms were stained with a rabbit polyclonal anti-Candida antibody (Biodesign International) conjugated with Alexa Fluor 568 (Invitrogen). Next, the endothelial cells were permeabilized with 0.5% Triton X-100, and then the cell-associated organisms (the adherent plus endocytosed organisms) were stained with the anti-Candida antiserum conjugated with Alexa Fluor 488. The coverslips were viewed by epifluorescence microscopy, and at least 100 cell-associated organisms per coverslip were scored for endocytosis. Each experiment was performed in triplicate on three separate occasions.
Endothelial cell damage assay.
The extent of endothelial cell damage caused by the C. glabrata strains was measured using our standard 51Cr release assay (3, 22). Briefly, HUVECs and HBMECs were grown to confluence in 96-well tissue culture plates, loaded with 51Cr overnight, and then infected with 2 × 105 cells of the C. glabrata strains. Parallel wells were incubated with a similar number of C. albicans cells or left uninfected as a positive or negative control, respectively. After a 16-h incubation, the amount of 51Cr that had been released into the medium and that remained associated with the endothelial cells was determined. Each experiment was performed three times in triplicate.
Endothelial cell production of IL-8.
The capacity of the different C. glabrata strains to stimulate endothelial cells to release interleukin 8 (IL-8) was determined using the same conditions as in the endothelial cell damage experiment. At the end of the 16-h incubation period, the medium above the cells was collected, centrifuged to remove cellular debris, and then stored at −80°C. At a later time, the IL-8 content of the conditioned medium was determined by commercial enzyme immunoassay (Hycult Biotech), following the manufacturer's instructions. Each experiment was performed in triplicate on three separate occasions.
Neutrophil fungicidal assay.
Isolation of neutrophils from healthy volunteers and the neutrophil fungicidal assay were performed as previously described (23). In brief, neutrophils were incubated in glass test tubes containing C. glabrata in RPMI 1640 medium with 10% pooled human serum. The neutrophil/fungus ratio was 5:1. Control tubes contained C. glabrata without neutrophils. After 1 h, the mixtures were sonicated to disrupt the neutrophils and then quantitatively cultured. The percentage of organisms killed was calculated by dividing the number of CFU in the tubes containing neutrophils by the number of CFU in tubes without neutrophils. Each experiment was performed in triplicate using neutrophils from different donors.
Animal studies.
The virulence of the C. albicans strains was analyzed by inoculating 10 or 11 male BALB/c mice per strain vial the lateral tail vein with 5 × 105 yeast-phase organisms and monitoring them three times daily for survival. To induce disseminated C. glabrata infection, groups of 7 or 8 mice were inoculated intravenously with 5 × 107 cells of each C. glabrata strain. After 1, 7, and 14 days, the mice were sacrificed, and the brains, livers, and kidneys were harvested. The organs were weighed and then homogenized in ice-cold phosphate-buffered saline (PBS) containing protease inhibitor cocktail (Sigma-Aldrich). An aliquot of the homogenate was quantitatively cultured, and the remainder was clarified by centrifugation and stored at −80°C for subsequent myeloperoxidase (MPO) and cytokine analysis. The organ fungal burden experiments were performed three times, and the results were combined. In some experiments, sections of the organs were removed prior to weighing, fixed in zinc-buffered formalin, embedded in paraffin, and cut into thin sections. These thin sections were then stained with Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS). In one experiment, kidneys and brain were harvested and snap-frozen in OCT, after which frozen sections were prepared. These sections were stained with the polyclonal anti-Als3 antibody (3) followed by an Alexa Fluor 568-labeled secondary antibody and then counterstained with the Alexa Fluor 488-labeled anti-Candida antiserum. The mouse studies were carried out in accordance with the National Institutes of Health guidelines for the ethical treatment of animals. This protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
MPO and whole-organ cytokine measurement.
MPO is made constitutively by granulocytes (24) and is also present in lesser amounts in monocytes and some tissue macrophages (25, 26). Thus, the MPO content of the organ homogenate was used as a marker for phagocyte recruitment. MPO levels were measured by enzyme immunoassay (Cell Sciences) (27, 28). The amounts of IL-6, IL-10, IL-12, gamma interferon (IFN-γ), tumor necrosis factor (TNF), and monocyte chemoattractant protein 1 (MCP-1) in the organ homogenates were measured using a mouse inflammation cytometric bead array kit (BD Biosciences). The MPO and cytokine levels were determined in the organ homogenates from a single experiment using 8 mice per C. glabrata strain.
Statistical analysis.
Differences between the interactions of the two strains of C. glabrata with host cells in vitro were analyzed using the Student t test. In the mouse studies, differences in organ fungal burden, MPO level, and cytokine content were analyzed using the Wilcoxon rank sum test. P values of <0.05 were considered significant.
RESULTS
ALS3 is dispensable for C. albicans virulence in mice.
The virulence of a C. albicans als3Δ/Δ null mutant was tested in the mouse model of disseminated infection. Consistent with the results of Cleary et al. (12), we found that the survival of mice infected with the als3Δ/Δ mutant was virtually identical to that of mice infected with the wild-type or als3Δ/Δ+ALS3 complemented strains (see Fig. S1 in the supplemental material). Thus, any contribution of Als3 to C. albicans virulence in this infection model was not detectable, possibly because the absence of Als3 was masked by the presence of other adhesins and/or invasins.
C. glabrata expressed Als3 in a functional manner.
We therefore sought to investigate the function of C. albicans Als3 in C. glabrata, which lacks a close ortholog of ALS3. C. albicans ALS3 was cloned into plasmid pGRB2.2, in which the gene was expressed under the control of the constitutive PGK1 promoter (17). This plasmid was introduced into C. glabrata BG14 to create strain Cg-Als3. First, we used flow cytometric analysis of cells labeled with a polyclonal anti-Als3 antibody to verify that Als3 was expressed on the cell surface of this strain. As expected, virtually all cells of strain Cg-Als3 had strong surface expression of Als3, whereas strain Cg-control, which contained only the backbone vector, did not (Fig. 1A).
Fig 1.
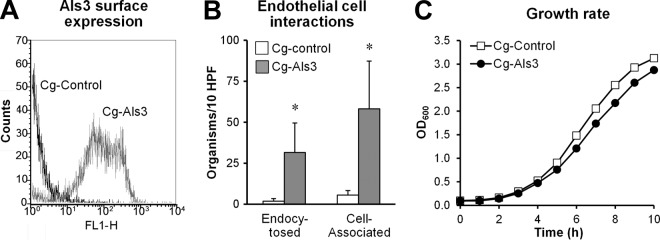
C. albicans Als3 is expressed on the surfaces of C. glabrata in a functional manner. (A) Flow cytometric analysis of C. glabrata expressing Als3 (Cg-Als3) or the wild-type control strain (Cg-control) after the organisms were stained with an anti-Als3 antibody. The histogram shows the results of analysis of 10,000 cells per strain. (B) Expression of Als3 in C. glabrata results in increased adherence to and endocytosis by endothelial cells in vitro. HUVECs were incubated with the indicated strains of C. glabrata for 90 min, after which the numbers of endocytosed and cell-associated organisms were determined using a differential fluorescence assay. Results are the means ± standard deviations of 3 experiments, each performed in triplicate. *, P < 0.001 versus Cg-control. HPF, high-power field. (C) Growth rate of the indicated strains of C. glabrata in minimal medium. Data are representative results of one of two independent experiments.
Next, to confirm that Als3 was functional when expressed in C. glabrata, we tested the interactions of strain Cg-Als3 with HUVECs in vitro. We found that the number of organisms that were associated with these endothelial cells (adherent plus endocytosed organisms) was 11-fold greater for strain Cg-Als3 than strain Cg-control (Fig. 1B). In addition, 21-fold more organisms of strain Cg-Als3 were endocytosed by these endothelial cells. Collectively, these data indicate that the adherence and invasion functions of Als3 were maintained when it was heterologously expressed in C. glabrata.
In addition, to investigate the effects of Als3 expression on the growth of C. glabrata, we analyzed the growth of the two strains in liquid culture. Strain Cg-Als3 grew slightly more slowly than strain Cg-control (Fig. 1C), likely due to the metabolic or fitness cost of expressing a foreign protein.
Expression of Als3 resulted in increased fungal trafficking to the brain and induction of an enhanced inflammatory response.
Next, we investigated the effects of expression of Als3 in the mouse model of disseminated C. glabrata infection. Although each mouse was inoculated with 5 × 107 C. glabrata cells, none of the animals infected with either strain exhibited signs of illness, and none died during the 14-day observation period.
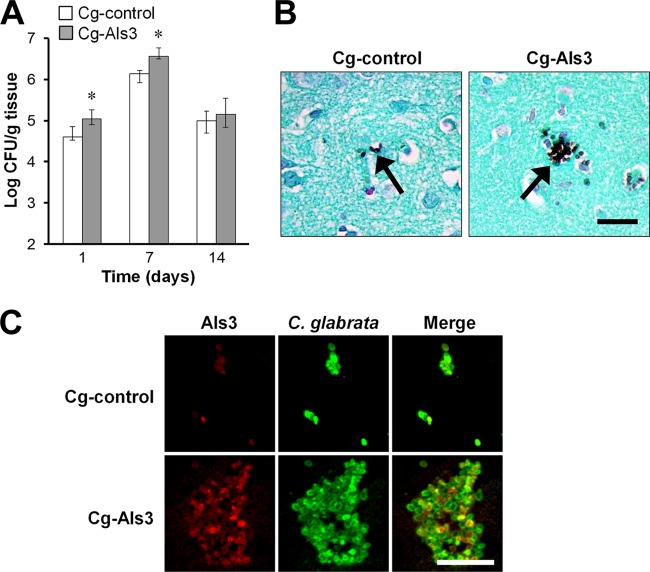
C. glabrata induced a different pattern of infection in each organ that was studied. For example, the liver fungal burden of mice infected with strain Cg-control was very high at day 1 and declined progressively over time (see Fig. S2 in the supplemental material). Moreover, the liver fungal burden of mice infected with Cg-Als3 was similar to that of mice infected with Cg-control at all 3 time points. In contrast, we found that the brain fungal burden of mice infected with Cg-control increased from day 1 to day 7 and then decreased on day 14 (Fig. 2A). Importantly, after 1 and 7 days of infection, mice infected with strain Cg-Als3 had a statistically significant, 3-fold increase in brain fungal burden compared to mice infected with Cg-control. However, by day 14, this difference had disappeared. The increased brain fungal burden induced by Cg-Als3 at days 1 and 7 was highly reproducible, as it was seen in 3 independent experiments. Elevated brain fungal burden in mice infected with strain Cg-Als3 was also evident on histopathologic analysis. Whereas only a few organisms of strain Cg-control were visible scattered throughout the brain, strain Cg-Als3 was observed to form microcolonies that contained numerous organisms (Fig. 2B and C). Thus, expression of Als3 in C. glabrata leads to increased trafficking to the brain.
Fig 2.
Mice infected with Cg-Als3 had increased brain fungal burdens. (A) Mice were infected intravenously with the indicated strains of C. glabrata and then sacrificed at the indicated times for brain fungal burden determination. Results are the medians ± interquartile ranges of 3 experiments, each consisting of 8 mice per strain of C. glabrata. *, P < 0.002 versus Cg-control. (B) Histopathology of the brains of mice infected for 7 days with the indicated strains of C. glabrata. Brain sections were stained with Gomori methenamine silver, and arrows indicate the organisms. Scale bar indicates 20 μm. (C) Expression of Als3 in vivo. Thin sections of the brains obtained after 7 days of infection were stained with an anti-Als3 antibody (red) and an anti-Candida antibody (green) and then imaged by confocal microscopy. Scale bar indicates 10 μm.
To verify that strain Cg-Als3 actually expressed Als3 in vivo, we stained thin sections of the brains of the infected mice with an anti-Als3 antibody. As expected, this antibody stained the Cg-control strain very weakly (Fig. 2C). In contrast, the anti-Als3 antibody stained most cells of strain Cg-Als3, although with varying intensities on different cells.
Next, we investigated whether the increased brain fungal burden of mice infected with strain Cg-Als3 was associated with an altered inflammatory response. After 7 and 14 days of infection, the brain MPO content, a marker of host phagocyte accumulation, was approximately 2-fold higher in the mice infected with Cg-Als3 than in those infected with Cg-control (Fig. 3A). Consistent with these results, the brain TNF, MCP-1, and IFN-γ levels were also significantly higher in mice infected with Cg-Als3 (Fig. 3B to D). There was no difference in the brain IL-6 or IL-12 levels in mice infected with either strain, and IL-10 levels were undetectable (data not shown). Collectively, these data demonstrate that by 7 days of infection, the increased brain fungal burden of mice infected with Cg-Als3 stimulated a stronger local inflammatory response. It is probable that by day 14 this response, in turn, caused the brain fungal burden to decrease.
Fig 3.
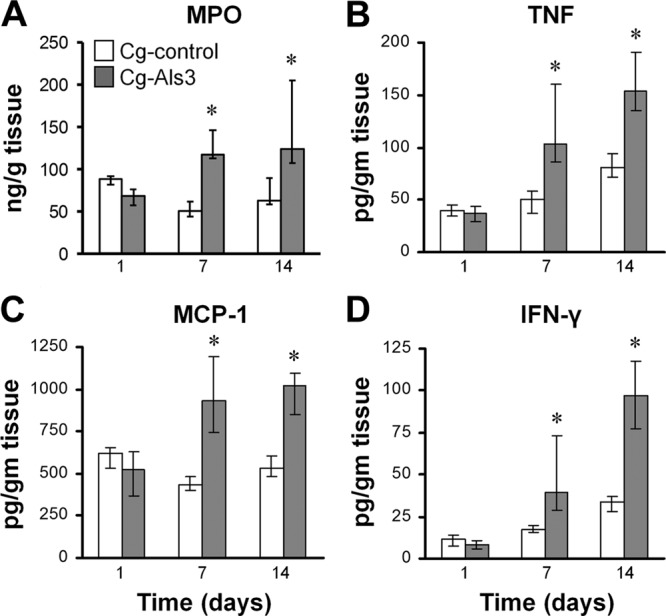
Infection with Cg-Als3 induced an increased inflammatory response in the brain. Mice were infected intravenously with the indicated strains of C. glabrata and then sacrificed at the indicated times. The levels of MPO (A), TNF (B), MCP-1 (C), and IFN-γ (D) in the brain homogenates were measured. Results are the medians ± interquartile ranges of 8 mice per strain of C. glabrata. *, P < 0.015 versus Cg-control.
The endothelial cells that line the blood vessels of the brain express unique proteins such as gp96 on their surface, whereas the endothelial cells that line other blood vessels do not (29). Furthermore, we have found previously that C. albicans Als3 binds to gp96 on brain endothelial cells (20). Therefore, we investigated the interactions of Cg-Als3 with HBMECs in vitro. We found that compared to the Cg-control strain, 6-fold more organisms of Cg-Als3 were associated with these endothelial cells and 69-fold more organisms were endocytosed by these cells (Fig. 4). This enhanced Als3-mediated adherence to and endocytosis by brain endothelial cells is likely the reason why Cg-Als3 had increased trafficking to the brain in vivo.
Fig 4.
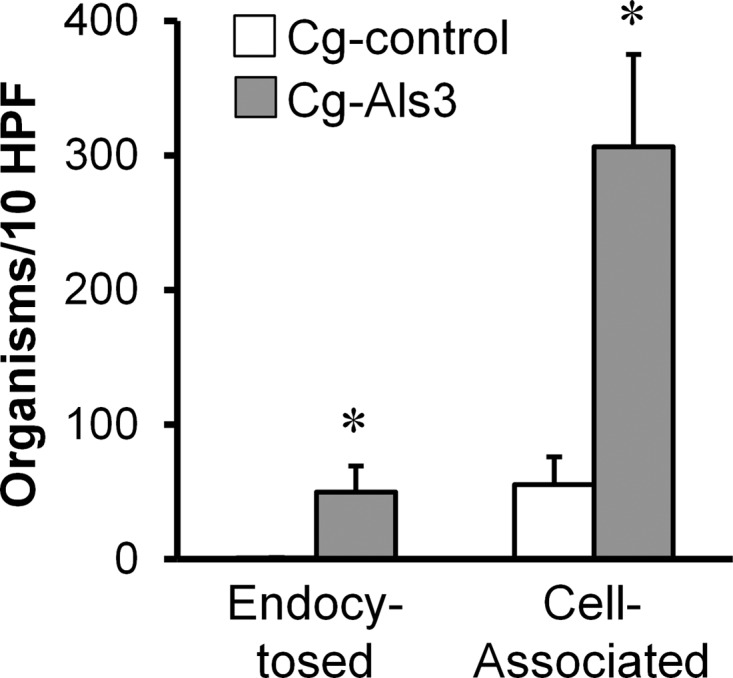
Expression of Als3 in C. glabrata resulted in increased adherence to and endocytosis by brain endothelial cells in vitro. HBMECs were incubated with the indicated strains of C. glabrata for 180 min, after which the numbers of endocytosed and cell-associated organisms were determined using a differential fluorescence assay. Results are the means ± standard deviations of 3 experiments, each performed in triplicate. *, P < 0.0001 versus Cg-control.
Expression of Als3 led to fungal persistence in the kidneys without increased inflammation.
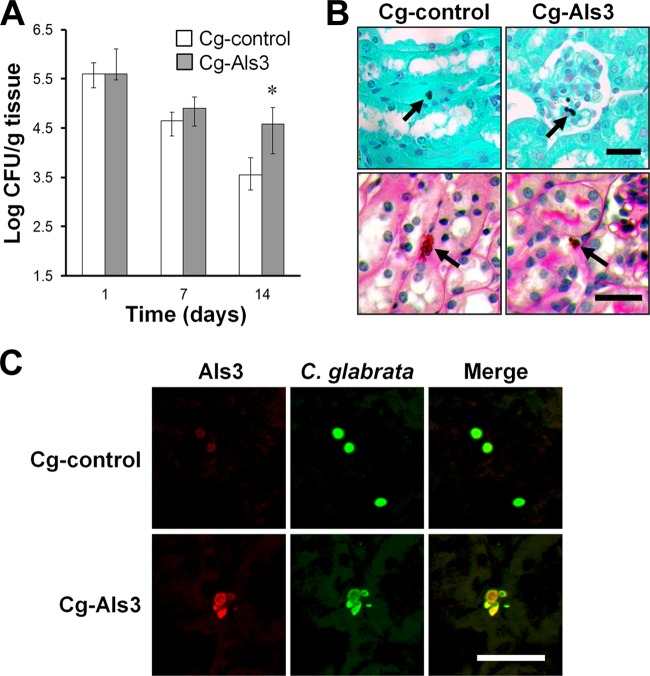
The kidney fungal burden of mice infected with the control strain of C. glabrata was high after 1 day of infection and then progressively declined at the subsequent time points (Fig. 5A). Although the kidney fungal burden of mice infected with strain Cg-Als3 was similar to that of mice infected with Cg-control after 1 and 7 days of infection, it was 9-fold higher on day 14. These results suggest that Als3 led to persistence of infection in the kidneys.
Fig 5.
Mice infected with Cg-Als3 had increased fungal persistence in the kidneys. (A) Mice were infected intravenously with the indicated strains of C. glabrata and then sacrificed at the indicated times for kidney fungal burden determination. Results are the medians ± interquartile ranges of 3 experiments, each consisting of 8 mice per strain of C. glabrata. *, P < 0.03 versus Cg-control. (B) Histopathology of the kidneys of mice infected with the indicated C. glabrata strains for 1 day. Kidney sections were stained with Gomori methenamine silver (top row) and periodic acid-Schiff (bottom row). Arrows indicate the organisms. Scale bar indicates 20 μm. (C) Expression of Als3 in vivo. Thin sections of the kidneys after 1 day of infection with the indicated strains were stained with an anti-Als3 antibody (red) and anti-Candida antibody (green) and then imaged by confocal microscopy. Scale bar indicates 10 μm.
Histopathologic analysis of GMS-stained kidneys obtained after 1 day of infection confirmed that the renal fungal burdens of mice infected with the Cg-control and Cg-Als3 strains were similar (Fig. 5B). However, the different strains appeared to travel to different regions of the kidneys. Strain Cg-control caused infection in both the cortex and medulla, and 19 of 46 (41%) fungal cells were found to be present in the renal cortex when the kidneys of 3 mice were viewed by a blinded observer. In contrast, strain Cg-Als3 had a distinct tropism for the renal cortex, and 42 of 63 (67%) fungal cells were visible in this region (P = 0.007 by Fisher's exact test). Some cells were even present in the glomeruli (Fig. 5B). At the day 1 time point, no organisms of either strain were visible in the collecting system. Unfortunately, as has been reported by others (30), the numbers of organisms that were visible in the kidney sections after 7 days of infection were too low to determine whether the tropism of Cg-Als3 for the renal cortex persisted over time. When the kidney sections were stained with PAS, cells of both C. glabrata strains were visible between the tubules and induced the accumulation of a modest mononuclear cell infiltrate (Fig. 5B). Of note, none of the organisms were observed to be within phagocytes. Staining of the kidney sections with the anti-Als3 antibody demonstrated that strain Cg-Als3 had strong surface expression of Als3 in vivo, whereas strain Cg-control did not (Fig. 5C).
Infection with Cg-control induced a strong initial inflammatory response in the kidneys that subsequently declined (Fig. 6). The kidneys of mice infected with this strain had high levels of MPO, MCP-1, and IFN-γ on day 1, and these levels fell progressively on days 7 and 14. The kidney inflammatory response induced by Cg-Als3 was very similar to that induced by Cg-control. The only exceptions were slight but statistically significant increases in MPO and IFN-γ content induced by Cg-Als3 on days 7 and 14, respectively. However, the magnitude of these increases was small and of uncertain biological significance. The similarity in the overall renal inflammatory response induced by strains Cg-Als3 and Cg-control at day 14 is striking, given that there were 9-fold more organisms present in the kidneys of mice infected with Cg-Als3 at this time point. This finding suggests that Cg-Als3 induced a markedly weaker inflammatory response in kidneys than did Cg-control.
Fig 6.
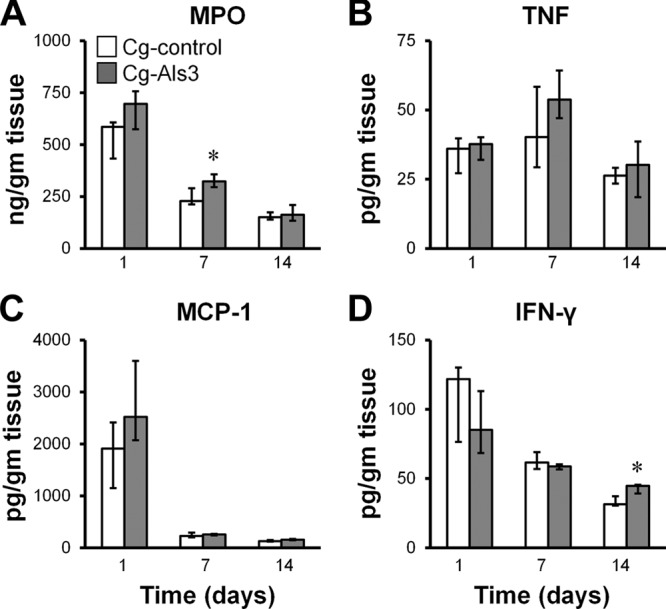
Mice infected with Cg-Als3 and Cg-control had similar profiles of kidney inflammatory response. Mice were infected intravenously with the indicated strains of C. glabrata and then sacrificed at the indicated times. The levels of MPO (A), TNF (B), MCP-1 (C), and IFN-γ (D) in the kidney homogenates were measured. Results are the medians ± interquartile ranges of 8 mice per strain of C. glabrata. *, P < 0.03 versus Cg-control.
One potential explanation for the persistence of Cg-Als3 in the kidneys is that expression of Als3 resulted in resistance to neutrophil killing. To test this hypothesis, we tested the susceptibility of both strains of C. glabrata to killing by human neutrophils. We found that 19% ± 12% fewer cells of Cg-Als3 were killed by neutrophils compared with the control strain (n = 9, P = 0.0008). Thus, increased resistance to neutrophil killing may have made a modest contribution to Als3-mediated persistence in the kidneys. However, the relatively low accumulation of phagocytes in the kidneys of mice infected with Cg-Als3 was probably the major factor that enabled this strain to avoid being cleared by the host.
Expression of Als3 had negligible effects on endothelial cell damage and stimulation in vitro.
In mice with hematogenously disseminated C. albicans infection, fungus-induced damage to host cells and stimulation of a strong host inflammatory response contribute to disease pathogenesis (31). In vitro, endothelial cell invasion is necessary for C. albicans to damage these cells and stimulate them to produce proinflammatory mediators such as leukocyte adhesion molecules and IL-8 (3, 19, 32). Therefore, we investigated whether strain Cg-Als3 had an increased capacity to damage endothelial cells and stimulate them to release IL-8 in vitro. Strain Cg-control did not cause detectable damage to HUVECs or HBMECs, even after 16 h of infection (Fig. 7A). Although the amount of endothelial cell damage (51Cr release) induced by strain Cg-Als3 was statistically greater than baseline, the absolute amount of damage was very low and not significantly greater than the amount of damage induced by Cg-control. As expected, C. albicans caused much greater damage than strain Cg-Als3 to both HUVECs and HBMECs (2, 22). These results indicate that expression of Als3 on C. glabrata had a minimal effect on endothelial cell damage in vitro.
Fig 7.
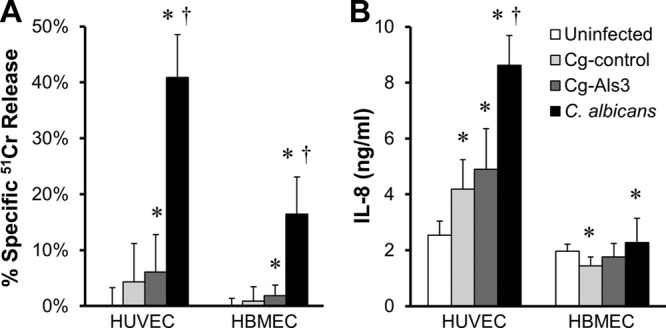
Expression of Als3 in C. glabrata had minimal effect on endothelial cell damage and stimulation of IL-8 release. HUVECs and HBMECs were incubated with Cg-control, Cg-Als3, or C. albicans for 16 h, after which the extent of endothelial cell damage (A) and secretion of IL-8 in to the medium (B) were determined. Results are the means ± standard deviations of 3 experiments, each performed in triplicate. *, P < 0.05 versus uninfected endothelial cells; †, P < 0.0001 versus Cg-Als3.
The C. glabrata strains had different effects on induction of IL-8 secretion by HUVECs compared to HBMECs. Both Cg-control and Cg-Als3 strains stimulated HUVECs to secrete approximately 1.8-fold more IL-8 than was released by uninfected endothelial cells (Fig. 7B). However, strain Cg-Als3 did not stimulate significantly more IL-8 secretion than Cg-control, and both strains stimulated less IL-8 secretion than did C. albicans. Infection of HBMECs with Cg-control actually caused a significant decrease in IL-8 production, whereas Cg-Als3 had no effect. Furthermore, C. albicans induced only a 1.2-fold increase in IL-8 secretion compared to uninfected HBMECs. Collectively, these results demonstrate that expression of Als3 on C. glabrata had little effect on the capacity of this organism to damage and stimulate endothelial cells in vitro. Thus, it is possible that the inability of Cg-Als3 to damage host cells and stimulate a strong proinflammatory response contributed to its low virulence in mice.
DISCUSSION
By employing a heterologous expression strategy, we found that C. albicans Als3 contributes to the pathogenesis of disseminated infection in vivo. Of interest, our data indicate that Als3 mediates pathogenicity by different mechanisms in different organs. For example, expression of Als3 in C. glabrata resulted in increased trafficking to the brain, but not the liver or kidneys, during disseminated infection in mice. In addition, the Als3-expressing strain had enhanced capacity to adhere to and invade HBMECs in vitro. Although the increase in brain fungal burden of mice infected with the Als3-expressing strain was relatively modest, the results with this strain are entirely consistent with our previous data obtained with C. albicans vps51Δ/Δ and slr1Δ/Δ mutants. Both of these C. albicans strains have increased surface expression of Als3, which results in enhanced tropism for the brain in the mouse model of disseminated infection and increased invasion of HBEMCs in vitro (20, 22). Thus, these combined data indicate that Als3 likely contributes to brain invasion during the initiation of disseminated candidiasis. A potential mechanism for this neurotropism is that Als3 binds to gp96, a heat shock protein that is expressed specifically on the surfaces of brain endothelial cells. Indeed, we have found previously that both C. albicans and a strain of S. cerevisiae that expresses C. albicans ALS3 are avidly endocytosed by human brain endothelial cells in a gp96-dependent manner (20).
Als3 had a different effect on fungal pathogenicity in the kidneys. Although expression of Als3 in C. glabrata did not affect the total number of organisms that trafficked to the kidneys, it enhanced trafficking to the renal cortex. A previous detailed histopathologic analysis of the kidneys of mice inoculated intravenously with wild-type C. albicans demonstrated that the initial infection is localized predominantly in the renal cortex (33). Furthermore, when mice are infected with a C. albicans efg1Δ/Δ cph1Δ/Δ mutant, which does not form hyphae or express Als3, the organisms do not infect the renal cortex but instead infect the medulla and pelvis (34). N-cadherin and E-cadherin are host cell receptors for Als3 (3, 4), and it has been reported that in rats, N-cadherin is preferentially expressed in the proximal tubules, which are in the renal cortex (35). Although the distribution of N-cadherin expression in the kidneys of mice has not been reported, E-cadherin is known to be expressed throughout the nephron, with the exception of the glomerulus (36). Therefore, it is possible that high level of expression of both N-cadherin and E-cadherin in the cortex contributed to the preferential localization of the Als3-expressinig C. glabrata.
An unexpected finding was that the Als3-expressing strain of C. glabrata persisted in much higher numbers than the control strain in the mouse kidney after 14 days of infection. One possible explanation for this result is that cells expressing Als3 are resistant to phagocyte killing. In support of this possibility, the Als3-expressing strain of C. glabrata was somewhat more resistant to being killed by human neutrophils in vitro than the control strain. More importantly, we found that even though the kidneys of mice infected with the Als3-expressing strain contained 9-fold more organisms than mice infected with the control strain, they did not have elevated levels of phagocytes or proinflammatory cytokines. These data suggest that Als3 may attenuate the host inflammatory response, either directly or indirectly.
Although C. glabrata is highly pathogenic in humans with hematogenously disseminated infection (37), wild-type strains generally have very low virulence in immunocompetent mice, and they rarely cause lethality after intravenous inoculation in the absence of immunosuppression (38, 39). This property led us to use C. glabrata as a heterologous host to study the contribution of C. albicans Als3 to virulence. It was notable that after intravenous inoculation into mice, even wild-type C. glabrata achieved an initial high level of infection in the brain, kidneys, and liver. Thus, the decreased virulence of C. glabrata in mice is probably not due to a major defect in invading the target organs. The relatively normal tissue invasion of wild-type C. glabrata is likely one reason why expression of Als3 increased brain fungal burden only modestly. In addition, the slightly lower growth rate of the Als3-expressing strain may have blunted its growth in the target organs compared to the control strain. Furthermore, the cell-to-cell variability in Als3 expression in vivo may have diminished the differences between the Als3-expressing and control strains of C. glabrata. Finally, the Als3-expressing strain of C. glabrata caused much less endothelial cell damage and stimulation of IL-8 secretion in vitro than did C. albicans. Thus, expressing Als3 in C. glabrata incompletely recapitulated the virulence properties of C. albicans, which is the likely explanation for the absence of mortality in mice infected with the Als3-expressing strain. Collectively, our results are consistent with a model that the virulence defect of C. glabrata in mice is multifactorial and thus unlikely to be completely rescued by the heterologous expression of a single cell surface protein.
In summary, the data presented herein indicate that Als3 contributes to trafficking to the brain and the renal cortex during disseminated infection in mice. In addition, expression of Als3 facilitates fungal persistence in the kidneys. These results indicate that Als3 does influence the pathogenesis of disseminated candidiasis in vivo, and they provide further insight into Als3 function. These data also demonstrate the utility of C. glabrata for elucidating the virulence function of C. albicans genes.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Villareal for assistance with tissue culture and Prasadarao Nemani at Childrens Hospital of Los Angeles for supplying the human brain microvascular endothelial cells. The human umbilical cords used as a source of endothelial cells in these studies were collected by the pediatric, perinatal, and mobile unit of the UCLA Clinical and Translational Science Institute at LA BioMed/Harbor-UCLA Medical Center (UL1TR000124).
This work was supported in part by grants R01AI054928, R01AI19990, and R01AI063382 from the National Institutes of Health, Bethesda, MD.
Footnotes
Published ahead of print 29 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00013-13.
REFERENCES
- 1. Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. 2008. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect. Immun. 76:4370–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Filler SG, Swerdloff JN, Hobbs C, Luckett PM. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Filler SG. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 5:e64. 10.1371/journal.pbio.0050064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Jr, Filler SG. 2005. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J. Biol. Chem. 280:10455–10461 [DOI] [PubMed] [Google Scholar]
- 5. Oh SH, Cheng G, Nuessen JA, Jajko R, Yeater KM, Zhao X, Pujol C, Soll DR, Hoyer LL. 2005. Functional specificity of Candida albicans Als3p proteins and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology 151:673–681 [DOI] [PubMed] [Google Scholar]
- 6. Seidl K, Solis NV, Bayer AS, Hady WA, Ellison S, Klashman MC, Xiong YQ, Filler SG. 2012. Divergent responses of different endothelial cell types to infection with Candida albicans and Staphylococcus aureus. PLoS One 7:e39633. 10.1371/journal.pone.0039633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279:30840–30849 [DOI] [PubMed] [Google Scholar]
- 8. Green CB, Zhao X, Hoyer LL. 2005. Use of green fluorescent protein and reverse transcription-PCR to monitor Candida albicans agglutinin-like sequence gene expression in a murine model of disseminated candidiasis. Infect. Immun. 73:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coleman DA, Oh SH, Zhao X, Zhao H, Hutchins JT, Vernachio JH, Patti JM, Hoyer LL. 2009. Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J. Microbiol. Methods 78:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mochon AB, Jin Y, Kayala MA, Wingard JR, Clancy CJ, Nguyen MH, Felgner P, Baldi P, Liu H. 2010. Serological profiling of a Candida albicans protein microarray reveals permanent host-pathogen interplay and stage-specific responses during candidemia. PLoS Pathog. 6:e1000827. 10.1371/journal.ppat.1000827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE., Jr 2006. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 194:256–260 [DOI] [PubMed] [Google Scholar]
- 12. Cleary IA, Reinhard SM, Miller CL, Murdoch C, Thornhill MH, Lazzell AL, Monteagudo C, Thomas DP, Saville SP. 2011. The Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology 157:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun BR, van Het Hoog M, d'Enfert C, Martchenko M, Dungan J, Kuo A, Inglis DO, Uhl MA, Hogues H, Berriman M, Lorenz M, Levitin A, Oberholzer U, Bachewich C, Harcus D, Marcil A, Dignard D, Iouk T, Zito R, Frangeul L, Tekaia F, Rutherford K, Wang E, Munro CA, Bates S, Gow NA, Hoyer LL, Kohler G, Morschhauser J, Newport G, Znaidi S, Raymond M, Turcotte B, Sherlock G, Costanzo M, Ihmels J, Berman J, Sanglard D, Agabian N, Mitchell AP, Johnson AD, Whiteway M, Nantel A. 2005. A human-curated annotation of the Candida albicans genome. PLoS Genet. 1:e1. 10.1371/journal.pgen.0010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. 10.1371/journal.ppat.0020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis D, Wilson RB, Mitchell AP. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cormack BP, Falkow S. 1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frieman MB, McCaffery JM, Cormack BP. 2002. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 46:479–492 [DOI] [PubMed] [Google Scholar]
- 18. Jaffe EA, Nachman RL, Becker CG, Minick CR. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phan QT, Belanger PH, Filler SG. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 68:3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. 2011. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 7:e1002305. 10.1371/journal.ppat.1002305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stins MF, Nemani PV, Wass C, Kim KS. 1999. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect. Immun. 67:5522–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ariyachet C, Solis NV, Liu Y, Prasadarao NV, Filler SG, McBride AE. 2013. SR-like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence. Infect. Immun. 81:1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spellberg BJ, Collins M, French SW, Edwards JE, Jr, Fu Y, Ibrahim AS. 2005. A phagocytic cell line markedly improves survival of infected neutropenic mice. J. Leukoc. Biol. 78:338–344 [DOI] [PubMed] [Google Scholar]
- 24. Schultz J, Kaminker K. 1962. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch. Biochem. Biophys. 96:465–467 [DOI] [PubMed] [Google Scholar]
- 25. Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. 2001. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 158:879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bos A, Wever R, Roos D. 1978. Characterization and quantification of the peroxidase in human monocytes. Biochim. Biophys. Acta 525:37–44 [DOI] [PubMed] [Google Scholar]
- 27. Ejzykowicz DE, Solis NV, Gravelat FN, Chabot J, Li X, Sheppard DC, Filler SG. 2010. Role of Aspergillus fumigatus DvrA in host cell interactions and virulence. Eukaryot. Cell 9:1432–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ejzykowicz DE, Cunha MM, Rozental S, Solis NV, Gravelat FN, Sheppard DC, Filler SG. 2009. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol. Microbiol. 72:155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prasadarao NV, Srivastava PK, Rudrabhatla RS, Kim KS, Huang SH, Sukumaran SK. 2003. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect. Immun. 71:1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brieland J, Essig D, Jackson C, Frank D, Loebenberg D, Menzel F, Arnold B, DiDomenico B, Hare R. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192:336–343 [DOI] [PubMed] [Google Scholar]
- 32. Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou A. 2005. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect. Immun. 73:4588–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lionakis MS, Lim JK, Lee CC, Murphy PM. 2011. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immun. 3:180–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen CG, Yang YL, Cheng HH, Su CL, Huang SF, Chen CT, Liu YT, Su IJ, Lo HJ. 2006. Non-lethal Candida albicans cph1/cph1 efg1/efg1 transcription factor mutant establishing restricted zone of infection in a mouse model of systemic infection. Int. J. Immunopathol. Pharmacol. 19:561–565 [DOI] [PubMed] [Google Scholar]
- 35. Prozialeck WC, Lamar PC, Appelt DM. 2004. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 4:10. 10.1186/1472-6793-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piepenhagen PA, Peters LL, Lux SE, Nelson WJ. 1995. Differential expression of Na(+)-K(+)-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am. J. Physiol. 269:C1417–C1432 [DOI] [PubMed] [Google Scholar]
- 37. Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745–4752 [DOI] [PubMed] [Google Scholar]
- 38. Graybill JR, Bocanegra R, Luther M, Fothergill A, Rinaldi MJ. 1997. Treatment of murine Candida krusei or Candida glabrata infection with L-743,872. Antimicrob. Agents Chemother. 41:1937–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobsen ID, Brunke S, Seider K, Schwarzmuller T, Firon A, d'Enfert C, Kuchler K, Hube B. 2010. Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy. Infect. Immun. 78:1066–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.