Abstract
The host deploys a subset of immune responses to expel helminths, which differs depending on the nature of the helminth. Strongyloides venezuelensis, a counterpart of the human pathogen S. stercoralis, naturally infects rodents and has been used as an experimental model. Here we show that induction of immunoglobulin G (IgG) and IgE is a prerequisite for rapid expulsion of S. venezuelensis during a primary infection. Activation-induced cytidine deaminase-deficient (AID−/−) mice, which lack the ability to switch IgM to other isotypes, normally developed T-helper 2 (Th2) cells and intestinal mastocytosis after infection with S. venezuelensis. Although AID−/− mice expelled Nippostrongylus brasiliensis normally, they required a much longer period to expel S. venezuelensis than wild-type (WT) mice. Adoptive transfers of immune sera from S. venezuelensis-infected but not N. brasiliensis-infected mice restored the ability of AID−/− mice to promptly expel S. venezuelensis. Immune serum-derived IgG and IgE induced worm expulsion via Fc γ receptor III (FcγRIII) and Fc ε receptor I (FcεRI), respectively, and a mixture of IgG and IgE showed collaborative effects. Whereas FcγRIII−/− mice or FcεRIα−/− mice normally could expel S. venezuelensis, FcγRIII−/− mice, when their IgE was neutralized by anti-IgE, or FcεRIα−/− mice, when their IgG binding to FcγRIII was blocked by anti-FcγRIII, showed a markedly reduced ability to expel S. venezuelensis. These data reveal that IgG and IgE play redundant roles but act in concert to accelerate S. venezuelensis expulsion. Mast cell-deficient mice, even those equipped with immune serum-derived IgG or IgE, failed to expel S. venezuelensis promptly, suggesting that mast cells are cellular targets of IgG and IgE.
INTRODUCTION
Intestinal helminth infections induce Th2-type immune responses characterized by goblet cell hyperplasia, mastocytosis, eosinophilia, and high serum levels of IgG1 and IgE. The host deploys a subset of these immune responses to expel intestinal helminths, which differs depending on the nature of the helminth. Nippostrongylus brasiliensis is a gut-dwelling nematode whose expulsion depends on interleukin-4 (IL-4)/IL-13 produced by Th2 cells. Increased IL-4/IL-13 levels in the intestinal milieu after infection drive goblet cell hyperplasia and smooth muscle cell contraction, leading to interferences of N. brasiliensis adherence and survival (1, 2). The presence of B cells and antibody (Ab) production are dispensable for N. brasiliensis expulsion during primary and challenge infections (3). In contrast, Ab production is an essential component of the immune responses against particular helminths. The IgE fraction in immune sera is regarded as a major player, because IgE-deficient mice show susceptibility to infections with Schistosoma mansoni (4), Trichinella spiralis (5, 6), or Brugia malayi (7). Administration of the IgG1 fraction from hyperimmune sera causes significant worm reduction in a primary infection with Heligmosomoides polygylus bakari (8). A recent study demonstrated that naturally existing IgG from naive mice suppresses the fecundity of adult worms during a primary Heligmosomoides polygylus bakari infection (9). These results have established the importance of Ig class switching in immune responses against particular helminth infections.
Murine strongyloidiasis has been used as an experimental model for human strongyloidiasis. Human strongyloidiasis is caused by Strongyloides stercoralis, which is distributed widely in tropical and subtropical areas. Although most infected people are asymptomatic, immunocompromised individuals could develop fatal hyperinfection and dissemination. Note that subjects treated with corticosteroids or other immunosuppressants, those coinfected with human T cell leukemia virus 1, diabetic patients, those with hematologic malignancies, organ transplant recipients, and malnourished infants are vulnerable (10).
The relevance of cellular and humoral immunity has been demonstrated in the host defense mechanism against murine strongyloidiasis induced by Strongyloides ratti and Strongyloides venezuelensis. A transfer of immune sera or mesenteric lymph node cells from infected mice confers resistance against S. ratti to naive wild-type (WT) mice (11). Immune sera exert a protective effect against the early-migrating tissue stage of larvae, and the IgG1-rich fraction shows the greatest protective activity among Ig isotypes (12). In contrast, a transfer of immune sera is insufficient to induce S. venezuelensis expulsion in hypothymic nude mice (13). These findings indicate that immune serum-mediated immunity against Strongyloides species is T cell dependent.
Mucosal mast cells in the intestinal wall have been shown to promote expulsion of S. venezuelensis (14–16). Proliferation of mucosal mast cells is induced by Th2 cells or their products (IL-3 and IL-9), and as we demonstrated previously, IL-18 also induces proliferation of mucosal mast cells by driving CD4+ T cells to produce IL-3 and IL-9 without inducing their development into Th2 cells (17). Thus, IL-3 and IL-9, which are derived from activated Th2 cells and IL-18-stimulated CD4+ T cells, play crucial roles in intestinal mastocytosis. Taken together, these observations indicate that the T cell dependence of Strongyloides species expulsion may be due largely to the T cell-derived growth factors for mucosal mast cells.
Fc ε receptor γ chain-deficient (FcRγ−/−) mice display intestinal mastocytosis after infection with S. venezuelensis. Nevertheless, they exhibit delayed kinetics of worm expulsion (18). This result clearly indicates that mast cell accumulation does not fully explain the immunity against S. venezuelensis and that FcRγ-mediated signaling also plays a relevant role in expelling S. venezuelensis. FcRγ is a widely expressed adaptor bearing an ITAM that transduces activation signals from various immunoreceptors, including Fc receptors, Ig-like receptors, and C-type lectins (19–21). This broad association precludes a simple interpretation of FcRγ-mediated S. venezuelensis expulsion.
We hypothesized that Abs are principal activators of FcRγ expressed on mucosal mast cells in promoting S. venezuelensis expulsion. To demonstrate this hypothesis, we employed AID−/− mice, which are devoid of Ig class switching. AID−/− mice produce more IgM than do WT mice, but they lack IgA, IgG, and IgE (22). Therefore, AID−/− mice are suitable recipients for passive transfers of class-switched Abs during the course of S. venezuelensis infection.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free C57BL/6 mice, BALB/c mice, WBB6F1+/+ mice, WBB6F1-W/Wv mice, Wistar rats, and Sprague-Dawley rats were purchased from SLC Japan (Hamamatsu, Japan). C57BL/6 AID−/− mice were obtained from Riken BRC through the National Bio-Resource Project of the MEXT, Japan (22). C57BL/6 Fc γ receptor III-deficient (FcγRIII−/−) mice were purchased from Oriental BioService (Kyoto, Japan). BALB/c Fc ε receptor Iα-deficient (FcεRIα−/−) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and backcrossed for 11 generations into the C57BL/6 background. BALB/c ΔdblGATA mice were purchased from Jackson Laboratory (23). C57BL/6 FcRγ−/− mice were described previously (24). All mice were bred under specific-pathogen-free conditions at the animal facilities of Hyogo College of Medicine, Nishinomiya, Japan, and were used at 7 to 12 weeks of age. All animal experiments were conducted according to the guidelines for animal experiments at Hyogo College of Medicine.
Antibodies.
Anti-IgE monoclonal antibody (MAb)-producing hybridoma 6HD5 was kindly provided by K. Okumura (Jundendo University, Tokyo, Japan) (25). Hybridoma JFP-1, producing an anti-green fluorescent protein MAb (RBRC-RCB2309), was provided by Riken BRC through the National Bio-Resource Project of the MEXT, Japan (26). MAbs were purified from ascites by affinity chromatography (HiTrap protein G HP; GE Healthcare, Little Chalfont, United Kingdom). Anti-2,4,6-trinitrophenyl (TNP) IgG1 (A111-3), anti-TNP IgE (C38-2), and biotinylated anti-IgE (R35-118) were purchased from BD Pharmingen (San Diego, CA). Anti-CD3 (2C-11), anti-CD28 (37.51), and anti-CD16/32 (93) were from Biolegend (San Diego, CA). Goat anti-IgG1, biotinylated goat anti-IgG1, and anti-IgE (23G3) were from Southern Biotech (Birmingham, AL).
Parasitological techniques.
A strain of Strongyloides venezuelensis was previously described (27) and maintained by serial passage in Wistar rats. The third-stage infective larvae (L3) were obtained from fecal culture by the filter paper method. Mice were infected by subcutaneous (s.c.) inoculation with 3,000 to 4,000 L3. The degree of infection was monitored by the number of eggs excreted per g feces or the recovery of adult worms from the small intestine. To obtain adult worms, the upper half of the small intestine from each infected mouse was removed, washed, cut open longitudinally, and incubated at 37°C in a 50-ml tube containing 30 ml of phosphate-buffered saline (PBS). Following 4 h of incubation, the tube was shaken vigorously, and worms that were shaken off the intestinal tissue were quantified under a microscope. Worm fecundity was calculated by dividing the number of eggs eliminated in feces by the number of worms recovered from the intestine of each mouse, as previously described (28). A strain of Nippostrongylus brasiliensis was maintained in our laboratory by serial passage in Sprague-Dawley rats. L3 were obtained from fecal culture by the filter paper method. Mice were infected by s.c. inoculation with 500 L3. To assess worm burdens, the whole small intestine from each infected mouse was removed and incubated on a steel mesh put on a beaker containing 100 ml PBS. Following 2 h at 37°C, worms that emerged from the intestinal tissue were quantified under a microscope.
Generation of immune sera.
WT C57BL/6 mice were infected s.c. with 3,000 S. venezuelensis L3 and bled by heart puncture on day 14 postinfection (p.i.). Sera were separated by centrifugation and used as the primary immune (1°) sera. To boost Ab production, WT C57BL/6 mice and BALB/c ΔdblGATA mice were infected twice with 3,000 L3 at 30-day intervals. Sera were collected 7 to 9 days after the second infection and were designated secondary immune (2°) sera for WT C57BL/6 mouse-derived sera and hyperimmune (HI) sera for BALB/c ΔdblGATA mouse-derived sera. Nonimmune (0°) sera were obtained from naive C57BL/6 mice or BALB/c mice. IgG (SvIgG) from HI sera was separated by affinity chromatography (HiTrap protein G), and its flowthrough fraction was collected as IgG-depleted (ΔIgG) sera. More than 90% depletion of IgG was achieved in the process. IgE (SvIgE) was purified from ΔIgG sera by affinity chromatography using a 6HD5 MAb-coupled HiTrap NHS column (GE Healthcare), and its flowthrough fraction was collected as IgG- and IgE-depleted (ΔIgG ΔIgE) sera. More than 96% depletion of IgE was achieved in the process. To obtain IgE-inactivated (ΔIgE) sera, HI sera were heated to 56°C for 30 min. Purified IgG contained 0 to 0.5 μg/ml IgE. Purified IgE contained no detectable IgG. To obtain immune sera against N. brasiliensis (Nb sera), ΔdblGATA mice were infected twice with 500 N. brasiliensis L3 at 30-day intervals and bled from the heart on day 7 or 8 after the second infection.
Measurement of IgG1, IgE, and total IgG.
Concentrations of IgG1 and IgE were measured by sandwich enzyme-linked immunosorbent assay (ELISA). The assay was performed on Costar 9018 96-well plates (Corning, New York, NY). The capture Abs (goat anti-mouse IgG1 for IgG1 and clone 23G3 for IgE) were used at a dilution of 1:200, and the biotinylated detection Abs (goat anti-mouse IgG1 for IgG1 and clone R35-118 for IgE) were used at 1:10,000. The signal was developed with TMB Microwell peroxidase substrate (KPL, Gaithersburg, MD), and the absorbance at 450 nm was recorded after H2SO4 addition, using a Benchmark Plus microplate spectrophotometer (Bio-Rad, Hercules, CA). Anti-TNP IgG1 and anti-TNP IgE were used for standardization of IgG1 and IgE, respectively. Concentrations of total IgG were measured by use of a mouse IgG enzyme immunoassay (EIA) kit (TaKaRa Bio Inc., Otsu, Japan). Anti-TNP IgG1 was used for standardization of total IgG.
T cell cytokine assay.
Mesenteric lymph nodes were harvested on day 10 p.i. with 4,000 L3, and CD4+ T cells were isolated using magnetically activated cell sorting (MACS) beads (Miltenyi Biotec). Isolated CD4+ T cells were resuspended in RPMI 1640 complete medium and stimulated on plates coated with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28. After 48 h of incubation, concentrations of IL-4 and gamma interferon (IFN-γ) in the culture supernatants were measured by DuoSet ELISA (R&D Systems).
Cytokine assays in vivo.
To measure IL-4 secretion in vivo, mice were injected intraperitoneally (i.p.) with 10 μg biotinylated rat anti-mouse IL-4 Ab, and sera were collected 20 h later. The serum levels of IL-4 were measured by an in vivo capture assay set (BD Biosciences, San Diego, CA).
Mucosal mast cell assessment.
Serum levels of mast cell protease 1 (MCPT-1) were measured with a mouse MCPT-1 ELISA kit (eBioscience, San Diego, CA). For histological examination of mucosal mast cells, a portion of the small intestine spanning 6 to 8 cm from the pyloric ring was removed, opened longitudinally, flattened on a filter paper, fixed in Carnoy's fluid, and stained with 0.1% alcian blue, pH 0.3. The number of mast cells in the epithelium and lamina propria under 10 villi was counted and presented per villous crypt unit (29).
FcγRII/III blocking and IgE neutralization in vivo.
To block FcγRII/III in vivo, 50 μg of anti-CD16/CD32 (93) was injected i.p. on days 6 and 8 p.i. with 4,000 L3. To neutralize IgE in vivo, 50 μg of anti-IgE (6HD5) was injected i.p. on days 6 and 8 p.i. with 4,000 L3. Anti-green fluorescent protein MAb JFP-J1 (rat IgG2a) was used as an isotype control.
Statistical analysis.
Statistical significance was examined by unpaired two-tailed Student's t test or one-way analysis of variance (ANOVA) followed by Tukey's or Dunett's post hoc test, as indicated in the text and the figure legends. Prism 5 (GraphPad, San Diego, CA) was used for data handling, analysis, and graphic representation. All significant comparisons are expressed as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
RESULTS
S. venezuelensis expulsion was impaired in the absence of AID.
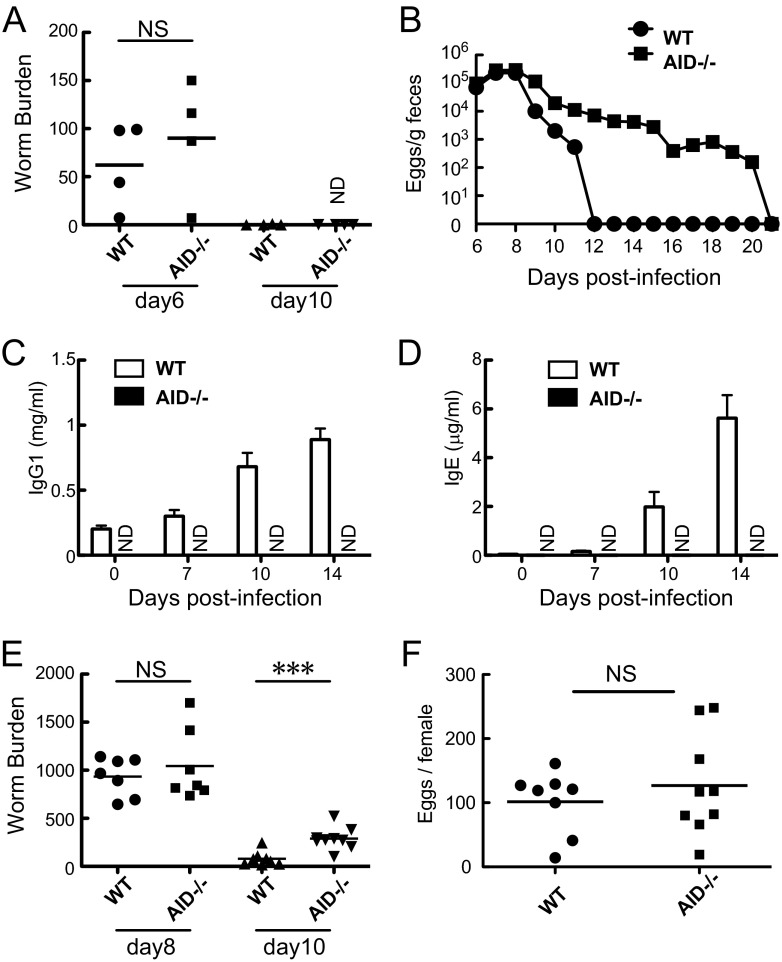
To examine the roles of Ab class switching during a primary infection with N. brasiliensis or S. venezuelensis, we took advantage of AID−/− mice. We infected WT mice and AID−/− mice with N. brasiliensis or S. venezuelensis L3 (Fig. 1A and B). Like WT mice, AID−/− mice completed expulsion of N. brasiliensis by day 10 p.i. (Fig. 1A). In contrast to the complete expulsion of S. venezuelensis by day 12 seen in WT mice, AID−/− mice required an additional 9 days to do so (Fig. 1B). These results revealed that AID−/− mice exhibited impairment in expelling S. venezuelensis but showed no defect in expelling N. brasiliensis. We then asked what essential component is required in the host defense mechanisms against S. venezuelensis. We suspected that class-switched Abs play a protective role during infection with S. venezuelensis. Whereas WT mice showed strong induction of IgG1 and IgE following S. venezuelensis infection, AID−/− mice were unable to produce IgG1 and IgE (Fig. 1C and D). Initial parasitization of adult worms in the small intestine was normal in AID−/− mice, because adult worms recovered from small intestines of AID−/− mice showed similar numbers to those from WT mice on day 8 p.i. (Fig. 1E). But eliminated eggs found in feces of AID−/− mice outnumbered those for WT mice around day 9 or day 10 p.i. (Fig. 1B). This was coincident with increased numbers of adult worms recovered from AID−/− mice compared to WT mice on day 10 p.i. (Fig. 1E) (on day 10, mean ± standard error of the mean [SEM] of 79 ± 26 worms [n = 8] versus 289 ± 38 worms [n = 9]; P = 0.0005 by Student's t test). No differences were observed in the fecundity of adult worms hosted in WT mice and AID−/− mice (Fig. 1F). Collectively, the class-switched Abs started working to expel S. venezuelensis around day 9, which coincided with the elevation of IgG1 and IgE.
Fig 1.
Nippostrongylus brasiliensis and Strongyloides venezuelensis infections in wild-type (WT) and activation-induced cytidine deaminase-deficient (AID−/−) mice. (A) Numbers of worms recovered from small intestines on days 6 and 10 after infection with 500 L3 of N. brasiliensis. Note that one worm was collected from one WT mouse on day 10. NS, not significant; ND, not detected. Results are representative of two independent experiments and are presented as mean values for four mice per group. (B) Fecal egg excretion in WT and AID−/− mice after infection with 4,000 L3 of S. venezuelensis. Results are representative of three independent experiments and are presented as mean values for four mice per group. (C and D) WT and AID−/− mice were bled on days 0, 7, 10, and 14 after infection with 4,000 L3 of S. venezuelensis. Serum levels of IgG1 (C) and IgE (D) were determined by ELISA. Results are representative of two independent experiments and are presented as mean values and SEM for four mice per group. (E) Numbers of worms recovered from small intestines on days 8 and 10 after infection with 4,000 L3 of S. venezuelensis. (F) Fecundity of each female adult worm on day 10 after infection with 4,000 L3 of S. venezuelensis. In panels E and F, the results are representative of three independent experiments and are presented as mean values for seven to nine mice per group. ***, P < 0.001 by Student's t test.
Th2-type immune responses and mucosal mast cell proliferation were intact in AID−/− mice.
We assessed the potency of AID−/− mice to provoke Th2-type immune responses following S. venezuelensis infection. CD4+ T cells were isolated from the mesenteric lymph nodes of WT mice and AID−/− mice on day 10 p.i. and were stimulated with anti-CD3 and anti-CD28 in vitro. CD4+ T cells from AID−/− mice produced levels of IL-4 and IFN-γ similar to those from WT mice (Fig. 2A and B). IL-4 production in vivo, which was detected by an in vivo cytokine capture assay, did not differ between WT mice and AID−/− mice on day 10 p.i. (Fig. 2C). We next examined the degree of intestinal mastocytosis in WT mice and AID−/− mice after S. venezuelensis infection. We also examined the serum levels of MCPT-1, as serum levels of MCPT-1 are parallel with the activation and proliferation of mucosal mast cells. Before infection, the serum levels of MCPT-1 were not detected (Fig. 2D), and very few mucosal mast cells could be seen in both WT mice and AID−/− mice (Fig. 2E, upper panels). AID−/− mice produced comparable levels of MCPT-1 to those by WT mice on day 10 p.i. and produced twice as much as did WT mice on day 14 p.i. (Fig. 2D). The accumulations of mast cells in AID−/− intestines also outnumbered those in WT controls on day 14 p.i. (Fig. 2E, lower panels) (33.3 ± 2.7/villous crypt unit [n = 7] versus 16.8 ± 1.4/villous crypt unit [n = 6]; P = 0.0004 by Student's t test). AID−/− mice had normal mast cell responses through day 10 but exacerbated mast cell responses on day 14 p.i., probably because the persistent infection continuously stimulated mast cell proliferation. These data showed that although Th2-type immune responses and mucosal mast cell proliferation occurred substantially in AID−/− mice, worm expulsion was retarded compared to that in WT mice.
Fig 2.
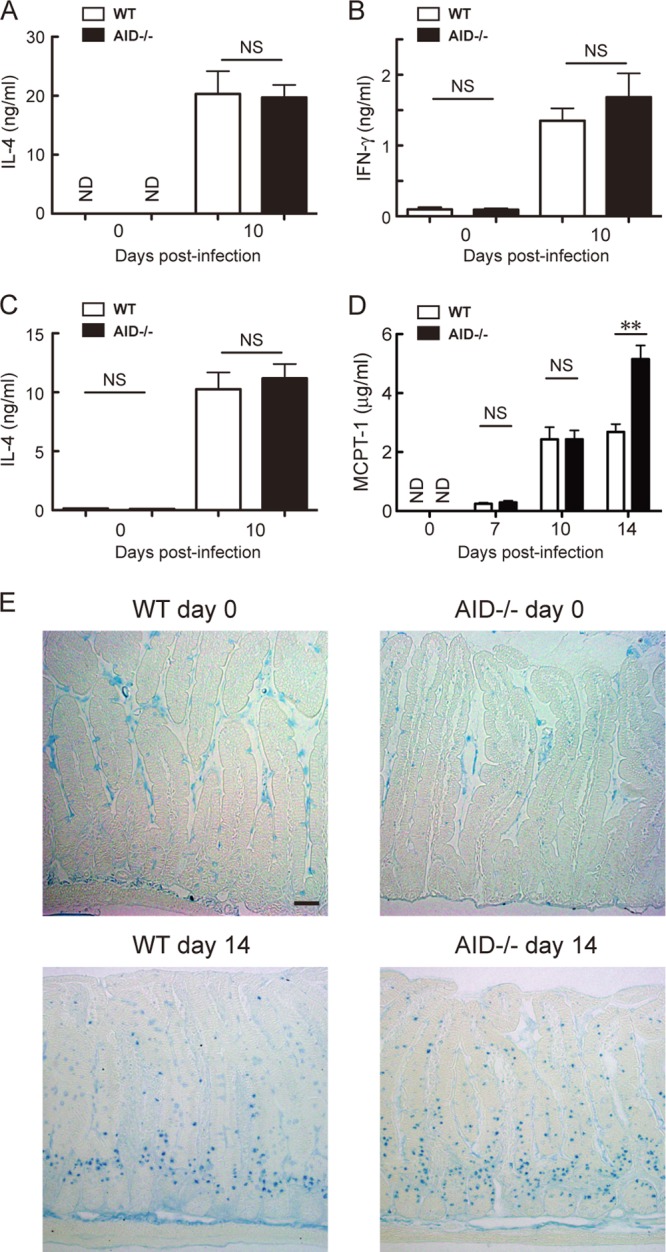
T-helper 2-type immune responses and mucosal mast cell proliferation after S. venezuelensis infection. (A and B) WT or AID−/− mice were infected with 4,000 L3 of S. venezuelensis. CD4+ T cells were isolated from the mesenteric lymph nodes on days 0 and 10 p.i. and were cultured on plates coated with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28 for 48 h. The concentrations of IL-4 and IFN-γ in the supernatants were measured by ELISA. Results are representative of two independent experiments and are presented as mean values and SEM for four mice per group. (C) IL-4 secretion was evaluated by an in vivo cytokine capture assay on days 0 and 10 after infection with 4,000 L3. Results are representative of two independent experiments and are presented as mean values and SEM for four mice per group. (D) WT and AID−/− mice were bled on days 0, 7, 10, and 14 after infection with 4,000 L3, and serum levels of MCPT-1 were measured by ELISA. Results are representative of two independent experiments and are presented as mean values and SEM for four to six mice per group. In panels A to D, statistical significance was evaluated by Student's t test. **, P < 0.01. (E) On days 0 and 14 p.i., a portion of the small intestine was stained with alcian blue for visualization of mucosal mast cells. Similar results were obtained in two independent infections. Bar, 50 μm.
Immune sera restored prompt worm expulsion in AID−/− mice.
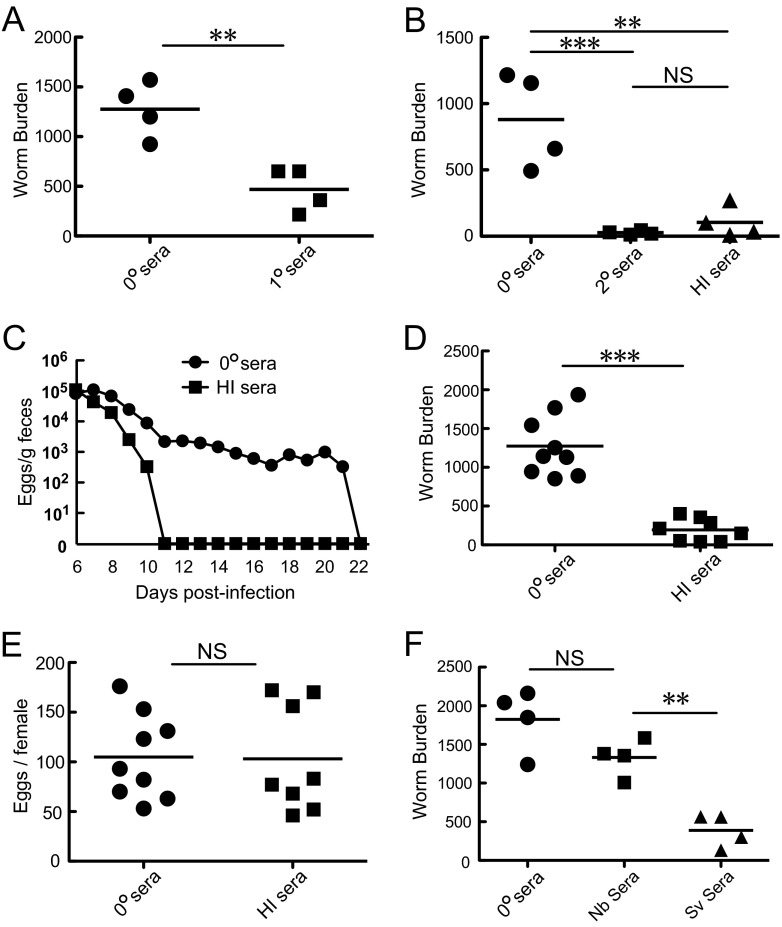
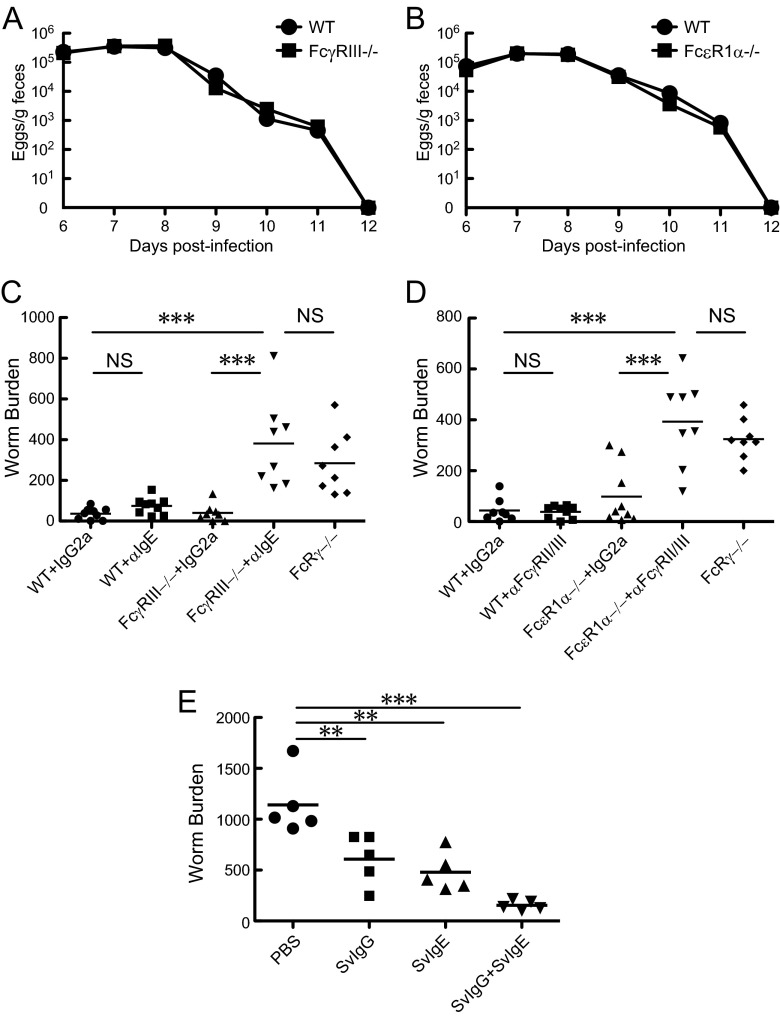
To investigate whether class-switched Abs can elicit S. venezuelensis adult worm expulsion, we passively transferred immune sera pooled from infected mice into AID−/− mice (Fig. 3) and several lines of mutant mice (see Fig. 4 to 6) following infection with S. venezuelensis. To accomplish this aim, we determined the timing of serum transfer. Although Fig. 1 indicated that class-switched Abs started working around day 9 p.i., endogenously produced Abs would have compromised the effects of passively transferred immune sera in class switching-competent mice if serum transfer had started at day 9. Therefore, we transferred immune sera on days 6 and 7 repeatedly or on day 7 once. We then sacrificed mice on day 8 to examine the serum effect on worm burdens. Initial trials of injecting 0.2 ml of immune sera pooled from primarily infected WT C57BL/6 mice failed to reduce worm burdens due to the shortage of the injected volume (data not shown). However, repeated i.p. injections with 1.5 ml of immune sera (1° sera) decreased worm burdens by more than 60% in AID−/− mice (Fig. 3A). To reduce the injection volume, Ab production was boosted by secondary infection at 30-day intervals. Repeated i.p. injections with 1 ml of the boosted immune sera (2° sera) decreased worm burdens by more than 90% (Fig. 3B). However, repeated injections of 1 ml were excessive for 20- to 25-g mice and might have unfavorable influences on homeostasis. Moreover, 2-fold volume injection was required when IgG- and IgE-depleted sera were administered because fractionation steps diluted the original sera (Fig. 4A). Therefore, Ab-rich sera were essential resources to avoid negative impacts on homeostasis potentially caused by volume overload. We empirically found that eosinophil-deficient BALB/c ΔdblGATA mice showed a 3.4-fold increase in IgG1 production and 3.7-fold increase in IgE production relative to WT C57BL/6 mice (for IgG1, 3.48 mg/ml versus 1.02 mg/ml; and for IgE, 38.7 μg/ml versus 10.5 μg/ml [data are means for 5 pools of ΔdblGATA mouse sera versus means for 2 pools of WT C57BL/6 mouse sera]). ΔdblGATA mice showed impairment in S. venezuelensis expulsion, which resulted in persistent infection like that in AID−/− mice (our unpublished findings). Persistent infection provided the host sufficient time to produce effective Abs in both quantity and quality. Only 0.1 ml of the sera obtained from infected ΔdblGATA mice showed comparable effects to 1 ml of the immune sera obtained from WT C57BL/6 mice (Fig. 3B). Therefore, we took advantage of these hyperimmune (HI) sera pooled from infected ΔdblGATA mice in all subsequent experiments. AID−/− mice transferred with the HI sera on days 6, 8, and 10 shortened the infection period from 22 days to 11 days (Fig. 3C). These effects were due to expulsion of adult worms from the small intestine but not to the suppression of fecundity (Fig. 3D and E). Next, we compared the activities of S. venezuelensis-immune sera (Sv sera) and N. brasiliensis-immune sera (Nb sera). Whereas Sv sera significantly reduced worm burdens, Nb sera exerted no notable effects on worm burdens (Fig. 3F). These data suggested that specific Abs against S. venezuelensis antigens played essential roles in expelling worms from the small intestine.
Fig 3.
Effects of passively transferred immune sera on S. venezuelensis expulsion in AID−/− mice. (A) Immune sera were collected from primarily infected WT C57BL/6 mice on day 14 after infection with 3,000 L3 of S. venezuelensis and were designated 1° sera. The IgE concentration of 1° sera was 3.5 μg/ml. AID−/− mice were injected i.p. with 1.5 ml of nonimmune sera (0° sera) or 1° sera on days 6 and 7, and adult worms were recovered from small intestines on day 8 after infection with 4,000 L3. Results are representative of two independent experiments and are presented as mean values for four mice per group. **, P < 0.01 by Student's t test. (B) Immune sera were collected from WT C57BL/6 mice and BALB/c ΔdblGATA mice, both of which were infected twice with 3,000 L3 at 30-day intervals, and the sera were designated 2° sera and HI sera, respectively. IgE concentrations of 2° sera and HI sera were 5.0 μg/ml and 50 μg/ml, respectively. AID−/− mice were injected i.p. with 1.0 ml of 0° sera, 1.0 ml of 2° sera, or 0.1 ml of HI sera on days 6 and 7, and adult worms were recovered from small intestines on day 8 after infection with 4,000 L3. Results are representative of two independent experiments and are presented as mean values for four mice per group. **, P < 0.01; ***, P < 0.001 by one-way ANOVA with Tukey's post hoc test. (C) Four AID−/− mice were injected i.p. with 0.1 ml of 0° sera or HI sera on days 6, 8, and 10 after infection with 4,000 L3, and eggs in feces were counted daily from day 6. Results are representative of two independent experiments and are presented as mean values for four mice per group. (D and E) AID−/− mice were injected i.p. with 0.1 ml of 0° sera or HI sera on days 6 and 7, and adult worms were recovered from small intestines on day 8 after infection with 4,000 L3. Feces were collected on day 8, and the fecundity of each adult worm was calculated. Results are representative of two independent experiments and are presented as mean values for eight or nine mice per group. ***, P < 0.001 by Student's t test. (F) AID−/− mice were injected i.p. with 0.1 ml of 0° sera, N. brasiliensis-immune sera (Nb sera), or S. venezuelensis-immune sera (Sv sera) on days 6 and 7 after infection with 4,000 L3, and adult worms were recovered on day 8. Nb sera and Sv sera contained 140 μg/ml of IgE and 50 μg/ml of IgE, respectively. Results are representative of two independent experiments and are presented as mean values for four mice per group. **, P < 0.01 by one-way ANOVA with Tukey's post hoc test.
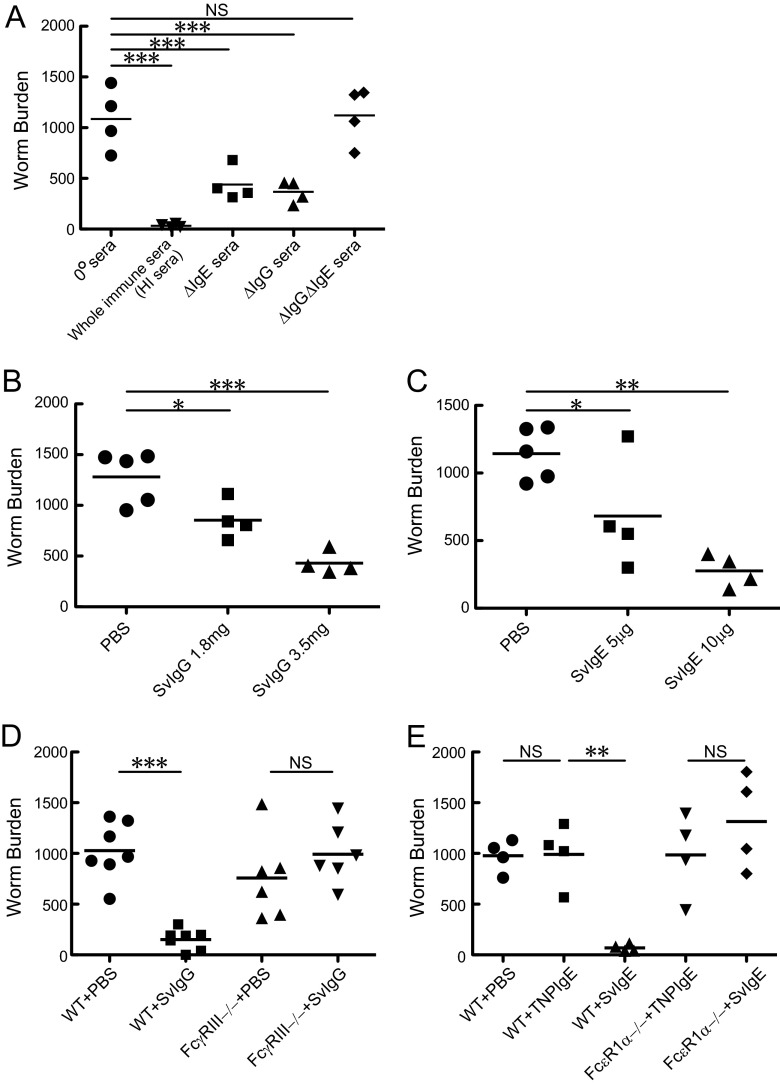
Fig 4.
Effects of fractionated immune sera on S. venezuelensis worm burdens. (A) Nonimmune sera (0° sera; 0.2 ml), whole immune sera (HI sera; 0.2 ml), IgE-inactivated immune sera (ΔIgE sera; 0.2 ml), IgG-depleted immune sera (ΔIgG sera; 0.22 ml), and IgG- and IgE-depleted immune sera (ΔIgG ΔIgE sera; 0.4 ml) were injected intravenously (i.v.) into AID−/− mice on days 6 and 7 after infection with 4,000 L3, and adult worms were recovered on day 8. Both 0.22 ml of ΔIgG sera and 0.4 ml of ΔIgG ΔIgE sera originated from 0.2 ml of HI sera. HI sera contained 7.0 mg/ml of IgG and 50 μg/ml of IgE. (B) PBS or HI serum-derived IgG (SvIgG) was injected i.v. into AID−/− mice on day 7 after infection with 4,000 L3, and adult worms were recovered on day 8. A total of 3.5 mg of SvIgG originated from 0.5 ml of HI sera and contained 2.0 mg of IgG1. (C) PBS or HI serum-derived IgE (SvIgE) was injected i.v. into AID−/− mice on day 7 after infection with 4,000 L3, and adult worms were recovered on day 8. Ten micrograms of SvIgE originated from 0.2 ml of HI sera. In panels A to C, results are representative of two independent experiments and are presented as mean values for four or five mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one-way ANOVA with Dunnett's post hoc test. (D) PBS or 1.8 mg of SvIgG was injected i.p. into WT mice or FcγRIII−/− mice on days 6 and 7 after infection with 4,000 L3, and adult worms were recovered on day 8. (E) PBS, 5 μg of anti-TNP IgE, or 5 μg of SvIgE was injected i.p. into WT mice or FcεRIα−/− mice on days 6 and 7 after infection with 4,000 L3, and adult worms were recovered on day 8. In panels D and E, results are representative of two independent experiments and are presented as mean values for four to seven mice per group. **, P < 0.01; ***, P < 0.001 by one-way ANOVA with Tukey's post hoc test.
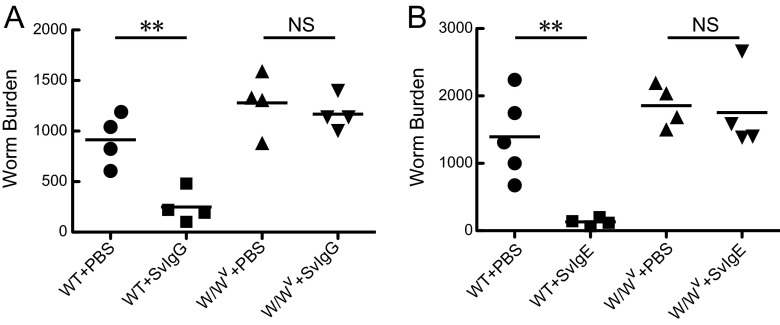
Fig 6.
Requirement of mast cells for IgG- and IgE-mediated S. venezuelensis expulsion. (A) PBS or 1.8 mg of SvIgG was injected i.v. into WT C57BL/6 mice or WBB6F1-W/Wv mice on days 6 and 7 after infection with 4,000 L3 of S. venezuelensis, and adult worms were recovered on day 8. (B) PBS or 5 μg of SvIgE was injected i.v. into WT C57BL/6 mice or WBB6F1-W/Wv mice on days 6 and 7 after infection with 4,000 L3, and adult worms were recovered on day 8. **, P < 0.01 by one-way ANOVA with Tukey's post hoc test.
Immune serum-derived IgG and IgE were able to induce worm expulsion.
Next, we examined which classes of Igs are able to induce worm expulsion. IgG depletion and IgE inactivation were achieved through affinity chromatography using protein G and through heat inactivation, respectively. Repeated intravenous injections of IgG-depleted (ΔIgG) sera or IgE-inactivated (ΔIgE) sera reduced worm burdens by about 60%, on average, compared to injections of nonimmune sera (0° sera) in S. venezuelensis-infected AID−/− mice (Fig. 4A). In contrast, whole immune sera (HI sera) decreased worm burdens by more than 90%, and the immune sera devoid of IgG and IgE (ΔIgG ΔIgE sera) showed no activities to promote worm expulsion (Fig. 4A). These results ruled out IgA as a relevant class of Ig in expelling worms and indicated that both IgG and IgE had the capability to exert S. venezuelensis expulsion. Furthermore, we fractionated IgG and IgE from immune sera (HI sera) to examine individual Ig activities. IgG fractionated from S. venezuelensis-immune sera (SvIgG) and IgE fractionated from S. venezuelensis-immune sera (SvIgE) induced worm expulsion in a dose-dependent manner (Fig. 4B and C). Additionally, we determined which Fc receptors play essential roles in IgG- and IgE-mediated worm expulsion. In mice, stimulatory IgG receptors are composed of FcγRI, FcγRIII, and FcγRIV (19). By analogy to allergen-induced anaphylaxis (30), the low-affinity IgG receptor FcγRIII is likely involved in IgG-mediated worm expulsion. SvIgG successfully induced worm expulsion in WT mice but not in FcγRIII−/− mice (Fig. 4D). We then examined if IgE-mediated activity is exerted through the high-affinity IgE receptor (FcεRI). As expected, SvIgE significantly reduced worm burdens in WT mice but not in FcεRIα−/− mice (Fig. 4E). Collectively, the anti-S. venezuelensis activity found in the immune sera was carried out by IgG via FcγRIII and by IgE via FcεRI.
Collaboration between IgG and IgE in S. venezuelensis expulsion.
Passive transfer experiments indicated the requirement of IgG and IgE for S. venezuelensis expulsion via FcγRIII and FcεRI, respectively. We examined whether FcγRIII−/− mice or FcεRIα−/− mice exhibit retardation in S. venezuelensis expulsion compared to WT mice. In contrast to our expectation, both types of mutant mice showed no retardation in worm elimination (Fig. 5A and B). We speculated that IgE compensated the loss of IgG and vice versa during S. venezuelensis infection. This speculation proved to be correct, based on experiments using MAbs for IgE and FcγRII/III. IgE depletion by anti-IgE MAb treatment significantly retarded worm expulsion on day 10 p.i. in FcγRIII−/− mice but not in WT mice (Fig. 5C). The worm burdens in IgE-depleted FcγRIII−/− mice were comparable to those of FcRγ−/− mice (Fig. 5C). Similar results were observed when IgG binding to FcγRIII was inhibited by anti-FcγRII/III MAb treatment of FcεRIα−/− mice (Fig. 5D). Additionally, we examined collaborative effects between SvIgG and SvIgE in S. venezuelensis expulsion. While a half-maximal-dose injection of SvIgG or SvIgE moderately accelerated worm expulsion, a combined administration of SvIgG and SvIgE significantly reduced worm burdens in S. venezuelensis-infected AID−/− mice (Fig. 5E). These data revealed that IgG and IgE played redundant roles but acted in concert to accelerate S. venezuelensis expulsion from infected animals.
Fig 5.
Collaboration between IgG and IgE in S. venezuelensis expulsion. (A) Fecal egg excretion in WT and FcγRIII−/− mice after infection with 4,000 L3. Results are representative of two independent experiments and are presented as mean values for five mice per group. (B) Fecal egg excretion in WT and FcεRIα−/− mice after infection with 4,000 L3. Results are representative of three independent experiments and are presented as mean values for five mice per group. (C) WT and FcγRIII−/− mice were injected i.p. with 50 μg of anti-IgE or an isotype control Ab on days 6 and 8 after infection with 4,000 L3. FcRγ−/− mice were infected with 4,000 L3 in parallel. Adult worms were recovered on day 10. (D) WT and FcεR1α−/− mice were injected i.p. with 50 μg of anti-FcγRII/III or an isotype control Ab on days 6 and 8 after infection with 4,000 L3. FcRγ−/− mice were infected with 4,000 L3 in parallel. Adult worms were recovered on day 10. In panels C and D, results are representative of combined data from two individual experiments and presented as mean values for eight or nine mice per group. ***, P < 0.001 by one-way ANOVA with Tukey's post hoc test. (E) PBS, 1.8 mg of SvIgG, 5 μg of SvIgE, or a mixture of 1.8 mg of SvIgG and 5 μg of SvIgE was injected i.v. into AID−/− mice on day 7 after infection with 4,000 L3, and adult worms were recovered on day 8. Results are representative of two independent experiments and are presented as mean values for five mice per group. **, P < 0.01; ***, P < 0.001 by one-way ANOVA with Dunnett's post hoc test.
Mast cells were indispensable for IgG- and IgE-mediated worm expulsion.
Previous studies demonstrated that mast cells play indispensable roles in S. venezuelensis expulsion from the small intestine (14–16) and that mast cells express FcγRIII and FcεRI (31). This evidence led us to predict that mast cells are cellular targets of IgG and IgE for eliciting worm expulsion. As expected, repeated injections of SvIgG or SvIgE were not able to promote worm expulsion in mast cell-deficient WBB6F1-W/Wv mice (Fig. 6A and B). Since adult worm numbers recovered from littermate WBB6F1+/+ mice were significantly lower than those from WBB6F1-W/Wv mice (99 ± 43 [n = 12] versus 1,470 ± 100 [n = 11]; P < 0.0001 by Student's t test) on day 8 p.i. under PBS-treated conditions, it was difficult to acquire reliable data from WBB6F1+/+ mice. Thus, WT C57BL/6 mice were employed as controls instead of WBB6F1+/+ mice (Fig. 6A and B). These data provided evidence that mast cells were indispensable for SvIgG- and SvIgE-mediated worm expulsion.
DISCUSSION
N. brasiliensis and S. venezuelensis are two typical gastrointestinal nematodes hosted by rodents, and the mechanisms by which these nematodes are expelled have been topical themes in parasite immunology. IL-4/IL-13 effects, including excessive mucus production from intestinal goblet cells and enhanced contractility of smooth muscles in the small intestine, are essential for N. brasiliensis expulsion (1, 2). In contrast, the presence of B cells is dispensable for N. brasiliensis expulsion during primary and secondary infections (3). Our data also demonstrated that Ig class switching-deficient AID−/− mice normally expelled N. brasiliensis (Fig. 1F). These findings confirm the requirement of IL-4/IL-13 for expulsion of N. brasiliensis and formally rule out the contribution of class-switched Abs to this expulsion. IL-3/IL-9-induced intestinal mastocytosis plays a major role in expelling S. venezuelensis (14–16). However, a previous report (18) and our data in this study indicate that intestinal mastocytosis is insufficient for promptly eliminating S. venezuelensis in the absence of FcRγ or AID.
We revealed that IgG and IgE accelerate S. venezuelensis expulsion and determined Fc receptors which play central roles in these processes. Immune serum-derived IgG and IgE induced worm expulsion via FcγRIII and FcεRI, respectively (Fig. 4D and E). However, the kinetics of worm expulsion observed in FcγRIII−/− mice or FcεR1α−/− mice were comparable in magnitude to those seen in WT mice (Fig. 5A and B). This discrepancy can be explained by a compensatory function exerted by IgE in the absence of IgG or by IgG in the absence of IgE. IgE neutralization in FcγRIII−/− mice or FcγRIII blockade in FcεR1α−/− mice retarded worm expulsion at a level equivalent to that in FcRγ−/− mice (Fig. 5C and D). These data indicate that dual defects in signaling from FcγRIII and FcεRI are responsible for the delayed worm expulsion observed in AID−/− mice as well as FcRγ−/− mice.
Our findings also provide evidence that mast cells are indispensable for IgG- and IgE-mediated S. venezuelensis expulsion (Fig. 6A and B). Mast cells express FcγRIII and FcεRI (31), both of which employ FcRγ to transduce signals from bound Igs, and mast cell-deficient mice were unable to expel S. venezuelensis in response to repeated administrations with immune serum-derived IgG or IgE (Fig. 6A and B). Collectively, these studies suggest that IgG and IgE act through FcRγ to fully activate mast cells to accelerate S. venezuelensis expulsion.
In mice, IgGs consist of four different subclasses: IgG1, IgG2a/c, IgG2b, and IgG3. IgG1, IgG2a/c, and IgG2b are able to bind to FcγRIII, but which subclasses dominate FcγRIII activation depends on the model systems studied (19). Since S. venezuelensis infection accompanies IgG1 induction, like the case for other helminth infections (Fig. 1C), IgG1 is the plausible activator of FcγRIII. However, further fractionations from the immune sera are needed to clarify this prediction.
A passive transfer of immune sera before infection suppresses fecal larval outputs or adult worm recoveries of S. ratti (11, 12). Additionally, secondary infection studies indicate that larvae are destroyed before reaching the small intestine (27, 32). Many of the migratory larvae are surrounded by eosinophils, neutrophils, and mononuclear cells and are disintegrated in the lung (33). These studies indicate that immune sera disable the migratory larvae at an early stage during the course of infection. Furthermore, a series of studies analyzing S. stercoralis L3 contained under the mouse skin has demonstrated that complement C3, IgM, and IgG consist of essential factors in humoral immunity elicited by S. stercoralis L3 immunization (34–36). Collectively, immune sera exert high activities to eliminate larvae migrating from the skin to the lung in a challenge infection. However, it remained unsolved whether the administration of immune sera accelerates adult worm expulsion from the small intestine.
Murrell reported that immune sera did not induce expulsion of S. ratti adult worms from the small intestines of rats when they were administered between day 1 and day 5 after infection (12). Since the half-life of IgE is 12 h in vivo (37) and the mucosal mast cell accumulation is insufficient at those time points, the timing of transferring immune sera might have been premature to see the immune sera's effect on adult worm expulsion. In our protocol, immune sera or immune serum-derived fractions were administered to the recipient mice on days 6 and 7 or on day 7 only. On day 6, living larvae have already left the lung, reached the small intestine, and become adult worms (27). Furthermore, the absence of AID had no influence on the initial habitation of adult worms in the small intestine (Fig. 1E), because induction of IgG1 and IgE did not occur before the larvae reached the small intestine during primary infection (Fig. 1C and D). Therefore, our findings clearly demonstrate that immune serum-derived IgG and IgE accelerate S. venezuelensis expulsion when adult worms reside in the small intestine. Nevertheless, since the host species and Strongyloides species differ between Murrell's study and ours, a combination of these different features may result in contradictory results.
The advantage of concurrent production of IgG1 and IgE during helminth infections remains elusive. Serum concentrations of IgG1 and IgE were about 1 mg/ml and 0.005 mg/ml, respectively, on average, on day 14 p.i. for S. venezuelensis (Fig. 1C and D). The quantitative gap between high concentrations of IgG1 and low concentrations of IgE seems to reflect a low affinity of IgG1 for FcγRIII and a high affinity of IgE for FcεRI, respectively. A combination of moderate doses of IgG (1.8 mg) and IgE (5 μg) revealed a high capability of expelling worms at similar levels to those with a high dose of IgG (3.5 mg) or IgE (10 μg) (Fig. 4B and C and 5E). These data imply that large quantities of IgG are unnecessary when small quantities of IgE are concurrently produced during S. venezuelensis infection, because small quantities of IgE have potential equal to that of large quantities of IgG in driving S. venezuelensis expulsion.
A high dose of IgG alone has a high capability to expel S. venezuelensis (Fig. 4B). Thus, what is the advantage of IgE class switching during S. venezuelensis infection? There may be a cut in expenditure of the immune system benefited by the high capability of IgE. Secretion of large quantities of IgG requires expansion of Ig-producing cells, accelerated production of Ig per cell, or both. IgE class switching could decrease the requisite amounts of total Ig for eliciting worm expulsion, which would lighten the burden imposed on the host immune system. Another possibility is that the host can avoid extremely high production of IgG, which may lead to excessive formation of immune complexes. Animals infected with S. venezuelensis may regulate to moderately produce IgG, which in combination with IgE could fully activate mast cells for eliciting S. venezuelensis expulsion.
Given the anti-Strongyloides activities of IgG and IgE in mice, asymptomatic patients may inhibit worm dissemination from latently infected sites by the activities of these Abs. Thus, immunoglobulins prepared from people living in areas of endemicity may become an adjunct to existing therapy to prevent or cure hyperinfection. Our findings also provide implications in developing vaccines against Strongyloides species.
In summary, we clearly demonstrated that IgG and IgE exert collaborative effects on S. venezuelensis expulsion and act through FcγRIII and FcεRI, respectively. We also propose that mast cells are cellular targets of IgG and IgE in expelling the worms. However, we know little about the mechanisms by which the activated mast cells could exert their functions against S. venezuelensis adult worms in the small intestine. We need further studies to clarify the elaborate processes by which S. venezuelensis is expelled by the host immune system.
ACKNOWLEDGMENTS
We thank T. Honjo for AID−/− mice, K. Okumura and T. Hirano for the 6HD hybridoma cell line, S. Kumasako and C. Minemoto for secretarial assistance, S. Yumikura, Y. Taki, and M. Nagata for technical support, and the joint-use research facilities at Hyogo College of Medicine for general support.
This work was supported by JSPS KAKENHI grants 22590384 and 23249022 and by a Grant-in-Aid for Researchers, Hyogo College of Medicine (2012).
We have no conflicting financial interests.
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr 2004. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201:139–155 [DOI] [PubMed] [Google Scholar]
- 2. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. 2011. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 208:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, Gaydo AG, Urban JF, Jr, Gause WC. 2010. B cells have distinct roles in host protection against different nematode parasites. J. Immunol. 184:5213–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King CL, Xianli J, Malhotra I, Liu S, Mahmoud AA, Oettgen HC. 1997. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J. Immunol. 158:294–300 [PubMed] [Google Scholar]
- 5. Dessein AJ, Parker WL, James SL, David JR. 1981. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J. Exp. Med. 153:423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. 2004. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 172:1139–1145 [DOI] [PubMed] [Google Scholar]
- 7. Spencer L, Shultz L, Rajan TV. 2001. Interleukin-4 receptor–Stat6 signaling in murine infections with a tissue-dwelling nematode parasite. Infect. Immun. 69:7743–7752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pritchard DI, Williams DJ, Behnke JM, Lee TD. 1983. The role of IgG1 hypergammaglobulinaemia in immunity to the gastrointestinal nematode Nematospiroides dubius. The immunochemical purification, antigen-specificity and in vivo anti-parasite effect of IgG1 from immune serum. Immunology 49:353–365 [PMC free article] [PubMed] [Google Scholar]
- 9. McCoy K, Stoel M, Stettler R, Merky P, Fink K, Senn B, Schaer C, Massacand J, Odermatt B, Oettgen H. 2008. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4:362–373 [DOI] [PubMed] [Google Scholar]
- 10. Marcos LA, Terashima A, Canales M, Gotuzzo E. 2011. Update on strongyloidiasis in the immunocompromised host. Curr. Infect. Dis. Rep. 13:35–46 [DOI] [PubMed] [Google Scholar]
- 11. Dawkins HJ, Grove DI. 1981. Transfer by serum and cells of resistance to infection with Strongyloides ratti in mice. Immunology 43:317–322 [PMC free article] [PubMed] [Google Scholar]
- 12. Murrell KD. 1981. Protective role of immunoglobulin G in immunity to Strongyloides ratti. J. Parasitol. 67:167–173 [PubMed] [Google Scholar]
- 13. Sato Y, Toma H. 1990. Effects of spleen cells and serum on transfer of immunity to Strongyloides venezuelensis infection in hypothymic (nude) mice. Int. J. Parasitol. 20:63–67 [DOI] [PubMed] [Google Scholar]
- 14. Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. 1993. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int. J. Parasitol. 23:551–555 [DOI] [PubMed] [Google Scholar]
- 15. Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392:90–93 [DOI] [PubMed] [Google Scholar]
- 16. Maruyama H, Yabu Y, Yoshida A, Nawa Y, Ohta N. 2000. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J. Immunol. 164:3749–3754 [DOI] [PubMed] [Google Scholar]
- 17. Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, Nakanishi K. 2005. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J. Exp. Med. 202:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onah DN, Uchiyama F, Nagakui Y, Ono M, Takai T, Nawa Y. 2000. Mucosal defense against gastrointestinal nematodes: responses of mucosal mast cells and mouse mast cell protease 1 during primary Strongyloides venezuelensis infection in FcRgamma-knockout mice. Infect. Immun. 68:4968–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nimmerjahn F, Ravetch JV. 2011. FcgammaRs in health and disease. Curr. Top. Microbiol. Immunol. 350:105–125 [DOI] [PubMed] [Google Scholar]
- 20. Maeda A, Kurosaki M, Kurosaki T. 1998. Paired immunoglobulin-like receptor (PIR)-A is involved in activating mast cells through its association with Fc receptor gamma chain. J. Exp. Med. 188:991–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanazawa N, Tashiro K, Inaba K, Miyachi Y. 2003. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J. Biol. Chem. 278:32645–32652 [DOI] [PubMed] [Google Scholar]
- 22. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553–563 [DOI] [PubMed] [Google Scholar]
- 23. Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519–529 [DOI] [PubMed] [Google Scholar]
- 25. Hirano T, Miyajima H, Kitagawa H, Watanabe N, Azuma M, Taniguchi O, Hashimoto H, Hirose S, Yagita H, Furusawa S, Ovary Z, Okumura K. 1988. Studies on murine IgE with monoclonal antibodies. I. Characterization of rat monoclonal anti-IgE antibodies and the use of these antibodies for determinations of serum IgE levels and for anaphylactic reactions. Int. Arch. Allergy Appl. Immunol. 85:47–54 [PubMed] [Google Scholar]
- 26. Hayashi K, Shirao T. 1999. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J. Neurosci. 19:3918–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato Y, Toma H. 1990. Strongyloides venezuelensis infections in mice. Int. J. Parasitol. 20:57–62 [DOI] [PubMed] [Google Scholar]
- 28. Negrao-Correa D, Souza DG, Pinho V, Barsante MM, Souza ALS, Teixeira MM. 2004. Platelet-activating factor receptor deficiency delays elimination of adult worms but reduces fecundity in Strongyloides venezuelensis-infected mice. Infect. Immun. 72:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller HR, Jarrett WF. 1971. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology 20:277–288 [PMC free article] [PubMed] [Google Scholar]
- 30. Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. 1997. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J. Clin. Invest. 99:901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. 1997. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. J. Clin. Invest. 99:915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandes A, Pereira AT, Eschenazi PD, Schilter HC, Sousa AL, Teixeira MM, Negrao-Correa D. 2008. Evaluation of the immune response against Strongyloides venezuelensis in antigen-immunized or previously infected mice. Parasite Immunol. 30:139–149 [DOI] [PubMed] [Google Scholar]
- 33. Dawkins HJ, Muir GM, Grove DI. 1981. Histopathological appearances in primary and secondary infections with Strongyloides ratti in mice. Int. J. Parasitol. 11:97–103 [DOI] [PubMed] [Google Scholar]
- 34. Brigandi RA, Rotman HL, Yutanawiboonchai W, Leon O, Nolan TJ, Schad GA, Abraham D. 1996. Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 82:279–289 [DOI] [PubMed] [Google Scholar]
- 35. Ligas JA, Kerepesi LA, Galioto AM, Lustigman S, Nolan TJ, Schad GA, Abraham D. 2003. Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 71:6835–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kerepesi LA, Hess JA, Nolan TJ, Schad GA, Abraham D. 2006. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 176:4315–4322 [DOI] [PubMed] [Google Scholar]
- 37. Vieira P, Rajewsky K. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18:313–316 [DOI] [PubMed] [Google Scholar]