Abstract
The exacerbated induction of innate immune responses in airways can abrogate diverse lung infections by a phenomenon known as stimulated innate resistance (StIR). We recently demonstrated that the enhancement of innate response activation can efficiently impair Bordetella pertussis colonization in a Toll-like receptor 4 (TLR4)-dependent manner. The aim of this work was to further characterize the effect of lipopolysaccharide (LPS) on StIR and to identify the mechanisms that mediate this process. Our results showed that bacterial infection was completely abrogated in treated mice when the LPS of B. pertussis (1 μg) was added before (48 h or 24 h), after (24 h), or simultaneously with the B. pertussis challenge (107 CFU). Moreover, we detected that LPS completely cleared bacterial infection as soon as 2 h posttreatment. This timing suggests that the observed StIR phenomenon should be mediated by fast-acting antimicrobial mechanisms. Although neutrophil recruitment was already evident at this time point, depletion assays using an anti-GR1 antibody showed that B. pertussis clearance was achieved even in the absence of neutrophils. To evaluate the possible role of free radicals in StIR, we performed animal assays using the antioxidant N-acetyl cysteine (NAC), which is known to inactivate oxidant species. NAC administration blocked the B. pertussis clearance induced by LPS. Nitrite concentrations were also increased in the LPS-treated mice; however, the inhibition of nitric oxide synthetases did not suppress the LPS-induced bacterial clearance. Taken together, our results show that reactive oxygen species (ROS) play an essential role in the TLR4-dependent innate clearance of B. pertussis.
INTRODUCTION
Despite high immunization rates in infants and children in many countries, the respiratory disease named whooping cough, or pertussis, remains endemic, with epidemics occurring every 2 to 5 years (1). Although this pattern has persisted since the prevaccine era (2), the incidence of the disease has been increasing steadily over the last 2 decades (3–6). Moreover, some countries and some states in the United States have reported a rise in cases, leading to numbers of cases similar to those observed in the 1940s and 1950s, when vaccination was just becoming widespread (4). In regard to the possible factors that contribute to this epidemiologic situation, much attention has been given to waning immunity associated mainly with the introduction of acellular vaccines, but another factor contributing to these outbreaks may be the adaptation of the causative agent, Bordetella pertussis, to vaccine selection pressure, as was proposed for the first time by Mooi et al. (7–9). In many countries, the genotypes that predominate within the current bacterial population are ptxP3, prn2, ptxA1, and fim3-2, which have been described as the major producers of pertussis toxin, in contrast to the old strains containing ptxP1 (9). Moreover, in some countries, bacteria that do not express the vaccine antigen pertactin have been isolated (10, 11). It was proposed that these modifications allow bacteria to subvert the immune response induced in the host by vaccination.
While the relevance of these factors in the epidemiologic situation of pertussis remains a matter of debate and study, the health system needs to revise and improve the current strategies used to control the disease. Basic knowledge, such as the mechanism by which bacterial clearance may be achieved in the host, is expected to contribute to the development of new strategies to improve the control of this disease. Recently, a number of studies have described the development of novel antimicrobial strategies that target the host immune response rather than the pathogen (12–14). These immunotherapeutics target host pathways that either directly activate effector cells or relieve pathogen-induced suppression of host killing mechanisms, resulting in the control and elimination of a wide variety of microorganisms (14). For example, a novel antimicrobial comprising cationic liposome DNA complexes and a crude membrane protein fraction derived from the attenuated Francisella tularensis strain LVS was described and effectively controlled in vivo and in vitro infections of the virulent F. tularensis strain SchuS4 in mice and human cells, respectively (15). The dramatic control of F. tularensis infection mediated by this novel antimicrobial was dependent on the stimulation of both reactive oxygen and nitrogen species (ROS and RNS, respectively) in vivo and in vitro (15). Ireland et al. (15) also demonstrated that the combination of such molecules is an effective antimicrobial agent against three other important bacteria: Burkholderia pseudomallei, Yersinia pestis, and Brucella abortus. Thus, the data presented herein represent an important step toward the development of novel, efficacious, broad-spectrum antimicrobial therapies directed against highly pathogenic microbes.
For B. pertussis, we recently found that stimulation of the lung innate immune response with lipopolysaccharide (LPS) confers a high level of protection against challenges with high doses of B. pertussis (16). This protective phenomenon, termed stimulated innate resistance (StIR), was observed by other researchers for diverse lung infections, such as influenza A virus, Streptococcus pneumoniae, and Aspergillus niger infections (17). Evans et al. (17) showed that intranasal administration of a Haemophilus influenzae bacterial lysate (nontypeable H. influenzae [NTHI] lysate) is able to block an otherwise deadly challenge with any of these pathogens. Furthermore, researchers have demonstrated that the action of the lysate can be mimicked by the use of a combination of Toll-like receptor 2 (TLR2) and TLR9 agonists (18). In the case of B. pertussis, the phenomenon of StIR was shown to be TLR4 dependent (16). In fact, this phenomenon was not observed in the mouse strain C3H/HeJ, which harbors a signaling defect in TLR4 that renders the mice hyporesponsive to LPS. In the present work, we have characterized this phenomenon in further detail, showing that pretreatment with the LPS of B. pertussis induces complete clearance of bacteria from the lungs if LPS is given 24 to 48 h before bacterial challenge. Similar results were observed both when the treatment and challenge were performed at the same time and when the treatment with B. pertussis LPS followed the challenge with B. pertussis. Consistent with previous findings reported for other pathogens (17, 19), we have shown that bacterial clearance does not depend on recruited neutrophils. The rapid bacterial clearance observed as a consequence of B. pertussis LPS treatment suggests that the StIR event should be mediated by fast-acting antimicrobial mechanisms. In fact, we demonstrated that the StIR phenomenon is associated with increased concentrations of ROS in the lung lining fluid.
These findings not only deepen the knowledge of StIR in B. pertussis but also provide an alternative mechanism by which B. pertussis bacteria can be cleared from the lungs; this information could be exploited to improve disease control.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The B. pertussis strain used in this study was Tohama I (CIP 8132; Collection of Institut Pasteur, France). The B. pertussis strain was grown on Bordet-Gengou (BG) agar plates containing 10% defibrinated sheep blood. For LPS extraction, subcultures were grown in Stainer-Scholte liquid medium for 20 h at 36.5°C until the optical density at 650 nm reached 1.0 (20).
Lipopolysaccharide extraction.
The LPS from B. pertussis was isolated by the hot phenol-water method (21), along with previously described modifications (22). The samples of LPS were dialyzed and lyophilized. Dry weight measures were used to determine the amounts of LPS obtained. The quality of each sample was checked by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (23; data not shown). We also verified that the effects observed in the experiments described below were due to LPS and not to nondetectable contaminants by performing animal assays with a TLR4-deficient mouse strain (C3H/HeJ), as previously described (16).
Assessment of bacterial clearance.
Specific-pathogen-free BALB/C, C3H/HeN, and C3H/HeJ mice used in these experiments were originally obtained from Jackson Laboratories (Bar Harbor, ME) or Harlan (The Netherlands). All of the mice were maintained in the animal care facilities of the Instituto Biológico Argentino (BIOL SAIC). All of the mouse procedures were performed in accordance with Argentine regulations. Inoculums (107 CFU/40 μl) were prepared, and the mice were inoculated intranasally as previously described (24). For bacterial counting, the mice were sacrificed by cervical dislocation, and their lungs were removed and homogenized in 1 ml of sterile phosphate-buffered saline (PBS). The appropriate dilutions were plated on BG blood agar plates and counted after 4 days of incubation at 36.5°C to determine the number of CFU per lung. A minimum of 5 mice per group were used in each of the 4 independent experiments performed.
The treatment of mice with LPS was performed by intranasal administration of 40 μl of PBS containing purified LPS (1 μg) 48 h and 24 h prior to, at the same time as, or 24 h after challenge with a suspension of B. pertussis (107 CFU/40 μl). The animals were sacrificed 24 h after the last treatment, and their lungs were removed aseptically for bacterial counting as described above.
Neutrophil depletion.
Neutrophils were depleted by intraperitoneal (i.p.) administration of a purified rat monoclonal antibody (RB6-8C5; 2.0 mg/ml) specific for Ly6G/Ly6C (Gr1) or an isotype control antibody (HB152). The dosing schedule consisted of administration of 400 μg of the antibody the day before intranasal inoculation with B. pertussis. The efficacy of the treatment with anti-Gr1 antibody was confirmed by a reduction of at least 95% of the neutrophil populations in the blood, spleen, and lungs.
ROS inhibition.
To block the induced enhancement of ROS, mice were treated with N-acetyl cysteine (NAC; Fluka) by intranasal administration (40 μl of NAC; 0.5 mM). NAC was coadministered with LPS (1 μg) 2 h before challenge with B. pertussis (107 CFU/40 μl). ROS inhibition was confirmed by measuring ROS in the bronchoalveolar lavage (BAL) fluid of the treated mice, as described below.
NOS.
To block the in vivo activity of nitric oxide synthetases (NOS), the mice were treated by the i.p. route with aminoguanidine salt (5 μg/kg of body weight; Sigma) 2 h before the B. pertussis challenge, as described above. NOS inhibition was confirmed by measuring the nitrites in the BAL fluid of the treated mice, as described below.
For all of the treatments described above, the pertinent controls were included. For each case, bacterial counting was performed as described above.
BAL fluid analysis.
The treated and PBS control mice were euthanized by intraperitoneal pentobarbital injection, and the thoracic cavity was dissected. To perform the BAL, the trachea was partially cut, and 1 ml of sterile PBS was flushed into the lungs and then withdrawn. This procedure was repeated 3 times. The BAL fluid was centrifuged at 600 × g for 15 min, and the pellet and supernatant were collected for posterior assays. The resultant cellular sediment was then washed, resuspended, and counted using a Neubauer chamber to determine the absolute number.
Cells from the BAL fluid were then stained with fluorescent antibodies for 1 h at 4°C, and flow cytometry analysis was performed using a FACSCalibur flow cytometer from Becton, Dickinson (NJ). Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, allophycocyanin (APC)- and peridinin chlorophyll protein (PerCP)-conjugated monoclonal specific antibodies for CD11c (clone N418; hamster IgG; eBioscience, San Diego, CA), Gr1 (clone RB6-8C5; rat IgG2b; eBioscience), CD11b (clone M1/70.15; rat IgG2b; Caltag Laboratories, CA), Ly6G (clone 1A8; rat IgG2a; BD Pharmingen), and Ly6C (AL-21; rat IgM; BD Pharmingen) were used to label the cells.
The BAL fluid supernatants were processed for ROS detection, and the nitrite concentrations were determined as described below.
ROS determination.
The levels of H2O2 were determined using an Amplex Red hydrogen peroxide/peroxidase assay kit (Invitrogen). Briefly, for each reaction, 50 μl of BAL fluid supernatant was mixed with 50 μl Amplex reagent (10 mM Amplex Red reagent stock solution, 10 U/ml horseradish peroxidase [HRP] stock solution, and 1× reaction buffer) and incubated at room temperature for 30 min. Fluorescence was measured in a FLUOstar Optima instrument (BMG Labtech), with an excitation range of 530 to 560 nm and with fluorescence emission detection at 590 nm. The concentration was calculated based on H2O2 standard curves.
Nitrite/nitrate measurement.
To assess the efficacy of NOS, the inhibition of nitrite/nitrate production was evaluated for the BAL fluid supernatants by a spectrophotometric method according to the technique described by Miranda et al., with the modifications proposed by Beda and Nedospasov (25, 26).
Statistical analysis.
Means ± standard errors (SE) were determined for all appropriate data. Two-tailed, unpaired Student t tests were used to determine the statistical significance of differences between groups. The results were also analyzed by one-way analysis of variance followed by the Bonferroni multiple-comparison test to determine significant differences between groups.
RESULTS
Protection against B. pertussis colonization.
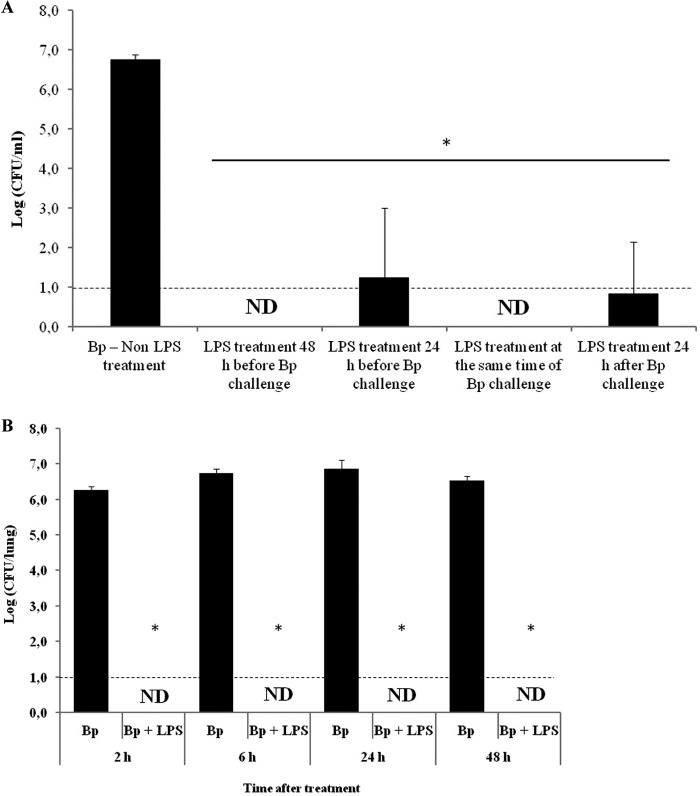
We previously found that profound inflammatory lung responses to LPS abrogated B. pertussis colonization in mice (16). The in vitro incubation of B. pertussis LPS with bacteria showed that LPS did not affect the viability of the bacteria, confirming that the observed effect was mediated by the LPS-induced response in the host, not by direct toxicity of LPS on the bacteria. To further characterize the observed phenomenon, we evaluated the LPS kinetics of action on B. pertussis Tohama phase I (challenge dose, 107 CFU of B. pertussis) mouse colonization (Fig. 1). To achieve this aim, the animals were pretreated with purified B. pertussis LPS (1 μg) 48 h and 24 h before the B. pertussis challenge. Experiments in which B. pertussis LPS (1 μg) was administered either at the same time as the challenge doses or 24 h after the challenge were also conducted. The results obtained were compared with those obtained for mice inoculated with B. pertussis Tohama phase I alone. Twenty-four hours after the last treatment, the lungs of the mice were collected for bacterial counting. Figure 1A shows that while the number of recovered colonies from the mice treated only with B. pertussis was 5.6 × 106 ± 1.6 × 106 CFU/lung, the number of CFU recovered from any of the mouse groups treated with LPS decreased by at least 5 orders of magnitude compared to that of the mice treated with B. pertussis alone (P < 0.001).
Fig 1.
(A) Bacterial colonization upon administration with B. pertussis LPS (1 μg) at different times before and after the B. pertussis challenge. (B) Bacterial clearance at different times postchallenge, induced by LPS coadministered with B. pertussis. In all cases, the nasal challenge of mice was performed with a sublethal dose (107 CFU/40 μl) of B. pertussis strain Tohama (Bp). The lungs of the infected mice were collected for bacterial counts 24 h after the last treatment (A) or 2, 6, 24, and 48 h after the B. pertussis challenge. The results show the average counts for five mice per group. The results presented are representative of at least four independent experiments. *, P < 0.001 in the Bonferroni test. ND, not detected.
We also evaluated the kinetics of B. pertussis clearance by counting the numbers of bacteria recovered from the lungs of mice simultaneously treated with LPS and B. pertussis at different times posttreatment (2, 6, 24, and 48 h). The results obtained again showed significant differences between the animals challenged with B. pertussis alone and the LPS-plus-B. pertussis-treated group (P < 0.001) (Fig. 1B). Interestingly, the treatment with B. pertussis LPS resulted in fast and total clearance of B. pertussis from the mouse lungs. B. pertussis LPS induced a total reduction in the lung pathogen burden even 2 h after treatment, whereas the sham-treated mice experienced progressive lung infections.
We confirmed that the observed effects were mediated mainly by LPS by performing similar experiments with a TLR4-defective mouse strain (C3H/HeJ). In accordance with our previously reported results (16), similar bacterial counts were recovered from the lungs of the mice simultaneously treated with LPS and B. pertussis and those of the mice treated only with B. pertussis (not shown).
Early bacterial clearance is not associated with neutrophil recruitment.
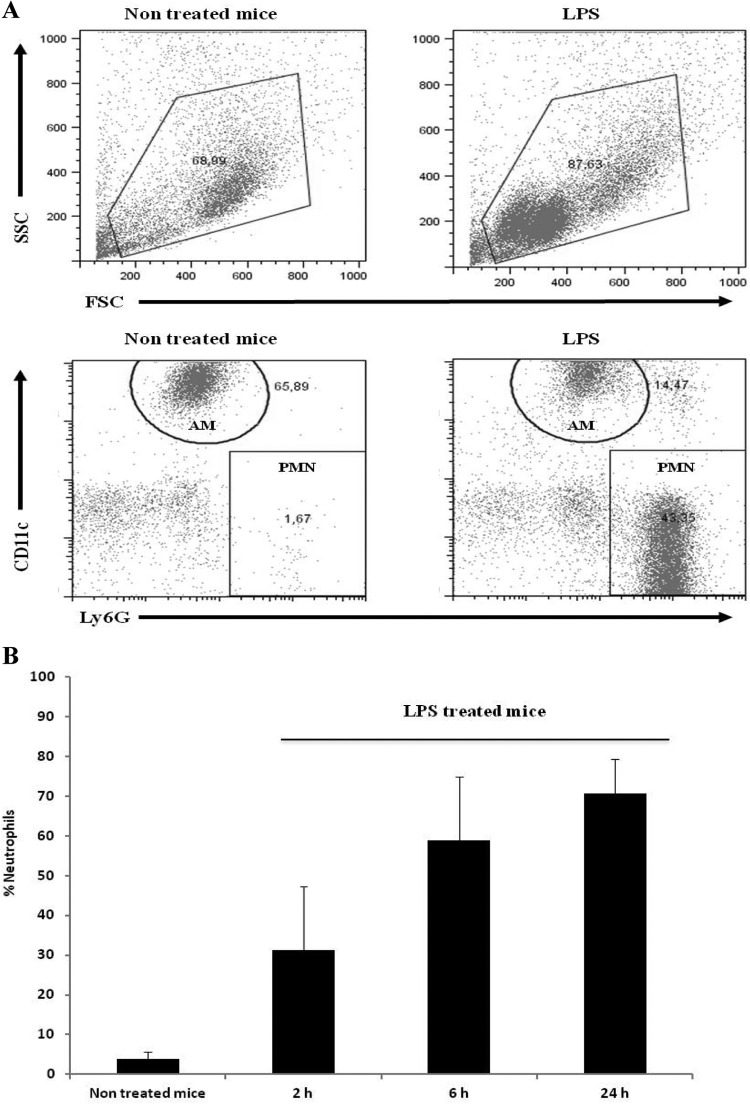
To identify the cell population that could be involved in the StIR phenomenon observed for B. pertussis, we analyzed leukocyte recruitment to the lungs and airways. Cells from BAL fluid were sampled at different times (2, 6, and 24 h) after LPS treatment and analyzed by flow cytometry. An example of the gating strategy used to select the cell population to be analyzed is included in Fig. 2A. Figure 2B shows that alveolar macrophages (AM), defined as CD11c+ CD11b− Ly6G− Gr1− cells, were the predominant cells in the non-LPS-treated animals. After LPS treatment, we did not observe significant changes in the population of alveolar macrophages (data not shown). In contrast, the neutrophils, characterized as CD11c− CD11b+ Ly6G+ Gr1+ cells, were largely recruited into the bronchoalveolar compartments of the LPS-treated mice (Fig. 2B). The recruitment into the airways was detected at 2 h, with the presence of approximately 9.5 × 102 neutrophils/BAL fluid sample (31% of total cells), and the presence continued to rise to more than 7.9 × 103 neutrophils/BAL fluid sample at 24 h postinfection (70% of total cells) (Fig. 2B). This cellular population recruitment was not observed in the noninfected lungs.
Fig 2.
Leukocyte recruitment into the airways after LPS treatment. The cellular populations in the airways were sampled by BAL and analyzed by flow cytometry at the indicated time points. (A) BAL fluid dot plots representative of PBS-treated mice (nontreated) and LPS-treated mice. The diagram corresponds to BAL fluid obtained 24 h after treatment. Alveolar macrophages (AM) were defined as CD11c+ CD11b+ Ly6G− GR1− cells, and neutrophils (PMN) were defined as CD11c− CD11b+ Ly6G+ GR1+ cells. (B) Quantitative analysis at different times after treatment of BAL fluid PMN obtained from PBS-treated animals (nontreated) and LPS-treated mice.
Because neutrophil infiltration could be essential to LPS action, we analyzed the impact of the depletion of Gr1+ cells on the onset of B. pertussis infection (Fig. 3). The antibody used depleted the Ly6G+ neutrophils as well as the proinflammatory monocytes (Ly6C+) (27). Remarkably, we found that Gr1+ cell depletion did not affect the LPS-induced B. pertussis clearance.
Fig 3.
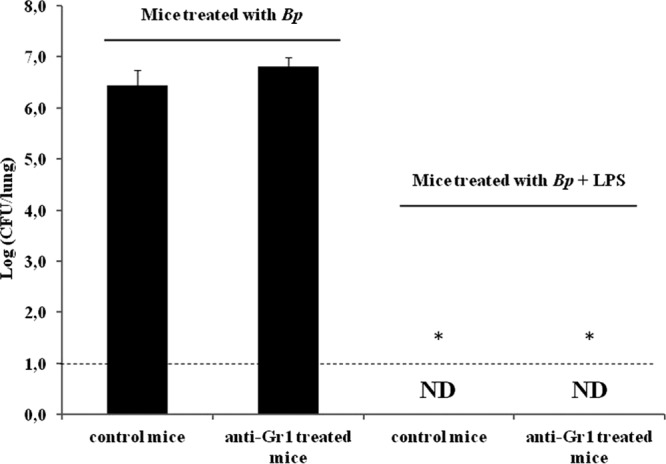
Lung bacterial counts in neutrophil-depleted mice. The nasal challenge of mice was performed with a sublethal dose (107 CFU/40 μl) of B. pertussis strain Tohama (Bp) coadministered with 1 μg of B. pertussis LPS. The lungs of the infected mice were collected for bacterial counts 24 h after the treatment. The results show the average counts for five mice per group. The results are representative of at least four independent experiments. *, P < 0.001 in the Bonferroni test. ND, not detected.
Bacterial clearance is associated with an increased level of reactive oxygen species.
To evaluate the possible role of free radicals in StIR, we inoculated mice intranasally with a suspension of B. pertussis supplemented with LPS and NAC, an antioxidant used therapeutically in inflammatory lung diseases. As a control, we first checked that NAC, LPS, or a combination of both molecules did not have an effect on bacterial growth. To this end, we incubated a bacterial suspension for 1 h at 37°C with NAC, LPS, or NAC plus LPS at the same ratio that we used in the in vivo experiments. After the incubation, the bacterial counts were determined on Bordet-Gengou agar plates. The bacterial counts obtained for all of the treatments analyzed were similar to those of the untreated bacterial suspension, indicating that direct interaction with NAC or LPS does not affect bacterial viability.
For the animal experiments, NAC was administered either alone or in combination with LPS 2 h before the B. pertussis treatment. The numbers of CFU recovered from the lungs of the animals treated with NAC, LPS, and B. pertussis were compared with those obtained from mice treated with LPS plus B. pertussis, NAC plus B. pertussis, or B. pertussis alone. The results obtained showed that NAC was able to block LPS's action when it was administered with LPS 2 h before the B. pertussis treatment (Fig. 4). These results strongly suggest that free radicals are involved in B. pertussis clearance by LPS administration. As an overall indicator of ROS generation, we evaluated the levels of H2O2 in the BAL fluid by using Amplex Red, which is converted to highly fluorescent resorufin upon oxidation (28, 29) (Fig. 5A). It was observed that ROS levels in the LPS-treated animals (3.70 ± 0.33 μM) were increased in comparison with the values detected in the BAL fluid of naïve animals (0.4 ± 0.04 μM). Moreover, the concentration of H2O2 was suppressed by the addition of NAC (0.57 ± 0.02 μM). To confirm that the observed effect on ROS was mediated mainly by B. pertussis LPS, similar experiments to those described above were performed in a TLR4-defective mouse strain (C3H/HeJ). The parental TLR4-competent mouse strain C3H/HeN was used as a positive control. Figure 5B shows that ROS levels were increased in the LPS-treated C3H/HeN animals (3.65 ± 0.37 μM) in comparison with the values detected in the BAL fluid of both the LPS-treated C3H/HeJ animals (1.24 ± 0.87 μM) and naïve C3H/HeJ animals (0.31 ± 0.05 μM) or naïve C3H/HeN animals (0.37 ± 0.23 μM).
Fig 4.
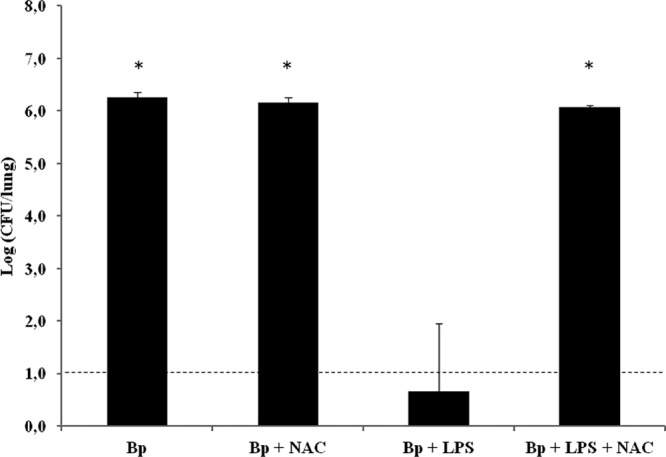
Bacterial colonization upon treatment with NAC. Mice were treated with 1 μg of B. pertussis LPS coadministered with NAC 2 h before challenge with B. pertussis (107 CFU/40 μl). The lungs of the infected mice were collected for bacterial counts 2 h after the challenge. The results show the average counts for five mice per group. The results are representative of at least four independent experiments. *, P < 0.001 in the Bonferroni test.
Fig 5.
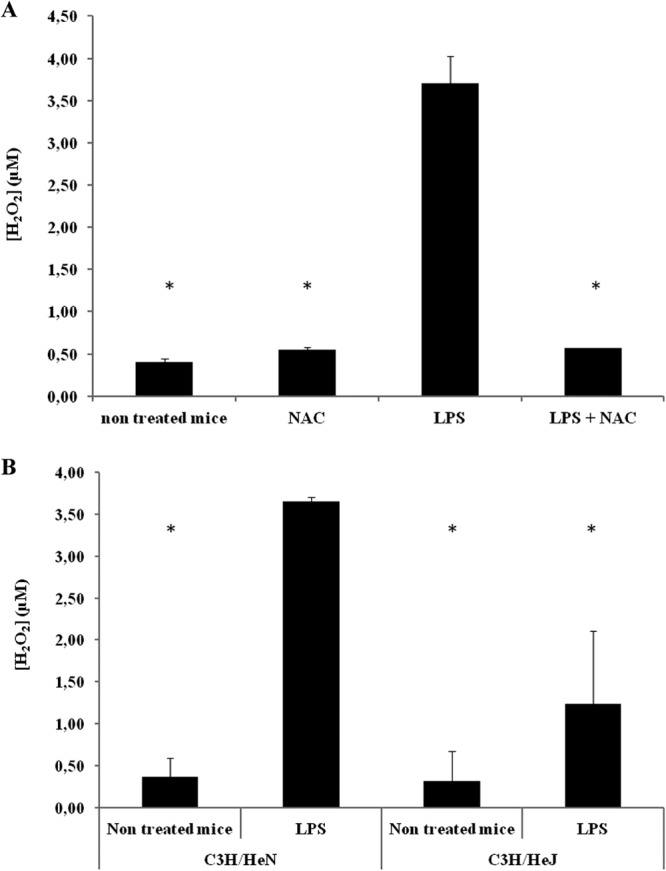
Quantification of H2O2 in BAL fluid from BALB/c mice (A) and C3H/HeN and C3H/HeJ mice (B). In all cases, the mice were treated with 1 μg of B. pertussis LPS alone or coadministered with NAC. BAL fluid samples were collected for H2O2 determination 2 h after treatment. The results show the average concentrations for five mice per group. *, P < 0.001 in the Bonferroni test.
The generation of ROS was also evaluated during B. pertussis infection. In particular, we analyzed the ROS levels 2 h and 24 h into the infection process. We observed that the ROS levels detected in the infected mice were similar to those of the noninfected mice: 1.52 ± 0.77 μM and 1.39 ± 0.42 μM in the infected mice at 2 h and 24 h, respectively, versus 1.23 ± 0.27 μM and 0.88 ± 0.13 μM in the noninfected mice at 2 h and 24 h, respectively.
To determine the putative contribution of reactive nitrogen species (RNS) to the StIR phenomenon, we evaluated the ability to clear B. pertussis infection by the administration of LPS in mice treated with aminoguanidine, a general inhibitor of NOS (Fig. 6). The inhibition of NOS activity by aminoguanidine treatment was confirmed in the BAL fluid obtained from LPS-treated mice versus LPS- and NOS inhibitor-treated mice by measuring the production of nitrites and nitrates. We observed that in the BAL fluid extracted from the LPS-treated mice, the level of nitrites was 15.8 ± 0.3 μM. In contrast, in the animals treated with aminoguanidine plus LPS, the level of nitrites was 6.1 ± 0.1 μM (P < 0.001) (Fig. 6A). Under these conditions, the blocking of NOS did not affect the ability of the LPS treatment to eliminate B. pertussis from the airways (P < 0.001) (Fig. 6B).
Fig 6.
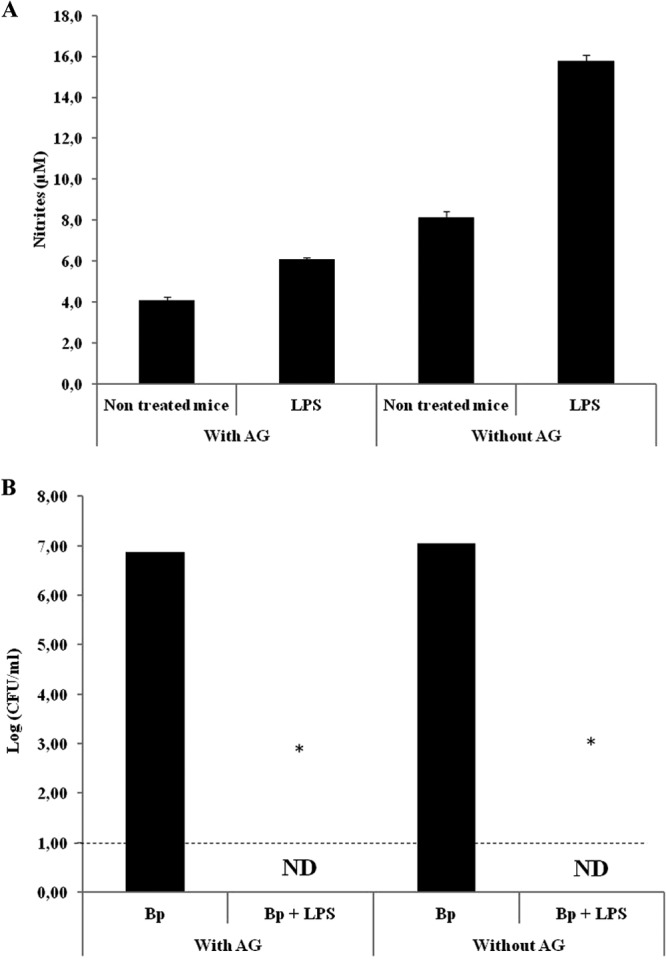
(A) Quantification of nitrites in BAL fluid from mice treated with 1 μg of B. pertussis LPS alone or coadministered with the NOS inhibitor aminoguanidine (AG). BAL fluid samples were collected for nitrite determination 2 h after treatment. The results show the average concentrations for five mice per group. P values were <0.001 in the Bonferroni test. (B) Bacterial colonization upon treatment with the NOS inhibitor. Mice were treated with 1 μg of B. pertussis LPS coadministered with the NOS inhibitor 2 h before challenge with B. pertussis (107 CFU/40 μl). The lungs of the infected mice were collected for bacterial counts 2 h after the challenge. The results show the average counts for five mice per group. The results are representative of at least four independent experiments. *, P < 0.001 in the Bonferroni test. ND, not detected.
DISCUSSION
The present study provides insight into the activity of LPS and the factors that affect the StIR phenomenon observed during B. pertussis infection. Interestingly, we found that bacterial infection was completely abrogated when the LPS of B. pertussis was added before (48 h or 24 h), after (24 h), or simultaneously with the B. pertussis challenge (Fig. 1A). Moreover, we observed that the effectors of bacterial clearance are fast acting, because at 2 h postinfection, the bacterial burden was diminished by several orders of magnitude (Fig. 1B). In the context of StIR, although neutrophils are recruited at early time points, they are not relevant for B. pertussis clearance (Fig. 3). These results are in agreement with those reported by Evans et al., who showed that neutrophil infiltration is not required for protection against different pathogens induced by StIR (17). The studies by Evans et al. provided the first insight into the cellular effectors of the resistant phenotype in the lung. Whereas neutrophils, mast cells, and alveolar macrophages are expendable in NTHI lysate-induced protection, Evans et al. showed in vitro that respiratory epithelial cells are sufficient to sense the treatment and to generate an antimicrobial response (17).
In the search for other putative fast-acting microbicidal effectors, we tested if StIR against B. pertussis can be blocked by the antioxidant NAC. Our results indicate that free radicals participate in the protection against B. pertussis (Fig. 4). In agreement with these results, we detected a rise in ROS in the bronchoalveolar lavage fluid after LPS treatment that could be suppressed by the coadministration of NAC (Fig. 5). The generation of highly toxic ROS within intracellular compartments such as the phagosome has been known for decades (30). It is accepted that different ROS are produced in this compartment by the action of NADPH oxidase, aiming to inactivate infective microorganisms that may have been phagocytosed upon cell activation. In recent years, novel oxidase enzyme members of the DUOX family have been described (31). Some members of this family are expressed at high levels in airway epithelial cells on the apical membrane and are thought to participate in microbial killing in the extracellular compartment (32, 33). In fact, DUOX enzymes are proposed to perform microbicidal activities in the airways, acting in concert with lactoperoxidase present in mucosal secretions, which is responsible for the generation of the highly toxic ion OSCN−, using H2O2 as an oxidant. Membrane-bound DUOX enzymes supply the H2O2 that allows this microbicidal mechanism to take place (34). We detected a significant rise in H2O2 in the BAL fluid upon intranasal LPS delivery in the TLR4-competent mouse strain but not the TLR4-deficient mouse strain, which indicates the activation of this mechanism upon TLR4 stimulation. In the nonstimulated animals, the presence of H2O2 in the BAL fluid corresponded to the basal quantities of this metabolite generated by the mechanisms described above (35).
Although DUOX expression is induced by proinflammatory cytokines, especially gamma interferon (36), the role of TLR4 ligands as triggers of H2O2 production in the airways, as reported here, has not been studied extensively. Recently, Moller and colleagues (37) showed that in healthy human volunteers, the inhalation of micrograms of Salmonella LPS triggers a fast airway response, with a peak of H2O2 production at 2 h poststimulation. According to the technique used, the authors concluded that H2O2 production under these conditions is maximal in the airways, although the alveoli also contribute to the generation of ROS. The results presented here are in agreement with the cited report. Although LPS can also trigger inducible NOS activation and RNS production, we showed that abrogation of NOS activity by treatment with aminoguanidine does not impair the LPS bactericidal effect. This result indicates that RNS are not major mediators of the observed effect.
The general StIR effect described by Evans and coworkers (17, 19) was triggered by a bacterial lysate containing LPS, among other pathogen-associated molecular patterns (PAMPs). They also showed that StIR is MyD88 dependent and can be triggered by a combination of TLR2 and TLR9 ligands. They did not test the effect of an MyD88-dependent TLR4 agonist such as LPS, although they tested MPL as a TLR4 agonist. These authors could not identify the antimicrobial mechanism operating in their system, although they showed that it could be produced by the airway epithelial cells. They found that epithelial cells, but not macrophages and dendritic cells, could be stimulated to kill bacteria in isolation and that this behavior was associated with the cytokine profiles, which were very similar to those noted in the whole lung system. The participation of highly reactive metabolites such as ROS, as proposed here, may also be a central part of this phenomenon. In fact, the participation of an ROS-dependent innate mechanism is crucial in the clearance of B. pertussis bacteria induced by LPS. This mechanism has not previously been described in the B. pertussis scenario. Like the case for other pathogens, we expect that the identification of specific clearance pathways for B. pertussis will aid in the development of novel therapeutics against this pathogen.
ACKNOWLEDGMENTS
We acknowledge Carlos Marra and his team for technical assistance in measuring nitrites/nitrates.
This work was supported by ANCPyT (Argentina) grants to D.H. and M.R. and a CICBA grant to D.H. D.H. is a member of the Scientific Career of CICBA. M.R. and G.M. are members of the Scientific Career of CONICET. E.Z., A.E., and M.O. have fellowships from CONICET.
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cherry JD. 2010. The present and future control of pertussis. Clin. Infect. Dis. 51:663–667 [DOI] [PubMed] [Google Scholar]
- 3. Hozbor D, Mooi F, Flores D, Weltman G, Bottero D, Fossati S, Lara C, Gaillard ME, Pianciola L, Zurita E, Fioriti A, Archuby D, Galas M, Binsztein N, Regueira M, Castuma C, Fingermann M, Graieb A. 2009. Pertussis epidemiology in Argentina: trends over 2004–2007. J. Infect. 59:225–231 [DOI] [PubMed] [Google Scholar]
- 4. CDC 2012. Pertussis epidemic—Washington, 2012. MMWR Morb. Mortal. Wkly. Rep. 61:517–522 [PubMed] [Google Scholar]
- 5. de Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rumke HC, Conyn-van Spaendonck MA. 2000. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg. Infect. Dis. 6:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mooi FR. 2010. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect. Genet. Evol. 10:36–49 [DOI] [PubMed] [Google Scholar]
- 7. Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 367:1012–1019 [DOI] [PubMed] [Google Scholar]
- 8. Mooi FR, van Loo IH, King AJ. 2001. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg. Infect. Dis. 7:526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mooi FR, Van Der Maas NA, De Melker HE. 2013. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol. Infect. [Epub ahead of print.] http://dx.doi.org/10.1017/S0950268813000071 [DOI] [PMC free article] [PubMed]
- 10. Hegerle N, Paris AS, Brun D, Dore G, Njamkepo E, Guillot S, Guiso N. 2012. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of bordetellae not expressing pertactin. Clin. Microbiol. Infect. 18:E340–E346 [DOI] [PubMed] [Google Scholar]
- 11. Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K, Kamachi K. 2012. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS One 7:e31985. 10.1371/journal.pone.0031985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gowen BB, Fairman J, Dow S, Troyer R, Wong MH, Jung KH, Melby PC, Morrey JD. 2009. Prophylaxis with cationic liposome-DNA complexes protects hamsters from phleboviral disease: importance of liposomal delivery and CpG motifs. Antiviral Res. 81:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krieg AM, Love-Homan L, Yi AK, Harty JT. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428–2434 [PubMed] [Google Scholar]
- 14. Panchal RG, Ulrich RL, Bradfute SB, Lane D, Ruthel G, Kenny TA, Iversen PL, Anderson AO, Gussio R, Raschke WC, Bavari S. 2009. Reduced expression of CD45 protein-tyrosine phosphatase provides protection against anthrax pathogenesis. J. Biol. Chem. 284:12874–12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ireland R, Olivares-Zavaleta N, Warawa JM, Gherardini FC, Jarrett C, Hinnebusch BJ, Belisle JT, Fairman J, Bosio CM. 2010. Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 6:e1000921. 10.1371/journal.ppat.1000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Errea A, Moreno G, Sisti F, Fernandez J, Rumbo M, Hozbor DF. 2010. Mucosal innate response stimulation induced by lipopolysaccharide protects against Bordetella pertussis colonization. Med. Microbiol. Immunol. 199:103–108 [DOI] [PubMed] [Google Scholar]
- 17. Evans SE, Scott BL, Clement CG, Larson DT, Kontoyiannis D, Lewis RE, Lasala PR, Pawlik J, Peterson JW, Chopra AK, Klimpel G, Bowden G, Hook M, Xu Y, Tuvim MJ, Dickey BF. 2010. Stimulated innate resistance of lung epithelium protects mice broadly against bacteria and fungi. Am. J. Respir. Cell Mol. Biol. 42:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzman Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. 2011. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J. Immunol. 186:5916–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, Dickey BF, Tuvim MJ. 2008. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 177:1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stainer DW, Scholte MJ. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211–220 [DOI] [PubMed] [Google Scholar]
- 21. West NP, Jungnitz H, Fitter JT, McArthur JD, Guzman CA, Walker MJ. 2000. Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect. Immun. 68:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hozbor D, Rodriguez ME, Samo A, Lagares A, Yantorno O. 1993. Release of lipopolysaccharide during Bordetella pertussis growth. Res. Microbiol. 144:201–209 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 24. Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, Graieb A, Rumbo M, Hozbor D. 2008. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26:4639–4646 [DOI] [PubMed] [Google Scholar]
- 25. Beda N, Nedospasov A. 2005. A spectrophotometric assay for nitrate in an excess of nitrite. Nitric Oxide 13:93–97 [DOI] [PubMed] [Google Scholar]
- 26. Miranda KM, Espey MG, Wink DA. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. 2009. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31:761–771 [DOI] [PubMed] [Google Scholar]
- 28. Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. 1997. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253:162–168 [DOI] [PubMed] [Google Scholar]
- 29. Mohanty JG, Jaffe JS, Schulman ES, Raible DG. 1997. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 202:133–141 [DOI] [PubMed] [Google Scholar]
- 30. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. 2005. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. (Warsz.) 53:199–206 [PubMed] [Google Scholar]
- 31. Lambeth JD. 2002. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr. Opin. Hematol. 9:11–17 [DOI] [PubMed] [Google Scholar]
- 32. Bae YS, Choi MK, Lee WJ. 2010. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 31:278–287 [DOI] [PubMed] [Google Scholar]
- 33. Leto TL, Geiszt M. 2006. Role of Nox family NADPH oxidases in host defense. Antioxid. Redox Signal. 8:1549–1561 [DOI] [PubMed] [Google Scholar]
- 34. Fischer H. 2009. Mechanisms and function of DUOX in epithelia of the lung. Antioxid. Redox Signal. 11:2453–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. 2003. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17:1502–1504 [DOI] [PubMed] [Google Scholar]
- 36. Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. 2005. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 579:4911–4917 [DOI] [PubMed] [Google Scholar]
- 37. Moller W, Heimbeck I, Hofer TP, Khadem Saba G, Neiswirth M, Frankenberger M, Ziegler-Heitbrock L. 2012. Differential inflammatory response to inhaled lipopolysaccharide targeted either to the airways or the alveoli in man. PLoS One 7:e33505. 10.1371/journal.pone.0033505 [DOI] [PMC free article] [PubMed] [Google Scholar]