Abstract
Clostridium perfringens type D causes disease in sheep, goats, and other ruminants. Type D isolates produce, at minimum, alpha and epsilon (ETX) toxins, but some express up to five different toxins, raising questions about which toxins are necessary for the virulence of these bacteria. We evaluated the contribution of ETX to C. perfringens type D pathogenicity in an intraduodenal challenge model in sheep, goats, and mice using a virulent C. perfringens type D wild-type strain (WT), an isogenic ETX null mutant (etx mutant), and a strain where the etx mutation has been reversed (etx complemented). All sheep and goats, and most mice, challenged with the WT isolate developed acute clinical disease followed by death in most cases. Sheep developed various gross and/or histological changes that included edema of brain, lungs, and heart as well as hydropericardium. Goats developed various effects, including necrotizing colitis, pulmonary edema, and hydropericardium. No significant gross or histological abnormalities were observed in any mice infected with the WT strain. All sheep, goats, and mice challenged with the isogenic etx mutant remained clinically healthy for ≥24 h, and no gross or histological abnormalities were observed in those animals. Complementation of etx knockout restored virulence; most goats, sheep, and mice receiving this complemented mutant developed clinical and pathological changes similar to those observed in WT-infected animals. These results indicate that ETX is necessary for type D isolates to induce disease, supporting a key role for this toxin in type D disease pathogenesis.
INTRODUCTION
Clostridium perfringens type D causes enterotoxemia in sheep and goats and, more rarely, in cattle and other animal species (1, 2). The clinical and pathological characteristics of the infection are quite different between sheep and goats (2). The infection in sheep is mostly characterized by systemic changes, including brain and lung edema, and hydropericardium, but usually with only minor and inconsistent intestinal changes (2). In contrast, the disease in goats typically involves enterocolitis, although systemic changes similar to those seen in sheep can occasionally be observed in the acute and subacute forms of the disease (3, 4).
C. perfringens type D isolates may be found in the intestine of normal animals, including sheep, goats, and cattle (2, 5). However, when the microbial balance of the gastrointestinal flora is disrupted, these bacteria proliferate in large numbers and produce disease, which is thought to be mediated mainly by toxins. These toxins can act locally in the intestine, as in chronic enterotoxemia of goats (4), or gain access to systemic circulation, as in all forms of sheep enterotoxemia and sometimes in the acute and subacute forms of the disease in goats (4, 5). C. perfringens type D enterotoxemia has been reproduced experimentally in sheep, goats, cattle, rats, and mice with a range of clinical signs and postmortem changes similar to those observed in natural disease (6–10).
By definition, type D isolates must produce both alpha (CPA) and epsilon (ETX) toxins, although some type D isolates can express up to five different toxins (11), which has raised questions about which toxin is most important for the virulence of these isolates (12). ETX, the third most potent clostridial toxin (after botulinum and tetanus toxins) and a National Institute of Allergy and Infectious Diseases (NIAID) class B priority toxin, is produced as an inactive prototoxin in the gastrointestinal tract of animals and then activated by proteolytic removal of the C-terminal 14 amino acids (13). Activated ETX is absorbed through the intestinal tract and transported to several target organs, including the brain, lungs, and kidneys (2). In the brain, ETX affects endothelial cells, producing perivascular edema, with consequent degeneration and necrosis of the surrounding cerebral parenchyma in animals that survive long enough to develop these lesions (14). It has also been suggested that ETX acts directly on neurons (15). Many clinical signs and postmortem changes of natural type D enterotoxemia in sheep and goats may be reproduced by intravenous injection of purified ETX (7, 16). However, while current evidence suggests that ETX is an important virulence factor for type D disease, there is no rigorous proof that, at natural disease production levels, ETX is an important virulence factor for type D disease. Since most C. perfringens type D isolates produce various levels of at least three different lethal toxins, some or all of those other potent toxins could suffice for type D disease.
Resolving the role of ETX in the pathogenesis of type D disease is important for the design and development of improved, new-generation human and veterinary vaccines and to devise therapeutic strategies for potential exposure to this toxin that may occur via terrorist attacks or other means. Reverse genetics have been used to provide definitive evidence of the action of several C. perfringens toxins, including CPA, beta toxin (CPB), NetB, and the enterotoxin (CPE) (12, 17–19). Previously, we generated an ETX null mutant (etx mutant) in the type D sheep disease isolate CN1020 (wild type [WT]) (20). In the current study, the etx mutant was complemented with the wild-type etx gene. The WT, the etx mutant, and the complemented derivative were used to evaluate the virulence contributions of ETX in three animal models of type D disease.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains and plasmids are described in Table 1. Escherichia coli strains were grown in 2× yeast-tryptone (YT) medium (21) supplemented with 150 μg/ml of erythromycin. C. perfringens strains were grown in fluid thioglycolate medium (Difco, Franklin Lakes, NJ), heart infusion (HI) broth (Oxoid, Basingstoke, United Kingdom) containing 0.375% (wt/vol) glucose and 0.1% (wt/vol) sodium thioglycolate, TPG broth (22), nutrient agar (23), or tryptose-glucose-yeast broth (TGY; Anaerobe Systems, Morgan Hill, CA) containing 3% (wt/vol) peptone, 2% (wt/vol) yeast extract, 5% (wt/vol) glucose, and 1% cysteine chloride. When required, clostridial medium was supplemented with 50 μg/ml of erythromycin and 20 μg/ml of thiamphenicol, depending on the strain to be propagated. Agar plates were incubated at 37°C for 24 to 48 h in an atmosphere of 10% (vol/vol) CO2 and 10% (vol/vol) H2 in N2. Growth was analyzed by measuring the optical density at 600 nm after standardization of the starting inoculum so that overnight cultures of the same relative turbidity were used to inoculate 90 ml of HI broth. Growth was monitored every 30 to 60 min for 8 h and then again at 24 h. Samples were taken at various time points for toxin quantitation.
Table 1.
Bacterial strains and plasmid characteristics
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| E. coli | ||
| JIR10750 | Top10(pJIR3872), Emr | Transformant |
| C. perfringens | ||
| CN1020 | Type D wild type (pJIR3118) | Burroughs-Wellcome collection via R. G. Wilkinson (20) |
| JIR4981 | CN1020(pJIR3120) etx::catP, Cmr/Thr | Transformant |
| JIR12604 | JIR4981(pJIR3120, pJIR3872), Cmr/Thr, Emr | |
| Plasmids | This study | |
| pJIR988 | E. coli-C. perfringens shuttle vector, Emr | 20 |
| pJIR3118 | Wild-type 48-kb ε-toxin plasmid from CN1020 | 20 |
| pJIR3120 | pJIR3118 etx::catP, Cmr/Thr | This study |
| pJIR3872 | pJIR988 Ω 1.4-kb etx+ PCR product, Emr |
Cmr/Thr, chloramphenicol and thiamphenicol resistance; Emr, erythromycin resistance.
All bacterial cultures and animal studies using the etx-complemented strain were carried out under biosafety level 3 (BSL3) conditions with the approval of the UC Davis Institutional Biosafety Committee and with Select Agent approval from the Centers for Disease Control and Prevention.
Construction of complementation vector.
The wild-type etx gene from strain CN1020 was PCR amplified along with 250 bp upstream and 170 bp downstream sequence using the forward primer 5′-CGGGATCCCGACTACTAAAAATTTATAGGC-3′, which included a BamHI site, and the reverse primer 5′-GGTCTAGAGACTATTTTACACTCTC-3′, which contained an XbaI site. PCR amplification resulted in the generation of a 1.4-kb fragment that was digested with BamHI and XbaI and ligated to the similarly digested vector, pJIR988, before introduction into the E. coli Top10 strain. This vector is an E. coli-C. perfringens shuttle vector that contains the replication regions from the low-copy-number E. coli plasmid pWSK29 and the C. perfringens plasmid pIP404, as well as the erm(B) gene for selection in C. perfringens. The resultant plasmid was subjected to restriction analysis and sequencing to confirm the insert. Construction of the etx-complemented strain was carried out in Australia under physical containment level 3 (PC3) conditions, in accordance with a license issued by the Office of the Gene Technology Regulator and with Select Agent approval from the Centers for Disease Control and Prevention.
Toxin assays.
Culture supernatants from HI or TGY broth cultures were obtained at late exponential growth phase, which is when ETX is maximally produced (12). For assessment of ETX levels, 1-ml samples were taken of each of the WT, etx mutant, and complemented strains, standardized to the same optical density, centrifuged at 14,000 × g for 5 min, and filtered through a 0.45-μm syringe filter (Sartorius, Goettingen, Germany). ETX production was determined as follows. The standardized, filtered culture supernatants were subjected to 12% SDS-PAGE before electroblotting onto nitrocellulose for Western analysis using the ETX monoclonal antibody 5B7 (12) or for a capture ETX enzyme-linked immunosorbent assay (ELISA) (see below). For all of these assays, a minimum of three independent cultures was used.
Molecular methods.
C. perfringens genomic (24) and plasmid DNA (25) was extracted as previously described. E. coli plasmid DNA was extracted using a Qiagen miniprep kit as described by the manufacturer. Plasmid DNA was introduced into C. perfringens by electroporation at 1.8 kV, 25 μF, and 200 Ω using a BTX ECM-630 Electro cell manipulator (BTX Laboratories) according to the method of Scott and Rood (26). PCR amplification of DNA sequences was conducted using Phusion DNA polymerase (New England BioLabs, Ipswich, MA) for amplification of etx fragments for cloning or Taq DNA polymerase (Roche, Penzberg, Germany) for probe generation, according to the manufacturer's instructions, using 30 cycles of denaturation (95°C), annealing (55°C), and extension (72°C) for between 1 and 1.5 min. PCR products were purified from agarose gels using the QIAquick gel extraction kit (Qiagen, Hilden, Germany) prior to cloning or production of probes to be used in Southern blotting. Restriction endonuclease digestion, ligations, and agarose and polyacrylamide gel electrophoresis were performed according to standard laboratory procedures (21).
Southern hybridization.
Genomic and plasmid DNA was digested with HindIII prior to separation through a 0.8% agarose gel and then transferred to a nylon membrane by capillary transfer. Washing, hybridization, chemiluminescent detection, and stripping prior to reprobing was performed according to the manufacturer's guidelines (Roche). Probes were generated using the digoxigenin (DIG) random labeling kit (Roche) according to the manufacturer's directions and included an etx probe generated using oligonucleotide primers etxF (5′-TACTCATACTGTGGGAACTTCGATACAAGC-3′) and etxR (5′-GGTCTAGAGACTATTTTACACTCTC-3′), an erm(B) probe from the primers ermF (5′-AATAAGTAAACAGGTAACGTCT-3′) and ermR (5′-GCTCCTTGGAAGCTGTCAGTAG-3′), and a catP probe derived from catF (5′-CTCAGTACTGAGAGGGAACTTAGATGGTAT-3′) and catR (5′-CCGGGATCCTTAGGGTAACAAAAAACACC-3′).
Production of type D cultures for animal infection.
For the sheep and goat experiments, the WT, the etx mutant (20), and its complemented derivative were grown for 8 h in TGY at 37°C. For each strain, a 10% (vol/vol) inoculum was transferred into 500 ml of TGY broth and grown at 37°C overnight. The mutant was grown in the presence of 14 μg/ml of chloramphenicol, while the complemented strain was grown in the presence of 14 μg/ml of chloramphenicol and 35 μg/ml of erythromycin. Fifty ml of a 1% (wt/vol) trypsin solution in peptone water (Difco) was added per 500 ml of culture before transferring the cultures to 500-ml empty sterile intravenous drip bags.
For the mouse experiments, the same strains were grown in ∼10 ml of TGY broth at 37°C overnight using the antibiotics described earlier. The cultures then were centrifuged for 30 min at 5,000 × g at 4°C. The supernatant was discarded, and fresh TGY was added to the pelleted cells to reach the desired dilution of 106 CFU/ml.
Animals.
Twenty-four healthy male or female Mulley sheep and 20 healthy male or female Boer and Toggenberg goats were used. All of the animals were between 3 and 4 months of age (20 to 30 kg of body weight) and had not been vaccinated against clostridial or other diseases. They had been weaned at the age of 10 to 14 weeks (an age at which most maternal antibodies have disappeared), placed in a warm, dry room, and fed alfalfa hay and water ad libitum for 1 or 2 weeks before the experiments. At that time, the animals were treated orally with amprolium (Merial, Duluth, GA) for coccidian parasite control. Fecal samples were collected twice at 2-day intervals before the animals were used, the second sample being collected 2 days before the beginning of the experiments. A fecal float analysis to detect intestinal parasite eggs and parasites was performed with each of these samples by following Standard Operational Procedures of the California Animal Health and Food Safety Laboratory, UC Davis. No parasites or parasite eggs were detected in any of the fecal samples tested. Mouse experiments involved 60 18- to 22-g healthy male or female BALB/c mice that were kept in an environment-controlled room for 2 days before and during the experiments. All procedures involving animals were reviewed and approved by the University of California—Davis Institutional Animal Care and Use Committee (permit number 16383).
Intraduodenal inoculation of C. perfringens strains or TGY into sheep and goats.
The animals were divided into four treatment groups: WT, etx mutant, the complemented strain, and TGY (negative control). The animals were fasted for 24 h before inoculation but were allowed access to water until 1 h before surgery. The preanesthetic induction was carried out by intravenous injection of xylazine (Lloyd, Shenandoah, IA) at 0.22 mg/kg, and anesthesia was maintained by intravenous injection of ketamine hydrochloride (Phoenix, St. Joseph, MO) at 11 mg/kg. Xylocaine (Sparhak, Lenexa, KS) (∼5 ml per animal) was injected locally in the area of incision. A laparotomy was performed via the right flank, and the pyloric area of the abomasum and the first portion of duodenum were exposed. Two hundred ml of a 20% (wt/vol) solution of cooking-grade wheaten corn flour in 0.85% (wt/vol) saline was injected into the abomasum of all animals in each group. Subsequently, 250 ml of a culture of the relevant strain containing ∼106 CFU/ml was inoculated into the duodenum through a drip over a period of approximately 10 min. Control animals received 250 ml of sterile TGY instead of bacterial culture. The abdominal incision was closed by separate muscle and skin sutures. The animals were allowed to recover from anesthesia and then clinically examined periodically after inoculation. Animals that showed severe clinical signs were euthanized as soon as possible after onset of clinical signs, unless spontaneous death occurred before euthanasia could be performed. Those animals that remained clinically healthy during that period and those that showed only mild or moderate clinical signs were euthanized 24 h after inoculation. Euthanasia was performed by an intravenous overdose of barbiturate.
Intraduodenal inoculation of C. perfringens strains into mice.
A mouse intraduodenal challenge model in sealed mice was used, as previously described (27). Briefly, the duodenum of mice was challenged with 106 CFU washed cells of one of the strains to be tested or with sterile TGY, under anesthesia, and the anus was sealed with glue. The animals were allowed to recover from anesthesia and then clinically examined periodically after inoculation. Animals that showed severe clinical signs were euthanized as soon as possible after onset of clinical signs, unless spontaneous death occurred before euthanasia could be performed. Those animals that remained clinically healthy during that period, and those that showed only mild or moderate clinical signs, were euthanized 48 h after inoculation.
Clinical and pathological evaluation.
A scoring system from 0 to 3 was used to characterize the overall clinical alterations in sheep, goats, and mice. The overall score for each animal was obtained by first calculating the individual scores for digestive, respiratory, and nervous clinical signs and then averaging them. A score of 0 was assigned to animals that did not show any clinical abnormalities during the experimental period. For digestive clinical signs, a score of 1 was assigned to animals that showed mild, transient diarrhea, a score of 2 to animals that showed moderate liquid, watery, green diarrhea, and a score of 3 to animals that showed moderate to severe diarrhea with or without blood and/or fibrin, with abdominal discomfort. For respiratory clinical signs, a score of 1 was assigned to animals that showed mild to moderate tachypnea, a score of 2 to animals that showed mild to moderate dyspnea, and a score of 3 to animals that showed severe dyspnea. For nervous clinical signs, a score of 1 was assigned to animals that showed mild ataxia, a score of 2 to animals that showed moderate to severe lethargy, ataxia, and/or incoordination, and a score of 3 to animals that showed severe depression, incoordination, recumbency, blindness, paddling, and/or spastic convulsions. The time between inoculation and the onset of clinical signs and the time between the onset of clinical signs and death was recorded for each animal. The assay endpoint included spontaneous death, development of severe clinical signs necessitating euthanasia, or survival without clinical alterations after 24 (sheep and goats) or 48 h (mice).
Postmortem examinations were performed either immediately or within 5 h after death of all animals. The main gross lesions were scored using a scale of 0 to 3, with 0 indicating no gross lesions and values between 1 and 3 indicating increasingly severe lesions. Samples of the gastrointestinal tract, brain, kidneys, lungs, heart, spleen, and liver were collected and fixed by immersion in 10% (vol/vol) formalin, pH 7.2, for a minimum of 24 h. The brains of the sheep and goats were then sliced into sections 0.5 cm thick and fixed in fresh formalin for 7 or more days before blocks were prepared from the corpus striatum, parietal cortex, and midbrain at the level of the thalamus and anterior coliculi, cerebellar peduncles, cerebellum, and medulla oblongata at the level of the obex. Mouse brains were cut into 4 or 5 equidistant cross sections. All tissues were processed routinely to obtain 4-μm-thick sections, which then were stained with hematoxylin and eosin. Selected sections were also stained with Gram, periodic acid of Schift (PAS), or phosphotungstenic acid hematoxylin (PTAH) stain. Other sections of the small intestine and the colon of sheep and goats were processed by an indirect immunoperoxidase staining technique for C. perfringens, as previously described (28), using the Dako EnVision kit (Dako, Carpenteria, CA) according to the instructions of the manufacturer. A rabbit polyclonal anti-C. perfringens antibody was used as the primary antibody (GenWay Bio, San Diego, CA). The main microscopic lesions were scored using a scale of 0 to 3, with 0 indicating no microscopic lesions and values between 1 and 3 indicating increasingly severe lesions.
ELISA for C. perfringens ETX.
Small and large intestinal contents of each animal were aseptically collected during postmortem examination and stored at −80°C until processed several weeks later for a capture ELISA to detect ETX. A commercial capture ETX ELISA kit (BIO-X, Brussels, Belgium) was used by following the manufacturer's instructions. The kit was slightly modified to quantify the amount of ETX present in each sample as follows. Serial dilutions of purified ETX (BEI Resources, ATCC, Manassas, VA) were used in positive-control wells; toxin was replaced by buffer in negative-control wells. The results were calculated according to the manufacturer's instructions, and the ETX concentrations were estimated by mathematical regression using five samples with known concentrations of ETX.
Viable counts and PCR analysis of strains isolated from inoculated animals.
To determine the number of CFU per gram, samples from the small intestine or colon content were aseptically collected during postmortem examination, serially diluted with TGY, spread onto CP ChromoSelect agar supplemented with Perfringens T.S.C. supplement (both from Sigma-Aldrich, Buchs, Switzerland), and incubated for 24 h at 37°C in anaerobiosis before the colonies were counted and the CFU per gram calculated.
To confirm the toxinotype of the isolates, ∼4 C. perfringens colonies isolated from the contents of the small and large intestine of each animal were subjected to PCR to amplify products specific for the genes encoding CPA, CPB, ETX, iota toxin (ITX), CPE, and beta2-toxin (CPB2), as previously described (29). Briefly, bacteria were grown on brain heart infusion agar (Hardy Diagnostics, Santa Maria, California), incubated anaerobically overnight at 37°C, and then processed for multiplex PCR using colony lysates as templates. The multiplex PCR products were then separated on 2% agarose gels, stained with ethidium bromide, and examined by UV light transillumination.
Statistical analyses.
CFU/g and ETX/ng were evaluated for normality using Shapiro-Wilk testing and transformed using Box-Cox transformation. The effects of inoculated strain, species, and location (small intestine or colon) on CFU/g and ETX/ng were evaluated using multiway analysis of variance (ANOVA) with the STATA 10.1 package (StataCorp LP, College Station, TX). Terms in the final ANOVA were evaluated using Wald testing to determine which values differed significantly from one another, using the Sidak correction to adjust the critical P value for multiple comparisons. For all inocula, the CFU/g and ETX/ng in each intestinal location for each inoculum in each species were compared using 2-sided t tests, with statistical significance at <0.05.
RESULTS
Construction and characterization of an etx complementation plasmid.
Previously, an etx null mutant was constructed in strain CN1020, and subsequent analysis indicated that this toxin gene was located on a conjugative plasmid (20). To use this mutant to determine the role of ETX in disease, it was essential to complement the etx mutation with the wild-type etx gene. This task proved to be very difficult, with numerous attempts being made with different PCR fragments and several high-copy-number E. coli-C. perfringens shuttle vectors. Invariably, these cloning experiments resulted in the isolation of deletion derivatives or point mutants that no longer produced a functional ETX protein, suggesting that overexpression of the wild-type etx gene was lethal in E. coli. Finally, the complementation vector pJIR3872 was constructed by introducing the amplified etx gene into the E. coli-C. perfringens shuttle vector pJIR988. This vector uses the low-copy-number pSC101 replicon in E. coli, an erm(B) erythromycin resistance gene for selection in C. perfringens, and the rep region from the C. perfringens plasmid pIP404. The use of this plasmid enabled us to clone the etx gene behind its native promoter for the first time. The etx region derived from pJIR3872 was sequenced, which confirmed that the etx promoter and gene sequences were intact.
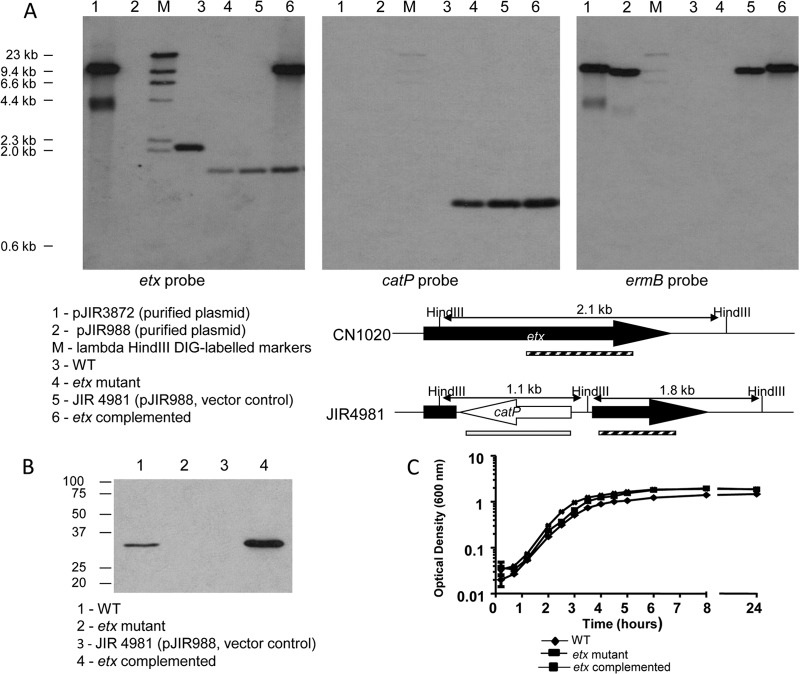
Under PC3 (equivalent to BSL3) containment conditions, purified pJIR3872 DNA was introduced into the CN1020 etx mutant JIR4981 by electroporation, selecting for erythromycin resistance. The resultant complemented strain, JIR12604, was confirmed by isolating plasmid DNA, using it to transform E. coli, and carrying out restriction analysis of the reisolated plasmid DNA. Southern hybridization analysis showed that JIR12604 had the expected profile, that of the etx deletion derivative, JIR4981, with the addition of the complementation plasmid (Fig. 1A). The chromosomal deletion in the native etx gene was still present, as demonstrated by a 1.8-kb etx-hybridizing band and a 1.1-kb catP-hybridizing band in JIR4981 and JIR12604 (Fig. 1A, lanes 4 and 6). The catP gene was used for the insertional inactivation of etx in the original construction of JIR4981 (20). The erm(B) probe was used to detect the complementation plasmid pJIR3872 in JIR12604 (Fig. 1A, lanes 5 and 6).
Fig 1.
Confirmation and characterization of etx complementation derivative. (A) Southern hybridization analysis of the WT (CN1020), etx mutant (JIR4981), complemented etx mutant (JIR12604), and a transformant carrying the vector plasmid pJIR3872. Genomic DNA was extracted and digested with HindIII before electrophoresis on a 0.8% Tris-acetate-EDTA gel. DNA was transferred to a nylon membrane and probed with DIG-labeled PCR products, as shown below the individual blots. The membrane was stripped and reprobed for subsequent probes. Marker sizes are indicated. Lane 1, pJIR3872, lane 2, pJIR988; lane M, DIG-labeled lambda HindIII; lane 3, CN1020; lane 4, JIR4981; lane 5, JIR4981(pJIR988); lane 6, JIR4981(pJIR3872) (i.e., JIR12604). A diagram of the expected fragments and position of the probes in the wild type (CN1020) and the mutant (JIR4981) is shown. The etx probe is indicated with a striped bar, and the position of the catP probe is indicated by the gray bar. (B) Western blot analysis of C. perfringens strains. Standardized, filtered culture supernatants were separated on a 12% acrylamide gel containing SDS prior to transfer onto a nitrocellulose membrane. ETX was detected using the 5B7 monoclonal antibody. Lane 1, CN1020; lane 2, JIR4981; lane 3, JIR4981(pJIR988); lane 4, JIR12604. Molecular sizes are indicated in kDa. (C) Analysis of the growth characteristics of CN1020, JIR4981, and JIR12604 in HI broth.
Western blot analysis (Fig. 1B) indicated that the complemented strain produced more ETX than the wild-type strain in vitro (Fig. 1B), presumably due to the presence of the etx gene on a multicopy plasmid in this strain. Similar results were also obtained by ELISA, with the WT strain producing 225 ng/ml of ETX and the etx-complemented strain producing 2,400 ng/ml of ETX. This difference was statistically significant (P < 0.05). The ETX level produced by the etx-complemented strain was still within the range observed for wild-type C. perfringens type D isolates (0 to 5,300 ng/ml) (12). The etx-complemented strain showed growth characteristics comparable to those of the etx mutant and the WT (Fig. 1C).
Epsilon toxin is required for virulence in sheep, goats, and mice.
The isogenic WT, etx mutant, and etx-complemented strains were examined for their ability to cause disease in our sheep, goat, and mouse disease models. Mice were used initially to determine whether there were differences in the virulence of these strains. After the initial mouse studies indicated that the etx mutant was attenuated for virulence, we moved to the sheep and goat animal models. These ruminants both are natural hosts for type D disease, although these species show different pathological changes during natural type D disease, as described earlier.
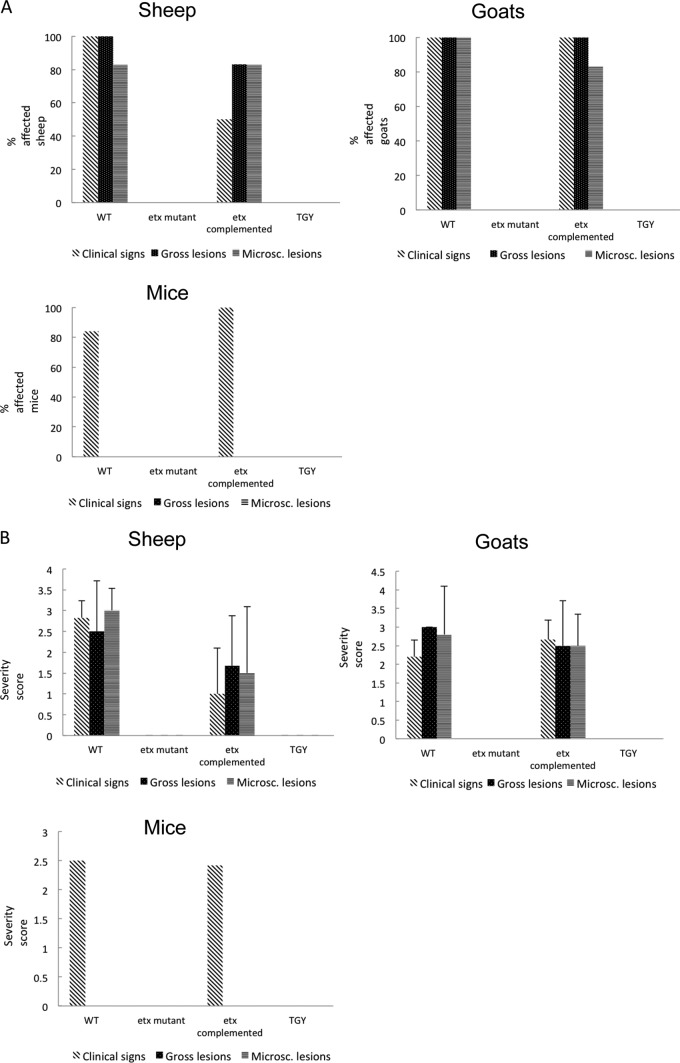
Most of the sheep, goats, and mice infected with the WT or with the complemented etx mutant showed clinical signs of disease (Fig. 2A), and most of the sheep and goats inoculated with these strains showed gross and microscopic changes (Fig. 2A). In contrast, no significant clinical alterations or gross or histological lesions were observed in any sheep, goat, or mouse receiving the etx mutant or the TGY negative control (Fig. 2A).
Fig 2.
Virulence of isogenic strains in sheep, goats, and mice. (A) Percentages of affected sheep, goats, and mice receiving the wild-type strain CN1020 (WT), the etx mutant JIR4081 (etx KO), its complemented derivative JIR12604 (etx complemented), or sterile, nontoxic culture medium (TGY) are shown. A total of 6 sheep, 5 goats, and 15 mice received each inoculum. (B) Severity score of clinical signs and gross and microscopic lesions in sheep, goats, and mice inoculated as described for panel A. Clinical signs, gross and microscopic lesions were scored using a scale of 0 to 3, with 0 indicating no clinical alterations or lesions and values between 1 and 3 indicating increasingly severe clinical signs or lesions. The averages ± standard deviations for all animals in each group are shown.
A summary of the overall severity scores observed for the clinical signs and the gross and microscopic lesions in sheep, goats, and mice is presented in Fig. 2B. Clinical signs observed in sheep infected with the WT or complemented strains consisted of one or more of the following: tachypnea, dyspnea, ataxia, lethargy depression, recumbency, blindness, paddling, and spastic convulsions. Clinical signs in similarly challenged goats included those mentioned for sheep, plus hemorrhagic diarrhea and abdominal pain. Clinical signs in mice infected with the WT or complemented strains included depression, ataxia, circling, and dyspnea. Clinical signs in the three species challenged with the WT strain were indistinguishable from those observed in equivalent animals infected with the complemented strain. This strain was not more virulent than the WT. In contrast, animals receiving the etx mutant or TGY (as a negative control) did not show any clinical abnormalities (Fig. 2B).
Gross changes in sheep infected with the WT or the complemented strain consisted of pulmonary edema, hydropericardium with strands of fibrin, and/or ascites. Gross changes in goats challenged with either of these two strains included those described in sheep plus the presence of hemorrhagic fluid with strands of fibrin in the jejunum, ileum, and colon, with incipient pseudomembrane formation, mucosal hemorrhage, and congestion in the latter. No gross abnormalities were observed in mice, sheep, or goats receiving the etx mutant or the TGY negative control alone. No gross abnormalities were observed in mice inoculated with any of the inocula.
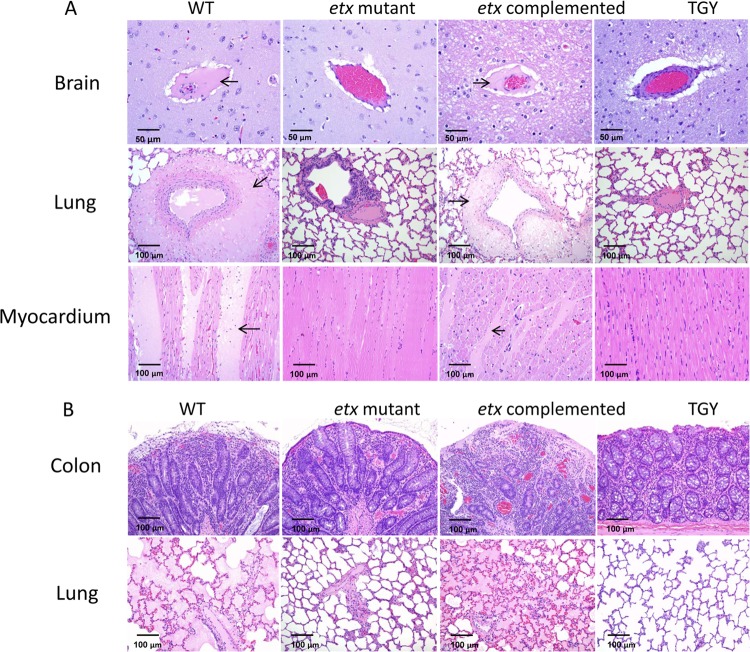
Microscopic lesions were observed only in sheep and goats challenged with the WT or complemented strains (Fig. 3A and B). In those sheep, histological lesions consisted of PAS- and PTAH-positive proteinaceous perivascular edema of the brain (mostly located in corpus striatum, thalamus, cerebellar peduncles, and cerebellar white matter) and the lung and interstitial edema of the myocardium and lung (Fig. 3A). In goats, histological lesions were mostly restricted to the intestine and consisted of pseudomembranous enterocolitis with severe necrosis of the colonic mucosa (Fig. 3B). Great numbers of large Gram-positive rods were seen in the lumen or lining the mucosa of the colon. Most of these bacilli stained positive for C. perfringens by immunohistochemistry. The only histological lesions observed outside the intestinal tract in goats challenged with the WT or the complemented strains consisted of interstitial perivascular and alveolar edema in the lungs (Fig. 3B). No significant histological abnormalities were observed in any tissues of the mice infected with the WT or complemented strains. No significant histological abnormalities were observed in any tissues of the three animal species receiving the etx mutant or TGY alone.
Fig 3.
Microscopic lesions in sheep, goats, and mice. (A) Microscopic lesions in sheep inoculated with the wild-type strain CN1020 (WT), the etx mutant JIR4081 (etx KO), its complemented derivative JIR12604 (etx complemented), or sterile, nontoxic culture medium (TGY) are shown. The brain and lungs of sheep inoculated with the WT or complemented strains show proteinaceous perivascular edema (arrows) indicating increased vascular permeability. There is also severe interstitial proteinaceous edema in the myocardium (arrows) of these animals. No significant histological lesions were observed in the brain, lungs, or myocardium of the sheep inoculated with the etx mutant or TGY. (B) Microscopic lesions in goats inoculated with the isogenic strains. Severe colitis with necrosis of superficial epithelium and lamina propria and neutrophilic infiltration was observed in animals inoculated with the WT or complemented strains. No significant histological lesions were observed in the colon or lungs of the goats inoculated with the etx mutant strain or TGY.
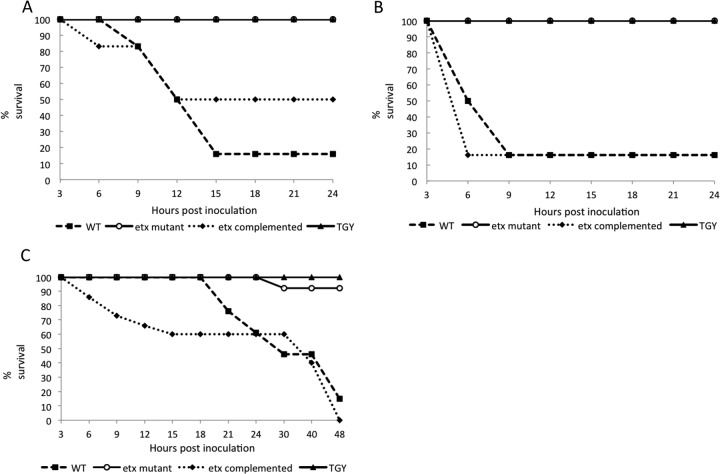
Lethality of strains.
After 24 h, the lethality varied between 50 and 83% in sheep challenged with the complemented and WT strains, respectively (Fig. 4A). This difference was statistically significant (P < 0.05). The lethality after 24 h in goats inoculated with either of these two strains was 83% (Fig. 4B), and the lethality after 48 h was 100 and 85% in mice inoculated with the same strains (nonstatistically significant difference; P < 0.05) (Fig. 4C). No lethality was observed in any sheep or goat receiving the etx mutant or TGY alone (Fig. 4A and B). Only 10% lethality was observed in mice challenged with the etx mutant, and no lethality was observed in mice receiving TGY alone (Fig. 4C).
Fig 4.
Kaplan-Meier survival curves. Progression of survival in a 24-h period in sheep (A) and goats (B) and in a 48-h period in mice (C) inoculated with the wild-type strain CN1020 (WT), the etx mutant JIR4081 (etx KO), its complemented derivative JIR12604 (etx complemented), or TGY. A total of 6 sheep, 5 goats, and 15 mice were treated with each inoculum.
Examination of intestinal contents of infected animals confirms expected phenotypes.
C. perfringens was cultured from the small and large intestinal contents of all of the sheep, goats, and mice challenged with the isogenic strains. Those isolates from animals challenged with the WT or complemented strains were identified as type D by the toxin genotyping multiplex PCR assay. Cells isolated from the animals inoculated with the etx mutant were identified as type A by this assay; this result is as expected, since their etx gene is disrupted by a large insertion. Isolates obtained from animals receiving only TGY were also identified as type A by PCR, as only the cpa gene was detected.
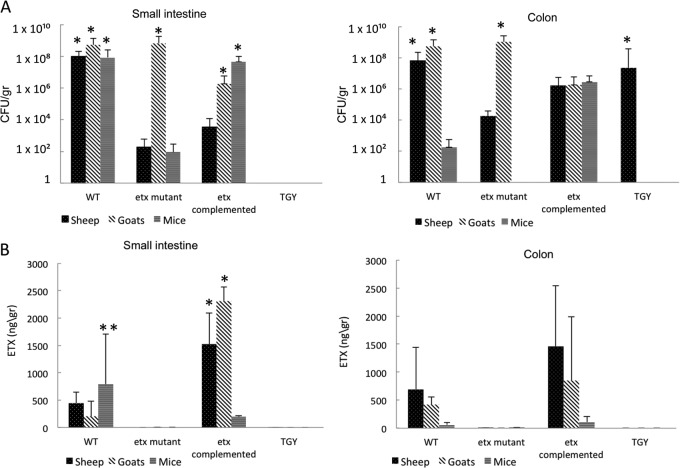
Various amounts of C. perfringens (CFU/g) were isolated from the small and large intestine of all three animal species following challenge with these strains (Fig. 5A). The CFU/g in most cases was statistically higher than the bacterial concentration in the corresponding inoculum (Fig. 5A). There were no statistically significant differences in the viable counts in the small intestine and colon of animals of the three species infected with any of the three isogenic strains. However, there was a statistically significant difference in viable counts in small intestine between sheep or mice versus goats infected with the etx mutant. There was also a statistically significant difference in viable counts in the small intestine between goats or mice versus sheep infected with the complemented strain. Except for the colon of sheep, no C. perfringens colonies were isolated from the small or large intestine of animals from any species receiving only TGY (Fig. 5A).
Fig 5.
Intestinal colonization of isogenic strains and ETX production in the intestine of infected animals. (A) Intestinal colonization (small intestine and colon) of sheep, goats, and mice inoculated with the isogenic strains or TGY are shown. Bars represent the average values from 6 sheep, 5 goats, or 15 mice ± SD. The asterisks indicate a statistically significant difference (P < 0.05) between the CFU/g in the inocula and in each of the specimens depicted. (B) Concentration of ETX (ng/ml) in the small intestine and colon of sheep, goats, and mice inoculated with the isogenic strains or TGY are shown. Bars represent averages from 6 sheep, 6 goats, or 15 mice ± SD. The single asterisks indicate a statistically significant difference (P < 0.05) between the amount of ETX detected in animals inoculated with the WT and the etx-complemented strains.
ETX was detected by ELISA in the small and large intestinal contents of all sheep, goats, and mice infected with the WT or the complemented mutant but not in intestinal contents of animals receiving the etx mutant or TGY alone (Fig. 5B). For sheep and goats, the amount of ETX detected in small intestinal content of animals infected with the complemented strain varied between animals but was higher than that detected in the same specimens of animals challenged with the WT (Fig. 5B). The small intestine of mice infected with the WT, however, had more ETX than the same specimens of mice challenged with the complemented strain (Fig. 5B). The same trends were observed in the colons from all three animal species, although overall the amount of ETX detected in the colon was not significantly different after challenge with the WT versus the complemented mutant. On average, the ETX levels measured in the colon were less than the amount of this toxin detected in the small intestine (Fig. 5B). Based on these PCRs, viable counts, and toxin assays, we concluded that in all of the infected animals the phenotypic profile obtained from the intestinal contents was in agreement with the profile expected from the specific infecting strain, thereby validating the virulence experiments.
DISCUSSION
The objective of this study was to evaluate the contribution of ETX to the pathogenesis of C. perfringens type D infections in three animal models, two of which (sheep and goats) are natural hosts. In this study, pathogenicity was eliminated when the etx gene was insertionally inactivated in a virulent wild-type C. perfringens type D strain. Virulence was restored when this etx mutant was complemented in trans with the wild-type etx gene, indicating that the loss of pathogenicity was not due to an unintended secondary mutation, thereby fulfilling molecular Koch's postulates. These data provide clear evidence that ETX is necessary for the virulence of C. perfringens type D in sheep, goats, and mice and represent the first definitive molecular study that shows an absolute requirement of ETX for virulence in the native hosts. This conclusion confirms previous studies suggesting that ETX is an essential virulence factor for C. perfringens type D and supports the use of ETX toxoids for vaccination against infection by this microorganism (1–5, 7, 8, 12).
The most consistent gross and histological finding in sheep challenged with the WT and complemented strains was hydropericardium and edema of brain, myocardium, and lungs, while in goats, the lesions were mostly restricted to the gastrointestinal tract, which showed necrotizing enterocolitis. Most of these observations are similar to those for the lesions described for spontaneous C. perfringens type D infections in these animal species (2, 16), thereby validating our sheep and goat models. The myocardial edema, which was consistently observed in most sheep infected with WT or complementing strains during this study, is a change that previously has only rarely been observed (27). It is possible that this lesion was responsible for at least some of the acute deaths observed in sheep if the edema affected the important areas of heart conduction, such as the atrioventricular or the sinoatrial nodes. The absence of gross and histological lesions in mice challenged with the WT and complemented strains is not surprising, since histological lesions are not observed consistently in this mouse model (27). The reason for the absence of gross and histological lesions in mice is unknown. Occasionally, no lesions are observed in sheep with acute or per-acute type D enterotoxemia (2). In those cases, it has been speculated that ETX affects neuron metabolism (2, 10), and that is the reason why some animals die without showing morphological alterations. It is possible that this was the case in the mice in our study.
In this study, sheep inoculated with the WT or the etx-complemented strains showed extensive systemic damage, while goats inoculated with either of these strains showed gross and histological changes that were mostly restricted to the gastrointestinal tract. The reason for this difference remains unknown, although it has been speculated in the past that absorption of ETX is faster in sheep; consequently, there is no time for development of intestinal lesions (6–8). Although this has never been proven, the fact that ligated small and large intestinal loops of sheep receiving high doses of ETX demonstrated lesions similar to those seen in the intestine of goats with natural type D enterotoxemia (30) seems to support this theory.
A presumptive diagnosis of C. perfringens type D disease can be achieved at necropsy by observing perivascular edema in the brain in sheep and occasionally in goats, and/or necrotizing enterocolitis or colitis in goats, followed by the isolation of C. perfringens type D (2). However, final confirmation of a type D infection should be based on the detection of ETX in the intestinal contents (2). In our experiments, in addition to observing perivascular edema of the brain in sheep and enterocolitis in goats, C. perfringens type D was isolated from the small and large intestinal contents of all animals inoculated with the WT and complemented strains. Furthermore, ETX was detected in the small and large intestinal content of these animals. Together, these findings confirmed that the clinical signs observed, gross and histological lesions, were produced by C. perfringens type D.
No ETX was detected in the intestinal contents of any of the three animal species challenged with the etx mutants or the TGY negative control. These experiments confirmed that the ETX detected in specimens from animals infected with the WT and complemented strains was indeed produced as a result of the bacterial inoculum. The small intestinal ETX levels detected in most of the sheep and goats inoculated with the complemented strain were significantly higher than those from the WT infections. This result is consistent with the in vitro results, which showed that the complemented strain produces more ETX than the parent strain, presumably as a consequence of the presence of the etx gene on a multicopy plasmid in the complemented strain. Lethality and severity scores of the lesions, however, were not greater in goats and sheep challenged with the etx-complemented strain versus the WT strain. This is probably an indication that the amount of ETX produced by the WT is already over the threshold level of this toxin needed to produce acute disease in these animals. Also, there was a large variation between individual animals regarding the amount of ETX present in the intestine, in particular the colon, where the ETX levels were highly variable and not statistically different between animals challenged with WT versus the etx-complemented strain. Some animals challenged with the etx-complemented strain had much less ETX than the average amount of ETX measured for the group (data not shown). These individual variations make correlations between ETX levels present in the intestine and lethality scores difficult.
Surprisingly, little information is available in the literature about the amount of ETX which is necessary in the intestines of animals to produce disease (8). This is probably due to the fact that few laboratories perform detection of ETX routinely in natural cases of enterotoxemia, and only a few experiments have been performed to reproduce this disease in the natural hosts (5, 8).
The C. perfringens type D viable counts from the small and large intestines of most animals inoculated with any of the three bacterial isolates were larger than the initial inoculum levels. This result, coupled with the relatively large amounts of ETX detected in the intestines of animals inoculated with the WT and complemented strains, confirmed that these animals suffered an active infection, where bacterial replication and toxin production occurred in the intestine. The C. perfringens cells isolated from the intestine of the animals challenged with the etx mutant were typed by PCR as type A, as expected from a derivative in which the etx gene had been insertionally inactivated.
In summary, for the first time we have shown that defined mutation of the etx gene leads to loss of ETX production and a concomitant loss of the lethality, clinical signs, and gross and histological lesions associated with C. perfringens type D infections in sheep and goats. This process was reversed by genetic complementation and restoration of the ability to produce ETX. Therefore, it is concluded that ETX is essential for the classical signs of C. perfringens type D infection in the natural hosts of these infections.
ACKNOWLEDGMENTS
National Institute of Allergy and Infectious Diseases grant AI056177 supported this research. This research also was supported by grants made by the Australian Research Council (ARC) to the ARC Centre of Excellence in Structural and Functional Microbial Genomics at Monash University.
We thank Jackie Parker and Jim Cravotta for excellent technical assistance.
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Barker IK, Van Dreumel AA, Palmer N. 1993. The alimentary system, p 1–318 In Jubb KF, Kennedy PC, Palmer N. (ed), Pathology of domestic animals, 4th ed, vol 2 Academic Press, San Diego, CA [Google Scholar]
- 2. Uzal FA, Songer JG. 2008. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J. Vet. Diagn. Investig. 20:253–265 [DOI] [PubMed] [Google Scholar]
- 3. Smith MC, Sherman DM. 2009. Digestive system, p 377–500 In Smith MC, Sherman DM. (ed), Goat medicine, 2nd ed Wiley-Blackwell, Ames, IA [Google Scholar]
- 4. Uzal FA, Kelly WR. 1996. Enterotoxaemia in goats: a review. Vet. Res. Comm. 20:481–492 [DOI] [PubMed] [Google Scholar]
- 5. Uzal FA, Kelly WR, Morris WE, Bermudez J, Biason M. 2004. The pathology of experimental Clostridium perfringens type D enterotoxemia in sheep. J. Vet. Diagn. Investig. 16:403–411 [DOI] [PubMed] [Google Scholar]
- 6. Blackwell TE, Butler DG, Prescott JF, Wilcock BP. 1991. Differences in signs and lesions in sheep and goats with enterotoxaemia induced by intraduodenal infusion of Clostridium perfringens type D. Am. J. Vet. Res. 52:1147–1152 [PubMed] [Google Scholar]
- 7. Uzal FA, Kelly WR. 1997. The effects of intravenous administration of Clostridium perfringens type D epsilon toxin on young goats and lambs. J. Comp. Pathol. 116:63–71 [DOI] [PubMed] [Google Scholar]
- 8. Uzal FA, Kelly WR. 1998. Experimental Clostridium perfringens type D enterotoxaemia in goats. Vet. Pathol. 35:132–140 [DOI] [PubMed] [Google Scholar]
- 9. Finnie JW. 1984. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 94:445–452 [DOI] [PubMed] [Google Scholar]
- 10. Finnie JW, Blumbergs PC, Manavis J. 1999. Neuronal damage in rat brains by Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 120:415–420 [DOI] [PubMed] [Google Scholar]
- 11. McClane BA, Uzal FA, Fernandez-Miyakawa ME, Lyerly DM, Wilkins T. 2006. The enterotoxigenic clostridia, p 698–752 In Dworkin M, Falkow S. (ed), The procaryotes, 3rd ed Springer-Verlag, New York, NY [Google Scholar]
- 12. Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, Uzal FA, McClane BA. 2005. Epsilon-toxin is required for most Clostridium perfringens genotype D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect. Immun. 73:7413–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527–535 [DOI] [PubMed] [Google Scholar]
- 14. Buxton D, Macleod NS, Nicolson TB. 1981. Focal symmetrical encephalomalacia in young cattle. Vet. Rec. 108:459. [DOI] [PubMed] [Google Scholar]
- 15. Finnie JW. 2003. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: a review. Aust. Vet. J. 81:219–221 [DOI] [PubMed] [Google Scholar]
- 16. Buxton D, Morgan KT. 1976. Studies of lesions produced in the brains of colostrum deprived lambs by Clostridium welchii (Clostridium perfringens) type D toxin. J. Comp. Pathol. 86:435–447 [DOI] [PubMed] [Google Scholar]
- 17. Awad MM, Bryant AE, Stevens DL, Rood JI. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191–202 [DOI] [PubMed] [Google Scholar]
- 18. Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4(2):e26. 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarker MR, Carman RJ, McClane BA. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946–958 [DOI] [PubMed] [Google Scholar]
- 20. Hughes ML, Poon R, Adams V, Sayeed S, Saputo J, Uzal FA, McClane BA, Rood JI. 2007. Epsilon-toxin plasmids of Clostridium perfringens type D are conjugative. J. Bacteriol. 189:7531–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed, vol 1–3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 22. Rood JI, Maher EA, Somers EB, Campos E, Duncan CL. 1978. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob. Agents Chemother. 13:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rood JI. 1983. Transferable tetracycline resistance in Clostridium perfringens strains of porcine origin. Can. J. Microbiol. 29:1241–1246 [DOI] [PubMed] [Google Scholar]
- 24. O'Connor JR, Lyras D, Farrow KA, Adams V, Powell DR. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61:1335–1351 [DOI] [PubMed] [Google Scholar]
- 25. Roberts I, Holmes WM, Hylemon PB. 1986. Modified plasmid isolation method for Clostridium perfringens and Clostridium absonum. Appl. Environ. Microbiol. 52:197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scott PT, Rood JI. 1989. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 82:327–333 [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Miyakawa ME, Sayeed S, Fisher DJ, Poon R, Adams V, Rood JI, McClane BA, Saputo J, Uzal FA. 2007. Development and application of an oral challenge mouse model for studying Clostridium perfringens type D infection. Infect. Immun. 75:4282–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diab SS, Kinde H, Moore J, Shahriar MF, Odani J, Anthenill L, Songer G, Uzal FA. 2012. Pathology of Clostridium perfringens type C enterotoxemia in horses. Vet. Pathol. 49:255–263 [DOI] [PubMed] [Google Scholar]
- 29. Bueschel D, Jost B, Billington S, Trinh H, Songer G. 2003. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121–129 [DOI] [PubMed] [Google Scholar]
- 30. Fernandez Miyakawa ME, Uzal FA. 2013. The early effects of Clostridium perfringens type D epsilon toxin in ligated intestinal loops of goats and sheep. Vet. Res. Comm. 27:231–241 [DOI] [PubMed] [Google Scholar]