Abstract
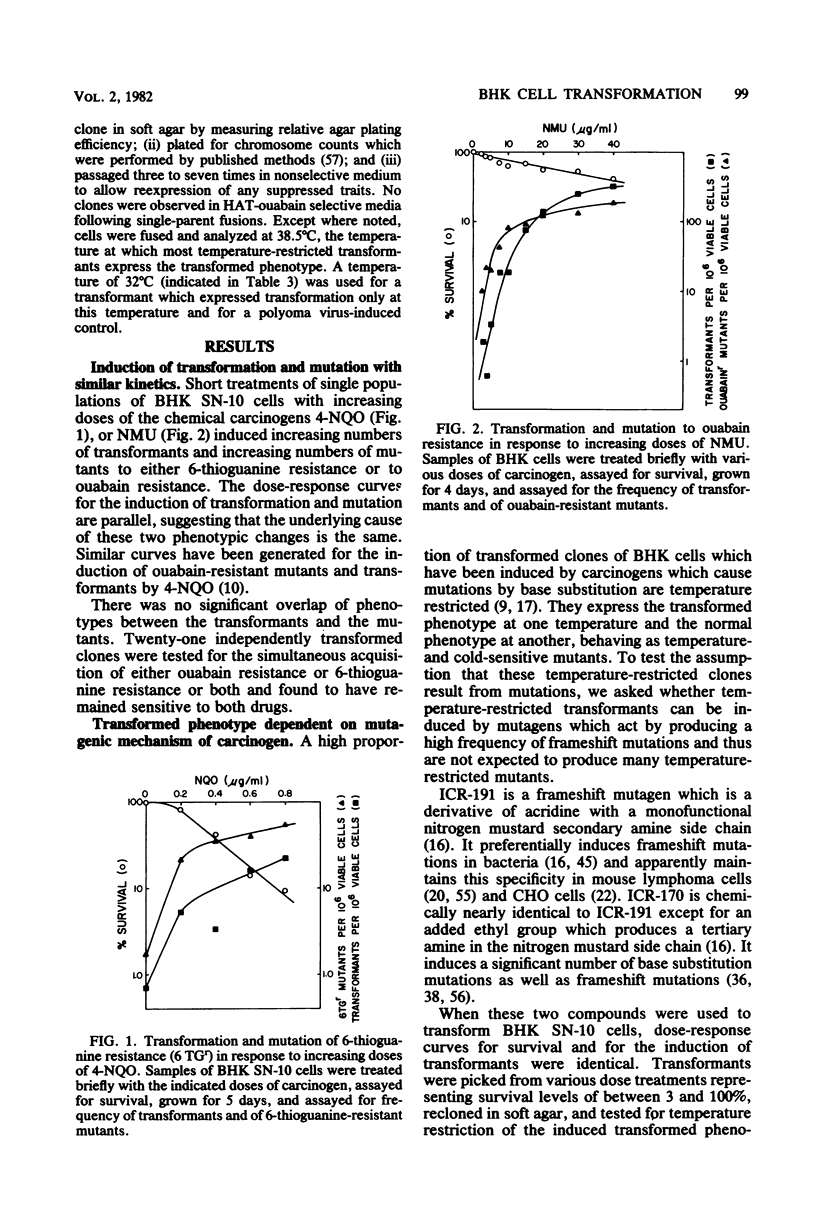
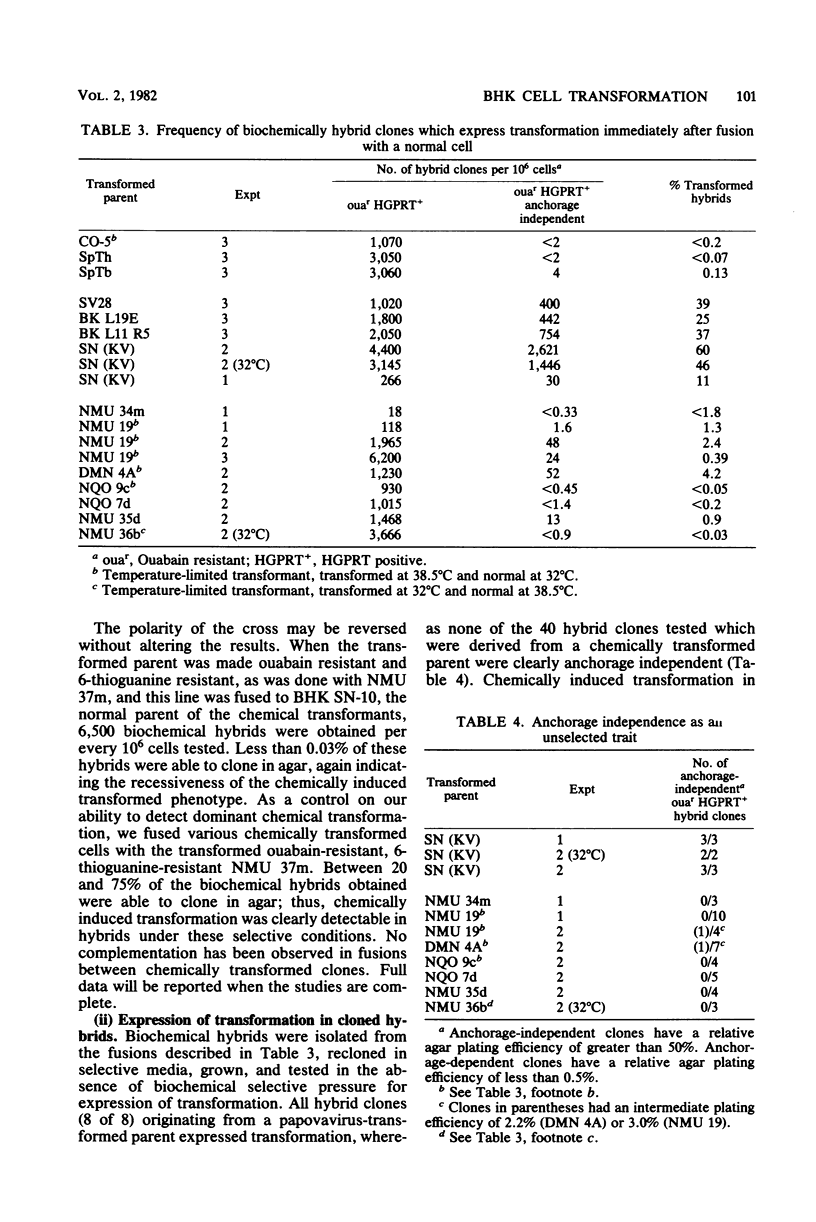
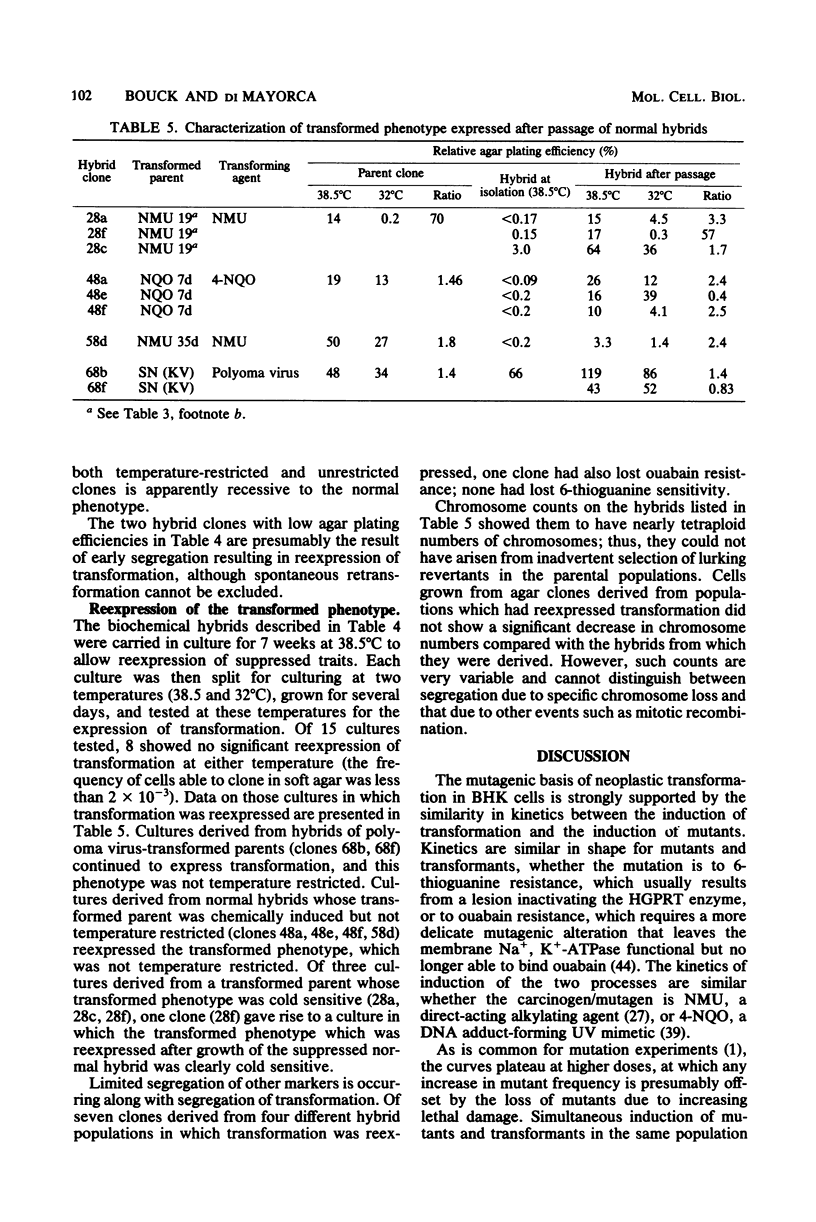
Treatment of BHK cells with mutagenic carcinogens induced neoplastic transformation in a single step. This transformation displayed the characteristics expected for a recessive mutation. Increasing doses of carcinogens induced transformants with kinetics similar to the kinetics with which they induced 6-thioguanine-resistant or ouabain-resistant mutants in the same population of cells. Transformants with temperature-restricted phenotypes were easily induced by carcinogens which cause mutations by base changes, but when ICR frameshift mutagens were used, the proportion of temperature-limited transformants was inversely related to the frequency with which a particular mutagen induced frameshift mutations. In hybrids between pseudodiploid isogenic strains of normal and transformed BHK cells, transformation was expressed as a dominant trait when the transformed parent was induced by a papovavirus, but was suppressed as a recessive trait when the transformed parent arose spontaneously or was chemically induced. Segregation of transformation was observed upon growth of suppressed normal hybrids, and the transformed phenotype which was reexpressed was in most cases characteristics of the original transformed parent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Turnbull D., Harcourt S. A., Lehmann A. R., Colella C. M. A comparison of the 8-azaguanine and ouabain-resistance systems for the selection of induced mutant Chinese hamster cells. Mutat Res. 1975 Dec;33(2-3):261–278. doi: 10.1016/0027-5107(75)90202-x. [DOI] [PubMed] [Google Scholar]
- Avilès D., Jami J., Rousset J. P., Ritz E. Tumor x host cell hybrids in the mouse: chromosomes from the normal cell parent maintained in malignant hybrid tumors. J Natl Cancer Inst. 1977 May;58(5):1391–1399. doi: 10.1093/jnci/58.5.1391. [DOI] [PubMed] [Google Scholar]
- Avilès D., Ritz E., Jami J. Chromosomes in tumors derived from mouse tumor x diploid cell hybrids obtained in vitro. Somatic Cell Genet. 1980 Mar;6(2):171–186. doi: 10.1007/BF01538794. [DOI] [PubMed] [Google Scholar]
- Barrett J. C. A preneoplastic stage in the spontaneous neoplastic transformation of syrian hamster embryo cells in culture. Cancer Res. 1980 Jan;40(1):91–94. [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Relationship between somatic mutation and neoplastic transformation. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3297–3301. doi: 10.1073/pnas.75.7.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Tsutsui T., Ts'o P. O. Neoplastic transformation induced by a direct perturbation of DNA. Nature. 1978 Jul 20;274(5668):229–232. doi: 10.1038/274229a0. [DOI] [PubMed] [Google Scholar]
- Berwald Y., Sachs L. In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst. 1965 Oct;35(4):641–661. [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Somatic mutation as the basis for malignant transformation of BHK cells by chemical carcinogens. Nature. 1976 Dec 23;264(5588):722–727. doi: 10.1038/264722a0. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Urlaub G. Chromosome-wide event accompanies the expression of recessive mutations in tetraploid cells. Science. 1975 Mar 21;187(4181):1091–1093. doi: 10.1126/science.1167702. [DOI] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Creech H. J., Preston R. K., Peck R. M., O'Connell A. P. Antitumor and mutagenic properties of a variety of heterocyclic nitrogen and sulfur mustards. J Med Chem. 1972 Jul;15(7):739–746. doi: 10.1021/jm00277a011. [DOI] [PubMed] [Google Scholar]
- Di Mayorca G., Greenblatt M., Trauthen T., Soller A., Giordano R. Malignant transformation of BHK21 clone 13 cells in vitro by nitrosamines--a conditional state. Proc Natl Acad Sci U S A. 1973 Jan;70(1):46–49. doi: 10.1073/pnas.70.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S. A., Worton R. G. Chromosome loss is responsible for segregation at the HPRT locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1977 Sep;3(5):539–551. doi: 10.1007/BF01539124. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Mondal S., Heidelberger C. Probabilistic view of the transformation of cultured C3H/10T1/2 mouse embryo fibroblasts by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7272–7276. doi: 10.1073/pnas.77.12.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U., Coffino P. Mutagenesis in S49 mouse lymphoma cells: induction of resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1977 Feb;74(2):679–683. doi: 10.1073/pnas.74.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. J., Harris H. Tumorigenicity of cells transformed by Simian virus 40 and of hybrids between such cells and normal diploid cells. J Cell Sci. 1979 Apr;36:223–240. doi: 10.1242/jcs.36.1.223. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic markers for quantitative mutagenesis studies in Chinese hamster ovary cells: characteristics of some recently developed selective systems. Mutat Res. 1980 Jan;69(1):113–126. doi: 10.1016/0027-5107(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Howell N., Sager R. Noncoordinate expression of SV40-induced transformation and tumorigenicity in mouse cell hybrids. Somatic Cell Genet. 1979 Jan;5(1):129–143. doi: 10.1007/BF01538791. [DOI] [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- Jami J., Ritz E. Tumor-associated transplantation antigens in immune rejection of mouse malignant cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2130–2134. doi: 10.1073/pnas.72.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha K. K., Cacciapuoti J., Ozer H. L. Expression of transformation in cell hybrids. II. Nonsuppression of the transformed phenotype in hybrids between a chemically transformed and nontransformed derivatives of Balb/3T3. J Cell Physiol. 1978 Nov;97(2):147–152. doi: 10.1002/jcp.1040970203. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics and etiology of human cancer. Adv Hum Genet. 1977;8:1–66. doi: 10.1007/978-1-4615-8267-0_1. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Malling H. V. The mutagenicity of the acridine mustard (ICR-170) and the structurally related compounds in Neurospora. Mutat Res. 1967 May-Jun;4(3):265–274. doi: 10.1016/0027-5107(67)90021-8. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Suppression of the transformed phenotype with retention of the viral 'src' gene in cell hybrids between Rous sarcoma virus-transformed rat cells and untransformed mouse cells. Exp Cell Res. 1980 Jun;127(2):373–384. doi: 10.1016/0014-4827(80)90442-5. [DOI] [PubMed] [Google Scholar]
- Munz P., Leupold U. Characterization of ICR-170-induced mutations in Schizosaccharomyces pombe. Mutat Res. 1970 Feb;9(2):199–212. doi: 10.1016/0027-5107(70)90058-8. [DOI] [PubMed] [Google Scholar]
- Nagao M., Sugimura T. Molecular biology of the carcinogen, 4-nitroquinoline 1-oxide. Adv Cancer Res. 1976;23:131–169. doi: 10.1016/s0065-230x(08)60545-x. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Hsie A. W. Chemical mutagenesis of mammalian cells can be quantified. Nature. 1977 Oct 27;269(5631):815–817. doi: 10.1038/269815a0. [DOI] [PubMed] [Google Scholar]
- Olive D. M., Lampert M., Major E. O. Comparison of wild-type BK virus DNA and BK virion DNA rescued from virus-transformed BHK cells. Virology. 1980 May;103(1):1–10. doi: 10.1016/0042-6822(80)90121-x. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Jha K. K. Malignancy and transformation: expression in somatic cell hybrids and variants. Adv Cancer Res. 1977;25:53–93. doi: 10.1016/s0065-230x(08)60632-6. [DOI] [PubMed] [Google Scholar]
- Poiley J. A., Raineri R., Pienta R. J. Two-stage malignant transformation in hamster embryo cells. Br J Cancer. 1979 Jan;39(1):8–14. doi: 10.1038/bjc.1979.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Baker R. M. (Na, K)ATPase activity in membrane preparations of ouabain-resistant HeLa cells. Biochemistry. 1977 Nov 15;16(23):5163–5168. doi: 10.1021/bi00642a600. [DOI] [PubMed] [Google Scholar]
- Roth J. R. Frameshift mutations. Annu Rev Genet. 1974;8:319–346. doi: 10.1146/annurev.ge.08.120174.001535. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: IV. Chromosome reduction and marker segregation in progeny clones from Chinese hamster cell hybrids. Somatic Cell Genet. 1979 Jul;5(4):491–502. doi: 10.1007/BF01538883. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles J. A. A method for detecting carcinogenic organic chemicals using mammalian cells in culture. Br J Cancer. 1977 Nov;36(5):558–563. doi: 10.1038/bjc.1977.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer C., Szpirer J. Suppression of the transformed phenotype of hepatoma cells after hybridization with normal diploid fibroblasts. Exp Cell Res. 1980 Feb;125(2):305–312. doi: 10.1016/0014-4827(80)90126-3. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]
- Wims L. A., Morrison S. L. ICR-191 and ethyl methanesulfonate induced mutagenesis at the immunoglobulin locus in the Y5606 cultured myeloma cell line. Mutat Res. 1981 Apr;81(2):215–228. doi: 10.1016/0027-5107(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Woodruff R. C., Gander R. M. The induction of temperature-sensitive mutations in Drosophila melanogaster by the acridine mustard ICR-170. Mutat Res. 1974 Dec;25(3):337–345. doi: 10.1016/0027-5107(74)90062-1. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C., Campbell C. E. Marker segregation without chromosome loss at the emt locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1980 Mar;6(2):199–213. doi: 10.1007/BF01538796. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]



