Abstract
Pseudomonas aeruginosa, a Gram-negative opportunistic human pathogen, is a frequent cause of severe hospital-acquired infections. Effectors produced by the type III secretion system disrupt mammalian cell membrane trafficking and signaling and are integral to the establishment of P. aeruginosa infection. One of these effectors, ExoS, ADP-ribosylates several host cell proteins, including Ras and Rab GTPases. In this study, we demonstrated that Rab5 plays a critical role during early stages of P. aeruginosa invasion of J774-Eclone macrophages. We showed that live, but not heat-inactivated, P. aeruginosa inhibited phagocytosis and that this occurred in conjunction with downregulation of Rab5 activity. Inactivation of Rab5 was dependent on ExoS ADP-ribosyltransferase activity, and in J744-Eclone cells, ExoS ADP-ribosyltransferase activity caused a more severe inhibition of phagocytosis than ExoS Rho GTPase activity. Furthermore, we found that expression of Rin1, a Rab5 guanine exchange factor, but not Rabex5 and Rap6, partially reversed the inactivation of Rab5 during invasion of live P. aeruginosa. These studies provide evidence that live P. aeruginosa cells are able to influence their rate of phagocytosis in macrophages by directly regulating activation of Rab5.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen capable of causing acute and chronic infections in immunocompromised individuals. P. aeruginosa infection is also a serious problem for patients hospitalized with AIDS, cancer, cystic fibrosis, and burns (1–4). The type III secretion (T3S) system allows Gram-negative bacteria to produce and translocate effector proteins into the cytoplasm of host cells. While the T3S system is conserved among distantly related pathogens, secreted effectors are pathogen specific (5). The secretion and translocation of T3S effectors into the cytosol of animal or plant cells initiates a biochemical cross talk between pathogen and host (6). Four T3S effectors have been identified in P. aeruginosa: ExoS, ExoT, ExoU, and ExoY. Each effector functions differently to help create an environment inside the human host that favors bacterial survival and propagation in tissue.
T3S effectors contribute to the ability of P. aeruginosa to invade tissue by breaking down physical barriers, damaging host cells, and conferring resistance to phagocytosis and host immune defenses (7, 8). Specifically, ExoS and ExoT are bifunctional effectors that have 76% homology, and both include Rho GTPase-activating (GAP) and ADP-ribosyltransferase (ADPr) activities (9). The GAP activities of ExoS and ExoT function similarly to inhibit P. aeruginosa internalization by inactivating Rho GTPases, Rho, Rac, and Cdc42, which regulate actin cytoskeleton structure (10–15). ExoS ADPr activity targets multiple specific substrates, including Ras family proteins, such as Ras, RalA, Rac1, and Rabs, to interrupt cell signaling (16–18). The substrate specificity of ExoT ADPr activity differs from that of ExoS ADPr activity and is limited to Crk-I (CT10 regulator of kinase I) and Crk-II adaptor proteins, which integrate protein tyrosine kinase signal transduction pathways (19–21). ExoU has been characterized as a necrotizing toxin with phospholipase activity (22) and has been found to block phagocyte-mediated clearance of infection (23). ExoY has adenylate cyclase activity and does not appear to play a major role in P. aeruginosa pathogenesis (24, 25).
Rab proteins, including Rab5, Rab7, Rab8, and Rab11, are known to be ADP-ribosylated by ExoS in vitro and in vivo (26). Rab proteins are a family of small GTP-binding proteins that regulate intracellular membrane trafficking of several pathogens, including Salmonella enterica serovar Typhimurium (27–29), Mycobacterium spp. (30), and Listeria monocytogenes (31). Rab5 also functions in the phagocytosis of IgG opsonized particles (32). In vitro studies have demonstrated that ExoS ADP-ribosylation of Rab5 diminishes the interaction between Rab5 and early endosome antigen 1 (EEA1) and fluid-phase uptake in intact cells Rab5, and its guanine exchange factors (GEFs), which include Rabex-5, Rin1, and Rap6 (also known as GAPex5) (33–36), play a critical role in intracellular membrane trafficking (37), including phagocytosis of apoptotic cells (38). Although Rab5 was found to be present on phagosomes following phagocytosis of several bacterial pathogens and latex beads, the functional role for Rab5 in phagocytosis of P. aeruginosa is not fully understood.
In this study, we demonstrate that Rab5 activity was regulated during early stages of P. aeruginosa phagocytosis in J774-Eclone macrophages. Expression of wild-type Rab5 (Rab5:WT) or a Rab5:Q79L, a GTP hydrolysis-defective mutant, increased invasion of heat-inactivated P. aeruginosa, while expression of Rab5:S34N, a GTP-binding-defective mutant, decreased phagocytosis. Rab5 was activated during invasion of heat-inactivated P. aeruginosa but was inactivated during invasion of live P. aeruginosa. Expression of constitutively active Rab5:Q79L overcame the suppressive effects of live P. aeruginosa on phagocytosis. Inactivation of Rab5 by live P. aeruginosa was dependent on ExoS ADPr activity, and in J774-Eclone cells, ExoS ADPr activity caused a more severe inhibition of phagocytosis than ExoS GAP activity. Finally, we found that expression of Rin1, a Rab5 GEF, interfered with the ability of live P. aeruginosa to inactivate Rab5. The ability of live P. aeruginosa to regulate phagocytosis by altering Rab5 activation provides further insight into how P. aeruginosa is able to manipulate the host during infection.
MATERIALS AND METHODS
Materials.
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. Primary and secondary antibodies used in immunoblotting were purchased from Cell Signaling Technology Inc. (Danvers, MA). Culture supplies were purchased from Invitrogen Life Technologies (Carlsbad, CA).
Cell culture.
J774-Eclone cells (39) were maintained under a 5% CO2 atmosphere in Dulbecco's minimum essential medium (DMEM), supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml of streptomycin. J774-Eclone cells were used for all P. aeruginosa phagocytosis studies. The Platinum-E retroviral packaging cell line (Plat-E cells) was purchased from Cell Biolabs, Inc. (San Diego, CA) and maintained in DMEM, 10% FCS, 1 μg/ml puromycin, 10 μg/ml blasticidin, 100 U/ml penicillin, and 100 mg/ml of streptomycin.
Bacterial strains.
P. aeruginosa strains PAO1 (a derivative of the original Australian isolate PAO), PA103 (which expresses ExoT and ExoU and is naturally devoid of ExoS and ExoY), and isogenic mutants of strain PA103, including PA103ΔExoU (PA103ΔU) (which expresses ExoT) and PA103 exoU exoT::Tc (PA103ΔTΔU) (a T3S effector null mutant), were provided by Dara Frank (Medical College of Wisconsin, Milwaukee, WI). P. aeruginosa P103ΔUΔT strains expressing (i) the pUCP vector (−), (ii) wild-type ExoS [ExoS(WT)], (iii) ExoS with active RhoGAP [ExoS(RhoG+)] but lacking ADPr activity due to E379A/E381A mutations, (iv)) ExoS with active ADPr activity [ExoS(ADPr+)] but lacking RhoGAP activity due to a R146A mutation, or (v) ExoS that lacks catalytic activity [ExoS(RhoG−/ADPr−)] due to E379A/E381A/R146A mutations were previously described (40). Bacteria were grown at 37°C in Luria broth with appropriate antibiotics. Prior to assay of phagocytosis, bacteria were grown to late log phase, diluted to a concentration of 107 CFU/ml, and added to cells at the indicated multiplicities of infection (MOIs).
Construction of recombinant pMX-puro retroviruses and cell lines.
cDNAs of Rab4, Rab5, Rab7, Rin1, Rabex5, and Rap6 were subcloned into the pMX-puro vector as described previously (41). cDNAs of ExoS and ExoS deletion mutants containing RhoGAP (1 to 232 amino acids) (ExoS:rRhoG) or ExoS ADPr (232 to 453 amino acids) (ExoS:ADPr) domains were subcloned into the pMX-puro vector at BamHI and NotI sites, respectively. The cDNAs were used in the Fugene6-mediated transfection of 90% confluent Plate-E cell monolayers. Cells were maintained at 37°C, and the medium containing released virus was harvested at 48 h after transfection. Viral stocks were aliquoted and frozen at −80°C until use. Cell lines were generated by infecting J774-Eclone cells with retrovirus encoding green fluorescent protein (GFP), Rab4, Rab5, Rab7, Rin1, Rabex5, Rap6, and ExoS domains, essentially as previously described (41).
Phagocytosis assay.
P. aeruginosa strains were cultured to late log phase and washed with phosphate-buffered saline (PBS) (pH 7.3) and then with NaHCO3 (pH 9) three times each. After washing, Alexa Fluor-594 (Invitrogen, Carlsbad, CA) was used to label live or heat-inactivated bacteria for 2 h at room temperature while protected from light. J774-Eclone cells (105 cells/ml) were plated on coverslips in 6-well plates and incubated overnight. Cells were washed once with PBS and then twice with Hanks balanced salt solution (HBSS)–2% bovine serum albumin BSA. Bacteria were added at a ratio of 200:1 and incubated for 30 min at 4°C. To initiate bacterial internalization, plates were placed in a 37°C water bath for 5 to 60 min. After this time, cells were placed on ice, washed three times with PBS, and then fixed for 20 min at room temperature using 3.7% paraformaldehyde. After fixation, cells were washed three times with PBS, incubated with 1% Triton X-100 at room temperature for 15 min, and incubated with 4′,6-diamidino-2-phenylindole (Roche Applied Science, Indianapolis, IN) to stain the nucleus. Coverslips were removed from the wells, washed, and mounted with Mowiol fluorescence mounting medium. The number of bacteria per cell was enumerated at a magnification of ×100 using a phase-contrast inverted fluorescence microscope. Two hundred cells were counted per slide, and each experiment was repeated three times. The phagocytic index refers to the number of bacteria inside each cell. An antibiotic protection assay, described previously (42), was used to analyze bacterial survival within macrophages. For this assay, following the indicated time of phagocytosis, cells were washed 3 times with PBS, and extracellular bacteria were killed by incubating cells with growth medium containing amikacin (400 μg/ml). After washing, cells were incubated at 37°C for an additional 30 and 60 min to examine bacterial survival within macrophages. Finally, cells were washed with PBS and lysed with 0.5% Triton X-100, lysates were plated on LB agar and incubated overnight, and bacterial colonies were enumerated.
Isolation of purified phagosomes.
Phagosome containing live or dead P. aeruginosa were isolated as described by Mukherjee et al. (27). Briefly, J774-Eclone cells were seeded at 1 ×108 cells/ml, and live or heat-inactivated P. aeruginosa was added to cells at a concentration of 2 × 109 bacteria/ml, followed by synchronization of cells and bacteria at 4°C for 1 h in HBSS buffer (27). Cells were then treated with prewarmed HBSS medium and incubated for 5 min at 37°C. Bacterial uptake was stopped by the addition of ice-cold HBSS medium. Unbound bacteria were removed by washing cells three times, with centrifugation at low speed (300 × g for 5 min) between washes. Washed cells were resuspended at a concentration of 2 × 108 cells/ml in homogenization buffer (250 mM sucrose, 0.5 mM EGTA, 20 mM HEPES-KOH, pH 7.2) and homogenized in a ball-bearing homogenizer at 4°C. Homogenates were centrifuged at a low speed (400 × g for 5 min) at 4°C to remove nuclei and unbroken cells. To obtain the phagosomal fraction, postnuclear supernatants were diluted with homogenization buffer (1:3), followed by centrifugation at 12,000 × g for 15 s at 4°C (27). Phagosomal fractions were resuspended in 100 ml of homogenization buffer containing protease inhibitors, loaded on a 1-ml 12% sucrose cushion, and centrifuged at 800 × g for 45 min at 4°C, and purified phagosomes were recovered from the bottom of the tube. Bacterial viability in the phagosomes was determined following selective lysis of the phagosomal membrane with solubilization buffer (50 mM Tris-HCl, 150 mM NaCl, and 0.5% NP-40) and plating lysates on LB agar plates. Bacterial colonies formed after overnight incubation were quantified as previously described (43).
Cell lysis and immunoblotting.
For immunoblot analysis, J774-Eclone cells were washed twice with PBS and then lysed with radioimmunoprecipitation assay (RIPA) cell lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) in the presence of protease and phosphatase inhibitors. Lysates were collected with cell scrapers and cleared by centrifugation. Prior to SDS-PAGE, cell lysates were resuspended in SDS sample buffer (60 mM Tris-HCl, 1% [wt/vol] SDS, 10% glycerol, 0.05% [wt/vol] bromophenol blue, pH 6.8, with 2% β-mercaptoethanol). Samples were subjected to SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting. Nitrocellulose membranes were incubated with blocking solution (Tris-buffered saline [TBS] containing 0.1% Tween 20 and 5% BSA) and were probed with the indicated antibodies.
RNAi sequences and transfection.
RNA interference (RNAi) sequences directed against mouse Rin1 (5′-UUAUACAUUUGCUUCACACCUAAGC-3′), mouse Rabex5 (5′-UUUAUAGAGACGCGUCAUGAUGUGC-3′), mouse Rap6 (5′-AAGAATCGATTACCTATAGCA-3′), mouse Rab5A (5′-AAGCACAGTCCTATGCAGATG-3′), mouse Rab5B (5′-AATCCGTGTGTTTAGATGACA-3′), or mouse Rab5C (5′-AAGCAGCCATTGTGGTCTATG-3′) were designed and synthesized by Ambion (Austin, TX). A scrambled RNAi sequence was designed as a control (5′-CACCUAAUCCGUGGUUCAA-3′). Prior to RNAi sequence transfection, J774-Eclone cells were plated in growth medium without antibiotics at 30 to 50% confluence. Transfection of RNAi sequences (20 nM final concentration) was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), as specified by Invitrogen. After transfection, cells were used for either immunoblotting, phagocytosis, or pulldown assays.
In vitro pulldown assays.
Cells were lysed using a buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM dithiothreitol (DTT), 5 mM MgCl2, 5% glycerol, and 1% Triton X-100, supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were incubated with 100 ml glutathione beads containing 10 μg of glutathione S-transferase (GST)–EEA1, followed by rocking for 1 h at 4°C. After incubation, beads were washed three times with PBS. The pulldown mixtures were subjected to SDS-PAGE and analyzed by immunoblotting using Rab5 antibodies.
Image quantification.
NIH Image J64 was used to quantify Western blots after images were scanned at a grayscale amplification of 600 dpi. Digital images of the Western blots from cell lines were captured and loaded into ImageJ64, and Rab5-specific bands, along with α/β-tubulin bands, were assessed in each sample using the Analyze → Gels function, which allows for background correction. The ratio of Rab5 signal to α/β-tubulin signal was calculated for each sample and served as an index of Rab5 expression. The indices of expression for other proteins examined in this study were derived in a similar manner.
Statistical analysis.
All samples in this study were analyzed in duplicate, and each experiment was repeated three times. Values represent the mean ± standard error of the mean (SEM) from three independent experiments. To compare two groups, Student's t test was used. A P value of < 0.05 was considered statistically significant.
RESULTS
Rab5 is required for phagocytosis of heat-inactivated P. aeruginosa by macrophages.
Previous studies have found that Rab GTPases, including Rab5, are manipulated by bacteria during phagocytosis (44). To investigate the involvement of Rab GTPases in P. aeruginosa phagocytosis, the pMX-puro retroviral expression system was used to express Rab proteins in J774-Eclone macrophages. Initially, to examine if the retroviral expression system altered phagocytosis of P. aeruginosa, control (nontransfected) or GFP-transfected J774-Eclone cells were incubated at 37°C with heat-inactivated strain PAO1 P. aeruginosa at a ratio of 200:1, and the phagocytic index was monitored (as described in Materials and Methods) over a 1-h period. Figure 1A shows that the rate of phagocytosis of heat-inactivated P. aeruginosa was not altered in GFP-expressing cells compared with nontransfected control cells. Similarly, the phagocytic index of heat-inactivated P. aeruginosa was not altered relative to increasing ratio of bacteria to cells in GFP-expressing cells compared with nontransfected control cells (Fig. 1A, inset). We concluded from these studies that phagocytosis of heat-inactivated P. aeruginosa by J774-Eclone macrophages was time and bacterial concentration dependent and was not altered by the pMX-puro retroviral expression system.
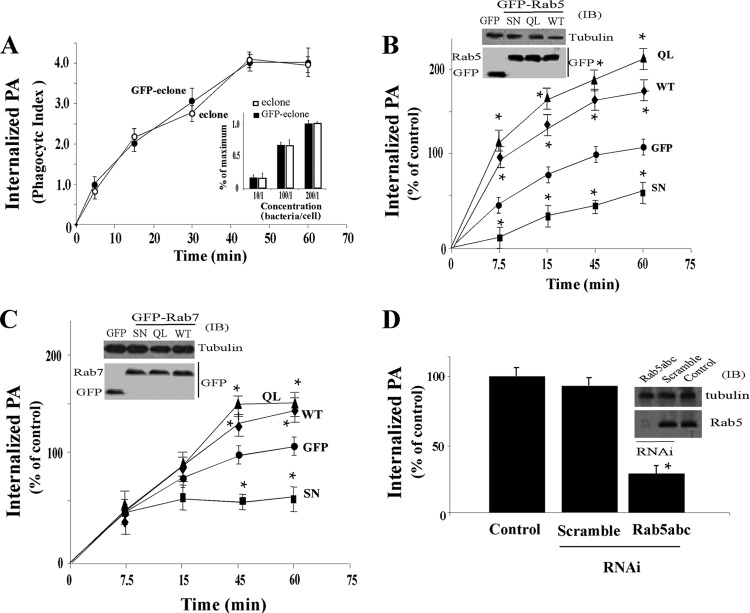
Fig 1.
Effect of Rab proteins on invasion of heat-inactivated P. aeruginosa in macrophages. (A) J774-Eclone cells alone or J774-Eclone cells expressing GFP were incubated in the presence of heat-inactivated strain PAO1 P. aeruginosa at a ratio of 200:1 (bacteria/cell). At the indicated times during a 60-min incubation period, cells were washed and fixed, and the phagocytic index of P. aeruginosa (PA) was determined as described in Materials and Methods. Inset, J774-Eclone or GFP–J774-Eclone cells were incubated with the indicated ratio of heat-inactivated P. aeruginosa for 60 min, and the phagocytic index was determined and is expressed as a percentage of the maximum, relative to the phagocytic index at the highest bacteria/cell ratio (200/1). (B) J774-Eclone cells expressing GFP or Rab5:WT (WT), Rab5:Q79L (QL), or Rab5:S34N (SN) were incubated with heat-inactivated P. aeruginosa at a ratio of 200:1, and the phagocytic index was determined at the indicated times and is expressed as percent internalization relative to the GFP control at 60 min. Inset, cells expressing the indicated constructs were lysed and subjected to immunoblotting (IB) with anti-GFP or antitubulin antibodies. (C) J774-Eclone cells expressing GFP or Rab7:WT, Rab7:Q67L (QL), or Rab7:S22N (SN) were incubated with heat-inactivated P. aeruginosa at a ratio of 200:1, and the phagocytic index was determined at the indicated times and is expressed as the percentage of the GFP control value at 60 min. Inset, cells expressing the indicated constructs were lysed and subjected to immunoblotting with anti-GFP or antitubulin antibodies. (D) Nontransfected J774-Eclone cells (control), or J774-Eclone cells transfected with a scramble RNAi sequence or RNAi sequences against Rab5 isoforms (Rab5 a, b, and c) were incubated with heat-inactivated P. aeruginosa at a ratio of 200:1 for 60 min, and the phagocytic index was determined and expressed as a percentage of the value for control cells. Inset, cells transfected with the indicated RNAi sequences were lysed and subjected to immunoblotting with antitubulin or anti-Rab5 antibodies. Results represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from control values (*, P < 0.05).
We next examined the effect of increased expression of Rab5 proteins on phagocytosis of heat-inactivated P. aeruginosa by J774-Eclone macrophages. Cells expressing GFP alone, GFP–wild-type Rab5 (Rab5:WT), GFP-Rab5:Q79L (QL) (a constitutively active GTP hydrolysis-defective mutant), or GFP-Rab5:S34N (a constitutively inactive GTP-binding-defective mutant) were incubated with heat-inactivated P. aeruginosa, and the phagocytic index was monitored over a 60-min period. Expression of Rab5:WT and the Rab5:Q79L mutant increased the rate of phagocytosis of heat-inactivated P. aeruginosa in relation to internalization of GFP-expressing control cells at 60 min (Fig. 1B). In comparison, expression of the Rab5:S34N mutant decreased the rate of phagocytosis of heat-inactivated P. aeruginosa compared to that of GFP-expressing cells (Fig. 1B). Enhanced expression of the indicated Rab5 construct was confirmed by immunoblot analysis in lysates of J774-Eclone cells, using tubulin as a reference protein (Fig. 1B, inset).
To examine the roles of other Rab GTPases in P. aeruginosa phagocytosis, cells expressing GFP alone, Rab7:WT, the Rab7:Q67L GTP hydrolysis-defective mutant, or the Rab7:S22N GTP-binding-defective mutant were incubated with heat-inactivated P. aeruginosa and monitored for phagocytosis over a 60-min period. The phagocytic index of J744-Eclone cells expressing Rab7:WT and Rab7:Q67L was greater than that of GFP-expressing cells after 30 min, while phagocytosis in cells expressing Rab7:S22N was halted beyond 15 min (Fig. 1C). Enhanced expression of the respective Rab7 construct in transfected J774-Eclone cells was confirmed by immunoblot analysis (Fig. 1C, inset). Unlike the case for Rab5 or Rab7, phagocytosis of heat-inactivated P. aeruginosa was not altered by transfection of Rab4 constructs. In analyses of Rab4, the relative phagocytic indices of J774-Eclone cells after 30 min were as follows: GFP, 100% ± 7%; Rab4:WT, 103% ± 6%; Rab4:Q67L, 98% ± 5%; and Rab4:S22N, 95% ± 5%.
We also examined the effect of depletion of Rab5 proteins by RNA interference (RNAi) on the uptake of heat-inactivated P. aeruginosa. Based on immunoblot analysis, greater than 95% of Rab5 protein was depleted by RNAi duplexes that targeted all three Rab5 isoforms (Fig. 1D, inset). Depletion of all, but not individual, Rab5 isoforms (data not shown) resulted in an 80% ± 6% reduction of internalization of heat-inactivated P. aeruginosa compared with that for control cells that were not RNAi treated or treated with a scramble RNAi sequence (Fig. 1D).
Collectively, these results show that Rab5 regulates early events in the phagocytosis of heat-inactivated P. aeruginosa and that effects of Rab5 on P. aeruginosa internalization differ kinetically from those of Rab7. In addition, the finding that constitutively active Rab5:Q79L increased the rate of phagocytosis while Rab5:S34N decreased the rate of phagocytosis provides evidence that Rab5 activation plays a role in the uptake of heat-inactivated P. aeruginosa in J774-Eclone macrophages.
Live P. aeruginosa blocks Rab5 activation in macrophages.
Evidence that Rab5 plays a role in phagocytosis of heat-inactivated P. aeruginosa led us to investigate the role of Rab5 during phagocytosis of live P. aeruginosa. Unexpectedly, internalization of live strain PAO1 into J774-Eclone macrophages was found to be 75% ± 4% lower than internalization of heat-inactivated or dead P. aeruginosa after 5, 15, or 30 min of infection.
We then examined whether enhanced expression of Rab5 proteins could overcome the suppressive effects of live P. aeruginosa on phagocytosis. For these studies, J774-Eclone cells expressing a GFP control or Rab5:WT, Rab5:Q79L, or Rab5:S34N were incubated with live or heat-inactivated P. aeruginosa and phagocytosis was analyzed after 15 min. A decrease in the internalization of live P. aeruginosa was observed in cells expressing GFP alone, Rab5:WT, or Rab5:S34N compared to internalization of heat-inactivated P. aeruginosa (Fig. 2B). However, in the cells expressing Rab5:Q79L, internalization of live P. aeruginosa closely approximated that of the heat-inactivated P. aeruginosa. Phagocytosis of live P. aeruginosa was also inhibited by silencing all Rab5 isoforms (Fig. 2C) but not by silencing individual Rab5 isoforms (not shown). Collectively, these results demonstrate that Rab5 exerts different effects on the internalization of live or heat-inactivated P. aeruginosa and that these differences are nullified by the expression of constitutively activated Rab5. The finding that interference of expression of all, but not individual, Rab5 isoforms inhibits phagocytosis of live P. aeruginosa also highlights the cooperative and redundant regulation of P. aeruginosa phagocytosis by the three Rab5 isoforms.
Fig 2.
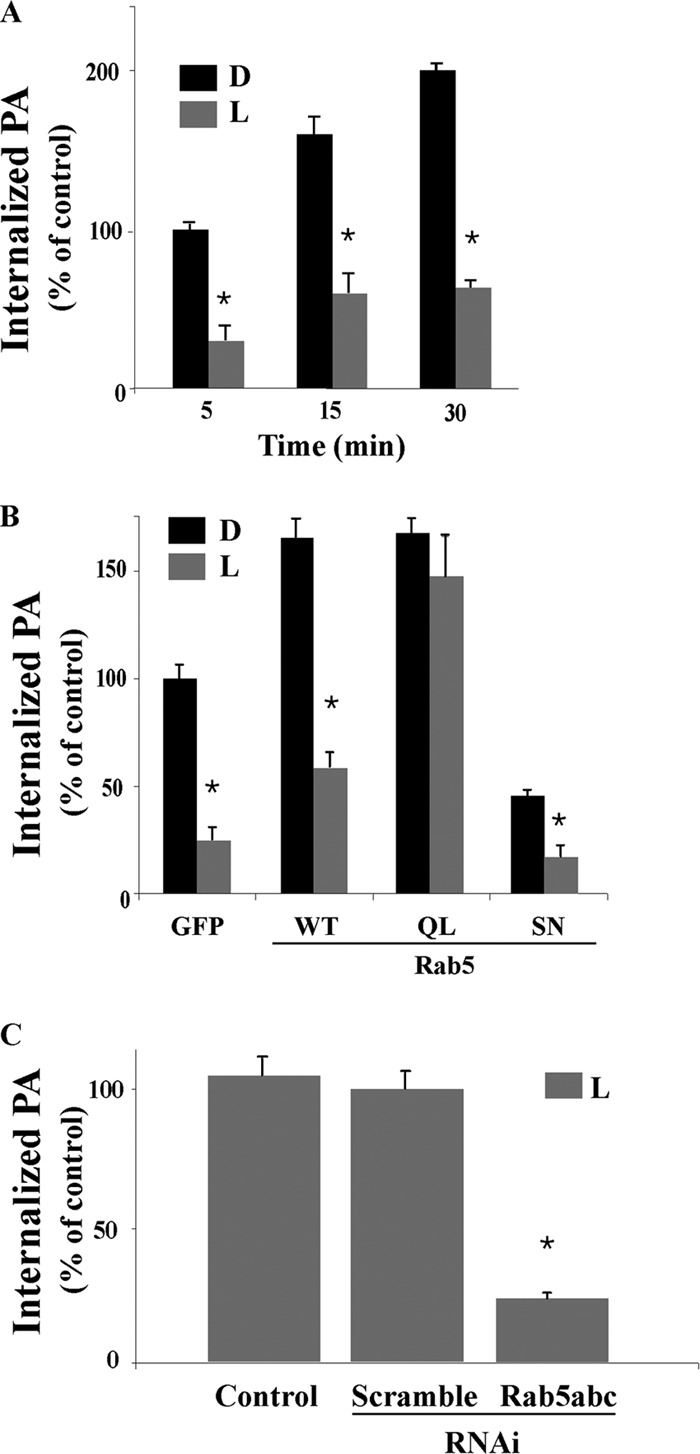
Effect of Rab5 on live P. aeruginosa invasion in macrophages. (A) J774-Eclone cells expressing GFP were incubated with live (L) or heat-inactivated, dead (D) strain PAO1 P. aeruginosa at a ratio of 200:1 for 5, 15, or 30 min. The phagocytic index was determined and is expressed as percent phagocytosis of heat-inactivated bacteria internalized in GFP control cells at 5 min. (B) J774-Eclone cells expressing GFP or Rab5:WT, Rab5:Q79L (QL), or Rab5:S34N (SN) were incubated with live or heat-inactivated P. aeruginosa at a ratio of 200:1 for 15 min at 37°C. After incubation, the phagocytic index was determined and is expressed as percent phagocytosis of heat-inactivated bacteria by GFP control cells at 15 min. (C) J774-Eclone cells alone (control) or cells transfected with a scramble RNAi sequence or RNAi sequences against Rab5 isoforms were incubated with heat-inactivated P. aeruginosa at a ratio of 200:1 for 30 min at 37°C. After incubation, the phagocytic index was determined and is expressed as the percent phagocytosis of control cells. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from control group values (*, P < 0.05).
Since the cycling of Rab5 between active and inactive states is integral to P. aeruginosa internalization, the question remains whether Rab5 activation is regulated by live P. aeruginosa as a defense mechanism against phagocytosis. To explore the ability of live P. aeruginosa to regulate Rab5 function, we first characterized the phagosomes carrying live or heat-inactivated P. aeruginosa during early phagocytosis. For these studies, cells were incubated with live or heat-inactivated strain PAO1 for 5 min at 37°C, and phagosomes containing P. aeruginosa were isolated and analyzed. Figure 3A shows that phagosomes containing live or heat-inactivated P. aeruginosa both recruited Rab5 protein on the phagosomal membrane but that Rab5 accumulation was significantly reduced in phagosomes containing live P. aeruginosa. Notably, Rab7 was not detected on phagosomes isolated after 5 min of internalization (Fig. 3A). A representative image showing relative levels of Rab5 and Rab7 in phagosomes, in relation to tubulin and actin reference proteins, is shown in Fig. 3A (inset).
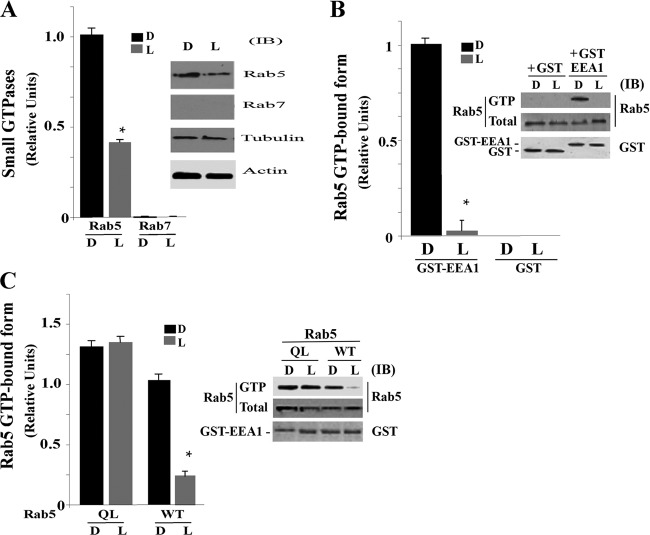
Fig 3.
Live P. aeruginosa inhibits Rab5 activation in macrophages. (A) J774-Eclone macrophages were incubated with live (L) or heat-inactivated (D) P. aeruginosa at a ratio of 200:1 for 5 min at 37°C. Cells were washed and resuspended in homogenization buffer, and phagosomes containing P. aeruginosa were isolated, as described in Materials and Methods. Phagosomal proteins were analyzed by immunoblot analysis and quantified by densitometry. Inset, representative immunoblot of isolated phagosomal proteins probed with anti-Rab5, anti-Rab7, antitubulin, or antiactin antibodies. (B) Cells were incubated with live or heat-inactivated P. aeruginosa as for panel A and then washed with ice-cold PBS, lysed, and incubated with glutathione beads in the presence of GST alone or GST-EEA1 at 4°C for 60 min. After incubation, the beads were washed and GTP-bound activated Rab5 was analyzed by immunoblotting with anti-Rab5. Inset, representative immunoblot of samples probed with anti-Rab5 or anti-GST antibodies. (C) Cells expressing Rab5:WT or Rab5:Q79L (QL) were incubated with live or heat-inactivated P. aeruginosa as described above, and activated Rab5 was assayed by incubating lysates with glutathione beads in the presence of GST-EEA1, followed by immunoblotting, as described for panel B. Inset, representative immunoblot of samples probed with anti-Rab5 or anti-GST antibodies. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from control group values (*, P < 0.05).
We then determined whether reduced accumulation of Rab5 on phagosomes containing live P. aeruginosa correlated with inhibition of Rab5 activation. In these studies, J774-Eclone macrophages were incubated with live or heat-inactivated P. aeruginosa for 5 min at 37°C, and active Rab5 in total cell lysates was assayed using a GST-EEA1 glutathione bead pulldown assay, detecting active GTP-Rab5 bound to EEA1 by immunoblot analysis using an anti-Rab5 antibody. As shown in Fig. 3B, incubation with live but not with heat-inactivated P. aeruginosa significantly inhibited Rab5 binding to GST-EEA1. A representative immunoblot (Fig. 3B, inset) shows lack of binding of lysates from live, but not heat-inactivated, P. aeruginosa treated cells to GST-EEA1, in relation to total Rab5 and GST proteins in lysates.
To examine the influence of live P. aeruginosa on activation of Rab5 during phagocytosis, J774-Eclone cells expressing Rab5:WT or the Rab5:Q79L constitutive mutant were incubated with live or heat-inactivated P. aeruginosa for 5 min at 37°C, and total lysates were analyzed for active GTP-Rab5 using the GST-EEA1 pulldown assay. As shown in Fig. 3C, incubation of cells with live P. aeruginosa, but not with heat-inactivated P. aeruginosa, inhibited Rab5 activation, and this inhibition was nullified by expression of constitutively active Rab5:Q79L. These results are shown in the representative immunoblot (Fig. 3C, inset). Together, these studies demonstrate that live P. aeruginosa regulates both recruitment and activation of Rab5 to early phagosomes.
Exotoxin S plays a critical role in Rab5 activation during phagocytosis of P. aeruginosa.
Our results show that phagocytosis of live P. aeruginosa by macrophages downregulates Rab5 activation. Previous studies found that ExoS can ADP-ribosylate Rab5 (18, 26), and in vitro studies confirmed that ADP-ribosylation of Rab5 by ExoS interfered with its interaction with EEA1 (45). These findings indicate an ability of ExoS to interfere with endosomal tethering during P. aeruginosa phagocytosis. To determine the relationship between ExoS expression and Rab5 activation during phagocytosis, P. aeruginosa strains lacking ExoS were compared with strain PAO1, which expresses ExoS, ExoT, and ExoY, for their ability to interfere with Rab5 activation during phagocytosis. P. aeruginosa strains that were examined included (i) PA103, which lacks ExoS but expresses ExoT and ExoU, (ii) PA103ΔU, which lacks ExoS, ExoY, and ExoU, and (iii) PA103ΔTΔU, which lacks all known T3S effectors.
When internalization of live or heat-inactivated P. aeruginosa was examined following incubation of J774-Eclone cells with these strains for 15 min, significant differences in the internalization of live strains were observed, whereas the phagocytic response was uniform for all the heat-inactivated strains (Fig. 4A). Live PAO1, which produces ExoS, caused the greatest inhibition of phagocytic uptake (75% ± 4%) relative to heat-inactivated strain PAO1. In comparison, live strain PA103, expressing ExoT and ExoU, inhibited phagocytosis by 40% ± 6%, which closely approximated the 34% ± 6% inhibition of phagocytosis caused by live strain PA103ΔU, which expresses only ExoT. Live strain PA103ΔTΔU, lacking all four T3S effectors, caused a 15% ± 3% inhibition of phagocytosis. These results provide evidence that ExoS and ExoT, produced by strain PAO1, have a more pronounced role than ExoT alone, produced by strain PA103, in inhibiting phagocytosis in J774-Eclone macrophages.
Fig 4.
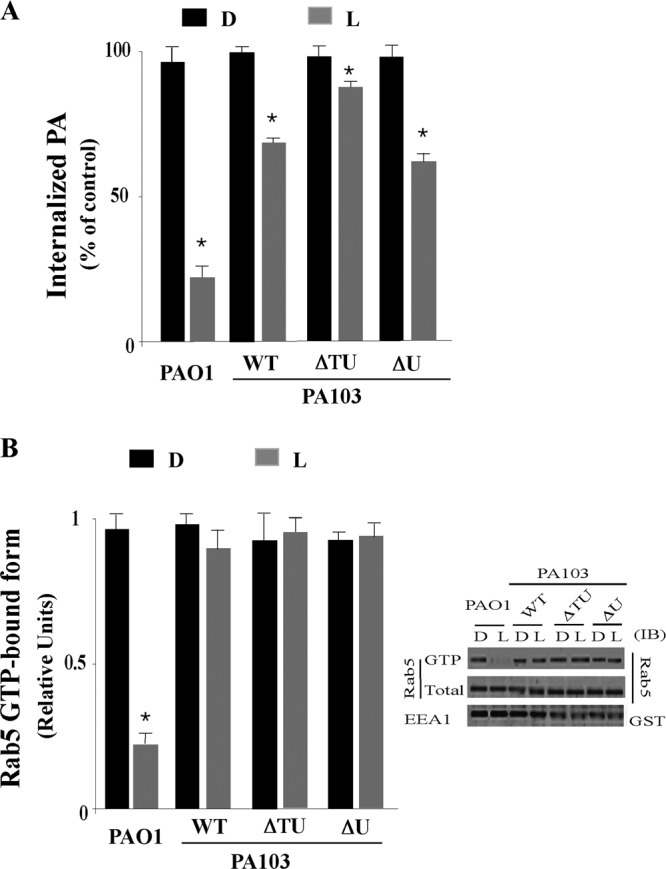
Selective effect P. aeruginosa exotoxins on Rab5 activation in macrophages. (A) J774-Eclone cells were incubated for 15 min with live (L) or heat-inactivated (D) P. aeruginosa strain PAO1:WT, PA103:WT, PA103 lacking both ExoT and ExoU (ΔTU), or PA103 lacking ExoU (ΔU), and the phagocytic index was determined. (B) J774-Eclone cells were incubated with live or heat-inactivated P. aeruginosa strains as for panel A and then lysed and examined for active GTP-bound Rab5 using the GST-EEA1 pulldown assay. Inset, representative immunoblot of active GTP-Rab5 and total Rab5 or GST-EEA1 in the indicated PAO1 or PA103 cell lysate. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from control group values (*, P < 0.05).
When the fate of bacteria internalized within J774-Eclone macrophages was examined, strain PA103 was found to be more sensitive to macrophage-mediated killing than strain PAO1. Survival rates of 53% ± 6% and 17% ± 3% were detected for internalized strain PA103 after 30 and 60 min, respectively, which compared with 75% ± 5% and 58% ± 5% survival rates for internalized strain PAO1 after 30 min and 60 min, respectively, relative to the total internalized bacteria after 15 min of phagocytosis (control, 100% ± 5%).
We then examined how the uptake and survival of PA103 and PAO1 strains within macrophages related to the ability of these strains to alter Rab5 activity. For these studies, cells were incubated with live or dead P. aeruginosa strains, and cell lysates were examined for active GTP-bound Rab5 in the GST-EEA1 pulldown assay, described above. As shown in Fig. 4B, incubation of cells with strain PAO1, but not with strain PA103, inhibited Rab5 activation, and as expected, internalization of heat-inactivated P. aeruginosa did not alter Rab5 activation. Interestingly, strains PA103 and PA103ΔU, which express ExoT, did not inhibit Rab5 activation (Fig. 4B), even though these strains inhibited P. aeruginosa internalization (Fig. 4A). A representative immunoblot (Fig. 4B, inset) shows lack of binding of J774-Eclone lysates obtained following exposure to live PAO1 to GST-EEA1, while all other lysates bound GST-EEA1. These results indicate that inhibition of J774-Eclone phagocytosis by ExoS-producing strain PAO1 occurs in conjunction with inhibition of Rab5 activation, while inhibition of phagocytosis by ExoT-producing PA103 strains occurs independently of alterations in Rab5 activation.
Several approaches were then used to further investigate the role of ExoS and Rab5 activation in the invasion of live P. aeruginosa in J774-Eclone cells. First, to assess the role of ExoS in P. aeruginosa internalization, we examined the ability of PA103ΔUΔT strains lacking ExoS [ExoS(−)], expressing ExoS [ExoS(WT)], or expressing ExoS with mutations that inactivate its GAP [ExoS (ADPr+)], its ADPr [ExoS (RhoG+)], or its GAP and ADPr [ExoS(ADPr−/RhoG−)] activities to be phagocytosed by J774-Eclone macrophages. For these studies, cells were incubated with each of these live P. aeruginosa strains for 5 min prior to washing and determination of the phagocytic index. As shown in Fig. 5A, greater than 80% inhibition (P < 0.05) was observed in the uptake of live PA103ΔUΔT expressing either ExoS(WT) or the ExoS(ADPr+) mutant compared to PA103ΔUΔT either lacking ExoS or expressing an ExoS(ADPr−/RhoG−) mutant. A significant (P < 0.05) but less pronounced inhibition of phagocytosis (∼40%) was caused by live PA103ΔUΔT expressing an ExoS(RhoG+) mutant. These results provide evidence that ExoS ADPr activity plays a more pronounced role in the antiphagocytic activity of live PA103ΔUΔT expressing ExoS than ExoS RhoG activity.
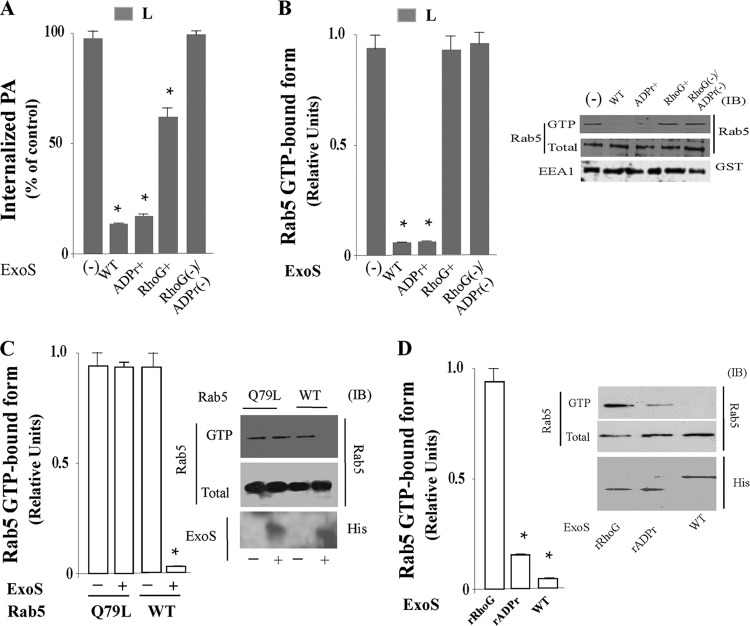
Fig 5.
Effect of ExoS GAP or ADPr activity on P. aeruginosa invasion and Rab5 activation in macrophages. (A) Live PA103ΔTΔU expressing a vector control (−), ExoS(WT), an ExoS(R146A) (ADPr+) mutant, an ExoS(E379A/E387A) (RhoG+) mutant, or an ExoS(R146A/E379A/E387A) (ADPr−/RhoG−) mutant were incubated with J774-Eclone macrophages at a ratio of 200:1 at 37°C for 5 min. After incubation, cells were washed and the phagocytic index was determined. (B) Live PA103 strains described for panel A were incubated with J774-Eclone macrophages for 30 min, and Rab5 activation was assayed as described above. Inset, representative immunoblot of active GTP-Rab5 and total Rab5 or GST-EEA1 in the lysates following incubation of cells with the indicated PA103 strains. (C) Cells expressing Rab5:WT or Rab5:Q79L were transfected with 6His-ExoS(WT) (ExoS), and lysates were assayed for active Rab5 as described above. Inset, representative immunoblot of samples probed with anti-Rab5 or anti-His tag antibodies. (D) Cells expressing Rab5:WT were transiently transfected with 6His-ExoS(WT), 6His-ExoS(rRhoG), or 6His-ExoS(rADPr). After transfection, the activated GTP-Rab5 was determined as described above. Inset, representative immunoblot of samples probed with anti-Rab5 or anti-His tag antibodies. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from control group values (*, P < 0.05).
We then examined how the antiphagocytic effects of these strains related to the formation of GTP-bound Rab5 during the invasion of live P. aeruginosa. A significant decrease (>90% inhibition) in the formation of the GTP-bound form of Rab5 was caused by live PA103ΔUΔT expressing ExoS(WT) or an ExoS(ADPr+) mutant compared to PA103ΔUΔT expressing an ExoS(ADPr−/RhoG−) mutant (Fig. 5B). Notably, PA103ΔUΔT expressing an ExoS(RhoG+) mutant did not inhibit formation of the GTP-bound form of Rab5 (Fig. 5B), indicating that the ADPr domain of ExoS is essential to inactivation of Rab5 during phagocytosis of live P. aeruginosa.
We then examined the roles of ExoS and ExoS domains in Rab5 activation upon coexpression of both Rab5 and ExoS constructs in J774-Eclone macrophages. As shown in Fig. 5C, when GTP-bound Rab5 was assayed in J774-Eclone cells expressing Rab5:WT or the Rab5:Q79L mutant and transiently cotransfected with ExoS, cells expressing both Rab5:WT and ExoS failed to produce GTP-bound Rab5, but activated GTP-bound Rab5 was detected in cells coexpressing constitutively active Rab5:Q79L and ExoS. When the effects of the individual ExoS domains on activation of Rab5 were examined, Rab5 activation was inhibited in cells expressing Rab5:WT and the ExoS-ADPr (rADPr) domain, while coexpression of Rab5:WT with ExoS-RhoGAP (rRhoG) did not interfere with Rab5 activation (Fig. 5D). Collectively, these results are consistent with ExoS ADP-ribosylation of Rab5 during infection with live P. aeruginosa leading to inactivation of Rab5 and interference with phagocytosis of P. aeruginosa.
Selective role of Rab5 GEFs during internalization of P. aeruginosa.
Rab5 cycling between its GTP-active and GDP-inactive form is regulated by Rab5 GEFs, including, Rin1, Rabex5, and Rap6. Since P. aeruginosa expressing ExoS ADPr activity was found to inhibit Rab5 activation in J774-Eclone macrophages, we hypothesized that the expression of Rab5 GEFs may overcome the inhibitory effect of live P. aeruginosa on Rab5 activation and P. aeruginosa invasion.
To test this hypothesis, cells expressing GFP, Rin1, Rabex5, or Rap6 were incubated in the presence of live or heat-inactivated strain PAO1, and P. aeruginosa internalization was assayed after 15 min. As shown in Fig. 6A, internalization of live and heat-inactivated P. aeruginosa was increased in Rin1-overexpressing cells compared to GFP control cells. Overexpression of Rap6 also increased the internalization of live and heat-inactivated cells, but to a lesser extent than that of Rin1. In contrast, overexpression of Rabex5 did not significantly alter internalization of live or dead P. aeruginosa. Phagocytosis of live or dead P. aeruginosa did not alter the expression of Rab5 GEF constructs (Fig. 6B).
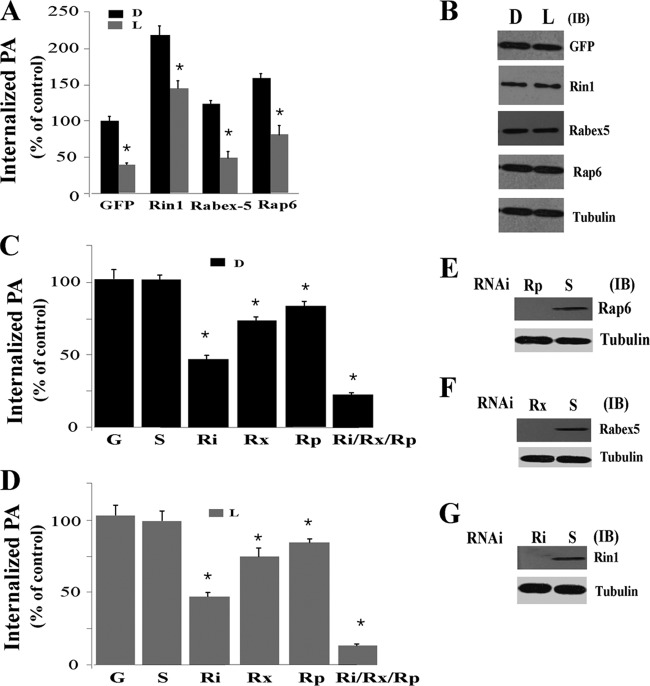
Fig 6.
Effect of Rab5-GEFs on the internalization of P. aeruginosa. (A) Live (L) or heat-inactivated (D) P. aeruginosa was incubated in the presence of cells expressing GFP or the indicated Rab5 GEF at the ratio of 200:1. The phagocytic index was determined after incubation for 15 min at 37°C. (B) J774-Eclone cells were transfected with the indicated Rab5-GEF construct, and cell lysates were immunoblotted with anti-GFP, anti-Rin1, anti-Rap6, anti-Rabex-5, or antitubulin antibodies. (C) Live (L) or heat-inactivated (D) P. aeruginosa was incubated with nontransfected J774-Eclone cells (G) or cells transfected with RNAi sequences against Rin1 (Ri), Rabex-5 (Rx), Rap6 (Rp), or all three Rab5-GEFs (Ri/Rp/Rx) or a scramble RNAi sequence (S). After transfection, cells were incubated for 24 h and then assayed for phagocytosis. (E to G) Cells were transfected with the indicated small interfering RNAs (siRNAs) and lysed at 24 h after posttransfection. Cell lysates were immunoblotted with anti-Rap6, anti-Rabex5, anti-Rin1, or antitubulin antibodies. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from the control group values (*, P < 0.05).
To further examine the role of Rab5-GEFs in P. aeruginosa internalization, we suppressed expression of Rab5-GEFs using RNAi sequences specific for Rin1, Rabex5, or Rap6. Interference of Rin1 expression caused a >60% inhibition of internalization of live or heat-inactivated P. aeruginosa compared to that for GFP control cells (Fig. 6C and D). In comparison, interference of Rabex5 and Rap6 expression caused only a small decrease in internalization of live or heat-inactivated P. aeruginosa compared to that for GFP control cells. Interference of expression of all three Rab5 GEFs, using a triple knockdown, caused >80% inhibition of internalization of live or heat-inactivated P. aeruginosa. Treating cells with each RNAi sequence depleted more than 95% of the targeted protein (Fig. 6E to G). These results indicate the ability of Rin1 to enhance invasion of live or heat-inactivated P. aeruginosa in J774-Eclone macrophages.
Rin1 expression partially reverses the negative effect of P. aeruginosa on Rab5 activation in macrophages.
Establishing a role of Rin1 in the internalization of live or dead P. aeruginosa led us to hypothesize that activation of Rab5 by Rin1 may, at least in part, be responsible for enhancing the internalization of live P. aeruginosa. To determine whether Rin1 activity increases internalization of live P. aeruginosa, J774-Eclone cells expressing GFP, Rin1, Rabex5, or Rap6 were incubated in the presence of live or heat-inactivated strain PAO1 P. aeruginosa for 5 min, and then the formation of the GTP-bound form of Rab5 was examined using a GST-EEA1 pulldown assay. In Fig. 7, we show that Rin1 expression increased the amount of active GTP-bound Rab5 formed during the internalization of live or heat-inactivated P. aeruginosa. This is consistent with our observation that the expression of Rin1 increased the internalization of live or heat-inactivated P. aeruginosa (Fig. 6A).
Fig 7.
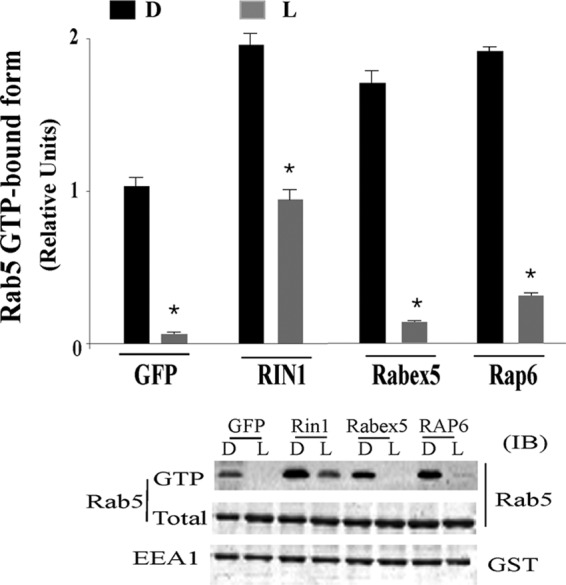
Effect of Rab5-GFEs on Rab5 activity during phagocytosis of P. aeruginosa. Live (L) or heat-inactivated (D) P. aeruginosa was incubated with cells expressing GFP alone or the indicated Rab5 GEF at a ratio of 200:1. P. aeruginosa phagocytosis was assayed after incubation at 37°C for 5 min. Cells were then washed and assayed for active GTP-Rab5 as described above. A representative immunoblot of samples probed with anti-Rab5 or anti-GST is shown. Data represent the mean ± SEM from three independent experiments. Asterisks represent statistically significant differences from the control group values (*, P < 0.05).
Interestingly, when Rabex5- or Rap6-expressing J774-Eclone cells were incubated with live P. aeruginosa, we observed a small amount of GTP-bound Rab5 in the GST-EEA1 pulldown assay for only Rap6-expressing J774-Eclone cells, but the increase in the Rab5 GTP-bound form was lower than that observed in Rin1-expressing cells (Fig. 7). Taken together, these results indicate that Rin1 plays a selective and critical role in the activation of Rab5 during live P. aeruginosa invasion, which is consistent with the observation that live P. aeruginosa inactivates Rab5, and this inhibitory effect can be partially reversed by the expression of Rin1.
DISCUSSION
The type III secretion system of P. aeruginosa is known to modulate host cell endocytosis (15, 45). In this study, we demonstrated that Rab5 plays a critical role during early steps in the phagocytosis of P. aeruginosa in J774-Eclone macrophages. We found that invasion of live, but not heat-inactivated, P. aeruginosa downregulates Rab5 activation and that inactivation of Rab5 during invasion requires expression of ExoS. In support of ExoS being a key effector in Rab5 activation, we confirmed that ExoS ADPr activity, but not ExoS RhoGAP, inhibits Rab5 activation. In addition, we found that overexpression of Rin1 partially reverses inactivation of Rab5 by ExoS ADPr activity during invasion of live P. aeruginosa. These observations led us to develop a model that portrays ExoS ADPr activity and its interference with Rab5 activation as integral to the diminished internalization of live, compared to heat-inactivated, P. aeruginosa in J774-Eclone macrophages.
While live P. aeruginosa was found to inactive Rab5 during P. aeruginosa phagocytosis, Rab5 also influenced phagocytosis of heat-inactivated P. aeruginosa. Both Rab5:WT and constitutively active Rab5:Q79L upregulated the phagocytic index of heat-inactivated P. aeruginosa by 2- to 4-fold, whereas expression of constitutively inactive Rab5:S34N reduced the phagocytic index by half, compared to that for control cells. Unlike Rab5, Rab4 and Rab7 did not alter P. aeruginosa internalization during early stages, up to 15 min, of phagocytosis, but the Rab7:S22N mutant was able to diminish P. aeruginosa internalization after 30 min. These observations indicate that Rab5 plays a key role during early stages, while Rab7 plays a role in later stages, of phagocytosis of heat-killed P. aeruginosa.
Rab5 is a substrate of ExoS ADPr activity and has been found to interfere with Rab5 function in vitro (45). P. aeruginosa strains lacking one or more of the four T3S effectors were used to examine the role of ExoS in phagocytosis in J774-Eclone macrophages. When the phagocytic indices of strains PA103, PA103ΔU, PA103ΔTΔU, and PAO1 strains were determined, we found that live strain PA103, lacking both ExoS and ExoY (46), was engulfed at a ∼3-fold-higher level than live strain PAO1, which expresses ExoS, ExoT, and ExoY. Studies performed in parallel found PAO1 survival within macrophages to be enhanced (58% ± 5% survival) in comparison to that of strain PA103 (17% ± 3% survival), indicating increased susceptibility of strain PA103 to macrophage-mediated killing. Notably, uptake of live PA103 was about 40% ± 6% less than that of heat-inactivated PA103, which is attributed to ExoT RhoGAP activity of strain PA103 (47). In this regard, uptake of strain PA103ΔU, lacking ExoS, ExoU, and ExoY but expressing ExoT, was 34% ± 6%, similar to that of strain PA103. We also observed that the phagocytic index of strain PA103ΔTΔU, which lacks known T3S effectors, was significantly lower than that of heat-inactivated PA103 (Fig. 4A). Consistent with factors other than T3S effectors playing a role in phagocytosis of live P. aeruginosa, exotoxin A, phospholipase C, alkaline protease, elastase, and cell surface lipopolysaccharides have all been shown to alter macrophage responses and hence impair bacterium internalization (48–50).
In examining the effect of P. aeruginosa on the activity of Rab5 proteins, we demonstrated that live P. aeruginosa, but not the heat-inactivated P. aeruginosa, is responsible for diminishing levels of active GTP-bound Rab5. The inhibitory effect of live P. aeruginosa on Rab5 activation was overcome by expression of the constitutively active Rab5:Q79L. Interestingly, we observed that live PAO1, but not live PA103, PA103ΔU, or PA103ΔTΔU, inhibited Rab5 activation (Fig. 4B). Because strain PA103 lacks ExoS and ExoY and because ExoY seems unlikely to affect Rab5 activation (51), we speculated that ExoS plays a role in the modulation of Rab5 activation during the internalization of live P. aeruginosa in J774-Eclone macrophages. Consistent with this idea, it was previously shown that ExoS ADPr activity blocked both HRP uptake and EGFR trafficking to lysosomes in CHO and HeLa cells, respectively (15, 45) In directly testing the role of ExoS in modulating Rab5 activation in J774-Eclone macrophages, we found that strain PA103ΔTΔU expressing ExoS(WT) or ExoS(ADPr) activity, but not ExoS(Rho-GAP) activity, diminished levels of GTP-bound Rab5, in conjunction with inhibition of P. aeruginosa internalization (Fig. 5A and B). Similarly, transient expression of ExoS(WT) and ExoS(rADPr), but not ExoS(rRhoG), within J774-Eclone cells was found to diminish levels of active GTP-bound Rab5 (Fig. 5D). Our results that ExoS anti-internalization activity was dependent mostly on its ADPr activity differ from those of previous studies in HeLa cells, where the ExoS anti-internalization function was attributed to RhoGAP activity (15, 52). One explanation for this discrepancy is our use of a different cell line, and cell line properties are known to influence the substrate specificity of ExoS (53). The ability of ExoS ADPr, but not its RhoGAP activity, to inactivate Rab5 (Fig. 5A) provides evidence for cell type-dependent mechanisms of phagocytosis, which can be differentiated by ExoS.
Previous studies found that Rab5 might undergo ADP-ribosylation on multiple arginine residues by ExoS (45). This observation, together with the facts that Rab5 is inactivated by ExoS:ADPr activity and that ADP-ribosylation interferes with Rab5 interaction with EEA1, indicates that key functional residues within the GTP-binding motif of Rab5 may be targeted by ExoS. Interestingly, Arg81 is located in switch II of Rab5 proteins, immediately downstream of the second GTP/GDP-binding motif (54), and mutation of Arg81 partially affects Rab5 function (55). Confirmation of Arg81 as well as other Arg residues in Rab5 as targets of ExoS ADPr activity is integral to understanding how ExoS affects Rab5 function.
Since the nucleotide status of Rab5 is integral for P. aeruginosa invasion, we examined the role of Rab5-GEFs, Rabex5, Rap6, and Rin1, in Rab5 activation during P. aeruginosa internalization. Overexpression of Rin1, and secondarily Rap6, enhanced internalization of heat-inactivated P. aeruginosa (Fig. 6A). Consistent with the importance of Rab5 activation to phagocytosis of heat-inactivated P. aeruginosa, Rab5 was activated when each of the Rab5-GEFs was overexpressed, albeit to different degrees (Fig. 7). However, when the nucleotide status of Rab5 was analyzed in the presence of live P. aeruginosa, only Rin1 significantly overcame the inactivation of Rab5 in macrophages (Fig. 7). This finding is in agreement with the significant increase in the internalization of live P. aeruginosa in cells overexpressing Rin1 proteins. It is worth noting that Rap6 and Rabex5 also increased levels of active GTP-Rab5, but it was significantly less than expression of Rin1 (Fig. 7). The involvement of Rin1 in Rab5 activation was corroborated by depletion of Rin1, which significantly inhibited the uptake of live P. aeruginosa (Fig. 6C). These results demonstrated for the first time that Rin1 is an essential regulator of Rab5 activation during phagocytosis of live P. aeruginosa.
In conclusion, we have demonstrated that live P. aeruginosa, but not heat-inactivated P. aeruginosa, downregulates Rab5 function in conjunction with inhibition of phagocytosis in J774-Eclone macrophages. Reduced phagocytosis of live P. aeruginosa by macrophages was overcome by expressing a constitutively active Rab5:Q79L mutant. ExoS ADPr activity mediated P. aeruginosa inactivation of Rab5, and unlike in previous studies, ExoS ADPr, rather than ExoS GAP, activity was a dominant inhibitor of P. aeruginosa internalization, highlighting cell line differences in mechanisms of P. aeruginosa internalization. Our studies support the hypothesis that increased Rab5 activity can accelerate phagocytosis of live P. aeruginosa and increase its degradation in macrophages. The exact mechanism of action of ExoS toward Rab5 function in macrophages is under investigation.
ACKNOWLEDGMENTS
We thank the Florida International University Foundation for financial support.
We thank Dara Frank for reagents and P. aeruginosa strains.
Footnotes
Published ahead of print 29 April 2013
REFERENCES
- 1. Domingo P, Ferre A, Baraldes MA, Ris J, Sanchez F. 1998. Pseudomonas aeruginosa bronchopulmonary infection in patients with AIDS, with emphasis on relapsing infection. Eur. Respir. J. 12:107–112 [DOI] [PubMed] [Google Scholar]
- 2. Maschmeyer G, Braveny I. 2000. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur. J. Clin. Microbiol. Infect. Dis. 19:915–925 [DOI] [PubMed] [Google Scholar]
- 3. Mendelson MH, Gurtman A, Szabo S, Neibart E, Meyers BR, Policar M, Cheung TW, Lillienfeld D, Hammer G, Reddy S, Choi K, Hirschman SZ. 1994. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin. Infect. Dis. 18:886–895 [DOI] [PubMed] [Google Scholar]
- 4. Pruitt BA., Jr 1974. Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J. Infect. Dis. 130(Suppl.):S8–S13 [DOI] [PubMed] [Google Scholar]
- 5. Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7:654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hueck CJ. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soong G, Parker D, Magargee M, Prince AS. 2008. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 190:2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyczak JB, Cannon CL, Pier GB. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051–1060 [DOI] [PubMed] [Google Scholar]
- 9. Barbieri JT, Sun J. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 152:79–92 [DOI] [PubMed] [Google Scholar]
- 10. Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25:1125–1139 [DOI] [PubMed] [Google Scholar]
- 11. Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369–36372 [DOI] [PubMed] [Google Scholar]
- 12. Krall R, Schmidt G, Aktories K, Barbieri JT. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066–6068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowell BA, Chen DY, Frank DW, Vallis AJ, Fleiszig SM. 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68:403–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rocha CL, Coburn J, Rucks EA, Olson JC. 2003. Characterization of Pseudomonas aeruginosa exoenzyme S as a bifunctional enzyme in J774A. 1 macrophages. Infect. Immun. 71:5296–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng Q, Barbieri JT. 2008. Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic 9:1948–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGuffie EM, Frank DW, Vincent TS, Olson JC. 1998. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect. Immun. 66:2607–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraylick JE, Riese MJ, Vincent TS, Barbieri JT, Olson JT. 2002. ADP-ribosylation and functional effects of Pseudomonas exoenzyme S on cellular RalA. Biochemistry 41:9680–9687 [DOI] [PubMed] [Google Scholar]
- 18. Henriksson ML, Sundin C, Jansson AL, Forsberg A, Palmer RH, Hallberg B. 2002. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Biochem. J. 367:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Yahr TL, Frank DW, Barbieri JT. 1997. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 179:1609–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun J, Barbieri JT. 2003. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 278:32794–32800 [DOI] [PubMed] [Google Scholar]
- 21. Deng Q, Sun J, Barbieri JT. 2005. Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. J. Biol. Chem. 280:35953–35960 [DOI] [PubMed] [Google Scholar]
- 22. Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama KK, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz MH, Shaver CM, King JD, Musunuri S, Kazzaz JA, Hauser AR. 2008. Pseudomonas aeruginosa induces localized immunosuppression during pneumonia. Infect. Immun. 76:4414–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vance RE, Rietsch A, Mekalanos JJ. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect. Immun. 73:1706–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U. S. A. 95:13899–13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fraylick JE, Rucks EA, Greene DM, Vincent TS, Olson JC. 2002. Eukaryotic cell determination of ExoS ADP-ribosyltransferase substrate specificity. Biochem. Biophys. Res. Commun. 291:91–100 [DOI] [PubMed] [Google Scholar]
- 27. Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. 2001. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem. 276:23607–23615 [DOI] [PubMed] [Google Scholar]
- 28. Madan R, Krishnamurthy G, Mukhopadhyay A. 2008. SopE-mediated recruitment of host Rab5 on phagosomes inhibits Salmonella transport to lysosomes. Methods Mol. Biol. 445:417–437 [DOI] [PubMed] [Google Scholar]
- 29. Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. 2008. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J. Cell Biol. 182:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clemens DL, Lee BY, Horwitz MA. 2000. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect. Immun. 68:2671–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alvarez-Dominguez C, Barbieri MA, Beron W, Wandinger-Ness A, Stahl PD. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834–13843 [DOI] [PubMed] [Google Scholar]
- 32. Duclos S, Diez R, Garin J, Papadopoulou B, Descoteaux A, Stenmark H, Desjardins M. 2000. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J. Cell Sci. 19:3531–3541 [DOI] [PubMed] [Google Scholar]
- 33. Olchowik M, Miaczynska M. 2009. Effectors of GTPase Rab5 in endocytosis and signal transduction. Postepy Biochem. 55:171–180 [PubMed] [Google Scholar]
- 34. Hunker CM, Galvis A, Kruk I, Giambini H, Veisaga ML, Barbieri MA. 2006. Rab5-activating protein 6, a novel endosomal protein with a role in endocytosis. Biochem. Biophys. Res. Commun. 340:967–975 [DOI] [PubMed] [Google Scholar]
- 35. Haas AK, Fuchs E, Kopajtich A, Barr F. 2005. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7:887–893 [DOI] [PubMed] [Google Scholar]
- 36. Goh T, Uchida W, Arakawa S, Ito E, Dainobu T, Ebine K, Takeuchi M, Sato K, Ueda T, Nakano A. 2007. VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell 19:3504–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg Zerial JM. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. 2006. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 28:8836–8842 [DOI] [PubMed] [Google Scholar]
- 39. Diment S, Leech MS, Stahl PD. 1987. Generation of macrophage variants with 5-azacytidine: selection for mannose receptor expression. J. Leukoc. Biol. 42:485–490 [DOI] [PubMed] [Google Scholar]
- 40. Bridge DR, Martin KM, Moore ER, Lee VM, Carroll JA, Rocha CL, Olson JC. 2012. Examining the role of actin-plasma membrane association in Pseudomonas aeruginosa infection and type III secretion translocation in migratory T24 epithelial cells. Infect. Immun. 80:3049–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barbieri MA, Fernandez-Pol S, Hunker C, Horazdovsky BH, Stahl PD. 2004. Role of rab5 in EGF receptor-mediated signal transduction. Eur. J. Cell Biol. 83:305–314 [DOI] [PubMed] [Google Scholar]
- 42. Goldova J, Ulrych A, Hercik K, Branny P. 2011. A eukaryotic-type signalling system of Pseudomonas aeruginosa contributes to oxidative stress resistance, intracellular survival and virulence. BMC Genomics 12:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Angus AA, Evans DJ, Barbieri JT, Fleiszig SM. 2010. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect. Immun. 78:4500–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brumell JH, Scidmore MA. 2007. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol. Mol. Biol. Rev. 71:636–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbieri AM, Sha Q, Bette-Bobillo P, Stahl PD, Vidal M. 2001. ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect. Immun. 69: 5329–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garrity-Ryan L, Shafikhani SS, Balachandran PP, Nguyen LL, Oza JJ, Jakobsen TT, Sargent J, Fang X, Cordwell S, Matthay MA, Engel JN. 2004. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 72:546–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wretlind B, Pavlovskis OR. 1983. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev. Infect. Dis. 5(Suppl.):S998–S1004 [DOI] [PubMed] [Google Scholar]
- 49. Ruhen RW, Holt PG, Papadimitriou JM. 1980. Antiphagocytic effect of Pseudomonas aeruginosa exopolysaccharide. J. Clin. Pathol. 33:1221–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pavlovskis OR, Pollack M, Callahan LT, 3rd, Iglewski BH. 1977. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect. Immun. 18:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hauser AR, Fleiszig S, Kang PJ, Mostov K, Engel JN. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krall R, Sun J, Pederson KJ, Barbieri JT. 2002. In vivo rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect. Immun. 70:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rucks EA, Fraylick JE, Brandt LM, Vincent TS, Olson JC. 2003. Cell line differences in bacterially translocated ExoS ADP-ribosyltransferase substrate specificity. Microbiology 149:319–331 [DOI] [PubMed] [Google Scholar]
- 54. Valencia A, Chardin P, Wittinghofer A, Sander C. 1991. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry 30:4637–4648 [DOI] [PubMed] [Google Scholar]
- 55. Li G, Stahl PD. 1993. Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 268:24475–24480 [PubMed] [Google Scholar]