Abstract
Depletion of arginine is a recognized strategy that pathogens use to evade immune effector mechanisms. Depletion depends on microbial enzymes such as arginases, which are considered virulence factors. The effect is mostly interpreted as being a consequence of successful competition with host enzymes for the substrate. However, both arginases and arginine deiminases (ADI) have been associated with pathogen virulence. Both deplete arginine, but their reaction products differ. An ADI has been implicated in the virulence of Giardia duodenalis, an intestinal parasite that infects humans and animals, causing significant morbidity. Dendritic cells (DC) play a critical role in host defense and also in a murine G. duodenalis infection model. The functional properties of these innate immune cells depend on the milieu in which they are activated. Here, the dependence of the response of these cells on arginine was studied by using Giardia ADI and lipopolysaccharide-stimulated human monocyte-derived DC. Arginine depletion by ADI significantly increased tumor necrosis factor alpha and decreased interleukin-10 (IL-10) and IL-12p40 secretion. It also reduced the upregulation of surface CD83 and CD86 molecules, which are involved in cell-cell interactions. Arginine depletion also reduced the phosphorylation of S6 kinase in DC, suggesting the involvement of the mammalian target of rapamycin signaling pathway. The changes were due to arginine depletion and the formation of reaction products, in particular, ammonium ions. Comparison of NH4+ and urea revealed distinct immunomodulatory activities of these products of deiminases and arginases, respectively. The data suggest that a better understanding of the role of arginine-depleting pathogen enzymes for immune evasion will have to take enzyme class and reaction products into consideration.
INTRODUCTION
Many pathogens are thought to compete with the host for arginine as part of their virulence mechanisms. This is best known for pathogens expressing arginases or inducing the respective host enzymes that compete for arginine with host nitric oxide (NO) synthases and thereby are considered to prevent antimicrobial NO formation (1, 2). However, other arginine-metabolizing enzymes have also been implicated in microbial virulence, in particular, arginine deiminases (ADI). The latter enzymes are thought to be relevant in several bacterial infections (3–5) and infections with the noninvasive gastrointestinal protozoan parasite Giardia duodenalis (6, 7), a medically significant cause of diarrheal syndromes and malabsorption (8, 9). In the latter case, ADI has been proposed as a virulence factor (10) possibly also interfering with NO-dependent antiparasite defense (11, 12).
Arginine is not only necessary for the generation of NO, but it also plays other important roles in the immune response. Lack of arginine was shown to inhibit T-cell function (13), and arginine levels affect signaling via the mammalian target of rapamycin (mTOR) pathway, as reported for other cells (14, 15). The mTOR pathway, in turn, was shown to contribute to the regulation of costimulatory surface marker levels on dendritic cells (DC) (1, 16, 17). These cells play a crucial role through interaction with other immune cells. Although DC are important for adaptive immunity to microbial infections, the effect of pathogen-mediated arginine depletion on their function is not known.
Arginine-dependent virulence mechanisms of pathogens can rely on multiple enzymes that may have different effects and lead to the formation of distinct metabolites. For example, arginases and deiminases both deplete arginine but generate ornithine and urea or citrulline and NH4+, respectively. Commonly, changes in immune cell responses due to different arginine levels have been studied by comparing responses in the presence or absence of arginine. However, this does not reflect the situation when arginine is depleted by an enzymatic reaction, as can be the case during infections. Yet, the combined effect of arginine depletion by an enzymatic reaction and the ensuing product formation on immune cells has largely been ignored.
Referring to G. duodenalis as a relevant model, we studied here the immunomodulatory effects of arginine depletion by exposing human monocyte-derived DC (moDC) to recombinant G. duodenalis ADI during DC activation with lipopolysaccharide (LPS). The effect of this treatment on interleukin-10 (IL-10), IL-12p40, and tumor necrosis factor alpha (TNF-α) secretion, as well as the cell surface expression of CD83 and CD86, was monitored. We show that both arginine depletion and NH4+ formation by the active parasite enzyme have an immunomodulatory effect on moDC, causing an increase in TNF-α production, as opposed to a decrease in IL-10 and IL12p40 production and a reduction of surface-located CD86 and CD83. In particular, the latter effect correlated with an inhibition of the mTOR pathway since phosphorylation of the mTOR S6 kinase (S6K) target protein was decreased. We furthermore show that NH4+ but not urea exacerbated the inhibition of IL-10 production and surface marker upregulation compared with arginine depletion alone, suggesting a difference between the immunomodulatory activities of the products of arginases and deiminases.
MATERIALS AND METHODS
Cell culture.
DC were cultured in RPMI 1640 (Sigma) supplemented with 10% fetal calf serum (FCS; Biochrom) or arginine-free RPMI (PanBiotech) supplemented with 10% dialyzed FCS (Biochrom). Both media contained 10 mM HEPES (Biochrom), penicillin (100 U/ml)-streptomycin (100 μg/ml) (PAA), and 50 μM 2-mercaptoethanol (Roth).
G. duodenalis strain WB-C6 (ATCC 50803) trophozoites were propagated in TYI-S-33 medium as previously described (18). Genomic DNA was isolated with the DNeasy Blood & Tissue kit (Qiagen).
Generation of recombinant proteins.
The ADI coding sequence (Gd-adi) was amplified from genomic DNA of G. duodenalis strain WB-C6 by PCR with Pfu polymerase (Fermentas) and specific primers (5′-AATGACTGACTTCTCCAAGGATAAAGA-3′ and 5′-TCCCTCACTTGATATCGACGCAGATGTCA-3′). The resulting 1.8-kb fragment was cloned into the expression vector pASG-IBA35 (StarGate cloning; IBA GmbH) in accordance with the manufacturer's manual to produce a recombinant protein with an N-terminal His6 tag. After transformation into Escherichia coli DH5αZ1 cells (19), the His6-tagged ADI (pASG-IBA35_ADI) was purified from cultures grown overnight at 37°C in LB medium supplemented with 100 μg/ml ampicillin (Roth) and 50 μg/ml spectinomycin (Sigma) without induction with anhydrotetracycline. Cells were harvested by centrifugation (8,200 × g, 10 min, 4°C) and then washed in ice-cold phosphate-buffered saline (PBS). The pellet was resuspended in 25 mM HEPES (Fluka)–150 mM NaCl (Merck)–5 mM imidazole (Merck) plus EDTA-free protease inhibitor cocktail (Roche), pH 7.5 (buffer A), and disrupted with a high-pressure homogenizer (EmulsiFlex; Avestin). After centrifugation (15,000 × g, 30 min, 4°C), the supernatant was passed through a 0.45-μm-pore-size filter (Sartorius) and loaded onto a HisTrap FF column (GE Healthcare) pre-equilibrated with buffer A on an Äkta fast protein liquid chromatography system (GE Healthcare). Protein was eluted in 25 mM HEPES–150 mM NaCl–200 mM imidazole, pH 7.5 (buffer B). Imidazole was removed by desalting with a PD-10 column (GE Healthcare), and residual LPS was removed with an EndoTrap kit (Hyglos). Recombinant protein in 1× PBS was concentrated with a Vivaspin concentrator (5-kDa polyethersulfone membrane; Sartorius). To obtain a catalytically inactive mutant form of ADI (ADIC424A), cysteine 424 was changed to alanine with the QuikChange mutagenesis kit (Stratagene). Site-directed mutagenesis PCR was performed with primers 5′-GTACGGCTCTCTGCACGCCGCATCTCAGGTTGTT-3′ and 5′-AACAACCTGAGATGCGGCGTGCAGAGAGCCGTAC-3′ and the vector pASG-IBA35_ADI as the template. Recombinant ADIC424A was expressed and purified as described for the wild-type enzyme. The purity of both recombinant proteins was verified by SDS-PAGE and Western blotting. Protein concentrations were determined with a BCA protein assay kit (Thermo Fisher Scientific). Purified proteins were stored in aliquots at −70°C. The enzymatic activity of thawed recombinant ADI was measured by colorimetric determination of citrulline formation as described previously (20) and was found to be stable for the duration of this study.
Production of polyclonal antibody.
An alpaca was immunized four times (days 0, 21, 35, and 49; Preclinics, Potsdam, Germany) with 300 μg of purified recombinant ADI resuspended in 100 μl PBS plus adjuvant (complete Freund's adjuvant for the first injection and incomplete Freund's adjuvant for subsequent booster injections). Preimmune serum was collected prior to the first immunization. After the last booster injection, the antiserum was obtained.
For Western blot analysis, alpaca antiserum was diluted 1:2,500 and the horseradish-peroxidase-conjugated goat anti-llama IgG secondary antibody (Bethyl Laboratories) was diluted 1:20,000, both in 5% nonfat dried milk (Roth)–1× PBS–0.05% Tween 20 (Roth).
Generation of human moDC.
Peripheral blood mononuclear cells were isolated from buffy coats of healthy volunteers (German Red Cross, Berlin, Germany) by density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare). Monocytes were separated by magnetic cell sorting with CD14 MicroBeads (Miltenyi Biotec). Typically, cells were thereby enriched to ≥90% CD14+ cells as determined by flow cytometry. To generate moDC, 3.0 × 106 monocytes were seeded into six-well tissue culture plates. DC growth medium additionally contained 1,000 U/ml human recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; Bayer) and 10 ng/ml human recombinant IL-4 (rIL-4; R&D Systems). After 6 days, immature DC were harvested and the cell population was characterized by analyzing an aliquot by flow cytometry. Staining of surface markers revealed low levels of CD14 and CD86 and high levels of HLA-DR, whereas CD25 and CD83 were not expressed.
DC stimulation and analysis of cytokine production and cell surface markers.
Immature DC were seeded into a 12-well tissue culture plate at 5 × 105/ml and cultured at 37°C either in DC growth medium or in arginine-free DC growth medium supplemented or not with 2 mM arginine (Merck) or citrulline (Sigma) and/or ammonium chloride (Sigma), respectively. Cells were exposed to different concentrations of recombinant ADI or ADIC424A, and 1 μg LPS/ml (E. coli 026:B6 from Sigma) was added at the same time to trigger DC maturation. Cell culture supernatants were collected after 24 h and frozen at −80°C until determination of TNF-α, IL-10, IL-12p70 (all from eBioscience), IL-23 (U-CyTech), and IL-12p40 (BioLegend) by enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturers' protocols. DC were stained with fluorescein isothiocyanate-conjugated anti-human CD14 (Immunotools), peridinin chlorophyll protein complex-conjugated CD86 (Abcam), phycoerythrin-conjugated HLA-DR (Immunotools), Alexa Fluor 488-conjugated CD83 (BioLegend), or Dyomics 647-conjugated CD25 (Immunotools) monoclonal antibody or the relevant isotype control (Immunotools). Data were acquired on an LSR II flow cytometer (Becton, Dickinson) and analyzed by FlowJo software (Tree Star, Inc.). Dead cells were excluded by gating according to forward scatter/side scatter characteristics.
Western blot analysis.
One million immature DC per milliliter were seeded into a 12-well tissue culture plate in arginine-free DC medium containing rGM-CSF and rIL-4 and further supplemented or not with 2 mM arginine, citrulline, and/or ammonium chloride. After 30 min of resting, cells were treated with 2 μM rapamycin (Sigma), recombinant ADI, or ADIC424A and further cultured at 37°C for 90 min. The immature cells were then exposed to 1 μg/ml LPS for 30 min. Cells were harvested and centrifuged at 300 × g at 4°C for 10 min, and supernatants were then collected for citrulline detection. Cell pellets were washed twice in ice-cold PBS before resuspension in lysis buffer (10 mM Tris-HCl [pH 7.2; Roth], 150 mM NaCl [Merck], 1% Triton X-100 [Merck], 1% sodium deoxycholate [Sigma], EDTA-free protease inhibitor cocktail [Roche]). To remove cell debris, samples were centrifuged for 5 min at 14,000 × g at 4°C. The protein concentration of the supernatant was determined with the BCA protein assay (Thermo Fisher Scientific) according to the manufacturer's protocol. A total of 50 μg protein was separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked for 1 h in 5% nonfat dried milk (Roth)–1× Tris-buffered saline (TBS)–0.1% Tween 20 (Roth) and incubated overnight at 4°C with primary antibodies (Cell Signaling) against p70-S6K (9202), phospho-p70-S6K (9205), eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (9452), phospho-E4-BP1 (9455), and β-actin (4967) diluted 1:1,000 in 5% BSA (Roth)–1× TBS–0.1% Tween 20. Bound antibodies were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch) diluted 1:2,000 in 5% BSA–1× TBS–0.1% Tween 20 and visualized on X-ray films (GE Healthcare) with the ECL Plus Western blotting detection system (GE Healthcare). Quantification of Western blot signals was performed with ImageJ 1.42q software (NIH).
Descriptive statistics.
Data are given as means ± standard deviations (SDs). Statistical significance was assessed by paired (two-tailed) t test. All analyses were performed with GraphPad Prism 5 software (GraphPad Software, Inc.) with a statistical significance level of P < 0.05.
RESULTS
Definition of experimental conditions for arginine depletion by recombinant, enzymatically active G. duodenalis ADI during DC activation in vitro.
The average daily intake of arginine by humans has been estimated to be 5 g, which corresponds to 28 mmol (21). It has been reported that an infected person sheds up to 109 G. duodenalis organisms per day (22). We first aimed to estimate the effect of ADI activity on the amount of arginine that may be available to G. duodenalis during an infection. Thus, we determined the ADI activity per million trophozoites to be equivalent to approximately 0.02 U by preparing lysates from different isolates and determining their ADI activities (data not shown). Therefore, at least 20 U of enzyme may be produced per day of infection. One unit corresponds to 1 μmol arginine metabolized per min; hence, >28 mmol could potentially be turned over in 1 day. Thus, infection with Giardia has the potential to substantially deplete the arginine in the gastrointestinal tract.
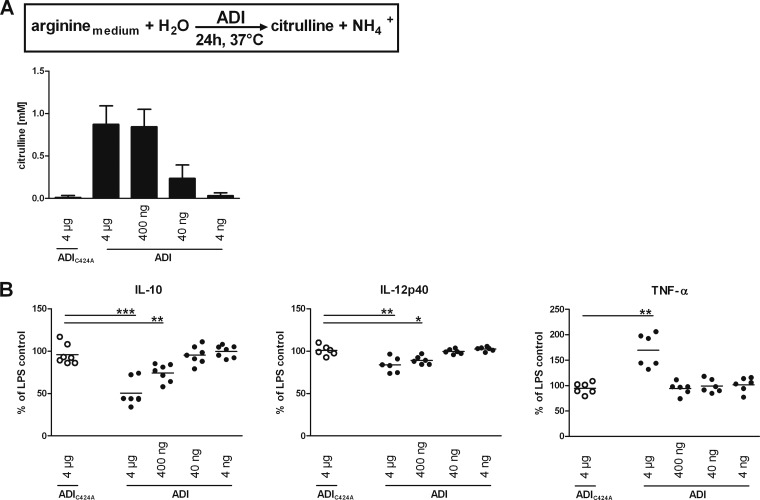
We cloned the adi gene present in strain WB-C6 and produced the respective recombinant enzyme, as well as an enzymatically inactive mutant form, ADIC424A (23), in which Cys at residue 424 was replaced with Ala, as hexahistidine-tagged proteins in E. coli. The recombinant proteins were purified, and the wild-type protein had an ADI activity level of 6.8 U/mg. Analysis by SDS-PAGE and Western blot assay of the two affinity-purified proteins demonstrated that the recombinant proteins were highly pure and behaved similarly to the endogenous ADI parasite protein of 66 kDa (Fig. 1). However, we could not confirm a previously described higher-molecular-mass 85-kDa form of native G. duodenalis ADI (24). To achieve arginine depletion by recombinant ADI in a standard 24-h DC stimulation assay enabling us to study the consequences of arginine depletion on DC, we calculated that 4 μg of active recombinant ADI (corresponding to 0.027 U and representing the equivalent of the enzymatic activity of two to three trophozoites per DC present in the assay) will convert all of the free arginine present in 43 min (culture volume of 1 ml RPMI containing 1.15 mM arginine), 10-fold less enzyme will achieve this in approximately 7 h, and 100-fold less enzyme would need nearly 72 h, assuming constant activity over time. Thus, we chose these amounts of the active enzyme to study dose dependence in the following DC activation experiments and used the inactive mutant form to control for possible effects unrelated to enzyme activity.
Fig 1.
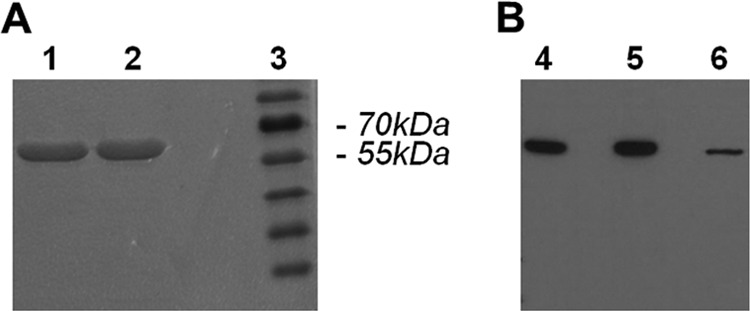
SDS-PAGE and Western blot analysis of purified recombinant G. duodenalis ADI. (A) SDS-PAGE and Coomassie staining of affinity-purified recombinant catalytically inactive ADIC424A (lane 1), recombinant enzymatically active ADI (lane 2), and molecular mass standards (lane 3). (B) Antigenic identification of 0.5 μg affinity-purified recombinant ADI (lane 4), 0.5 μg catalytically inactive ADIC424A (lane 5), and native ADI in 2.9 μg of G. duodenalis strain WB-C6 lysate (lane 6) by Western blotting with alpaca polyclonal antiserum raised against ADI. Preimmune serum, used as a control, did not react with any G. duodenalis protein (data not shown).
Enzymatic arginine depletion by ADI modifies pro- and anti-inflammatory cytokine secretion of LPS-activated moDC.
In the intestine, DC project cellular extensions between epithelial cells into the lumen of the gut to sample antigens (25, 26). Through these projections, they are thought to recognize microbe-associated molecular patterns by pattern recognition receptors and become stimulated to mature and migrate to peripheral lymph nodes, where they initiate antigen-specific immunity (27, 28). Maturing DC upregulate cell surface markers (e.g., major histocompatibility complex, CD83, CD86) and release cytokines (e.g., IL-12, IL-10, TNF-α) to enable communication with and activation of immune cells (29). To model such conditions and investigate potential immunomodulatory effects of arginine limitation on DC function, we used human moDC, which can be readily prepared in the necessary quantities; stimulated them with LPS; and exposed the cells to ADI. The citrulline concentrations in the culture supernatants were determined at the end of the 24-h assay period and confirmed the calculated effects of the ADI dilutions (see above and Fig. 2A) on arginine turnover. As expected, addition of ADIC424A at all of the concentrations tested did not result in any specific citrulline formation (Fig. 2A and data not shown). Cytokine concentrations in these supernatants were then determined by ELISA (Fig. 2B). LPS activation of cells stimulated roughly a 100-fold increase in IL-12p40, TNF-α, and IL-10 levels in arginine-replete medium compared to those in nonactivated controls (data not shown). In contrast, LPS-stimulated moDC exposed to 4 μg/ml of ADI produced significantly less IL-10 and IL-12p40. These values were 45% (P < 0.001, n = 7) and 17% (P < 0.01, n = 6) lower than those of control cells exposed to mutant ADIC424A, respectively. In contrast, TNF-α secretion by ADI-treated and LPS-activated moDC was increased and values were, on average, 74% (P < 0.01, n = 6) higher than the values of LPS-stimulated, mutant enzyme-exposed control cells. Of note, decreased IL-10 and IL-12p40 levels were also observed with 10-fold less ADI present whereas increased TNF-α was observed only at the higher enzyme concentration, indicating that the time required by different doses of the enzyme to deplete arginine was relevant. These data show that arginine depletion by ADI modulates cytokine secretion of LPS-activated moDC and, importantly, that the effects differ, depending on the cytokine analyzed.
Fig 2.
Enzymatic arginine depletion by ADI modulates cytokine secretion of LPS-activated human moDC. Immature moDC (5 × 105 per sample) were exposed to the indicated amounts of recombinant ADI or, as a control, corresponding levels of ADIC424A (only the largest amount is shown), and 1 μg/ml LPS was added. Citrulline content was determined as a measure of the cumulative ADI activity and arginine depletion over the 24-h assay time (A). DC cytokine secretion into supernatants was assessed by ELISA and is expressed as a percentage of the amount secreted by LPS-stimulated cells not exposed to any ADI protein (B). Bars in panel A represent the mean ± SD from experiments with DC prepared from seven different donors. Symbols in panel B represent values from individual donors, and means are indicated by horizontal lines. Differences between the amounts of cytokines secreted by cells exposed to mutant (control) or active ADI were analyzed by paired (two-tailed) t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Enzymatic arginine depletion by ADI reduces the upregulation of surface CD83 and CD86 levels of LPS-activated moDC.
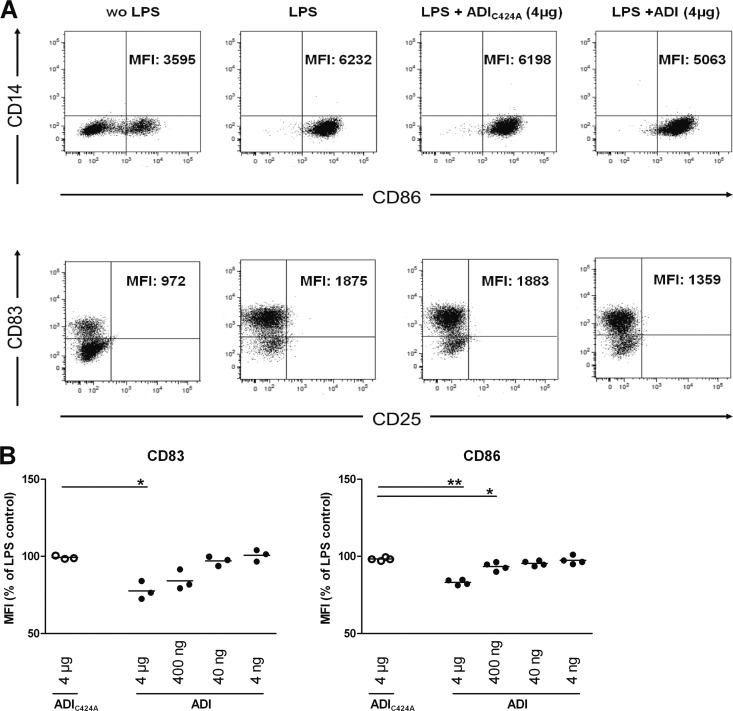
To investigate the influence of arginine depletion by ADI on the phenotype of maturing DC, moDC were stimulated with LPS and treated with ADI or the mutant form ADIC424A as described above and analyzed for selected surface marker proteins levels by flow cytometry (Fig. 3A). Activation of immature moDC with LPS induced the upregulation of CD83 and CD86 (data not shown) as expected. In contrast, LPS-stimulated moDC treated with arginine-depleting levels of ADI expressed significantly less CD83 and CD86 than did control cells. Surface CD83 was, on average, 22% (P < 0.05, n = 3) and CD86 was 15% (P < 0.01, n = 4) lower than on LPS-treated, mutant enzyme-exposed control cells. The effects were again ADI dose dependent (Fig. 3B). The reduced upregulation of surface CD83 and CD86 unlikely reflects a general effect on surface protein levels, since HLA-DR abundance on LPS-activated moDC was not affected by ADI (data not shown).
Fig 3.
Enzymatic arginine depletion by ADI reduces CD83 and CD86 surface marker induction by LPS activation on moDC. Cells were treated as described in the legend to Fig. 1, and all of the cells, except nonstimulated control cells, were treated with 1 μg/ml LPS. After incubation for 24 h at 37°C, moDC were harvested, stained with cell surface marker-specific antibodies, and analyzed by flow cytometry. (A) Representative dot plots for CD14/CD83 (top) and CD25/CD86 (bottom) expression with the respective mean fluorescence intensity (MFI) values for CD86- and CD83-positive populations (cells in the lower right and top left quadrants of panel A) for CD86 and CD83, respectively) are shown. wo, without. (B) Relative MFI values as percentages of those of control LPS-stimulated cells for CD83 (n = 3) and CD86 (n = 4) with moDC from different donors are shown. Horizontal lines correspond to mean values, and differences between the respective MFI values on cells exposed to mutant (control) and active ADI were analyzed by paired (two-tailed) t test. *, P < 0.05; **, P < 0.01.
ADI immunomodulatory effects on LPS-activated moDC result from both arginine depletion and product formation.
We asked next whether the modulatory effects of ADI on the moDC functional phenotype resulted from arginine depletion and/or the formation of the ADI reaction products citrulline and/or NH4+. To investigate possible cause-effect relationships, moDC were generated as before, harvested, and then seeded into arginine-free culture medium that was only subsequently supplemented with arginine, citrulline, and/or ammonium chloride, respectively. Cells were then treated as before with 4 μg of ADI or ADIC424A and activated with LPS. After 24 h of incubation, supernatants were collected and citrulline content and cytokine concentration were determined. NH4+ formation by ADI was also determined in culture supernatants, and levels were comparable to those observed after supplementation with ammonium chloride (data not shown). Furthermore, moDC were harvested and cell surface markers were analyzed.
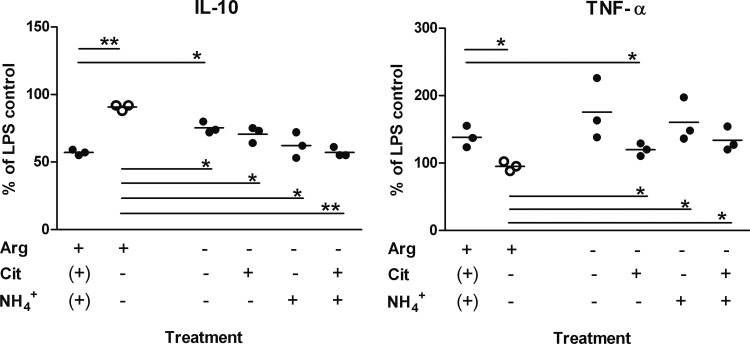
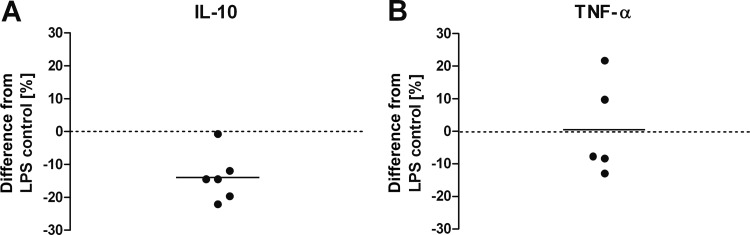
IL-10 and TNF-α secretion of cells grown in arginine-replete medium were modulated as described above; i.e., ADI-treated LPS-activated moDC produced less IL-10 and more TNF-α than did control cells (Fig. 4). MoDC stimulated in the absence of arginine showed a small decrease in IL-10 production, but levels remained significantly higher than those of cells treated with ADI in the presence of arginine, where citrulline and NH4+ could be formed (Fig. 4). The addition of the ADI products, in particular, of NH4+ in the form of NH4Cl, reduced IL-10 levels to those observed in the presence of arginine and ADI. Similarly, CD83 and CD86 surface levels were also reduced, although this did not reach statistical significance for the latter marker (data not shown). In contrast, the increase in TNF-α production was driven mainly by arginine depletion. LPS-activated DC from all of the donors tested showed significantly increased TNF-α production in the absence of arginine (P < 0.01; n = 8), with cells from some donors reacting particularly strongly, producing nearly three times as much as the respective control DC (data not shown and Fig. 4). The increase due to arginine depletion was not affected by NH4+ but reduced by citrulline, indicating that citrulline could substitute for arginine in this respect (Fig. 4). Supplementation of DC with NH4+ during stimulation in the presence of arginine but with no ADI present also reduced IL-10 but had no effect on TNF-α production (Fig. 5).
Fig 4.
Depletion of arginine and formation of ADI reaction products citrulline and ammonium ions modulate the moDC response to LPS activation. Immature moDC after harvesting were seeded into arginine-free growth medium. The medium was then supplemented as indicated with arginine (Arg), citrulline (Cit), and/or ammonium chloride (2 mM each; brackets indicate addition of ADI to arginine-replete medium to reflect formed, not supplemented products). Cells were activated with LPS and treated with ADI (●) or mutant ADIC424A (○) as a control. Values correspond to percentages of the respective parameter determined for cells stimulated with LPS only in arginine-replete medium. After 24 h, supernatants were collected and cytokine concentrations were determined by ELISA. Horizontal lines correspond to mean values, and differences between the respective parameter determined with cells exposed to mutant (control) and active ADI were analyzed by paired (two-tailed) t test. *, P < 0.05; **, P < 0.01.
Fig 5.
NH4+ reduces IL-10 secretion by LPS-stimulated moDC. Immature moDC were prepared and stimulated with LPS as described in the legend to Fig. 4 in medium supplemented with either arginine alone (control) or arginine plus NH4+Cl. After 24 h, supernatants were taken and cytokine concentrations were determined by ELISA. Cytokine concentrations are expressed as percent differences from the cytokine amounts produced by control cells. Each dot represents an independent experiment with moDC prepared from an individual donor. Significance was tested against the null hypothesis that addition of NH4+ had no effect by paired (two-tailed) t test. P values were significant (P < 0.01) for IL-10 levels and nonsignificant (P > 0.05) for TNF-α levels.
Thus, the immunomodulation of moDC by ADI resulted from a combination of distinct effects of arginine depletion and/or citrulline and NH4+ product formation.
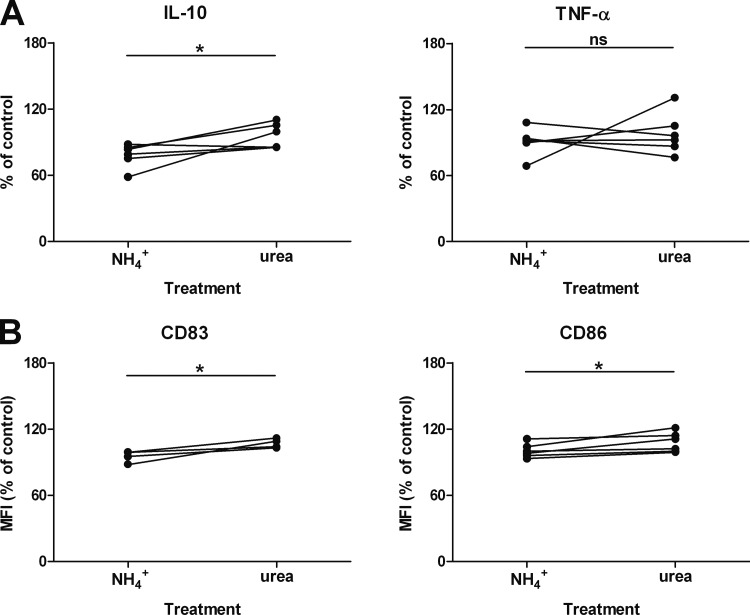
NH4+ and urea, the reaction products of ADI and arginases, differ in their effects on cytokine secretion and the surface marker profile of LPS-stimulated moDC.
As mentioned before, many pathogens are thought to evade NO-mediated immune clearance through arginine depletion by arginases, which will result in the formation of ornithine and urea (2). Immunomodulatory effects may thus be different from conditions where deiminases are relevant. We were therefore interested in comparing the immunomodulatory effects of NH4+ and urea. LPS-stimulated cells were incubated for 24 h in medium devoid of arginine but supplemented with ammonium chloride or urea, and then supernatants were collected for the detection of IL-10 and TNF-α by ELISA (Fig. 6A) and of CD83 and CD86 proteins by flow cytometry (Fig. 6B). Notably, cells stimulated in medium supplemented with urea produced significantly more IL-10 and displayed significantly higher levels of CD83 and CD86 surface proteins than did cells stimulated in the presence of NH4+. In contrast and corroborating our finding that TNF-α production was not affected by NH4+ (Fig. 4), no differences between cells treated with NH4+ and cells treated with urea were noted with respect to TNF-α release.
Fig 6.
Immunomodulation of LPS-activated moDC undergoing arginine starvation is different between NH4+ and urea. MoDC were prepared as described in the legend to Fig. 4 and stimulated in arginine-free medium supplemented with either 2 mM ammonium chloride or urea. After 24 h, supernatants were collected and cytokine concentrations were determined by ELISA (A). The moDC were harvested, and surface marker proteins were analyzed by flow cytometry (B). Dots represent values from experiments with cells from six (A) or four (B) individual donors and are expressed as percentages of control values obtained with LPS-activated cells with no supplement. *, P < 0.05 (paired [two-tailed] t test); ns, nonsignificant.
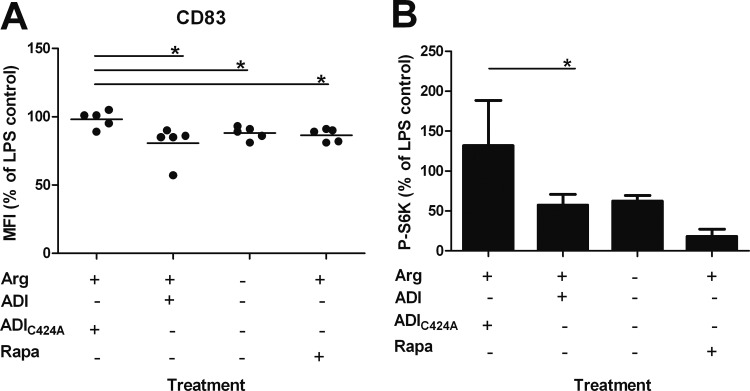
Arginine turnover by ADI results in decreased phosphorylation of the mTOR signaling pathway target S6K in LPS-activated moDC.
Previous studies suggested an inhibitory effect of branched-chain amino acids on the mTOR/S6K signaling pathway, resulting in impaired maturation of moDC and particularly affecting CD83 expression (30). mTOR is a serine/threonine kinase that is present in two distinct protein complexes, mTORC1 and mTORC2. Activation of mTORC1 leads to the phosphorylation of the proteins S6K and 4E-BP1, which are both involved in the regulation of protein translation (31). Arginine levels have been shown to affect mTOR signaling in T cells (14, 15). Since ADI by arginine depletion had immunomodulatory effects on moDC, we hypothesized that this may also involve the mTOR pathway in moDC. MoDC were seeded into arginine-free medium and then supplemented or not with arginine. The cells were treated with recombinant ADI or catalytically inactive ADIC424A or with the mTOR inhibitor rapamycin as a positive control and activated with LPS. Cells were incubated for 24 h before determination of surface CD83 levels (Fig. 7A). In the presence of arginine, CD83 levels were lower on cells treated with ADI than on cells exposed to the mutant enzyme, as shown before. MoDC stimulated in the absence of arginine or in the presence of arginine and rapamycin also showed reduced levels of CD83 protein (Fig. 7A).
Fig 7.
ADI-mediated arginine depletion decreases mTOR signaling in LPS-stimulated moDC. Immature moDC (106 in arginine [Arg]-free growth medium) were seeded into each well of a 12-well tissue culture plate. Arginine-free medium (ΔArg) was supplemented or not with 2 mM arginine, and cells were treated or not with 4 μg of ADI, 4 μg of ADIC424A, or 2 μM rapamycin (Rapa). For activation, 1 μg/ml LPS was added to each sample after 2 h. (A) MoDC CD83 surface marker levels 24 h after stimulation and those of stimulated control cells are compared. Bars represent the mean ± SD of independent experiments with five different donors. *, P < 0.05 (paired [two-tailed] t test compared with the respective control). (B) In parallel, 30 min after LPS stimulation, cells were harvested, washed, and lysed. A 50-μg sample of cell extract was separated by SDS-PAGE, and p70-S6K, phospho-p70-S6K, and β-actin were detected by immunoblotting and quantified by image analysis. Phosphorylated p70-S6K levels were normalized relative to β-actin and compared to those of stimulated control cells. Levels of total p70-S6K were not significantly different between the different experimental groups. Bars represent the mean ± SD from five individual experiments with cells from different donors. *, P < 0.05; **, P < 0.01 (compared with the respective control by paired [two-tailed] t test).
To investigate whether the reduced CD83 surface levels caused by ADI activity correlated with mTOR signaling, treated cells were harvested 30 min after LPS stimulation to assess mTOR-dependent phosphorylation events. Cells were lysed, and equal amounts of total protein were separated by SDS-PAGE. The abundance of phosphorylated mTOR target protein S6K was then determined by Western blotting, and results were quantified. Suppression of S6K phosphorylation in moDC correlated with reduced CD83 surface protein levels, and this depended on arginine levels (Fig. 7B). Control cells treated with rapamycin showed the expected suppression of S6K phosphorylation. Phosphorylation of 4E-BP1, however, did not depend on arginine availability (data not shown).
These data suggest that arginine levels, similar to what has been shown for branched-chained amino acids (30), affect mTOR activity in moDC but also indicate a difference between amino acids.
DISCUSSION
We show here that arginine depletion by G. duodenalis ADI modulates the surface markers and cytokine response of in vitro-activated human moDC. The immunomodulation depended on both the depletion of arginine and the formation of ADI reaction products, in particular, NH4+. By investigating the consequences of arginine depletion, we found that the mTOR pathway is implicated in the molecular signaling process and leads to the modulation of DC responses. These findings are further evidence that supports the hypothesis suggested by others (10) that ADI is a molecularly defined virulence and pathogenicity factor of G. duodenalis. Arginine-depleting enzymes of pathogens have been implicated in immune evasion mechanisms because they compete for the same substrate as host NO synthases. To date, little attention has been paid to effects beyond this inhibition of microbial effector function. Our data imply that arginine-metabolizing enzymes of pathogens are more widely involved in immunomodulation and suggest distinct roles for the reaction products of different enzyme classes in this process.
Currently, the relevance of our in vitro findings for the understanding of the pathogenesis of giardiasis must remain speculative and further studies are required. Nonetheless, we will discuss their potential implications. First, and as mentioned before, DC in the intestine project extensions between epithelial cells into the lumen of the gut to sample antigens (25, 26). Although only two studies with murine cells have been published to date that investigated the interaction of Giardia parasites with DC (32, 33), they provided evidence for the importance of DC in the control of Giardia infection (33). In addition, human epithelial cells, upon interaction with Giardia, have been found to produce DC-attracting chemokines such as CCL20 (34). The ability to modulate DC function could thus provide a selective advantage to the parasite, and our calculations suggest that arginine depletion may indeed occur in situ.
Second, arginine-dependent response modulation would be consistent with observations on the pathophysiology of human giardiasis. Atrophy of villi has been detected microscopically in intestinal biopsy specimens from chronically infected patients, and symptomatic disease has been correlated with a dysfunction of the epithelial barrier (35) but the process leading to this is not understood. These sequelae can, however, also be observed when intestinal biopsy specimens are treated with TNF-α (36). TNF-α may thus have pathogenic properties in this context. Studies with mice showed that peak Giardia parasite load levels were around 10-fold higher in animals devoid of TNF-α (37); hence, it was proposed that it has a protective function in giardiasis. However, in the same study, transepithelial resistance was reduced to the same extent despite a much lighter parasite burden in TNF-α-responsive mice. An explanation consistent with the results from human biopsy specimens exposed to TNF-α (35) could be that reduced epithelial integrity during giardiasis is due to parasite factors and TNF-α, the latter playing dual (protective and pathogenic) roles.
Third, children suffering from symptomatic giardiasis were shown to have increased mucosal levels of proinflammatory cytokines, including TNF-α, which decreased after antiparasitic treatment and resolution of symptoms while local levels of IL-10 increased after treatment (38). This is in agreement with our in vitro findings and may indicate that parasite ADI-mediated arginine depletion impaired IL-10 and enhanced TNF-α secretion by mucosal DC in situ in these children. The relative abundance of IL-10 and TNF-α is recognized as a critical parameter in intestinal diseases in mice (39) and in humans (40, 41).
Fourth, although G. duodenalis ADI is found intracellularly as part of the arginine dehydrolase (ADH) pathway and this pathway is thought to exploit arginine as an energy source to produce ATP in these amitochondrial organisms (42), the protein is also released by the parasite. Microarray analysis of the transcriptional response to host cell contact had revealed an upregulation of adi mRNA (10), and ADI and ornithine carbamoyl transferase (OCT, the next enzyme in the ADH pathway) were released by the parasite in contact with intestinal cells in vitro and in vivo (43; S.B. unpublished data). The facts that ADI and OCT were detected in these assays and are also immunodominant antigens during infection (44, 45) indicate that significant amounts of the enzymes are extracellular, and therefore, free citrulline may even be further metabolized to ornithine. It is tempting to speculate that this may exacerbate TNF-α production because the negative feedback of citrulline on this parameter would be reduced. Thus, release of ADH compounds could have evolved in part because of selective pressure by the host's immune response.
To our knowledge, this is the first report showing an immunomodulatory effect of arginine depletion and NH4+ formation on the response of DC other than on NO formation. Precedence for a clinically relevant role for amino acids in the modulation of immune responses exists (46, 47). For DC, it has been shown that branched-chain amino acids affect maturation, and it has been proposed that this modulation is a consequence of inhibition of the mTOR pathway (30). Our results are consistent with this hypothesis but extend the concept to arginine. Of note, NH4+ has recently been reported to modulate mTOR activity in yeast cells (48). This suggests that reaction products formed by arginine-depleting enzymes could further affect mTOR signaling. However, additional signaling pathways are likely to be involved in the mediation of the distinct effects of arginine depletion alone and NH4+ formation. The latter has recently also been invoked in the T cell inhibition mediated by Salmonella l-asparaginase II (49). Further studies are required to understand this comprehensively.
In summary, we describe immunomodulatory effects of arginine depletion on human DC. Using ADI from G. duodenalis, we show that this immunomodulation depends on arginine depletion and the products formed by the enzyme. This reveals novel facets of DC response modulation by arginine-metabolizing enzymes and may have implications for the general understanding of the arginine-dependent virulence mechanisms of pathogens.
ACKNOWLEDGMENTS
We thank V. Rickerts for a constructive critique of the manuscript. The technical support of Petra Gosten-Heinrich is gratefully acknowledged.
This work was in part supported by a grant to T.A. (01KI1019) from the National Research Platform for Zoonosis, which is funded by the German Federal Ministry of Education and Research (BMBF), and a grant from the Georg and Agnes Blumenthal Stiftung to T.A. and F.S.
Footnotes
Published ahead of print 15 April 2013
REFERENCES
- 1. Bronte V, Zanovello P. 2005. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5:641–654 [DOI] [PubMed] [Google Scholar]
- 2. Das P, Lahiri A, Lahiri A, Chakravortty D. 2010. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 6:e1000899. 10.1371/journal.ppat.1000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fulde M, Willenborg J, de Greeff A, Benga L, Smith HE, Valentin-Weigand P, Goethe R. 2011. ArgR is an essential local transcriptional regulator of the arcABC operon in Streptococcus suis and is crucial for biological fitness in an acidic environment. Microbiology 157:572–582 [DOI] [PubMed] [Google Scholar]
- 4. Benga L, Goethe R, Rohde M, Valentin-Weigand P. 2004. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell. Microbiol. 6:867–881 [DOI] [PubMed] [Google Scholar]
- 5. Ryan S, Begley M, Gahan CG, Hill C. 2009. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ. Microbiol. 11:432–445 [DOI] [PubMed] [Google Scholar]
- 6. Rópolo AS, Touz MC. 2010. A lesson in survival, by Giardia lamblia. ScientificWorldJournal 10:2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stadelmann B, Merino MC, Persson L, Svard SG. 2012. Arginine consumption by the intestinal parasite Giardia intestinalis reduces proliferation of intestinal epithelial cells. PLoS One 7:e45325. 10.1371/journal.pone.0045325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faubert G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35–54, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ankarklev J, Jerlstrom-Hultqvist J, Ringqvist E, Troell K, Svard SG. 2010. Behind the smile: cell biology and disease mechanisms of Giardia species. Nature reviews. Microbiology 8:413–422 [DOI] [PubMed] [Google Scholar]
- 10. Ringqvist E, Avesson L, Soderbom F, Svard SG. 2011. Transcriptional changes in Giardia during host-parasite interactions. Int. J. Parasitol. 41:277–285 [DOI] [PubMed] [Google Scholar]
- 11. Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164:1478–1487 [DOI] [PubMed] [Google Scholar]
- 12. Andersen YS, Gillin FD, Eckmann L. 2006. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect. Immun. 74:2473–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC. 2004. l-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell. Immunol. 232:21–31 [DOI] [PubMed] [Google Scholar]
- 14. Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, Huang R, Zhang J, Tan B, Wang W, Wu G. 2008. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 138:867–872 [DOI] [PubMed] [Google Scholar]
- 15. Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. 2004. Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int. J. Mol. Med. 13:537–543 [PubMed] [Google Scholar]
- 16. Weichhart T, Saemann MD. 2009. The multiple facets of mTOR in immunity. Trends Immunol. 30:218–226 [DOI] [PubMed] [Google Scholar]
- 17. Bi Y, Liu G, Yang R. 2011. mTOR regulates T-cell differentiation and activation in immunity and autoimmunity. Crit. Rev. Eukaryot. Gene Expr. 21:313–322 [DOI] [PubMed] [Google Scholar]
- 18. Keister DB. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487–488 [DOI] [PubMed] [Google Scholar]
- 19. Lutz R, Bujard H. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knodler LA, Schofield PJ, Edwards MR. 1995. l-Arginine transport and metabolism in Giardia intestinalis support its position as a transition between the prokaryotic and eukaryotic kingdoms. Microbiology 141(Pt 9):2063–2070 [DOI] [PubMed] [Google Scholar]
- 21. Böger RH. 2007. The pharmacodynamics of l-arginine. J. Nutr. 137:1650S–1655S [DOI] [PubMed] [Google Scholar]
- 22. Danciger M, Lopez M. 1975. Numbers of Giardia in the feces of infected children. Am. J. Trop. Med. Hyg. 24:237–242 [DOI] [PubMed] [Google Scholar]
- 23. Li Z, Kulakova L, Li L, Galkin A, Zhao Z, Nash TE, Mariano PS, Herzberg O, Dunaway-Mariano D. 2009. Mechanisms of catalysis and inhibition operative in the arginine deiminase from the human pathogen Giardia lamblia. Bioorg. Chem. 37:149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Touz MC, Rópolo AS, Rivero MR, Vranych CV, Conrad JT, Svard SG, Nash TE. 2008. Arginine deiminase has multiple regulatory roles in the biology of Giardia lamblia. J. Cell Sci. 121:2930–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361–367 [DOI] [PubMed] [Google Scholar]
- 26. Chieppa M, Rescigno M, Huang AY, Germain RN. 2006. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203:2841–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pulendran B, Palucka K, Banchereau J. 2001. Sensing pathogens and tuning immune responses. Science 293:253–256 [DOI] [PubMed] [Google Scholar]
- 28. Münz C, Steinman RM, Fujii S. 2005. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 202:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 30. Kakazu E, Kanno N, Ueno Y, Shimosegawa T. 2007. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J. Immunol. 179:7137–7146 [DOI] [PubMed] [Google Scholar]
- 31. Carrera AC. 2004. TOR signaling in mammals. J. Cell Sci. 117:4615–4616 [DOI] [PubMed] [Google Scholar]
- 32. Kamda JD, Singer SM. 2009. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infect. Immun. 77:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamda JD, Nash TE, Singer SM. 2012. Giardia duodenalis: dendritic cell defects in IL-6 deficient mice contribute to susceptibility to intestinal infection. Exp. Parasitol. 130:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roxström-Lindquist K, Ringqvist E, Palm D, Svard S. 2005. Giardia lamblia-induced changes in gene expression in differentiated Caco-2 human intestinal epithelial cells. Infect. Immun. 73:8204–8208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD. 2007. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 56:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. 1999. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 112(Pt 1):137–146 [DOI] [PubMed] [Google Scholar]
- 37. Zhou P, Li E, Shea-Donohue T, Singer SM. 2007. Tumour necrosis factor alpha contributes to protection against Giardia lamblia infection in mice. Parasite Immunol. 29:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maciorkowska E, Kaczmarski M, Kemona A. 2005. The role of cytokines in giardiasis in children. Med. Wieku Rozwoj. 9:665–673 (In Polish.) [PubMed] [Google Scholar]
- 39. Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, Davis R, Flavell R, Kollias G. 2001. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20:3760–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ordás I, Mould DR, Feagan BG, Sandborn WJ. 2012. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 91:635–646 [DOI] [PubMed] [Google Scholar]
- 41. Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. 2011. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 1246:102–107 [DOI] [PubMed] [Google Scholar]
- 42. Zúñiga M, Perez G, Gonzalez-Candelas F. 2002. Evolution of arginine deiminase (ADI) pathway genes. Mol. Phylogenet. Evol. 25:429–444 [DOI] [PubMed] [Google Scholar]
- 43. Ringqvist E, Palm JE, Skarin H, Hehl AB, Weiland M, Davids BJ, Reiner DS, Griffiths WJ, Eckmann L, Gillin FD, Svard SG. 2008. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol. Biochem. Parasitol. 159:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Téllez A, Palm D, Weiland M, Aleman J, Winiecka-Krusnell J, Linder E, Svard S. 2005. Secretory antibodies against Giardia intestinalis in lactating Nicaraguan women. Parasite Immunol. 27:163–169 [DOI] [PubMed] [Google Scholar]
- 45. Palm JE, Weiland ME, Griffiths WJ, Ljungstrom I, Svard SG. 2003. Identification of immunoreactive proteins during acute human giardiasis. J. Infect. Dis. 187:1849–1859 [DOI] [PubMed] [Google Scholar]
- 46. Calder PC. 2006. Branched-chain amino acids and immunity. J. Nutr. 136:288S–293S [DOI] [PubMed] [Google Scholar]
- 47. Evoy D, Lieberman MD, Fahey TJ, III, Daly JM. 1998. Immunonutrition: the role of arginine. Nutrition 14:611–617 [DOI] [PubMed] [Google Scholar]
- 48. Santos J, Sousa MJ, Leao C. 2012. Ammonium is toxic for aging yeast cells, inducing death and shortening of the chronological lifespan. PLoS One 7:e37090. 10.1371/journal.pone.0037090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kullas AL, McClelland M, Yang HJ, Tam JW, Torres A, Porwollik S, Mena P, McPhee JB, Bogomolnaya L, Andrews-Polymenis H, van der Velden AW. 2012. l-Asparaginase II produced by Salmonella typhimurium inhibits T cell responses and mediates virulence. Cell Host Microbe 12:791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]