Abstract
The need for a vaccine against botulism has increased since the discontinuation of the pentavalent (ABCDE) botulinum toxoid vaccine by the Centers for Disease Control and Prevention. The botulinum toxins (BoNTs) are the primary virulence factors and vaccine components against botulism. BoNTs comprise three domains which are involved in catalysis (LC), translocation (HCT), and host receptor binding (HCR). Recombinant HCR subunits have been used to develop the next generation of BoNT vaccines. Using structural studies and the known entry properties of BoNT/A, an HCR subunit vaccine against BoNT/A that contained the point mutation W1266A within the ganglioside binding pocket was designed. HCR/A(W1266A) did not enter primary neurons, and the crystal structure of HCR/A(W1266A) was virtually identical to that of wild-type HCR/A. Using a mouse model, experiments were performed using a high-dose vaccine and a low-dose vaccine. At a high vaccine dose, HCR/A and HCR/A(W1266A) elicited a protective immune response to BoNT/A challenge. At the low-dose vaccination, HCR/A(W1266A) was a more protective vaccine than HCR/A. α-HCR IgG titers correlated with protection from BoNT challenge, although titers to block HCR/A entry were greater in serum in HCR/A-vaccinated mice than in HCR/A(W1266A)-vaccinated mice. This study shows that removal of receptor binding capacity enhances potency of the subunit HCR vaccine. Vaccines that lack receptor binding capacity have the added property of limited off-target toxicity.
INTRODUCTION
Botulinum neurotoxins (BoNTs) are produced by several Clostridium botulinum species and are the most toxic proteins for humans (1). BoNTs act at peripheral motor neurons to inhibit acetylcholine secretion across synaptic clefts to muscle (2). BoNT paralysis of respiratory musculature can last for several months and may be lethal (3). Due to this extreme potency and neuronal specificity, BoNTs are also used in local injections for treatment of spastic muscle disorders and, more recently, regulation of autonomic cholinergic junctions and pain management (reviewed in references 4–6). BoNT/A was the first serotype utilized to treat human neurological diseases. BoNT/B has also been approved for several human therapies, and other BoNT serotypes are being evaluated as potential therapeutics.
Two factors drive BoNT specificity for motor neurons: neuronal receptors and cleavage of neuronal substrates. BoNTs are 150-kDa di-chain proteins comprising a 50-kDa light chain (LC) and a 100-kDa heavy chain (HC) linked by a disulfide bond (7, 8) (Fig. 1A). The HC has two domains: the N-terminal translocation domain (HCT) and the C-terminal receptor binding domain (HCR) (9). For BoNT/A, the HCR binds a ganglioside and synaptic vesicle glycoprotein 2 (SV2) as receptors to enter neurons (10–12). The BoNT-receptor complex is endocytosed into the synaptic vesicle cycling pathway, where the HCT delivers the LC into the neuronal cytosol via a pH-dependent mechanism (13, 14). LC/A cleaves a synaptosome-associated protein of 25 kDa (SNAP25) which is required for synaptic vesicle fusion to the plasma membrane (15, 16).
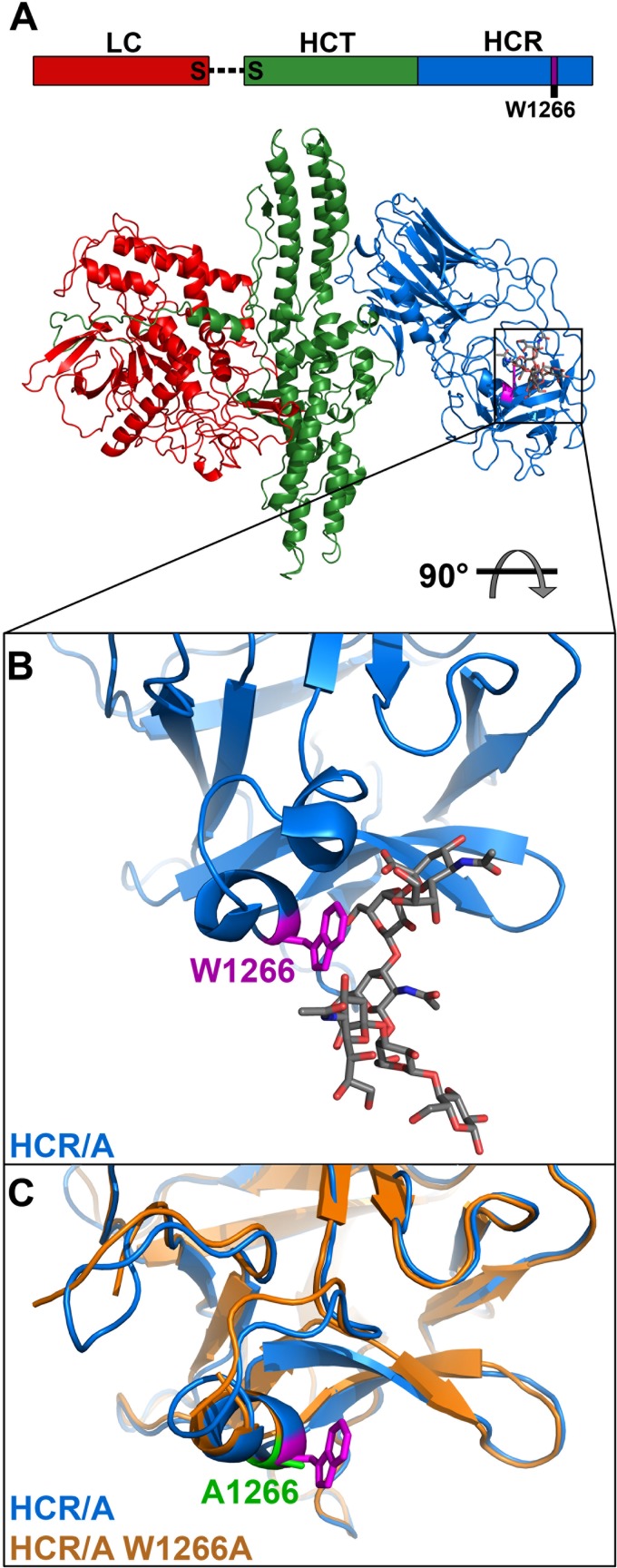
Fig 1.
HCR/A(W1266A) structure. (A) Linear schematic of HCR domains and color-coded crystal structures with ganglioside modeled into the binding site. (B) Ganglioside binding pocket of HCR/A, with tryptophan 1266 colored fuchsia, cocrystallized ganglioside in gray, oxygen atoms in red, and nitrogen atoms in blue. (C) Overlay of wild-type HCR/A from holotoxin structure (W1266 in fuchsia) and HCR/A(W1266A) in orange (alanine 1266 in green). RMSD = 0.4Å.
BoNT/A binds the ganglioside GT1b via the terminal 5′-sialic acid and galactose through eight residues, identified in the structure of a complex of HCR/A with the GT1b sugar moiety (17). The ganglioside binding pocket (GBP) is lined with residues Y1117, E1203, F1252, H1253, S1264, W1266, S1275, and R1276. The imidazole ring of H1253 makes a hydrogen bond with the 6′-OH of Gal4, while the indole ring of W1266 stacks on the adjacent side of the galactose ring. Y1117 and R1276 interact with the terminal 5′-sialic acid, while Y1267 and G1279 line the back of the GBP but do not contact the ganglioside directly. Several of these residues comprise the E…H…SXWY…G motif identified in most BoNT serotypes and the tetanus toxin (18). Mutation of residues within the GBP affects ganglioside binding affinity. For example, BoNT/A(W1266L) had 0.7% of the toxicity of wild-type BoNT/A in a paralytic half-time assay (18). Mutations in the analogous tryptophan in the lactose binding pocket (GBP equivalent) of tetanus toxin also reduced ganglioside binding affinity (19, 20).
BoNT toxoid vaccines have been used for individuals at risk for exposure, including health care providers, researchers, first responders, and military personnel (21). The earliest vaccines were generated by formalin inactivation, similar to the current tetanus vaccine (22) and the pentavalent (ABCDE) botulinum toxoid vaccine (23). However, the pentavalent vaccine has lost efficacy during storage and was recently discontinued after more than 30 years of use (24). In 2002, Torii et al. reported the generation of an ABEF toxoid vaccine, which is currently used to immunize at-risk workers in Japan (25, 26).
In humans that generate antibody responses to BoNT/A therapy, most of the immune epitopes are located within the HC (27, 28). Animal vaccination studies also implicated major immune epitopes within the HC (28, 29). Recombinant HCR subunit vaccines are currently being evaluated (29, 30). The recombinant botulism vaccine for BoNT/A and BoNT/B (rBV A/B) consists of the HCR domains that are expressed in Pichia pastoris (31). This vaccine is currently in early clinical trials; passive immunization in animal models from neutralizing antibodies generated in humans improved survival with BoNT challenges (32). Previously, our lab engineered a vaccine composed of the HCR domains of each of the seven BoNT serotypes which protected against challenge by each respective holotoxin (33). A lack of cross-neutralization is consistent with the observation that immunoreactive epitopes appear to be distinct for different serotypes, as reported for BoNT/A and BoNT/B (30, 34).
Efforts to improve upon tetanus vaccines involved deletion and subsequent site-directed mutation to the ganglioside binding residues (35). In addition, evidence exists that the HCR C-terminal subunit, which still binds GT1b, is inefficient at eliciting protective immunity (36). More recently, HCR/A(W1266L/Y1267S) was shown to protect mice from BoNT challenge (37). While the study made important findings for understanding the entry pathways for BoNT/A across mucosas and neurons, the di-mutations used may have had perturbed structural integrity within the ganglioside binding site (17) and the high-dose vaccinations complicated assessment of vaccine potency. The current study utilizes a single-point-mutated HCR/A, HCR/A(W1266A), which lacked detectable binding to neurons without perturbing protein structure. HCR/A(W1266A) elicited a more protective immune response to BoNT/A challenge than HCR/A in a mouse model of intoxication. Thus, disruption of ganglioside binding improves the effectiveness of recombinant HCR/A as a vaccine against botulism while limiting the possibility of off-target toxicity.
MATERIALS AND METHODS
Construction of HCR expression vectors.
DNA encoding the HCR domain of BoNT/A subtype A1 (residues 870 to 1,296) was synthesized (EZBiolab, Inc., Westfield, IN) with optimal codon bias for Escherichia coli expression. The DNA fragment was amplified and subcloned into unique KpnI and NotI restriction sites of a modified pET28 vector (Novagen) containing either a 3×FLAG epitope or a triple hemagglutinin (HA) epitope downstream of the His (6) epitope. A point mutation (W1266A) was made by site-directed mutagenesis (Agilent Technologies, Santa Clara, CA). pET28-3×FLAG-His6-HCR/A and pET28-3×FLAG-His6-HCR/A(W1266A) were transformed into BL21(DE3) (Agilent Technologies, Santa Clara, CA) for protein expression.
HCR purification.
E. coli BL21(DE3) pET28a-3×FLAG-HCR/A, pET28a-3HA-HCR/A, and pET28a-3×FLAG-HCR/A(W1266a) were grown overnight on LB plates with 50 μg/ml kanamycin and inoculated into LB medium (400 ml) containing the same antibiotic at 30°C for 2 h at 250 rpm to an optical density at 600 nm (OD600) of ∼0.6 when 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added, followed by an overnight culture at 250 rpm and 16°C. Cells were harvested, and pellets were suspended in 20 mM Tris-HCl (pH 7.9), 0.5 M NaCl, and 5 mM imidazole. Cells were lysed with a French press and clarified by centrifugation and filtration (cellulose acetate). HCR/A proteins were purified from the filtered lysate using Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen) equilibrated in lysis buffer. The column was washed with 20 mM Tris-HCl (pH 7.9), 0.5 M NaCl, and 10 mM imidazole, followed by another wash with 20 mM Tris-HCl (pH 7.9), 0.5 M NaCl, and 20 mM imidazole. Proteins were eluted with 20 mM Tris-HCl (pH 7.9), 0.5 M NaCl, and 250 mM imidazole. The NTA eluent was purified on a S200HR size exclusion Sephacryl (600-ml column; Sigma) equilibrated in 20 mM Tris-HCl (pH 7.9), 0.2 M NaCl, and 0.1% Triton X-100. Peak fractions were pooled and applied to a fresh Ni2+-NTA resin (Qiagen) equilibrated in lysis buffer. This column was washed, and HCRs were eluted with the buffers described above. Eluent was then dialyzed into 10 mM Tris-HCl (pH 7.9), 0.2 M NaCl, and 40% (vol/vol) glycerol and stored at −20°C.
HCR binding and entry into neurons.
E18 rat cortical neurons (BrainBits, LLC) were dissociated and plated on poly-d-lysine- and laminin-coated glass-bottom dishes (MatTek). Cells were cultured in neurobasal medium (Life Technologies) supplemented with 0.5 mM GlutaMAX-I (Life Technologies), 2% B27 supplement (Life Technologies), and Primocin (InvivoGen). Cells were cultured for 10 to 14 days to allow morphological differentiation.
(i) HCR/A binding and entry.
Cells were washed in Dulbecco's phosphate-buffered saline (DPBS), and 40 nM HCR was incubated in 500 μl buffer (15 mM HEPES, 145 mM NaCl, 2.2 mM CaCl2, 0.5 mM MgCl2, pH 7.4) supplemented with either low potassium (5.6 mM KCl) or high potassium (56 mM KCl).
(ii) Serum neutralization.
HA-HCR at 40 nM was preincubated with serum dilutions of 1:50 to 1:400 for 30 min at 4°C and then warmed to 37°C prior to incubation with neurons. HCRs were incubated with neurons for 5 min. Cells were washed, fixed with PBS and 4% (wt/vol) paraformaldehyde, permeabilized with 0.1% Triton X-100 and 4% (vol/vol) formalin, and incubated with 150 mM glycine. For immunofluorescence, cells were blocked in 10% (vol/vol) fetal bovine serum, 2.5% (wt/vol) cold fish skin gelatin, 0.1% Triton X-100, 0.05% Tween 20 in DPBS, and the primary antibodies mouse α-FLAG (Sigma), rat α-HA (Roche), or guinea pig α-synaptophysin (Synaptic Systems) and incubated overnight at 4°C in 5% (vol/vol) fetal bovine serum, 1% (wt/vol) cold fish skin gelatin, 0.1% Triton X-100, and 0.05% Tween 20 in DPBS. Cells were washed 3 times and incubated for 1 h in the secondary antibodies goat α-mouse Alexa561 or goat α-rat Alexa561 and goat α-guinea pig Alexa488 (Life Technologies). Finally, cells were washed and fixed and mounting reagent CitiFluor AF3 (Electron Microscopy Sciences) was added to each well. Images were captured on a Nikon TE2000 total internal reflection fluorescence (TIRF) microscope equipped with a CFI Plan Apo VC ×100 oil, numerical aperture (NA) 1.49-type objective using a Photometrics CoolSnap HQ2 camera. Image analyses were performed using ImageJ.
Crystallization and structure of HCR.
HCR/A(W1266A) was dialyzed and concentrated to 10 mg/ml in 10 mM Tris-HCl (pH 7.6) and 50 mM NaCl. The hanging drop method of vapor diffusion was employed for crystallization of protein. Crystals were grown at 19°C. Crystals were grown in buffer containing 100 mM HEPES (pH 7.5), 12.5% (wt/vol) polyethylene glycol 3350 (PEG 3350), and 100 mM NaCl. Diffraction data of HCR/A(W1266A) were collected using an R-AXIS IV++ with a MicroMax 007 generator at 100 K. HKL2000 (38) was used for data processing. Data collection and processing statistics are summarized in Table 1. The structure of HCR/A(W1266A) was solved by the molecular replacement method using the PHASER (39) program and using the structure of HCR/A (residues 875 to 1,295; protein data bank [PDB] code 3FUO) (40) as the search model. The initial structure obtained from molecular replacement trials was refined using the program CNS (41). The refinement procedure consisted of rigid body and positional refinement followed by a simulated annealing protocol. Iterative refinement rounds were followed by manual fitting and rebuilding with COOT (42) using 2Fo-Fc and Fo-Fc difference Fourier maps. The final model was completed with Rcrystal/Rfree values of 0.207/0.246.
Table 1.
Data collection and refinement statistics
| Diffraction data and refinement statistics | Value for HCR/A(W1266A)a |
|---|---|
| Diffraction data | |
| Resolution range (Å) | 50–2.3/2.38–2.30 |
| No. of total reflections | 104,368 |
| No. of unique reflections | 21,639/1,601 |
| Completeness (%) | 95.2/72.5 |
| Redundancy | 4.8/3.9 |
| I/σ(I) | 21.4/6.4 |
| Unit cell a, b, and c (Å) | 44.2, 80.6, 138.5 |
| Space group | P212121 |
| Rsymm | 0.06/0.20 |
| Vm (Å3/Da)/solvent content (%) | 2.44/47.8 |
| No. of monomers in an asymmetric unit | 1 |
| Refinement statistics | |
| Rcrystal/Rfree | 0.205/0.245 |
| RMSD bond length/bond angle | 0.006/1.4 |
| No. of protein atoms | 3,440 |
| No. of water molecules | 186 |
| No. of glycerol atoms | 12 |
| Avg B factor | |
| Protein atoms (Å2) | 27.9 |
| Water molecules (Å2) | 29.6 |
| Glycerol (Å2) | 33.0 |
| Ramachandran statistics | |
| Most-favored regions (%) | 85.3 |
| Additional allowed regions (%) | 14.7 |
| Generously allowed regions (%) | 0 |
| Disallowed regions (%) | 0 |
PDB code 4IQP.
HCR vaccine challenge.
Female ICR mice (18 to 22 g) were immunized intraperitoneally with 1.0 μg or 0.1 μg each of either HCR/A or HCR/A(W1266A) mixed with an equal volume of alhydrogel as an adjuvant on days 1 and 14. Vaccinated mice were challenged with BoNT/A on day 28 with 1,000 50% lethal doses (LD50). Sera were collected 4 days prior to challenge. These experiments were approved by the Animal Care and Use Committee at the University of Wisconsin—Madison.
Solid-phase determination of IgG titers following HCR immunization.
HCR/A or HCR/A(W1266A) (0.5 μg) was spotted in triplicate onto nitrocellulose strips (Millipore). Spots were dried, blocked with 2% dried milk in 20 mM Tris-HCl (pH 7.4) and 140 mM NaCl with 0.1% Tween 20 (TBST) at room temperature (RT) for 20 min, and then incubated with sera from mice vaccinated with either HCR/A or HCR/A(W1266A) at a 1:20,000 final dilution in 2% dried milk in TBST for 1 h at RT. Strips were washed in Tris-buffered saline (TBS)-0.5% Tween 20, incubated with goat α-mouse IgG-horseradish peroxidase (HRP) (Pierce; 1:20,000 dilution) in TBST for 1 h at RT, washed with TBS-0.5% Tween 20, and incubated in Super Signal (Pierce) for 5 min at RT. Light emission was imaged with a digital camera (Cell Bioscience). AlphaView software (ProteinSimple) was used to convert the images to numerical values using densitometry. For each serum, signals obtained from the control proteins 3×F-HCR/Tetanus toxin (3×F-HCR/TeNT) and LC/Tetanus toxin (LC/TeNT) were subtracted. 3×F-HCR/TeNT was used to negate cross-reactivity to the 3×F tag, and LC/TeNT was used to blank cross-reactivity. The control serum signal varied with each serum and was <10% of the signal detected for HCR/A derivatives. Signals were proportional to serum dilutions in a dose range and time of imaging, allowing a determination of the relative HCR-specific IgG signal as a function of survival to BoNT challenge. Values reported were uniformly divided by 100 and are reported as arbitrary units. Statistical analysis between sets of data was assessed by the Student t test (GraphPad Prism 5).
RESULTS
The crystal structure of HCR/A(W1266A) is the same as that of wild-type HCR/A.
The crystal structure of HCR/A in complex with the sugar moieties of GT1b (17) revealed the residues that contact/interact with the ganglioside, including W1266, which is located within the GBP region (Fig. 1B). The crystal structure of HCR/A(W1266A) was solved to 2.3 Å. The electron density for the entire HCR/A(W1266A) molecule except the first five N-terminal residues (plus the FLAG/His6 epitopes) and two internal residues located in the loop areas is visible in the structure, indicating that the long FLAG/His6 tag is flexible and does not influence the structure of the rest of the protein. This also ensures that the FLAG tag is exposed for the immunofluorescence assays. The folding of HCR/A(W1266A) is virtually identical to that of wild-type HCR/A. Furthermore, the GBP architecture was also conserved despite the absence of the indole R group (Fig. 1C). The root mean square deviation (RMSD) value between HCR/A and HCR/A(W1266A) was 0.4 Å for 416 Cα atoms, indicating conservation of overall structure (Table 1). This predicted that the linear and conformational epitopes, except those that included W1266, should be conserved between wild-type HCR/A and HCR/A(W1266A).
HCR/A(W1266) does not enter neurons.
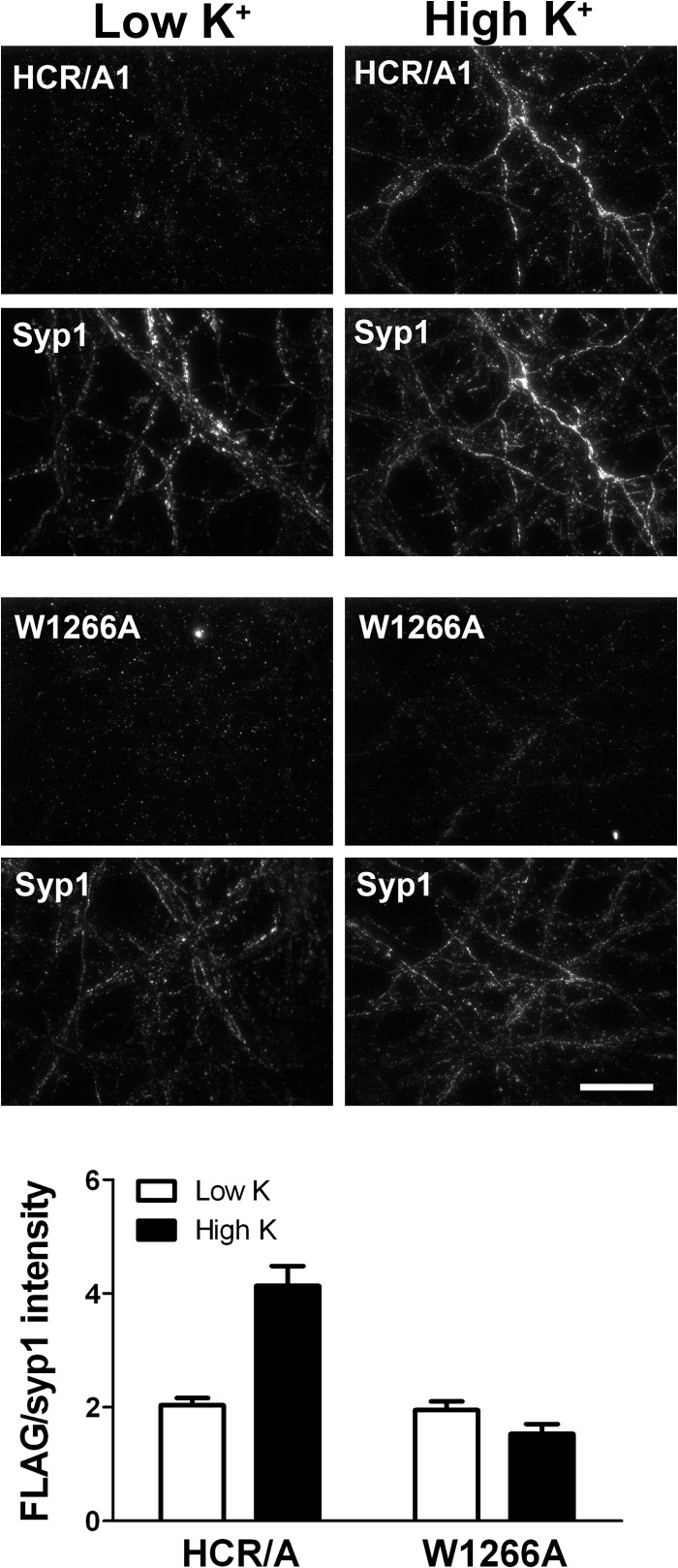
HCR/A entry into neurons was tested using cultured embryonic day 18 rat cortical neurons. Dong et al. showed that BoNT/A enters cells through synaptic vesicle exocytosis that was stimulated by membrane depolarization with a high-potassium buffer containing CaCl2 (12). Therefore, cells were incubated with either HCR/A or HCR/A(W1266A) for 5 min in either a low-potassium buffer (resting conditions) or a high-potassium buffer to stimulate membrane depolarization. While HCR/A uptake was significantly greater when incubated in high-potassium buffer than when incubated in low-potassium buffer, HCR/A(W1266A) binding did not increase upon incubation in high-potassium buffer (depolarizing conditions) (Fig. 2). HCR/A colocalization with the synaptic vesicle marker synaptophysin1 (Syp1) was measured using Pearson's correlation coefficients. HCR/A colocalization with Syp1 significantly increased upon incubation in high-potassium buffer, indicating entry into neurons using the synaptic vesicle pathway (data not shown) (43). In contrast, HCR/A(W1266A) colocalization with Syp1 was low under resting or depolarizing buffer conditions. This showed that HCR/A(W1266A) cell association was significantly impaired compared to that of wild-type HCR/A.
Fig 2.
W1266 is required for HCR entry into neurons. (Top) HCR/A or HCR/A(W1266A) at 40 nM was incubated with rat primary cortical neurons for 5 min in either low potassium (5.6 mM) as a resting condition or high potassium (56 mM) to stimulate synaptic vesicle cycling. Cells were stained for 3×FLAG (HCR) and synaptophysin1. (Bottom) Ten fields were imaged, and the average intensity of 3×FLAG-HCR was normalized to the synaptic vesicle marker synaptophysin1.
HCR/A(W1266A) elicits a protective immune response to BoNT/A challenge.
Mice were vaccinated with 1.0 μg or 0.1 μg of HCR/A or HCR/A(W1266A) on days 1 and 14 and challenged with 1,000 LD50 of BoNT/A on day 28. Survival was scored after 4 days (Table 2). Upon high-dose vaccination, mice vaccinated with either HCR/A or HCR/A(W1266A) survived BoNT/A challenge. In contrast, low-dose vaccination with wild-type HCR/A protected only a fraction of mice (13 of 20) from challenge while HCR/A(W1266A) yielded full protection (20 of 20) to challenge. This indicates that HCR/A(W1266A) elicited a stronger protective immune response than did wild-type HCR/A.
Table 2.
HCR/A(W1266A) vaccine protection to BoNT/A challengea
| Dose and HCR/A immunogen | No. of mice who survived BoNT/A challenge of 1,000 LD50/total no. of mice |
|---|---|
| 1.0 μg | |
| A | 4/4 |
| A(W1266A) | 4/4 |
| 0.1 μgb | |
| A | 13/20 (6/10, 7/10) |
| A(W1266A) | 20/20 (10/10, 10/10) |
Mice were vaccinated with 1.0 μg or 0.1 μg of either HCR/A or HCR/A(W1266A) on days 1 and 14 and challenged with 1,000 LD50 of BoNT/A on day 28. Survival was scored after 4 days. Controls showed that adjuvant only did not protect mice from BoNT/A. At 0.1 μg of HCR, results shown are pooled from two separate experiments, the data of which are shown independently in parentheses.
Survival rates with BoNT/A challenge for the two experiments were pooled and found to be statistically different between HCR/A and HCR/A(W1266A) vaccinations (Fisher exact P value of <0.05).
IgG titers following HCR immunization.
IgG titers were compared between mice vaccinated with HCR/A and mice vaccinated with HCR/A(W1266A), using a dot blot to detect antibodies that bound both linear and conformational epitopes. Both HCR/A and HCR/A(W1266A) were spotted onto nitrocellulose membrane to use as probes for reactivity from sera from each vaccine group. Under the experimental conditions used, antibody titers were proportional to the dilution for each vaccine group, allowing assessment of antibody titers elicited by each vaccination. Serum titers from the high-dose vaccination (1.0 μg) with HCR/A and HCR/A(W1226A) were similar (Fig. 3A).
Fig 3.
IgG titers to HCR/A and HCR/A(W1266A) following high- and low-dose HCR/A vaccination. (A) A total of 0.5 μg of HCR/A(W1266A) (black boxes) or HCR/A (gray boxes) was spotted, in triplicate, onto nitrocellulose strips. Each strip was individually probed with serum (1:20,000) from mice vaccinated with 1.0 μg (A1 vaccination) or HCR/A(W1266A) (W1266A vaccination) from mice who survived BoNT/A challenge. Strips were incubated with goat α-mouse IgG-HRP and incubated in Super Signal, and light emission was imaged. Backgrounds were subtracted using 3×FLAG-HCR/TeNT for FLAG epitope tag reactivity and LC/TeNT as a control protein. Results are presented as arbitrary units of HRP reactivity using the mean and standard error of the mean (SEM). Significance was not observed between IgG titers. Output signals were proportional to serum dilutions in the dose range and time of imaging used in this analysis, allowing relative HCR-specific IgG titers to be determined. (B) Experiment was performed as in panel A except HCR was probed with sera from mice receiving 0.1 μg HCR/A or HCR/A(W1266A) vaccine. In the HCR/A vaccination, 13 of 20 mice survived BoNT/A challenge. Sera from animals that did not survive challenge were assayed separately (columns labeled “Dead”). All animals immunized with HCR/A(W1226A) survived BoNT/A challenge. In HCR/A(W1266A) vaccination, 20 of the 20 mice survived 0.1-μg challenge. ns, not significant; *, P value of 0.05.
Serum titers from the low-dose vaccination (0.1 μg) with HCR/A and HCR/A(W1226A) correlated with the survival to challenge by BoNT/A. Serum titers of mice vaccinated with HCR/A that did not survive BoNT/A challenge were approximately 1,000-fold lower than those of mice that survived challenge. This indicates that for 7 of 20 mice, protective immunity was not generated or the response had not yet matured (Fig. 3B). Mice vaccinated with 0.1 μg of HCR/A(W1266A) survived BoNT/A challenge. Of note, the antibody titers were not significantly different for the surviving mice vaccinated with either wild-type HCR/A or HCR/A(W1266A). Therefore, while HCR/A was a less-efficacious vaccine than HCR/A(W1266A) in a population study, mice vaccinated with HCR/A that survived BoNT/A challenge generated a protective IgG titer similar to that of mice vaccinated with HCR/A(W1266A) (Fig. 3B). With a low-dose vaccination, regardless of the vaccine administered, dot blot analysis shows that serum titers measured using HCR/A(W1266A) as an antigen were significantly higher than those using HCR/A as an antigen (Fig. 3B). This trend was not observed in sera from mice vaccinated with a high (1.0 μg) dose (Fig. 3A).
Antibodies generated against HCR/A and HCR/A(W1266A) block the binding of HCR/A to neurons.
Sera from 2 mice vaccinated with the high dose of HCR/A or HCR/A(W1266A) that survived BoNT/A challenge were pooled to give identical α-HCR IgG titers. Sera were tested for potency to block the binding of HCR/A to neurons. Serum dilutions (1:50 to 1:400) were preincubated with 40 nM wild-type HCR/A and added to neurons in high-potassium buffer to stimulate HCR/A entry. At each dilution tested, the potencies of the two antisera to block HCR/A entry were similar, except at 1:200, for which blockage of HCR/A entry by α-HCR/A sera was ∼3-fold more potent than that by the sera from α-HCR/A(W1266A)-vaccinated mice (Fig. 4). Preimmune sera did not reduce HCR/A entry into neurons. This indicates that both HCR/A and HCR/A(W1266A) stimulated the production of antibodies that blocked receptor binding.
Fig 4.
HCR vaccine neutralization of HCR-receptor interactions. HA-tagged HCR/A at 40 nM in high-potassium buffer was incubated with serial dilutions of vaccine sera from mice receiving 1 μg HCR/A or HCR/A(W1266A) or preimmune sera at the highest dilution for 30 min at 4°C and incubated with rat primary cortical neurons for 5 min (37°C). Cells were fixed and HCR detected with α-HA antibody and secondary Alexa 488. Intensity was normalized to synaptophysin1. ***, significance between HCR/A signals from HCR/A vaccine sera and HCR/A(W1266A) vaccine sera.
DISCUSSION
In this study, we determined that elimination of receptor binding by a single amino acid substitution (W1266A) improved efficacy of the subunit HCR/A vaccine against BoNT/A challenge. Vaccine protection paralleled quantitative antibody titers. HCR/A(W1266A) elicited a potent protective response without eliciting a higher neutralizing antibody titer to HCR-receptor binding than that elicited by wild-type HCR/A. This suggests that neutralizing capacity for BoNT intoxication need not parallel potency to neutralize HCR-receptor interactions. While recombinant HCRs are candidate BoNT vaccines, each variant retains an ability to bind and enter neurons that is similar to that of the native holotoxin (29, 33, 42), and possible physiological consequences of recombinant HCRs entering motor neurons are unknown. By eliminating receptor binding, HCR/A retains its protective ability and even appears to be a more protective immunogen than wild-type HCR while eliminating chance side effects and sequestration from immune detection. The basis for the increased efficacy of the modified HCR is likely due to increased circulating immunogen due to the elimination of receptor binding, since the W1266A mutation did not cause an apparent change in the protein structure (Fig. 1). This would stimulate a quicker immune response relative to that of the wild-type HCR.
The W-to-A mutation was chosen for these studies based upon the desire to eliminate all R group interactions with galactose of GT1b (Fig. 1B). Since W is conserved within the GBPs of BoNT serotypes A, B, E, F, G and tetanus toxin (17, 20, 44–47), we propose that this mutation can be introduced into each HCR serotype to generate a potentially less reactive but more potent vaccine. In addition, since BoNT/C and BoNT/D possess comparable aromatic residues within another ganglioside binding loop within the HCR (48, 49), we propose that the respective W and F may be modified to A to reduce potential reactivity and enhance vaccine potency. The inadvertent hazard of retaining ganglioside binding was reported during the development of the cholera toxin B subunit in adjuvant development (50).
Antibody-mediated protection to BoNTs works by three mechanisms: (i) IgA antibodies generated at mucosal surfaces prevent transcytosis across gut epithelia and block entry into circulation, (ii) IgA and IgG bind BoNTs in circulation and mark toxins for clearance by phagocytic immune cells, and (iii) specific antibodies bind in the vicinity of the receptor binding sites and block neuronal receptor interaction (termed a neutralizing antibody) (51–53). In addition, neutralizing antibodies that target the light chain of BoNT/A through another mechanism, potentially by preventing translocation of the LC but not endocytosis of the holotoxin, have been found (54–56). Numerous epitopes on both the N- and C-terminal regions of HCR/A have been described, and neutralizing activity is absent from antibodies that target the N terminus, distal to the GBP (17, 30, 57, 58).
The greater titer of the α-HCR/A sera for blocking HCR binding may reflect a higher affinity of α-HCR/A antibody for the homologous HCR relative to α-HCR(W1266A) antibody, since the two sera had similar IgG titers to HCR/A. This also suggests that BoNT neutralizing epitopes may not exclusively block the binding of HCR to receptor. Therefore, antibodies generated to the HCR/A(W1266A) vaccine likely mark circulating BoNT/A for clearance and inhibit receptor recognition. This is consistent with the observation that BoNTs can be cleared by combinations of monoclonal antibodies that do not neutralize toxin activity (59). Furthermore, work by Atassi and his coworkers identified the peptide composing the GPB as a minor yet reproducible immunological epitope, though dominant epitopes varied by species (58).
HCR/A(W1266A) is a candidate BoNT/A vaccine, since this protein elicited a more potent immune response than wild-type HCR/A at a low-dose vaccination. The inability of HCR/A(W12266A) to enter neurons reduces the potential for off-target toxicity via retrograde trafficking to the central nervous system (60), enhancing the utility of a vaccine.
ACKNOWLEDGMENTS
J.T.B., J.-J.P.K., and E.A.J. acknowledge membership in the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research Program.
J.T.B., J.-J.P.K., and E.A.J. acknowledge support from the Great Lakes Regional Center of Excellence, grant number U54 AI057153.
Footnotes
Published ahead of print 13 May 2013
REFERENCES
- 1. Gill DM. 1982. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torda C, Wolff HG. 1947. On the mechanism of paralysis resulting from toxin of Clostridium botulinum; the action of the toxin on acetylcholine synthesis and on striated muscle. J. Pharmacol. Exp. Ther. 89:320–324 [PubMed] [Google Scholar]
- 3. Davletov B, Bajohrs M, Binz T. 2005. Beyond BOTOX: advantages and limitations of individual botulinum neurotoxins. Trends Neurosci. 28:446–452 [DOI] [PubMed] [Google Scholar]
- 4. Johnson EA. 1999. Clostridial toxins as therapeutic agents: benefits of nature's most toxic proteins. Annu. Rev. Microbiol. 53:551–575 [DOI] [PubMed] [Google Scholar]
- 5. Evidente VG, Adler CH. 2010. An update on the neurologic applications of botulinum toxins. Curr. Neurol. Neurosci. Rep. 10:338–344 [DOI] [PubMed] [Google Scholar]
- 6. Kaji R. 2011. New and emerging indications of botulinum toxin therapy. Parkinsonism Relat. Disord. 17:S25–S27 [DOI] [PubMed] [Google Scholar]
- 7. Lacy DB, Stevens RC. 1999. Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 291:1091–1104 [DOI] [PubMed] [Google Scholar]
- 8. Montecucco C, Schiavo G. 1995. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 28:423–472 [DOI] [PubMed] [Google Scholar]
- 9. Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. 1998. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Biol. 5:898–902 [DOI] [PubMed] [Google Scholar]
- 10. Takamizawa K, Iwamori M, Kozaki S, Sakaguchi G, Tanaka R, Takayama H, Nagai Y. 1986. TLC immunostaining characterization of Clostridium botulinum type A neurotoxin binding to gangliosides and free fatty acids. FEBS Lett. 201:229–232 [DOI] [PubMed] [Google Scholar]
- 11. Marxen P, Fuhrmann U, Bigalke H. 1989. Gangliosides mediate inhibitory effects of tetanus and botulinum A neurotoxins on exocytosis in chromaffin cells. Toxicon 27:849–859 [DOI] [PubMed] [Google Scholar]
- 12. Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. 2006. SV2 is the protein receptor for botulinum neurotoxin A. Science 312:592–596 [DOI] [PubMed] [Google Scholar]
- 13. Hoch DH, Romero-Mira M, Ehrlich BE, Finkelstein A, DasGupta BR, Simpson LL. 1985. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. U. S. A. 82:1692–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koriazova LK, Montal M. 2003. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 10:13–18 [DOI] [PubMed] [Google Scholar]
- 15. Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. 1993. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 335:99–103 [DOI] [PubMed] [Google Scholar]
- 16. Simpson LL. 2004. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 44:167–193 [DOI] [PubMed] [Google Scholar]
- 17. Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. 2008. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b: insight into the toxin-neuron interaction. PLoS Pathog. 4:e1000129. 10.1371/journal.ppat.1000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rummel A, Mahrhold S, Bigalke H, Binz T. 2004. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol. 51:631–643 [DOI] [PubMed] [Google Scholar]
- 19. Rummel A, Bade S, Alves J, Bigalke H, Binz T. 2003. Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J. Mol. Biol. 326:835–847 [DOI] [PubMed] [Google Scholar]
- 20. Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. 2009. Gangliosides as high affinity receptors for tetanus neurotoxin. J. Biol. Chem. 284:26569–26577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Byrne MP, Smith LA. 2000. Development of vaccines for prevention of botulism. Biochimie 82:955–966 [DOI] [PubMed] [Google Scholar]
- 22. Velikanov I. 1934. Experimental immunization of man against botulism. Klin. Med. 12:1802–1806 [Google Scholar]
- 23. Smith LA, Rusnak JM. 2007. Botulinum neurotoxin vaccines: past, present, and future. Crit. Rev. Immunol. 27:303–318 [DOI] [PubMed] [Google Scholar]
- 24. CDC 2011. Notice of CDC's discontinuation of investigational pentavalent (ABCDE) botulinum toxoid vaccine for workers at risk for occupational exposure to botulinum toxins. MMWR Morb. Mortal. Wkly. Rep. 60:1454–1455 [PubMed] [Google Scholar]
- 25. Torii Y, Tokumaru Y, Kawaguchi S, Izumi N, Maruyama S, Mukamoto M, Kozaki S, Takahashi M. 2002. Production and immunogenic efficacy of botulinum tetravalent (A, B, E, F) toxoid. Vaccine 20:2556–2561 [DOI] [PubMed] [Google Scholar]
- 26. Smith LA. 2009. Botulism and vaccines for its prevention. Vaccine 27(Suppl 4):D33–D39 [DOI] [PubMed] [Google Scholar]
- 27. Park JB, Simpson LL, Anderson TD, Sataloff R. 2003. Immunologic characterization of spasmodic dysphonia patients who develop resistance to botulinum toxin. J. Voice 17:255–264 [DOI] [PubMed] [Google Scholar]
- 28. Dertzbaugh MT, West MW. 1996. Mapping of protective and cross-reactive domains of the type A neurotoxin of Clostridium botulinum. Vaccine 14:1538–1544 [DOI] [PubMed] [Google Scholar]
- 29. Clayton MA, Clayton JM, Brown DR, Middlebrook JL. 1995. Protective vaccination with a recombinant fragment of Clostridium botulinum neurotoxin serotype A expressed from a synthetic gene in Escherichia coli. Infect. Immun. 63:2738–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolimbek BZ, Aoki KR, Steward LE, Jankovic J, Atassi MZ. 2007. Mapping of the regions on the heavy chain of botulinum neurotoxin A (BoNT/A) recognized by antibodies of cervical dystonia patients with immunoresistance to BoNT/A. Mol. Immunol. 44:1029–1041 [DOI] [PubMed] [Google Scholar]
- 31. Shearer JD, Manetz TS, House RV. 2012. Preclinical safety assessment of recombinant botulinum vaccine A/B (rBV A/B). Vaccine 30:1917–1926 [DOI] [PubMed] [Google Scholar]
- 32. Shearer JD, Vassar ML, Swiderski W, Metcalfe K, Niemuth N, Henderson I. 2010. Botulinum neurotoxin neutralizing activity of immune globulin (IG) purified from clinical volunteers vaccinated with recombinant botulinum vaccine (rBV A/B). Vaccine 28:7313–7318 [DOI] [PubMed] [Google Scholar]
- 33. Baldwin MR, Tepp WH, Przedpelski A, Pier CL, Bradshaw M, Johnson EA, Barbieri JT. 2008. Subunit vaccine against the seven serotypes of botulism. Infect. Immun. 76:1314–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atassi MZ, Jankovic J, Steward LE, Aoki KR, Dolimbek BZ. 2012. Molecular immune recognition of botulinum neurotoxin B. The light chain regions that bind human blocking antibodies from toxin-treated cervical dystonia patients. Antigenic structure of the entire BoNT/B molecule. Immunobiology 217:17–27 [DOI] [PubMed] [Google Scholar]
- 35. Qazi O, Sesardic D, Tierney R, Söderbäck Z, Crane D, Bolgiano B, Fairweather N. 2006. Reduction of the ganglioside binding activity of the tetanus toxin HC fragment destroys immunogenicity: implications for development of novel tetanus vaccines. Infect. Immun. 74:4884–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh MK, Gupta G, Boopathi M, Gupta P, Chauhan V, Tomar A, Singh L, Dhaked RK. 2012. Deletion mutant comprising 198 residues of BoNT/A toxin receptor binding domain retained gt1b binding property but failed to induce protective antibody response in a mouse model. Protein Pept. Lett. 19:530–537 [DOI] [PubMed] [Google Scholar]
- 37. Elias M, Al-Saleem F, Ancharski DM, Singh A, Nasser Z, Olson RM, Simpson LL. 2011. Evidence that botulinum toxin receptors on epithelial cells and neuronal cells are not identical: implications for development of a non-neurotropic vaccine. J. Pharmacol. Exp. Ther. 336:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307–326 [DOI] [PubMed] [Google Scholar]
- 39. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J. Appl. Crystallogr. 40:658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu Z, Chen C, Barbieri JT, Kim JJ, Baldwin MR. 2009. Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 48:5631–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brunger AT. 2007. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2:2728–2733 [DOI] [PubMed] [Google Scholar]
- 42. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 43. Blum FC, Chen C, Kroken AR, Barbieri JT. 2012. Tetanus toxin and botulinum toxin A utilize unique mechanisms to enter neurons of the central nervous system. Infect. Immun. 80:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swaminathan S, Eswaramoorthy S. 2000. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 7:693–699 [DOI] [PubMed] [Google Scholar]
- 45. Kumaran D, Eswaramoorthy S, Furey W, Navaza J, Sax M, Swaminathan S. 2009. Domain organization in Clostridium botulinum neurotoxin type E is unique: its implication in faster translocation. J. Mol. Biol. 386:233–245 [DOI] [PubMed] [Google Scholar]
- 46. Benson MA, Fu Z, Kim JJ, Baldwin MR. 2011. Unique ganglioside recognition strategies for clostridial neurotoxins. J. Biol. Chem. 286:34015–34022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Binz T. 2013. Clostridial neurotoxin light chains: devices for SNARE cleavage mediated blockade of neurotransmission. Curr. Top. Microbiol. Immunol. 364:139–157 [DOI] [PubMed] [Google Scholar]
- 48. Karalewitz AP, Kroken AR, Fu Z, Baldwin MR, Kim JJ, Barbieri JT. 2010. Identification of a unique ganglioside binding loop within botulinum neurotoxins C and D-SA. Biochemistry 49:8117–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kroken AR, Karalewitz AP, Fu Z, Kim JJ, Barbieri JT. 2011. Novel ganglioside-mediated entry of botulinum neurotoxin serotype D into neurons. J. Biol. Chem. 286:26828–26837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Ginkel FW, Jackson RJ, Yoshino N, Hagiwara Y, Metzger DJ, Connell TD, Vu HL, Martin M, Fujihashi K, McGhee JR. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 73:6892–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ravichandran E, Al-Saleem FH, Ancharski DM, Elias MD, Singh AK, Shamim M, Gong Y, Simpson LL. 2007. Trivalent vaccine against botulinum toxin serotypes A, B, and E that can be administered by the mucosal route. Infect. Immun. 75:3043–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simpson LL. 2007. Balancing the benefits and risks of a botulinum toxin vaccine. Expert Rev. Vaccines 6:883–886 [DOI] [PubMed] [Google Scholar]
- 53. Ravichandran E, Gong Y, Saleem FHA, Ancharski DM, Joshi SG, Simpson LL. 2006. An initial assessment of the systemic pharmacokinetics of botulinum toxin. J. Pharmacol. Exp. Ther. 318:1343–1351 [DOI] [PubMed] [Google Scholar]
- 54. Adekar SP, Takahashi T, Jones RM, Al-Saleem FH, Ancharski DM, Root MJ, Kapadnis BP, Simpson LL, Dessain SK. 2008. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS One 3:e3023. 10.1371/journal.pone.0003023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xie L, Mark Jones R, Glass TR, Navoa R, Wang Y, Grace MJ. 2005. Measurement of the functional affinity constant of a monoclonal antibody for cell surface receptors using kinetic exclusion fluorescence immunoassay. J. Immunol. Methods 304:1–14 [DOI] [PubMed] [Google Scholar]
- 56. Takahashi T, Joshi SG, Al-Saleem F, Ancharski D, Singh A, Nasser Z, Simpson LL. 2009. Localization of the sites and characterization of the mechanisms by which anti-light chain antibodies neutralize the actions of the botulinum holotoxin. Vaccine 27:2616–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corbett CR, Ballegeer E, Weedmark KA, Elias MD, Al-Saleem FH, Ancharski DM, Simpson LL, Berry JD. 2011. Epitope characterization of sero-specific monoclonal antibody to Clostridium botulinum neurotoxin type A. Hybridoma 30:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Atassi MZ, Dolimbek BZ, Hayakari M, Middlebrook JL, Whitney B, Oshima M. 1996. Mapping of the antibody-binding regions on botulinum neurotoxin H-chain domain 855-1296 with antitoxin antibodies from three host species. J. Protein Chem. 15:691–700 [DOI] [PubMed] [Google Scholar]
- 59. Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. U. S. A. 99:11346–11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pirazzini M, Rossetto O, Bertasio C, Bordin F, Shone CC, Binz T, Montecucco C. 2013. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins C and D in neurons. Biochem. Biophys. Res. Commun. 430:38–42 [DOI] [PubMed] [Google Scholar]