Abstract
Legionella pneumophila is an intracellular bacterium that resides within amoebae and macrophages in a specialized compartment termed the Legionella-containing vacuole (LCV). As well as providing an intracellular niche for replication, the LCV helps to prevent the release of bacterial components into the cytoplasm. Recognition of these components as danger signals by the host activates immune responses leading to clearance of the bacterium. Here, we examined the role of two important virulence factors of L. pneumophila, the potent danger signal flagellin and the translocated Dot/Icm type IVB secretion system effector SdhA, which is crucial to maintain LCV integrity, in the Galleria mellonella infection model. We demonstrate that flagellin expression does not contribute to virulence, replication, or induction of clearance mechanisms. Conversely, SdhA expression is important for virulence. We found that in the absence of SdhA, the LCV in hemocytes showed signs of instability and leakage. Furthermore, in contrast to wild-type L. pneumophila, a ΔsdhA mutant caused a transient depletion of hemocytes and reduced mortality. Analysis of the ΔsdhA mutant in the A/J mouse model also showed a significant replication defect. Together, our data underline the crucial importance of SdhA in infection across different model organisms.
INTRODUCTION
Legionella pneumophila is a facultative intracellular bacterial pathogen found in environmental aquatic reservoirs and human-made water systems. Accidental inhalation of contaminated aerosols carries bacteria into the human lung, where they establish a severe, potentially fatal, pneumonia in susceptible populations, called Legionnaires' disease (1). Alveolar macrophages constitute the first line of the airway immune defense (2) and play a key role in L. pneumophila infection. Usually these professional phagocytes take up bacteria deposited on the alveolar surface and degrade them in phago-lysosomes as a primary bactericidal mechanism. However, L. pneumophila avoids trafficking to the lysosome by creating a replicative vacuole that resembles the rough endoplasmic reticulum, termed the Legionella-containing vacuole (LCV) (3). Formation of the LCV is dependent on a type IVB secretion system (T4SS) known as the defective in organelle trafficking/intracellular multiplication (Dot/Icm) secretion system (4, 5). The Dot/Icm T4SS translocates more than 300 effectors into the host cell, where they manipulate a plethora of host cell signaling processes, including vesicular trafficking and innate immune signaling (6–9).
Although wild-type (WT) bacteria remain within a membrane-bound vacuole, pathogen-associated molecular patterns (PAMPs) leaking into the cytoplasm from the LCV activate cytosolic danger signal sensors, such as Naip5/Ipaf, and trigger an inflammatory response (10). In C57BL/6 mice, the Naip5/Ipaf inflammasome detects bacterial flagellin and induces a caspase-1-dependent response (11–13). A/J mice harbor a polymorphism in the Naip5 allele that renders the strain more susceptible to L. pneumophila infection (14, 15). Moreover, L. pneumophila activates additional inflammatory signaling factors, including NF-κB, RIG-I, and AIM2 (16–18).
One of the few Dot/Icm effectors that are crucial for the replication of L. pneumophila in mouse macrophages is SdhA (19). SdhA is a highly conserved effector found in L. pneumophila, Legionella longbeachae, and Legionella drancourtii. SdhA is required for maintaining the integrity of the LCV; deletion of sdhA results in destabilization of the LCV and release of the bacterium into the cytoplasm (17, 20). The molecular basis of the LCV-stabilizing function of SdhA is not yet clear; however, there is evidence that SdhA counterbalances the effect of the secreted L. pneumophila phospholipase PlaA. A ΔsdhA mutant induces type I interferon signaling and caspase-1 activation, culminating in the death of infected host cells (16, 17, 20). Notably, activation of these responses is not dependent on flagellin, but rather is triggered by bacterial nucleic acids (16, 17).
We recently established that the larvae of the wax moth Galleria mellonella are an effective model for L. pneumophila infection (21). L. pneumophila forms LCVs in insect phagocytic cells, known as hemocytes, in a Dot/Icm-dependent manner. Although infection of the larvae induces an immune response, as judged by the activation of the phenoloxidase cascade, nodule formation, and upregulation of antimicrobial peptides, larvae ultimately succumb to L. pneumophila infection. It is currently unknown which bacterial PAMPs and Dot/Icm T4SS effectors contribute to L. pneumophila pathogenesis in the G. mellonella model. Here we sought to determine the role of flagellin and SdhA in L. pneumophila-induced larval death and, in the case of SdhA, compare the findings to virulence in A/J mice.
MATERIALS AND METHODS
Bacterial strains and G. mellonella larvae.
L. pneumophila serogroup 1 strain 130b is a spectinomycin-resistant clinical isolate from the Wadsworth Veterans Administration Hospital, Los Angeles, CA (22). The L. pneumophila ΔdotA strain is a dotA insertion mutant (kanamycin resistance) of L. pneumophila strain 130b (23). G. mellonella larvae were obtained from Livefood UK and stored at room temperature in the dark.
Plasmid and strain construction.
The p4HA-SdhA (pICC1340) complementation plasmid was created using standard molecular biology techniques. Briefly, sdhA was amplified by PCR from L. pneumophila 130b chromosomal DNA using the primers GNS414 5′-CAG TCC CGG GAT ATT TCA GAA AAG ATC AAG CTT TTA GAA T-3′ and GNS415 5′-CTA TCC CGG GTT ATG CTG ATG GCG CTA ATT GG-3′, digested using XmaI, and ligated into the cleaved pICC562 vector (pMMB207C-HAx4 [24]). The correct orientation and sequence identity of the sdhA insert were confirmed by DNA sequencing, and the p4HA-SdhA plasmid was transformed into L. pneumophila by electroporation.
To generate an sdhA mutant, a 2.3-kb fragment of L. pneumophila 130b genomic DNA encoding SdhA was amplified by PCR using the primers 5′-GAC CTG GAG CAT GTC AAA GGG-3′ and 5′-CCG CTA AAG GAT GTA ACA GGC-3′. The amplified product was cloned into pGEM-Teasy and digested with BamHI, removing an internal fragment of sdhA, and ligated with a kanamycin resistance gene cassette. This construct was introduced into L. pneumophila 130b by natural transformation for homologous recombination (25). Briefly, bacteria were incubated in ACES yeast extract (AYE) broth at 30°C with 10 μg/ml of pGEMTeasy::sdhA::km until exceeding an optical density at 600 nm greater than 1.5. These cultures were then spread on plates with charcoal-yeast extract (CYE) with kanamycin, and kanamycin-resistant colonies were confirmed to have lost the pGEM-Teasy backbone based on ampicillin sensitivity. The insertion mutation was confirmed by PCR using the primers outside the construct (5′-CCC TAA ATA ATG AAA AGC TGG-3′ and 5′-CAC ATA TCA TTC GAA TAT GTG C-3′) as well as one primer outside the construct and one in the deleted region (5′-ACT ATA AGG GAA TAA AAC CAG-3′).
To generate a deletion mutant of flaA, a mutant copy of flaA carrying an in-frame deletion was generated by PCR and inserted into a unique SalI restriction enzyme site of the mutagenesis vector pSR47s (26). The mutant copy was generated by overspan PCR using the primers FlaA(1) (5′-AGC TAG GTC GAC AAA ATT ACA AGA TGG GCA AAC C-3′) and FlaA(4) (5′-AGC TAG GTC GAC CTG ACC CTA CGC CTG GTG-3′) and the PCR products produced using the primers FlaA(1)/FlaA(2) (5′-ATT GCG TTG GGC TGT AAG-3′) and FlaA(3) (5′-ATG CTT ACA GCC CAA CGC AAT GTA TTA TCG TTG TTA GGT CGA-3′)/FlaA(4) as the templates. The suicide mutagenesis vector pSR47s carries kanamycin resistance, and the sacB gene of Bacillus subtilis results in sucrose sensitivity. After transformation into L. pneumophila 130b via electroporation, isolation of the cointegrates was achieved by selection for the plasmid-carried kanamycin resistance. Loss of the sacB gene carried by the cointegrated plasmid was selected by culture on CYE plates containing 5% sucrose. The result of the second homologous recombination event is either gene replacement or reconstitution of the wild type. A PCR spanning the flaA gene was used to distinguish mutant and wild-type alleles.
Infection of G. mellonella.
L. pneumophila strains were cultured on CYE plates at 37°C for 4 days, then inoculated into AYE, and G. mellonella larvae were infected as previously described (21, 27). Briefly, 10 G. mellonella larvae were injected with 10 μl of 109 CFU/ml bacteria in Dulbecco's phosphate-buffered saline (D-PBS; unless otherwise indicated) and incubated at 37°C in the dark for up to 72 h, and time of death was recorded. At least three independent replicates of each experiment were performed.
Intracellular growth assay and hemocyte depletion assay.
At 0, 5, 18, and 24 h postinfection (p.i.) hemolymph was extracted from three infected larvae and pooled, and the CFU per 0.1 g of hemolymph was determined as previously described (21). Similarly, at the indicated time points, the hemocyte concentration was determined in extracted hemolymph by counting (21).
Indirect immunofluorescence on infected cells.
Hemolymph from infected G. mellonella was extracted at 5 and 18 h p.i. Cells were fixed and permeabilized as previously described (21) and stained with rabbit anti-L. pneumophila antibody (PA1-7227; Affinity BioReagents), donkey anti-rabbit IgG–Alexa Fluor 488–conjugated antibody (711-485-152; Jackson ImmunoResearch), and mouse anti-hemagglutinin (HA) conjugated to tetramethyl rhodamine isothiocyanate (TRITC; H9037; Sigma) and 5 μg ml−1 of 4′,6-diamidino-2-phenylindole (DAPI) to visualize DNA. Samples were analyzed using an Axio M1 Imager microscope, and images were processed with the Axiovision software (Carl Zeiss).
Transmission electron microscopy (TEM).
Hemocytes were extracted from infected and control G. mellonella at 5 h p.i. The cells were transferred to 35-mm dishes and centrifuged at 5,000 rpm for 5 min. The cells were washed 3 times with 1× PBS and cooled on ice before fixation with 0.5% glutaraldehyde (Sigma) in 200 mM sodium cacodylate (TAAB) for 5 min on ice, then at room temperature for a further 25 min. The cells were washed with 200 mM sodium cacodylate before postfixation in 1% osmium tetroxide–1.5% potassium ferrocyanide for 1 h. The cells were washed in double-distilled water (ddH2O) and stained overnight at 4°C with 0.5% uranyl acetate. The cells were washed with ddH2O before serial dehydration in ethanol and embedded flat in Epon resin. Ultrathin sections (∼70 nm) were cut parallel to the surface of the dish by using a Leica ultramicrotome. The sections were collected onto Formvar-coated 50 mesh EM grids and stained for 30 s with Reynold's lead citrate before imaging. Samples were viewed by using an FEI Tecnai G2 electron microscope with a Soft Imaging System Megaview III charged-coupled-device camera. Images were collected at 1,376 by 1,032 by 16 pixels using AnalySIS version Docu software (Olympus Soft Imaging Solutions).
Infection of mice.
All mouse procedures were approved by the University of Melbourne Animals Ethics Committee. The comparative virulence of L. pneumophila 130b wild type (WT) and the ΔsdhA and ΔflaA mutant strains were examined as described previously (28). Briefly, 6- to 8-week-old male or female A/J or C57BL/6 mice were anesthetized and inoculated intranasally with approximately 2.5 × 106 CFU of each L. pneumophila strain under investigation. At 72 h after inoculation, mice were killed and their lung tissue isolated. Tissue was homogenized, and complete host cell lysis was achieved by incubation in 0.1% saponin for 15 min at 37°C. Serial dilutions of the homogenate were plated onto both plain and antibiotic selective CYE agar plates to determine the number of viable bacteria and the ratio of WT to mutant bacteria colonizing the lung. For single infections, 2 groups of mice were inoculated separately with approximately 2.5 × 106 CFU each of L. pneumophila 130b WT and the ΔsdhA mutant strain. Lung CFU were determined 72 h after infection as described above on plain CYE agar.
RESULTS
Flagellin is dispensable for L. pneumophila virulence in G. mellonella.
Given the propensity of L. pneumophila flagellin to stimulate inflammatory host cell death, we investigated its role in the G. mellonella model. Toward this end, we created an L. pneumophila 130b ΔflaA mutant. Pulmonary infection of nonpermissive C57BL/6 mice showed that the 130b ΔflaA mutant replicated to higher numbers (P = 0.0003; Mann-Whitney) than wild-type bacteria in mouse lungs (Fig. 1A), which is consistent with phenotypes reported for the L. pneumophila Lp01 and Lp02 ΔflaA mutants (12, 29). We then infected G. mellonella larvae with 107 wild-type L. pneumophila 130b or ΔflaA mutant and monitored survival over 72 h. No differences between the two strains were observed over the course of the infection (Fig. 1B). In order to determine the viable bacterial load, we extracted hemolymph from infected larvae and determined the CFU per 0.1 g of tissue at 0, 5, 18, and 24 h p.i. This revealed that the ΔflaA strain replicated as efficiently as wild-type L. pneumophila (Fig. 1C), indicating that flagella do not promote L. pneumophila virulence in the G. mellonella model.
Fig 1.
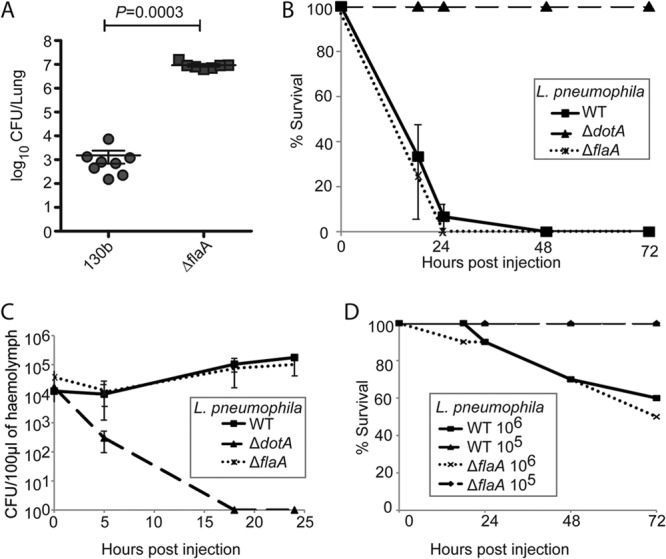
Motility and flagellin expression are dispensable for L. pneumophila virulence in G. mellonella. (A) In single pulmonary infections of C57BL/6 mice, the L. pneumophila 130b ΔflaA strain replicated to higher numbers at 72 h p.i. than the parental wild-type strain (P = 0.0003; Mann-Whitney). (B) Ten G. mellonella larvae were infected with 107 CFU of WT, ΔdotA, or ΔflaA L. pneumophila 130b, and mortality was monitored for 72 h. Deletion of the flaA gene did not affect mortality compared to WT-infected larvae. (C) Hemolymph from larvae infected as described above was extracted at 0, 5, 18, and 24 h p.i., and the CFU/0.1 g of extracted hemolymph were enumerated. The WT and ΔflaA strains replicated similarly, and no significant difference was observed. The ΔdotA strain was cleared by 18 h p.i. Results are means of three independent experiments ± standard deviations. (D) G. mellonella larvae were infected with 105 or 106 CFU of the WT or ΔflaA strains, and mortality was monitored. For both strains, no mortality was observed when larvae were infected with 105 CFU. In larvae infected with 106 CFU, some death was seen; however, no significant differences between strains were observed. Results are representative of two independent experiments.
As infection of larvae with 107 CFU of L. pneumophila causes very rapid death, any increase in virulence of the ΔflaA mutant as observed in C57BL/6 mice could have been masked. Therefore, we infected the larvae with infectious doses of 105 or 106 bacteria, which we have previously shown are less pathogenic (106) and nonpathogenic (105) for the insect, respectively (21). At these doses, the mortality induced by the L. pneumophila ΔflaA mutant was indistinguishable from the wild type (Fig. 1D). This suggests that in G. mellonella the induction of an antimicrobial response and clearance of low infectious doses of L. pneumophila are not dependent on detection of flagellin.
SdhA localizes to and promotes stability of the LCV in infected G. mellonella hemocytes.
Recently it has emerged that the LCV is an important barrier that prevents the release of PAMPs into the cytoplasm, thereby shielding L. pneumophila against cytoplasmic bactericidal immune mediators (17, 20). SdhA plays a key role in maintaining the integrity of the LCV. In the absence of SdhA, macrophages undergo rapid cell death due to the activation of pyroptosis, which prematurely ends intracellular replication of the mutant, explaining the inability of this strain to efficiently replicate in isolated primary mouse macrophages and human macrophage cell lines (17, 19). Our previous work suggested that efficient intracellular replication and the ability to cause hemocyte depletion are important for virulence of L. pneumophila in G. mellonella larvae (21). Given the importance of SdhA in macrophages, we investigated its role following infection of G. mellonella with L. pneumophila.
We first generated an L. pneumophila 130b ΔsdhA deletion mutant and a complementation plasmid that expressed the effector fused to four N-terminal HA tags (4HA-SdhA). Using immunofluorescence microscopy, we confirmed that the 4HA-SdhA protein was translocated into G. mellonella hemocytes during infection. At 5 h p.i., SdhA localized to the membrane of the LCV, often displaying strong staining close to the bacterial poles (Fig. 2A). By 18 h p.i., SdhA was found all around the LCV (Fig. 2B), similar to results obtained in mouse macrophages (19).
Fig 2.
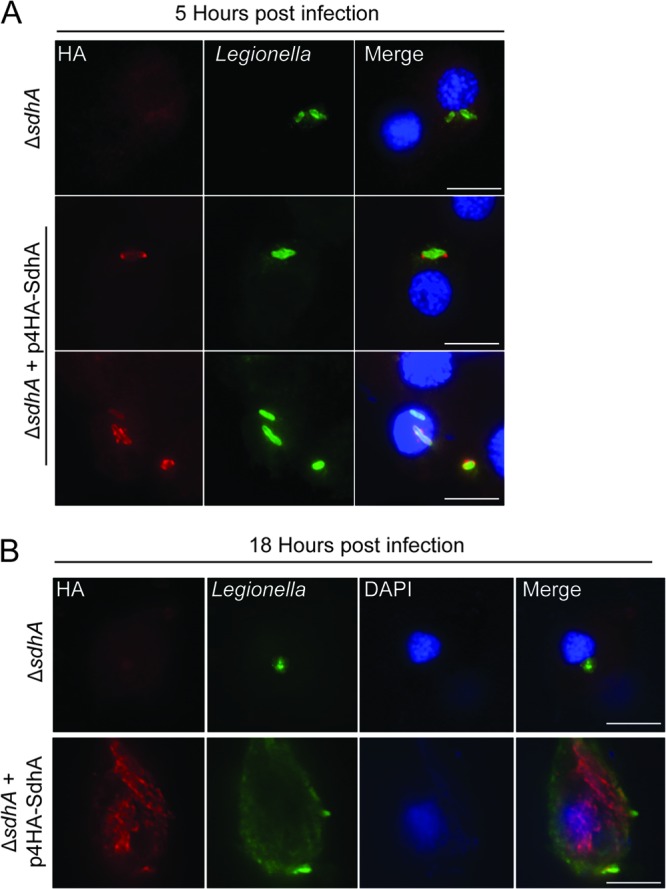
4HA-SdhA is found on LCVs in infected hemocytes. Hemocytes were extracted from G. mellonella larvae infected with ΔsdhA or ΔsdhA plus p4HA-SdhA L. pneumophila 130b at 5 or 18 h p.i. and stained using anti-HA (red) and anti-Legionella (green) antibodies. 4′,6-Diaimidino-2-phenylindole (DAPI) was used to visualize DNA (blue). (A) At 5 h p.i., 4HA-SdhA localized to the LCV membrane and was concentrated at the poles of the bacteria in approximately 45% of infected cells. (B) At 18 h p.i., 4HA-SdhA could be seen surrounding the bacteria. Results are representative of three independent experiments. Bar, 5 μm.
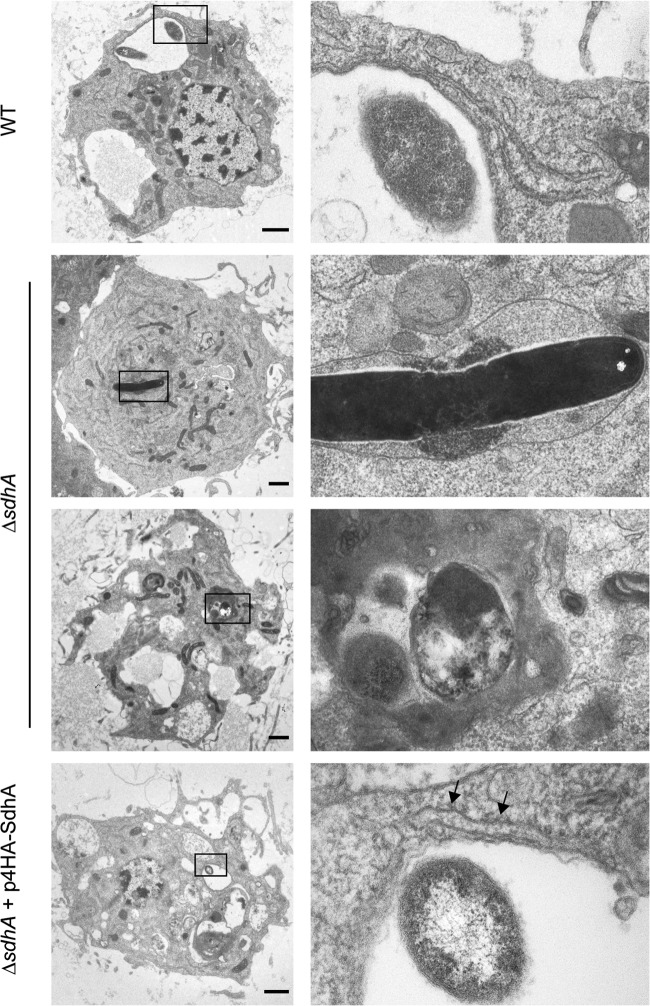
To determine if SdhA is important to LCV maintenance in G. mellonella hemocytes, we infected larvae with wild-type L. pneumophila 130b, ΔsdhA, or ΔsdhA expressing 4HA-SdhA, isolated hemocytes after 5 h of infection, and analyzed their ultrastructure by TEM (Fig. 3). The LCVs of wild-type bacteria were similar in morphology to those described previously (21), by 5 h p.i. intracellular bacteria were contained within large vacuoles to which endoplasmic reticulum (ER)-derived membranes and ribosomes were recruited. However, the LCV of the ΔsdhA mutant showed signs of instability, with electron-dense material apparent within the vacuole. This may have been due to infiltration of the cytoplasm or release of bacterial contents into the LCV. The LCVs of hemocytes infected with the ΔsdhA mutant expressing 4HA-SdhA showed a similar morphology to those infected with wild-type bacteria. These observations indicated that SdhA is important for maintenance of LCV stability within G. mellonella hemocytes.
Fig 3.
LCVs of L. pneumophila ΔsdhA in hemocytes, showing signs of instability. G. mellonella larvae were infected with WT, ΔsdhA, or ΔsdhA plus p4HA-SdhA L. pneumophila 130b for 5 h. Hemocytes were extracted and processed for transmission electron microscopy. Hemocytes infected with WT bacteria contained LCVs of similar morphology to those previously described. Recruitment of the ER to the LCV is indicated (black arrows). The LCVs of the ΔsdhA mutant contained electron-dense material, which may be cytoplasmic or bacterial in origin, indicating loss of stability of the vacuole. The LCVs of the complemented strain were of comparable morphology to those of WT bacteria. Images are representative of two independent experiments. Bar, 1 μm.
Lack of SdhA results in accelerated hemocyte depletion.
Lack of SdhA has been associated with rapid macrophage cell death, with early signs of apoptosis appearing as soon as 30 min p.i. (19, 20). We previously showed that infection of G. mellonella with L. pneumophila causes hemocyte depletion and high larval mortality at 18 h p.i. (21). In order to determine if deletion of sdhA alters hemocyte cytotoxicity, larvae were infected as described above, hemolymph was extracted at 2, 5, 10, 18, and 24 h p.i., and the number of hemocytes was determined by cell counting (Fig. 4). As previously shown, infection with the avirulent ΔdotA strain resulted in no change in hemocyte density over the course of this experiment. In contrast, infection with the ΔsdhA strain resulted in a significantly higher loss of hemocytes between 2 and 5 h p.i. compared to larvae infected with the WT or the ΔdotA mutant strain (Fig. 4) (P < 0.0001, unpaired t test). In combination with the apparent instability of LCVs containing the ΔsdhA strain as observed by TEM, the data indicate that hemocytes, similarly to macrophages, induce rapid cell death in response to cytosolic ΔsdhA bacteria.
Fig 4.
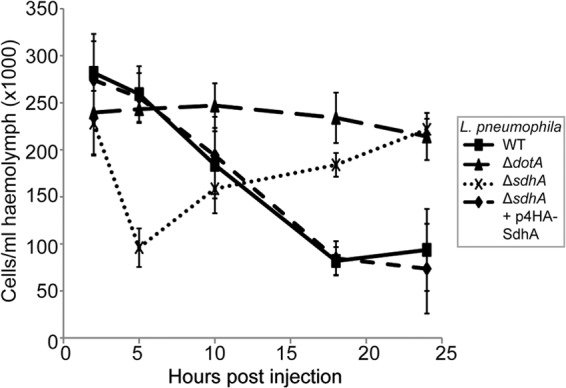
L. pneumophila ΔsdhA causes rapid, transient hemocyte depletion. Hemocytes from larvae infected with WT, ΔdotA, ΔsdhA, or ΔsdhA plus p4HA-SdhA L. pneumophila were extracted at 2, 5, 10, 18, and 24 h p.i., and viable cells were counted. Infection with the ΔsdhA mutant, but not with WT or complemented strains, caused significant (P < 0.0001) hemocyte depletion at 5 h p.i. By 18 h p.i., the hemocyte levels had almost recovered to the 2-h p.i. levels in larvae infected with the ΔsdhA strain, while hemocyte depletion was seen in the larvae infected with WT or ΔsdhA plus p4HA-SdhA (P < 0.0001 compared to ΔsdhA strain). Results are means of at least 4 experiments ± standard deviations.
Interestingly, between 5 and 24 h p.i., hemocyte levels in ΔsdhA-infected G. mellonella gradually recovered to levels comparable to those in larvae infected with the avirulent ΔdotA strain (Fig. 4). In contrast, wild-type L. pneumophila and the complemented ΔsdhA strain caused significant hemocyte depletion between 10 and 24 h p.i. compared to ΔsdhA- or ΔdotA-infected larvae (P < 0.0001, unpaired, t test). These results showed that hemocyte depletion can be transient and suggested that rapid hemocyte depletion can be segregated from larval mortality.
The L. pneumophila ΔsdhA mutant is attenuated in G. mellonella.
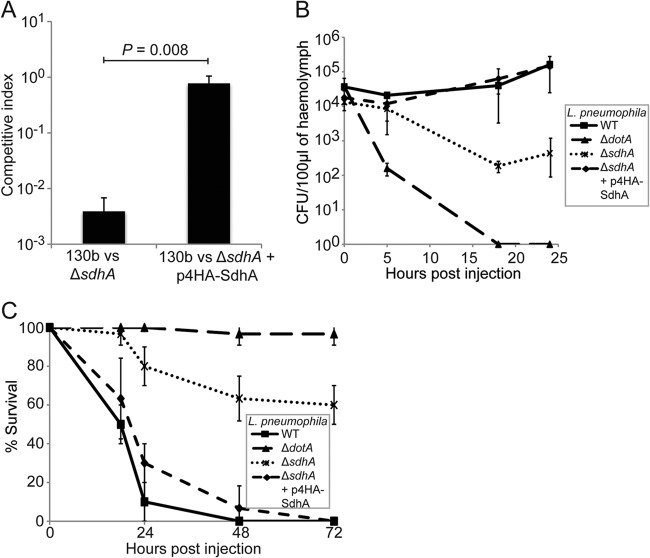
To further analyze how the observed transient hemocyte depletion correlated with the replication of the ΔsdhA strain and to determine the importance of SdhA for replication in vivo, we examined the competitive fitness of this strain during mixed infection of G. mellonella. Larvae were infected with a 1:1 ratio of wild type:ΔsdhA or wild type:ΔsdhA plus p4HA-SdhA. Hemolymph was extracted at 24 h p.i., and the ratio between the wild type and mutant was determined and expressed as competitive index (CI) (Fig. 5A). The ΔsdhA strain was significantly outcompeted by 24 h p.i., while the complemented strain replicated as well as the wild type (P = 0.008, unpaired t test), showing that SdhA has an important role for L. pneumophila fitness in the larvae.
Fig 5.
L. pneumophila ΔsdhA is severely attenuated in infection of G. mellonella larvae. (A) G. mellonella larvae were infected with a 1:1 ratio of WT:ΔsdhA or WT:ΔsdhA plus p4HA-SdhA, hemolymph was plated, and CFU were counted at 24 h p.i. to calculate the competitive index. The WT strain significantly outcompeted the ΔsdhA strain (CI, 0.002); fitness of the mutant could be restored by expression of 4HA-SdhA (CI, 0.88). (B) Hemolymph from larvae infected with WT, ΔdotA, ΔsdhA, or ΔsdhA plus p4HA-SdhA was extracted at 0, 5, 18, and 24 h p.i., and the CFU/0.1 g of extracted hemolymph were enumerated. The ΔsdhA strain did not replicate; however, it persisted over 24 h, unlike the ΔdotA strain, which was cleared by 18 h p.i. Results are means of at least three independent experiments ± standard deviations. (C) Larvae were infected with WT, ΔdotA, ΔsdhA, or ΔsdhA plus p4HA-SdhA L. pneumophila, and mortality was monitored for 72 h. Infection with WT resulted in 100% mortality within 24 h p.i., while the ΔsdhA strain only killed 40% of infected larvae by 72 h p.i.
In order to analyze the growth attenuation in G. mellonella in more detail, larvae were infected with the individual strains, and the viable CFU per 0.1 g of hemolymph were determined. While the wild-type bacterial load increased steadily over the course of the experiment, the ΔsdhA strain did not replicate, and its counts decreased more than 10-fold in the first 18 h p.i. but then remained stable at this level (Fig. 5B). The ΔdotA strain was completely cleared by 18 h p.i. Replication of the ΔsdhA mutant could be restored by complementation with the 4HA-SdhA expression plasmid.
To characterize how the different hemocyte depletion kinetics and inefficient replication of the ΔsdhA strain affect larval mortality, 10 G. mellonella larvae were infected with wild-type, ΔsdhA, or ΔsdhA expressing 4HA-SdhA L. pneumophila 130b. Infection with ΔdotA was used as a control. The ΔsdhA strain was significantly attenuated for induction of larval mortality compared to the wild-type strain; by 72 h p.i., only 40% of infected larvae had succumbed to infection, compared to 100% for the wild-type strain (Fig. 5C). This phenotype was complemented by the addition of 4HA-SdhA on a plasmid. The ΔsdhA strain caused increased mortality compared to ΔdotA, which was avirulent under these conditions, as reported previously (21). Taken together, these data show that substantial hemocyte depletion alone does not trigger death of G. mellonella larvae. In fact, induction of larval death seems to rather be determined by the bacterial load at the time of hemocyte depletion, with increased numbers of wild-type L. pneumophila after efficient intracellular replication overwhelming the larvae.
SdhA is required for bacterial replication in the lungs of A/J mice.
In order to validate the role of SdhA in L. pneumophila virulence, we analyzed the competitive fitness and replication of the ΔsdhA mutant in the lungs of A/J mice. In mixed infections, the ΔsdhA mutant was strongly outcompeted by the wild-type strain (mean CI of 0.03 for ΔsdhA versus wild type) (Fig. 6A), showing that SdhA contributes to the fitness of L. pneumophila in murine infection. The determination of bacterial CFU after 72 h of single infections with WTor ΔsdhA confirmed that the replication of the ΔsdhA strain in mouse lungs was significantly reduced in comparison to the wild-type strain (Fig. 6B) (P = 0.0001, Mann-Whitney U). Each data point represents bacterial counts from one animal. Together, these results showed that SdhA is important for the virulence of L. pneumophila in the A/J mouse infection model as well as G. mellonella.
Fig 6.
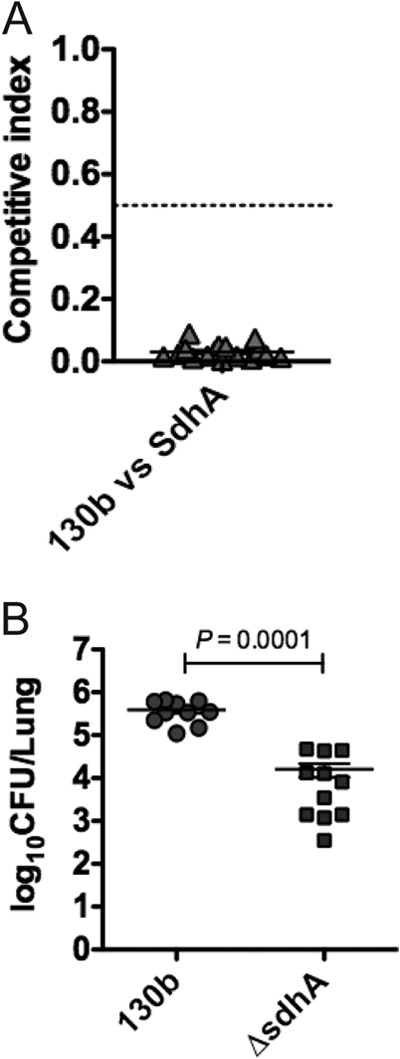
Pulmonary infections of A/J mice with L. pneumophila. (A) In mixed infections, wild-type L. pneumophila strain 130b and the ΔsdhA strain were introduced into the lungs of A/J mice by intranasal inoculation at a 1:1 ratio. At 72 h p.i., the competitive index was calculated (as the ratio of mutant to wild-type bacteria in the lungs divided by the ratio of mutant to wild-type bacteria in the inoculum). The wild-type strain significantly outcompeted the ΔsdhA strain by 72 h p.i. (CI, 0.03). (B) In single infections, the ΔsdhA strain was significantly attenuated at 72 h p.i. compared to wild-type L. pneumophila (P = 0.0001, Mann-Whitney U test). Each data point represents the bacterial count from the lungs of one animal; means ± standard errors of the means are shown (although the error bar is not visible for 130b).
DISCUSSION
We recently established the larvae of the wax moth G. mellonella as a model for L. pneumophila infection (21, 30). After injection into the larvae, L. pneumophila replicates in an LCV in insect hemocytes. Infection triggers an antibacterial immune response; however, it ultimately results in drastic depletion of hemocytes and the death of the larvae. Virulence of L. pneumophila in G. mellonella depends on the Dot/Icm T4SS, and our previous observations suggested that replication, hemocyte depletion, and death of the larvae are linked. However, the contribution to virulence of specific T4SS effectors and other virulence factors remained unknown.
We hypothesized that virulence in the insect might be influenced by the same virulence factors as in macrophages. To test this hypothesis and further compare cell culture and in vivo models, we analyzed the role of flagellin and SdhA, which both impact L. pneumophila-induced cell death and replication in macrophages.
Infection of the larvae with an L. pneumophila ΔflaA nonmotile mutant showed that flagellum-based motility or adhesion appear not to be required for bacterial replication or killing of the insects. In contrast, previous studies found that flagella contributed significantly to the virulence of the human pathogens Listeria monocytogenes and Campylobacter jejuni in G. mellonella (42, 43). In vitro infection experiments showed that flagella promoted contact and invasion by L. pneumophila into tissue culture cells; however, they were dispensable for establishment of the LCV or intracellular replication (44). Our data suggest that in G. mellonella, these crucial steps of L. pneumophila infection are mediated by other virulence factors.
L. pneumophila flagellin plays a crucial role in the immune response and growth restriction of L. pneumophila in mice (12, 29, 31). In murine macrophages, flagellin acts as an important PAMP that triggers inflammasome activation and immune signaling (11, 12). L. pneumophila lacking flagellin showed enhanced virulence in mice (31). Conversely, in G. mellonella infection, we found that lack of flagellin did not increase the virulence of L. pneumophila, demonstrating that flagellin is not an essential trigger for clearance of the bacterium by the insect immune response. This represents an important difference between the L. pneumophila mouse and G. mellonella models. Host specificity of virulence factors has previously been documented in L. pneumophila (32), suggesting that the wide host range for L. pneumophila has resulted in differential requirements for virulence factors. Our knowledge about specific G. mellonella immune-signaling pathways is still limited. PAMP recognition receptors, including Toll-like receptors (33), have been identified in Lepidopteran hemocytes (reviewed in reference 34). However, if and how G. mellonella responds to flagellin remain to be characterized.
Aside from flagellin, other bacterial components can act as PAMPs and activate immune responses. Creation of a protective intravacuolar niche to avoid detection by danger signal sensors is a strategy employed by several intracellular pathogens (35, 36). Recently, it was shown that SdhA has a crucial role in stabilizing the LCV membrane, ensuring bacterial survival and efficient replication (20). Here we confirmed that in infected hemocytes SdhA localizes around the bacterial vacuole, reminiscent of the localization described previously in macrophages (19). Breakdown of the LCV membrane and degradation of cytoplasmic L. pneumophila ΔsdhA has previously been inferred by immunofluorescence microscopy with the use of markers for vacuolar lysis (20). The TEM images of the ultrastructure of L. pneumophila ΔsdhA-infected cells presented here suggest that lack of SdhA leads to release of the bacteria and possibly bacterial contents into the cytoplasm. This verified that SdhA is a major mediator of LCV stability in different host cells, including insect hemocytes.
Lack of SdhA and vacuolar instability have been associated with rapid cell death and defective replication (17, 19, 20). However, these phenotypes and their effects on virulence of L. pneumophila ΔsdhA had not been investigated in in vivo infection models. Infection of G. mellonella with the ΔsdhA strain resulted in rapid, but transient, hemocyte depletion by 5 h p.i. This correlates with results from primary mouse macrophages in which the ΔsdhA strain caused significant cell death by 6 h p.i. (19). As observed in infected macrophages, rapid cell death resulted in inefficient replication of the bacteria in the larvae. Interestingly, between 10 and 24 h p.i., the number of hemocytes increased in larvae infected with the ΔsdhA mutant, likely because of the observed lack of bacterial replication. In contrast, hemocyte depletion upon infection with wild-type L. pneumophila culminated between 18 and 24 h p.i., after which most larvae had succumbed to the infection.
We and others have previously proposed that hemocyte depletion could cause death of the larvae (21, 37). The transient, nonfatal hemocyte depletion by L. pneumophila ΔsdhA suggests that different mechanisms of larval death and hemocyte depletion exist. Furthermore, our results suggest that hemocyte depletion per se is not sufficient to kill the larvae. Rapid nodule formation by circulating hemocytes to neutralize invading pathogens has been reported as a general response to infection and cause of a transient depletion of hemocytes (38). However, as we did not observe a similar, early decrease in response to infection with wild-type L. pneumophila, it is more likely that the drop observed for the ΔsdhA strain is not due to a general, nonspecific antibacterial response. Considering the well-demonstrated induction of rapid macrophage death described for L. pneumophila ΔsdhA (17, 19, 20), a similar rapid death response in hemocytes seems the likely explanation.
Alternatively, the bacterial load in the hemolymph might be decisive for tipping the balance toward either transient or complete, fatal depletion of hemocytes. As in macrophages, rapid hemocyte death might prematurely suppress intracellular replication. This is in line with our observation that the ΔsdhA strain did not replicate but did persist in the insect. The numbers of the ΔsdhA bacteria after the first incomplete round of replication could be too low to infect all remaining hemocytes, allowing replenishment and control of the infection. Similar to this model, renewal of the epithelium in response to infection was recently described as a major parameter in ensuring the gut homeostasis and host defense of Drosophila melanogaster (39). In contrast, after efficient replication, the WT bacteria could overwhelm and destroy all hemocytes in the next rounds of infection. Ultimately, the combination of full hemocyte depletion and additional, adverse effects due to the higher bacterial load result in death of the larvae.
Taken together, our study demonstrates the suitability of the G. mellonella model to investigate the role of Dot/Icm T4SS effectors in L. pneumophila virulence. Importantly, phenotypes observed in cell culture infection can be reproduced in hemocytes. In addition, we also analyzed replication of the ΔsdhA mutant in the lungs of A/J mice. The ΔsdhA strain showed reduced bacterial loads in single infections and was outcompeted by WT bacteria in mice, showing a good correlation to the G. mellonella model.
Previously, only very few Dot/Icm effectors have been shown to have a significant role in in vivo models of infection. AnkB is required for intrapulmonary proliferation in mice (40), while deletion of LubX results in enhanced virulence in D. melanogaster (41). We have presented here evidence that an L. pneumophila ΔsdhA mutant is strongly attenuated in G. mellonella and A/J mice, underpinning the fundamental importance of this effector and the maintenance of LCV integrity for the intracellular lifestyle and virulence of L. pneumophila.
ACKNOWLEDGMENTS
Transmission electron microscopy was performed at the Henry Wellcome Imaging Centre, Division of Infectious Diseases, St. Mary's Hospital Campus, Imperial College London. This work was supported by grants from the Wellcome Trust, the Medical Research Council, the Australian National Health and Medical Research Council, and the Australian Research Council.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM, McDade JE, Shepard CC, Brachman PS. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189–1197 [DOI] [PubMed] [Google Scholar]
- 2. Delclaux C, Azoulay E. 2003. Inflammatory response to infectious pulmonary injury. Eur. Respir. J. Suppl. 42:10s–14s [DOI] [PubMed] [Google Scholar]
- 3. Swanson MS, Isberg RR. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Segal G, Shuman HA. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 30:197–208 [DOI] [PubMed] [Google Scholar]
- 5. Vogel JP, Andrews HL, Wong SK, Isberg RR. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873–876 [DOI] [PubMed] [Google Scholar]
- 6. Ge J, Shao F. 2011. Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors. Cell. Microbiol. 13:1870–1880 [DOI] [PubMed] [Google Scholar]
- 7. Ge J, Xu H, Li T, Zhou Y, Zhang Z, Li S, Liu L, Shao F. 2009. A Legionella type IV effector activates the NF-κB pathway by phosphorylating the IκB family of inhibitors. Proc. Natl. Acad. Sci. U. S. A. 106:13725–13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo ZQ. 2012. Legionella secreted effectors and innate immune responses. Cell. Microbiol. 14:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, Vance RE. 2011. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 7:e1001289. 10.1371/journal.ppat.1001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontana MF, Vance RE. 2011. Two signal models in innate immunity. Immunol. Rev. 243:26–39 [DOI] [PubMed] [Google Scholar]
- 11. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9:1171–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203:1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600 [DOI] [PubMed] [Google Scholar]
- 14. Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. 1994. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J. mice. A murine model of human Legionnaires' disease. Am. J. Pathol. 145:1537–1546 [PMC free article] [PubMed] [Google Scholar]
- 15. Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF. 2003. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13:27–36 [DOI] [PubMed] [Google Scholar]
- 16. Monroe KM, McWhirter SM, Vance RE. 2009. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 5:e1000665. 10.1371/journal.ppat.1000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ge J, Gong YN, Xu Y, Shao F. 2012. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc. Natl. Acad. Sci. U. S. A. 109:6193–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartfeld S, Engels C, Bauer B, Aurass P, Flieger A, Bruggemann H, Meyer TF. 2009. Temporal resolution of two-tracked NF-κB activation by Legionella pneumophila. Cell. Microbiol. 11:1638–1651 [DOI] [PubMed] [Google Scholar]
- 19. Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. 2006. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl. Acad. Sci. U. S. A. 103:18745–18750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Creasey EA, Isberg RR. 2012. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc. Natl. Acad. Sci. U. S. A. 109:3481–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harding CR, Schroeder GN, Reynolds S, Kosta A, Collins JW, Mousnier A, Frankel G. 2012. Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. 80:2780–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edelstein PH. 1986. Control of Legionella in hospitals. J. Hosp. Infect. 8:109–115 [DOI] [PubMed] [Google Scholar]
- 23. Sansom FM, Newton HJ, Crikis S, Cianciotto NP, Cowan PJ, d'Apice AJ, Hartland EL. 2007. A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell. Microbiol. 9:1922–1935 [DOI] [PubMed] [Google Scholar]
- 24. Dolezal P, Aili M, Tong J, Jiang JH, Marobbio CM, Lee SF, Schuelein R, Belluzzo S, Binova E, Mousnier A, Frankel G, Giannuzzi G, Palmieri F, Gabriel K, Naderer T, Hartland EL, Lithgow T. 2012. Legionella pneumophila secretes a mitochondrial carrier protein during infection. PLoS Pathog. 8:e1002459. 10.1371/journal.ppat.1002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newton HJ, Sansom FM, Bennett-Wood V, Hartland EL. 2006. Identification of Legionella pneumophila-specific genes by genomic subtractive hybridization with Legionella micdadei and identification of lpnE, a gene required for efficient host cell entry. Infect. Immun. 74:1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merriam JJ, Mathur R, Maxfield-Boumil R, Isberg RR. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schroeder GN, Petty NK, Mousnier A, Harding CR, Vogrin AJ, Wee B, Fry NK, Harrison TG, Newton HJ, Thomson NR, Beatson SA, Dougan G, Hartland EL, Frankel G. 2010. Legionella pneumophila strain 130b possesses a unique combination of type IV secretion systems and novel Dot/Icm secretion system effector proteins. J. Bacteriol. 192:6001–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newton HJ, Sansom FM, Dao J, Cazalet C, Bruggemann H, Albert-Weissenberger C, Buchrieser C, Cianciotto NP, Hartland EL. 2008. Significant role for ladC in initiation of Legionella pneumophila infection. Infect. Immun. 76:3075–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2:e18. 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aurass P, Schlegel M, Metwally O, Harding CR, Schroeder GN, Frankel G, Flieger A. 2013. The Legionella pneumophila Dot/Icm-secreted effector PlcC/CegC1 together with PlcA and PlcB promotes virulence and belongs to a novel zinc metallophospholipase C family present in bacteria and fungi. J. Biol. Chem. 288:11080–11092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. 2011. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J. Immunol. 187:6447–6455 [DOI] [PubMed] [Google Scholar]
- 32. Tyson JY, Pearce MM, Vargas P, Bagchi S, Mulhern BJ, Cianciotto NP. 2013. Multiple Legionella pneumophila type II secretion substrates, including a novel protein, contribute to differential infection of amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis. Infect. Immun. 81:1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng TC, Zhang YL, Liu C, Xu PZ, Gao ZH, Xia QY, Xiang ZH. 2008. Identification and analysis of Toll-related genes in the domesticated silkworm, Bombyx mori. Dev. Comp. Immunol. 32:464–475 [DOI] [PubMed] [Google Scholar]
- 34. Marmaras VJ, Lampropoulou M. 2009. Regulators and signalling in insect haemocyte immunity. Cell. Signal. 21:186–195 [DOI] [PubMed] [Google Scholar]
- 35. Roy CR. 2012. Vacuolar pathogens value membrane integrity. Proc. Natl. Acad. Sci. U. S. A. 109:3197–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar Y, Valdivia RH. 2009. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe 5:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bergin D, Brennan M, Kavanagh K. 2003. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 5:1389–1395 [DOI] [PubMed] [Google Scholar]
- 38. Ratcliffe NA, Gagen SJ. 1977. Studies on the in vivo cellular reactions of insects: an ultrastructural analysis of nodule formation in Galleria mellonella. Tissue Cell 9:73–85 [DOI] [PubMed] [Google Scholar]
- 39. Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5:200–211 [DOI] [PubMed] [Google Scholar]
- 40. Price CT, Al-Quadan T, Santic M, Jones SC, Abu Kwaik Y. 2010. Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J. Exp. Med. 207:1713–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kubori T, Shinzawa N, Kanuka H, Nagai H. 2010. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 6:e1001216. 10.1371/journal.ppat.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Champion OL, Karlyshev AV, Senior NJ, Woodward M, La Ragione R, Howard SL, Wren BW, Titball RW. 2010. Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J. Infect. Dis. 201:776–782 [DOI] [PubMed] [Google Scholar]
- 43. Joyce SA, Gahan CG. 2010. Molecular pathogenesis of Listeria monocytogenes in the alternative model host Galleria mellonella. Microbiology 156:3456–3468 [DOI] [PubMed] [Google Scholar]
- 44. Dietrich C, Heuner K, Brand BC, Hacker J, Steinert M. 2001. Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect. Immun. 69:2116–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]