Abstract
Acinetobacter baumannii is an opportunistic pathogen that causes nosocomial infections. Due to the ability to persist in the clinical environment and rapidly acquire antibiotic resistance, multidrug-resistant A. baumannii clones have spread in medical units in many countries in the last decade. The molecular basis of the emergence and spread of the successful multidrug-resistant A. baumannii clones is not understood. Bacterial toxin-antitoxin (TA) systems are abundant genetic loci harbored in low-copy-number plasmids and chromosomes and have been proposed to fulfill numerous functions, from plasmid stabilization to regulation of growth and death under stress conditions. In this study, we have performed a thorough bioinformatic search for type II TA systems in genomes of A. baumannii strains and estimated at least 15 possible TA gene pairs, 5 of which have been shown to be functional TA systems. Three of them were orthologs of bacterial and archaeal RelB/RelE, HicA/HicB, and HigB/HigA systems, and others were the unique SplT/SplA and CheT/CheA TA modules. The toxins of all five TA systems, when expressed in Escherichia coli, inhibited translation, causing RNA degradation. The HigB/HigA and SplT/SplA TA pairs of plasmid origin were highly prevalent in clinical multidrug-resistant A. baumannii isolates from Lithuanian hospitals belonging to the international clonal lineages known as European clone I (ECI) and ECII.
INTRODUCTION
Toxin-antitoxin (TA) systems, widespread in bacteria and archaea, consist of a stable “toxin” component and an unstable “antitoxin” (1, 2). Both components usually form a complex where the activity of the toxin is inhibited by the antitoxin. Under some conditions, a labile antitoxin is degraded and a more stable toxin comes into action by inhibiting essential cellular processes such as translation, replication, and biosynthesis of ATP and cell wall (2–4). TAs are encoded by plasmids or reside in bacterial chromosomes (5). Toxins of plasmid-borne TAs mediate postsegregational killing of cells that have lost plasmids, and such TAs have been proposed to function as plasmid stabilization elements (6). The role of TAs encoded by bacterial chromosomes is far less understood (7). Similarly to plasmid-encoded TAs, they have been suggested to stabilize various genetic elements (pathogenicity islands and prophages) or function as stress-responsive elements by modulating bacterial growth and death (8–10). Bioinformatic searches have demonstrated a tremendous abundance of TAs in bacterial and archaeal genomes (1, 5). TA systems are currently grouped into five types (types I to V) according to the nature of the antitoxin and the mode of interaction of the toxin and antitoxin (11, 12). In all cases, toxins are proteins, while an antitoxin is either RNA (types I and III) or a protein (types II, IV, and V). In type I TAs, an antitoxin suppresses the toxicity of a toxin protein by binding to its mRNA. In type II and III TAs, a toxin is neutralized by the direct binding of an antitoxin protein and antitoxic RNA, respectively. In type IV TAs, an antitoxin modifies and protects the target of the toxin, while in type V TAs, an antitoxin is an RNase, which specifically cleaves mRNA of the toxin (11, 12).
The role of TA systems in the life of bacterial pathogens is not yet understood. It was shown that free-living bacteria and pathogens existing under variable conditions have numerous TA systems (13, 14). TAs have been suggested to mediate bacterial persistence by generating slowly growing cells tolerant to antibiotics and environmental changes (15). Moreover, TAs were shown to promote biofilm formation through programmed cell death (16).
Multidrug-resistant A. baumannii is an emerging Gram-negative nosocomial pathogen worldwide (17). It is characterized by the capacity to withstand harsh environmental conditions such as dryness and heat, the ability to form biofilms on abiotic surfaces (18), and the ability to acquire foreign DNA via mobile genetic elements and recombination mechanisms (19, 20). These features place A. baumannii among the most important nosocomial infection agents (17, 18). Of special concern is the dramatic increase in the resistance of A. baumannii to broad-spectrum antibiotics such as carbapenems in recent years (21). The successful A. baumannii clonal lineages associated with carbapenem resistance, known as European clone I (ECI) and ECII, have been disseminated globally (17).
Data on the presence, diversity, and role of TA systems in A. baumannii physiology and pathogenicity are lacking. In this study, we have undertaken a bioinformatic search for putative A. baumannii TA loci and found at least five functional TA systems. The toxins of these systems all inhibited translation and caused RNA degradation to different levels. Three of the newly described A. baumannii TAs were homologous to the well-known RelBE and HicAB TA families and the less-well-described HigBA family (GP49/Cro domain proteins), while the two others represented the so-far-unique SplTA (DUF497/COG3514 domain proteins) and CheTA (HTH/GNAT domain proteins) TAs. We have also shown that the plasmid-borne HigBA and SplTA systems were prevalent among multidrug-resistant A. baumannii isolates from Lithuanian hospitals, belonging to ECI and ECII clones.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
The Escherichia coli strain and plasmids used in this work are listed in Table 1. The strains were grown in liquid or solid Luria-Bertani (LB) medium at 37°C if not mentioned otherwise. Antibiotics were added at the following concentrations: ampicillin at 100 μg/ml, kanamycin at 60 μg/ml, and chloramphenicol at 33 μg/ml. For protein overexpression, LB medium was supplemented with arabinose and/or isopropyl-β-d-thiogalactopyranoside (IPTG) at different concentrations, as indicated below.
Table 1.
Strains and plasmids
| Strain or plasmid | Genotype or description | Reference |
|---|---|---|
| Strain | ||
| E. coli BW25113 F′ | BW25113/F′ proA+B+ lacIqΔ(lacZ)M15 zzf::mini-Tn10 (Kanr) | 25 |
| Plasmids | ||
| pBAD30 | Expression plasmid; Ampr | 36 |
| pUHE25-2 | Expression plasmid; Ampr | 37 |
| pUHEcat | Expression plasmid; Cmlr; originally named pUHE25-2(cat) | 25 |
| pUHEcat-“antitoxin” series plasmids | ||
| pUHEcat-RelBAb | A. baumannii RelBAb gene cloned into pUHEcat through PaeI and HindIII sites | This work |
| pUHEcat-HicBAb | A. baumannii HicBAb gene cloned into pUHEcat through PaeI and HindIII sites | This work |
| pUHEcat-CroAb | A. baumannii CroAb gene cloned into pUHEcat through PaeI and HindIII sites | This work |
| pUHEcat-COG3514Ab | A. baumannii COG3514Ab gene cloned into pUHE25cat through PaeI and HindIII sites | This work |
| pUHEcat-GNATAb | A. baumannii GNATAb gene cloned into pUHEcat through PaeI and HindIII sites | This work |
| pUHEcat-XAb | A. baumannii XAb gene cloned into pUHE2cat through PaeI and HindIII sites | This work |
| pBAD-“toxin” series plasmids | ||
| pBAD-RelEAb | A. baumannii RelEAb gene cloned into pBAD30 through EcoRI and HindIII sites | This work |
| pBAD-HicAAb | A. baumannii HicAAb gene cloned into pBAD30 through EcoRI and HindIII sites | This work |
| pBAD-GP49Ab | A. baumannii GP49Ab gene cloned into pBAD30 through EcoRI and HindIII sites | This work |
| pBAD-DUF497Ab | A. baumannii DUF497Ab gene cloned into pBAD30 through EcoRI and HindIII sites | This work |
| pBAD-HTHAb | A. baumannii HTHAb gene cloned into pBAD30 through EcoRI and HindIII sites | This work |
| pBAD-ZetaAb | A. baumannii ZetaAb gene cloned into pBAD30 through XbaI and HindIII sites | This work |
PCR reagents, DNA-modifying enzymes, and kits for molecular biology experiments were obtained from Thermo Scientific. All the enzymes were used as recommended by the supplier. The oligonucleotide primers used in this study were obtained from Metabion and are listed in Table S1 in the supplemental material.
Bioinformatic search for TA pairs.
The sequences of currently known experimentally validated TA proteins (see Table S2 in the supplemental material) were used as an input for the PSI-BLAST and TBLASTN programs. The searches were performed against the accessible A. baumannii genomes and plasmids and Acinetobacter phages (listed in Table S3 in the supplemental material), with the following parameters: BLOSUM62 matrix, a word size of 3, and an E value of >0.01. PSI-BLAST was executed with 3 iterations. Extensive analyses were performed by using the RASTA-Bacteria tool (22), and the resulting gene pairs were manually searched for homology by using PSI-BLAST against the microbial genomes. The TADB online tool was also consulted for possible TA components (23). Protein similarity and identity were evaluated by global alignment using the Needleman-Wunsch alignment algorithm with the Needle (EMBOSS) tool. The results of the bioinformatic search were last updated in November 2012.
Detection and screening of TA system genes in clinical A. baumannii isolates.
DNAs of A. baumannii isolates (n = 476), recovered from various clinical specimens of patients hospitalized in Lithuanian regional medical centers during the years 2010 and 2011 and genotyped as described previously (24), were used for detection and screening of TA systems. A. baumannii cell lysates were prepared by boiling in water for 10 min. Putative TA genes were detected by PCR using the DNAs of the selected A. baumannii isolates, corresponding to all the genotype groups (24). The screening of validated A. baumannii TAs in the whole collection of clinical A. baumannii isolates was undertaken by PCR using the Tecan Freedom EVO robotic system. The primers for PCR (see Table S1 in the supplemental material) were designed to match the conserved gene sequences using the primer BLAST tool (NCBI).
Cloning of putative A. baumannii TA genes.
The primers used for cloning of the putative TA systems (see Table S1 in the supplemental material) were designed based on the A. baumannii TCDC-AB0715 genome (GI:385235550). The DNA of a representative clinical A. baumannii isolate (number 35 [ECII]) containing all five putative TA pairs was used as a matrix for cloning. All the genes were first cloned into pUHE25-2 series vectors to generate equal Shine-Dalgarno (SD) sites. Subsequently, the genes coding for the putative toxins were recloned into pBAD series vectors. To obtain E. coli strains with A. baumannii toxin-antitoxin gene pairs, E. coli strain BW25113 F′ was transformed first with pUHEcat-“antitoxin” plasmids and then with pBAD-“toxin” plasmids (Table 1).
Assays for DNA, RNA, and protein synthesis rates in vivo.
The E. coli BW25113 F′ pUHEcat-“antitoxin” pBAD-“toxin” strains were grown in M9 minimal medium, and replication, transcription, and translation rates were measured by the incorporation of [2-14C]thymidine (1 μCi/ml, final concentration), [2-14C]uridine (1 μCi/ml), and l-[35S]methionine (3 μCi/ml), respectively, as previously described (25). For the control experiments, effects of the replication inhibitor ciprofloxacin (10 μg/ml), the transcription inhibitor rifampin (100 μg/ml), and the translation inhibitor gentamicin (100 μg/ml) were measured.
Kill-rescue assay.
The E. coli BW25113 F′ pUHEcat-“antitoxin” pBAD-“toxin” strains were grown in LB medium until the stationary phase, diluted 1:500 into fresh medium containing 0.2% glucose (for full repression of the pBAD system), and grown until the early logarithmic phase (optical density at 600 nm [OD600] = 0.1). The culture was then pelleted and resuspended in fresh medium with the respective inducers for toxins and antitoxins, arabinose (pBAD plasmids) and/or IPTG (pUHE plasmids), or water (control). The cultures were then inoculated onto a microplate and incubated in a Tecan Infinite M200 Pro plate reader at 37°C with shaking. The OD600 was measured every 15 min.
Northern analysis.
The E. coli BW25113 F′ pUHEcat-“antitoxin” pBAD-“toxin” strains were grown in LB medium at 37°C until an OD600 of 0.1 was reached, and arabinose was then added to a final concentration of 0.2% for toxin induction. The samples were collected before and after different times of induction and subjected to RNA extraction with hot phenol, as described previously (26). Fifteen micrograms of RNA per sample was loaded onto a 2% agarose gel. After electrophoresis, the gel was visualized, transferred onto a SensiBlot Plus (Thermo) nylon membrane, hybridized with lpp- or ssrA-specific DNA probes, labeled with the Biotin DecaLabel DNA labeling kit (Thermo), and visualized with the Biotin Chromogenic Detection kit (Thermo). The primers used for PCR amplification of DNA probes are listed in Table S1 in the supplemental material.
Statistical analysis.
For the comparison of categorical variables, Fisher's exact test was used (GraphPad Software, San Diego, CA, USA). A P value of <0.05 was considered to be statistically significant.
RESULTS
Bioinformatic search for candidate A. baumannii TA pairs.
We have applied two different approaches to evaluate possible TA systems encoded in currently available complete sequences of 11 chromosomes and 25 plasmids of A. baumannii and 8 Acinetobacter phages (see Table S3 in the supplemental material). The search based on homology with the currently known validated TA pairs (see Table S2 in the supplemental material) using BLAST and TBALSTN tools resulted in a conspicuous variety of RelE superfamily toxins, accompanied mostly by RelB family antitoxins and a few HipAB and HicAB modules (Fig. 1). The BLAST tools also suggested candidate Zeta toxins, which were notably larger (360 amino acids) than their currently known functional orthologs (∼270 amino acids). A. baumannii putative Zeta toxins fell into the group of longer Zeta-like proteins, which had not been tested for their functionality (27). Open reading frames (ORFs) preceding the possible Zeta toxins had no conserved structural domains (Fig. 1).
Fig 1.
Putative TA systems encoded by A. baumannii chromosomes and plasmids. Names for the putative TA pairs were assigned according to their similarity to the validated toxins and antitoxins or conserved domains. X represents a protein that exhibited no homology or conserved domain. TA variations from different chromosomes or plasmids were grouped under the same TA system if both components shared at least 50% similarity and 30% identity with other pairs in this group. The arrangement of genes coding for the putative TAs is represented by gray and light gray arrows for the putative toxins and antitoxins, respectively. The overlaps between ORFs coding for the truncated versions of toxins or antitoxins and ORFs coding for the rest of these proteins are indicated by darker shading. Additional ORFs that overlap the putative TA genes are shown as transparent arrows. The sizes of the arrows and gaps or overlaps are drawn to scale. The presence and absence of the putative TA pairs in the clinical A. baumannii isolates are indicated by “+” and “−,” respectively, under a separate column named “Clin. Is.” Similarly, the functionality of TAs is indicated under the column “Funct.” The gene pairs that were not tested are denoted “nt.” GI numbers of the candidate TAs are given in Table S4 in the supplemental material.
BLASTing against the recently widely expanded list of functional TA pairs (5) suggested the A. baumannii GP49-like protein as a candidate toxin to form a TA pair with a Cro-like regulator protein as an antitoxin. This pair was among the most prevalent in the A. baumannii plasmids found in the databases. It was sometimes followed by a third open reading frame, coding for a putative protein (Fig. 1). Another significantly different pair, which also contained a GP49 domain protein and an HTH domain protein, was much less abundant (Fig. 1). The pairs consisting of GP49 domain proteins and HTH domain proteins of the Xre/Cro family were previously described and named the Tad-Ata TA system (1, 28). It was suggested that these loci might be considered a distinct subclass of the higBA family (28), which is characterized by a modest toxin similarity to RelE superfamily proteins and by the inverted gene order, with the toxin gene being the first gene in the TA operon (29). In agreement with such a description, we refer to the pair consisting of a GP49 domain protein and a Cro or HTH protein as HigBAb/HigAAb.
Next, we used the RASTA tool to evaluate all ORFs according to their size and pairing with other ORFs (22). By using this approach, we identified additional putative A. baumannii TA pairs, DUF497/COG3514 and two variants of HTH/GNAT and DUF1044/RelB, which have never been shown to be functional. The DUF497/COG3514 pair was widespread among small, ∼10-kb plasmids (Fig. 1), and its protein and nucleotide sequences were absolutely conserved. Additionally, pairs consisting of an HTH domain protein and N-acetyltransferases of the GCN5-related (GNAT) superfamily were observed in almost all A. baumannii chromosomes analyzed (Fig. 1). Another novel pair consisted of the DUF1044 protein and RelB antitoxin. In the Pfam collection of protein families, the DUF1044 protein belonged to the “plasmid toxin clan” together with GP49, Txe, and other toxins. In the bioinformatic search, this pair was found only once (Fig. 1) and was not chosen for further investigation.
Overall, both searches suggested 15 candidate A. baumannii TA pairs: 9 of them encoded solely by plasmids and 4 encoded solely by chromosomes. The remaining two TA pairs were encoded mainly by chromosomes, but each TA also had a version in the plasmid (Fig. 1). No candidate TA pairs were found on Acinetobacter phages.
Cloning and expression of A. baumannii TA systems in E. coli.
To confirm the functionality of TA systems found in A. baumannii, we chose six putative TA modules: several homologs of characterized TAs (RelBEAb, HicABAb, HigBAAb, and XAb/ZetaAb) as well as the newly found candidate TA pairs DUF497Ab/COG3514Ab and HTHAb/GNATAb. They were all found to be present in clinical A. baumannii isolate number 35 from our collection, the DNA of which was used for cloning. The putative toxin and putative antitoxin genes were individually cloned into the compatible E. coli plasmids pBAD30 and pUHEcat (Table 1). Notably, we were unable to clone the toxin genes alone: DNA sequencing of the transformants revealed single-nucleotide deletions that caused a shift of the reading frame and truncation of the product due to the emerged stop codon. To overcome this, E. coli strain BW25113 F′ was transformed first with the plasmid harboring an A. baumannii candidate antitoxin and subsequently with the plasmid harboring the respective toxin. We obtained BW25113 F′ pUHEcat-“antitoxin” pBAD-“toxin” strains for all six candidate TA pairs.
A. baumannii codes for at least four functional TA pairs.
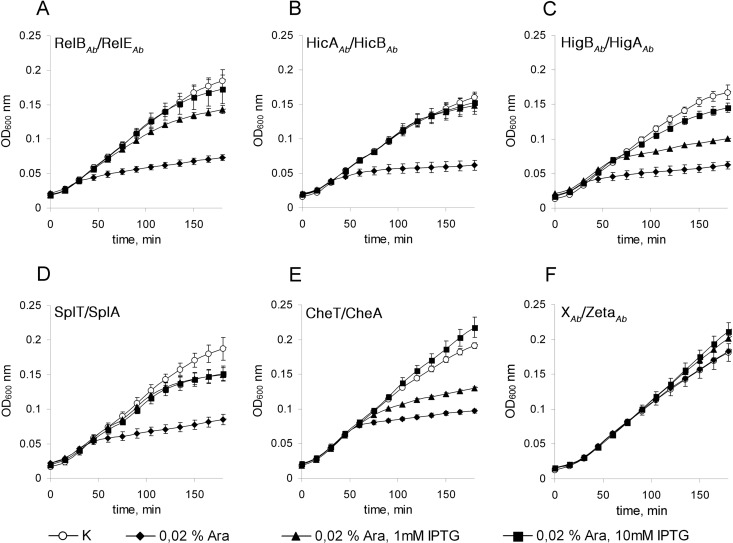
Six cloned TA pairs were subjected to the “kill-rescue” assay in E. coli cells. The strains harboring TA pairs encoded on two independent plasmids were grown until the early logarithmic phase (OD600 = 0.1) in LB medium with 0.2% glucose. The culture was then pelleted and resuspended in fresh medium with the respective inducers for toxins and antitoxins. For most of the putative toxins, except for ZetaAb, induction with 0.02% arabinose was sufficient to inhibit the growth of E. coli (Fig. 2A to E). Meanwhile, some antitoxins required induction with up to 10 mM IPTG to take over the toxins' effect. Increasing the concentration of arabinose or decreasing the concentration of IPTG caused an incomplete restoration of E. coli growth (Fig. 2A, C, and E).
Fig 2.
Growth of E. coli BW25113 F′ harboring the putative TA components of A. baumannii. The E. coli strains harboring the pUHE25-2cat-“antitoxin” and pBAD30-“toxin” plasmids were grown until the early logarithmic phase (OD600 = 0.1) with 0.2% glucose, pelleted, and resuspended in fresh medium with the inducers. The data represent averages of data from at least three independent experiments with three replicates each time; the error bars show the standard deviations.
Surprisingly, the HTHAb/GNATAb TA pair appeared to function oppositely than expected: the A. baumannii HTH domain protein arrested bacterial growth when induced, whereas the GNAT domain protein was able to abolish this effect. To fit the general scheme, the HTHAb-encoding gene was recloned into the pBAD plasmid, and the GNATAb-encoding gene was recloned into the pUHEcat plasmid. Such manipulations did not change the mode of action of these proteins (Fig. 2E). Therefore, based on our results, we consider HTHAb to be a toxin and GNATAb to be its antitoxin, and we named this pair CheTA (for switched-element toxin-antitoxin system). We also propose that the DUF497Ab/COG3514Ab pair be renamed SplTA (for small-plasmid toxin-antitoxin) according to its prevalence in small, ∼10-kb plasmids of A. baumannii. Neither the ZetaAb gene nor the neighboring putative gene, the XAb gene, was toxic to E. coli at any concentration of inducer tested (Fig. 2F).
A. baumannii TA system toxins inhibit translation in E. coli.
Next, we asked what the mode of action of toxins of the functional A. baumannii TA systems is. For this, we measured the incorporation of radiolabeled precursors of replication, transcription, and translation after the induction of toxins with 0.2% arabinose in E. coli cells. As can be seen in Fig. 3A, the overexpression of all five toxins caused a significant reduction in the incorporation of [35S]methionine. The incorporation of the precursor of RNA biosynthesis, [2-14C]uridine, was much less affected, whereas the incorporation of the DNA precursor [2-14C]thymidine remained almost unaltered (Fig. 3A). The toxins SplT and CheT did not cause immediate inhibition of translation compared to RelEAb, HicAAb, and HigBAb (Fig. 3B). While all five A. baumannii toxins mostly inhibited protein synthesis, the effect on RNA synthesis was also evident (Fig. 3A).
Fig 3.
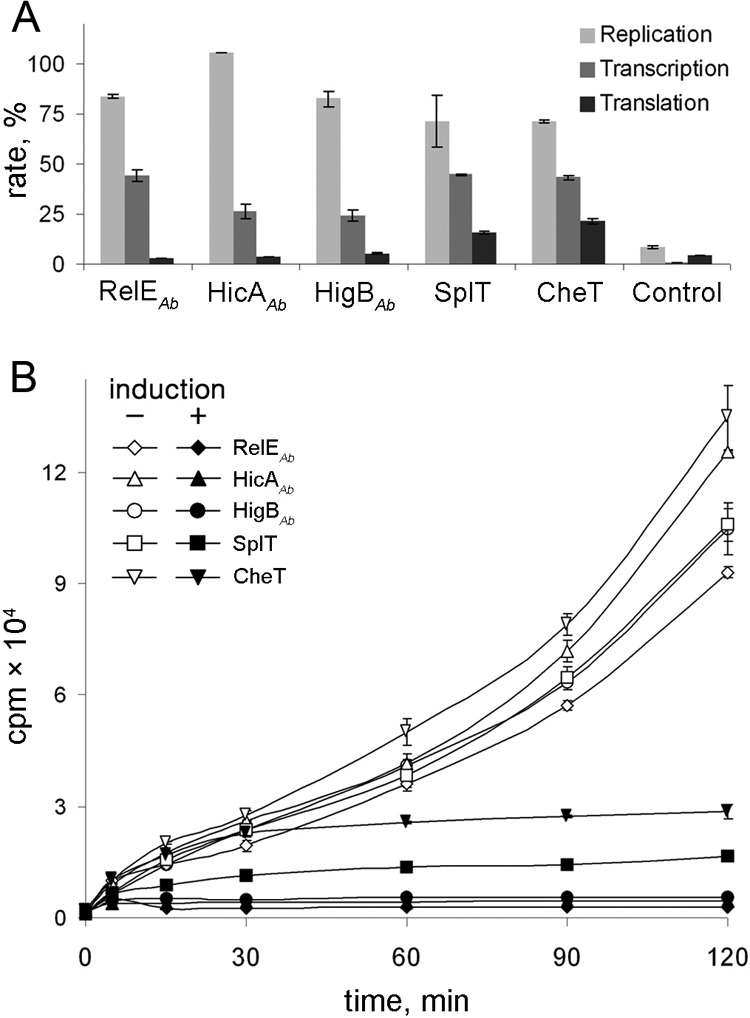
Effect of the overexpression of A. baumannii toxins on protein, DNA, and RNA syntheses in E. coli. The E. coli BW25113 F′ pBAD-“toxin” pUHEcat-“antitoxin” series strains were grown in M9 medium at 37°C and induced as described in Materials and Methods. (A) The incorporation of [2-14C]thymidine, [2-14C]uracil, and [35S]methionine was measured after 2 h of toxin induction. The RNA synthesis inhibitor rifampin, the DNA synthesis inhibitor ciprofloxacin, or the protein synthesis inhibitor gentamicin served as a positive control. Toxin induction and controls were normalized to the measurements of the same strain without induction (taken as 100%). (B) Dynamics of the incorporation of [35S]methionine in the absence (indicated by white symbols) or in the presence (indicated by black symbols) of 0.2% of arabinose.
A. baumannii TA system toxins cause RNA degradation in E. coli.
Most of the translation inhibitors from currently known TA pairs inhibit translation due to the degradation of mRNA (10). We have performed Northern analysis of total RNA isolated after the induction of A. baumannii toxins in E. coli cells (see Materials and Methods). lpp mRNA and transfer-messenger RNA (tmRNA), also known as ssrA (30), which are abundant and relatively stable E. coli RNAs, were chosen for analysis. The induction of A. baumannii RelE, HicA, and HigB toxins caused rapid degradation of lpp mRNA (Fig. 4). The effect of the induction of SplT and CheT toxins on lpp mRNA was less pronounced. The induction of RelEAb, HicAAb, and HigBAb not only caused tmRNA cleavage (Fig. 4, white arrow) but also impaired its processing, as can be seen from the accumulation of the precursor ssrA RNA (Fig. 4, black arrow). Similarly to the effect on lpp mRNA, induction of SplT and CheT toxins did not cause immediate degradation of tmRNA; however, after 1 h of induction, cleavage was evident (Fig. 4).
Fig 4.

Effect of overexpression of A. baumannii toxins on E. coli lpp mRNA and tmRNA. The E. coli BW25113 F′ pBAD-“toxin” pUHEcat-“antitoxin” series strains were grown as described in Materials and Methods. The samples were taken before (time zero) and at different times after the induction of the toxin with 0.2% arabinose. Total RNA was isolated, and 15 μg of RNA per sample was subjected to Northern analysis. The membranes were probed with lpp- or ssrA-specific probes labeled with biotin. Black and white arrows indicate ssrA transcript before maturation (precursor of tmRNA) and the cleavage product of ssrA, caused by the induction of toxins, respectively.
Noncanonical HigBA, CheTA, and SplTA toxin-antitoxin pairs are abundant in clinical A. baumannii isolates.
We have performed a PCR-based search for TA systems, validated in this work, with a collection of clinical A. baumannii isolates (n = 476) from Lithuanian hospitals. Most of these isolates belonged to the multidrug-resistant clonal lineages known as European clone I (ECI) and ECII; others were sporadic or nontypeable isolates (N/S) according to a previous pulsed-field gel electrophoresis (PFGE) analysis (24).
Despite the variety of RelBE, HicAB, and other currently known TA family homologs found by a bioinformatic search (Fig. 1), the actual RelBEAb and HicABAb TA systems were least abundant (Fig. 5). The RelBEAb system was found in 7 to 11% of isolates in all groups. HicABAb was almost limited to the ECII group (69.2% prevalence in ECII isolates, compared to 1 to 11% prevalence in other groups). Interestingly, the most frequently found TAs were the TAs that did not belong to currently described TA families (Fig. 5). Except for HigBAAb, the SplTA and CheTA systems are so far unique: none of their homologs have previously been shown to be functional. Chromosome-borne CheTA was the most prevalent TA system and was found in 76.5% of isolates, showing a high prevalence in all groups. However, within the A. baumannii isolates belonging to the ECI and ECII lineages, the most prevalent were the plasmid-borne SplTA and HigBAAb TA systems (88.6% and 89.6% frequencies, respectively) (Fig. 5).
Fig 5.
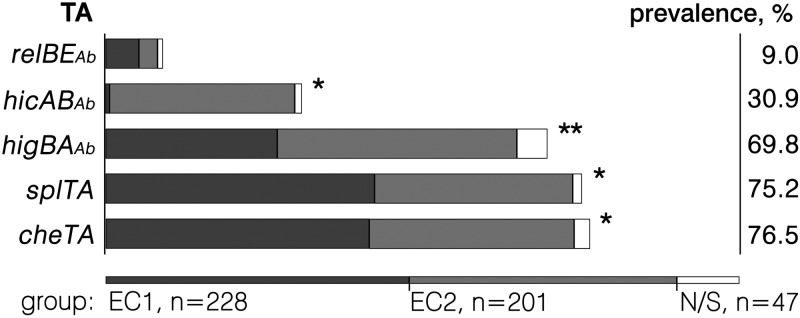
Occurrence of the validated TA systems in clinical A. baumannii isolates. PCR-based screening of the validated TA systems in clinical A. baumannii isolates was undertaken as described in Materials and Methods. The isolates tested (n = 476) comprised genotype groups belonging to the ECI and ECII lineages and to a group of nontypeable or sporadic (N/S) isolates (represented by different-shaded bars). Statistically significant (P < 0.05) differences in the prevalence of TA systems in all three groups (pairwise comparison) are marked with an asterisk; a statistically significant difference between the ECII group and other groups, but not between the ECI and N/S groups, is marked with a double asterisk. The percentage given on the right represents the prevalence of TAs in all the A. baumannii strains tested.
DISCUSSION
At first glance, the bioinformatic search showed a rather limited variety of A. baumannii type II TA systems. However, a more thorough analysis using the RASTA tool (22) revealed a larger repertoire. Our search was limited to the currently assembled genomes (see Table S3 in the supplemental material), but a peek into scaffolds of the newly sequenced genomes revealed at least a few more A. baumannii TA families. These families included a pair consisting of the putative MazE antitoxin and the putative PIN domain toxin in the A. baumannii Naval-82 chromosome (GI:421789021 and GI:421789037, respectively) and also the Doc family toxin, overlapping hypothetical proteins in at least three A. baumannii chromosomes: ABNIH1 (GI:417872276 and GI:417872275, respectively), Naval-82 (GI:421788562 and GI:421788565, respectively), and 6013150 (GI:332852538 and GI:332852537, respectively). We also found a number of orphan antitoxins and presumed antitoxin-antitoxin pairs. Some pairs, not included in the list of A. baumannii TAs, consisted of toxin or antitoxin homologs and unrelated proteins, for example, a pair consisting of the MazF toxin and a DedA family protein in the 1656-2 chromosome (GI:384129960:128964–129218) or a very abundant pair consisting of a Phd antitoxin and a permease of the DMT family (GI:323518892 and GI:323518893, respectively).
One of the most outstanding properties of A. baumannii TA systems, predicted and validated in this study, was their “reverse organization.” While the TA operons, with some exceptions, code for the antitoxin gene located upstream of the toxin gene (2), most of the A. baumannii TAs did not obey this rule (Fig. 1). Moreover, A. baumannii TA genes were separated by distances greater than the typical distance in the range of a few base pairs (10). Other TA genes overlapped by as much as 38 bp (Fig. 1). Also, the distance between genes coding for the TA components was largely variable in A. baumannii TAs. This is most evident for the HicAAb/HicBAb and HigBAb/HigAAb pairs, where the distance varies from overlap to separation (Fig. 1).
We have observed that, in some cases, the genes coding for A. baumannii TA components were truncated and restored by overlapping ORFs coding for the rest of the protein (Fig. 1). An interesting example is a gene coding for the putative A. baumannii Zeta toxin, which is truncated and restored at different places in plasmids pABTJ1 and pACICU2. The largest overlap (191 bp) was observed in a toxin gene of the (HipBAb/HipAAb)2 pair (where the subscript 2 indicates the second of two homologous modules), which resulted in a large repetition of a protein fragment (Fig. 1). Whether such TA components are functional is questionable. Such breaking could be thought as an example of the evolution of three-component TA systems, where an additional third ORF codes for a regulator of the system. The homologs of such three-component systems are known to exist in the form of two-component TA systems (31–33).
We have shown that A. baumannii possesses functional TAs, the toxins of which, when expressed ectopically, inhibited translation by degrading RNA in E. coli. Toxins of the HicAAb/HicBAb and RelBAb/RelEAb modules caused rapid degradation of RNA, as expected from their homology to the well-documented TA systems. Overexpression of the HigBAb toxin had a similar effect on translation (Fig. 3B) and RNA degradation (Fig. 4). Such observations are consistent with the proposal that Tad homologs (GP49 domain proteins) belong to the RelE superfamily and should be classified as HigBs. The newly confirmed SplT/SplA and CheT/CheA systems revealed some notable differences in their impact on the growth of E. coli and also in the different degrees of mRNA and tmRNA degradation. This raised the question about the possible different modes of action of the respective toxins of the SplT/SplA and CheT/CheA modules.
Based on the conserved motifs, it was suggested that DUF497 (or COG2929) and COG3514 (or COG5304) proteins might act as a toxin and an antitoxin, respectively (1). We have shown that DUF497Ab/COG3514Ab (renamed SplTA) is a functional TA system and that its toxin, SplT, inhibits translation. However, both translation inhibition and RNA degradation were less pronounced than those of other toxins tested in our study. The detailed mechanism of this toxin as well as other newly identified toxins remains to be investigated.
The ability of GNAT proteins to form TA pairs with DNA-binding proteins containing an HTH or RHH domain was considered controversial (1); however, some of them were annotated TAs. Indeed, we have shown in this study that HTHAb/GNATAb (renamed CheTA) did act as a TA module but oppositely than expected: the HTH domain protein acted as a toxin, while the GNAT domain protein neutralized its effect. However, the delayed action of the CheT toxin could suggest a different mode of its activity or a different interaction with the antitoxin. Despite the fact that CheA clearly abolished the toxicity of CheT, according to the sequence analysis, it is a putative N-acetyltransferase of the GCN5-related (GNAT) superfamily. Some GNAT proteins are known to regulate translation by acetylation of ribosomal proteins (34). GNAT proteins also contribute to bacterial antibiotic resistance (35) and are annotated mostly as putative antibiotic resistance genes in A. baumannii. Whether an antitoxin could possibly use an acetylation activity remains to be elucidated.
A. baumannii TAs differed in their localization and abundance and showed clear association with plasmids. Approximately two-thirds of the candidate TAs were solely plasmid borne according to the bioinformatic search. The most TA-rich plasmid, the A. baumannii 94-kb plasmid p3ABAYE (GI:169147050), possibly carries five TAs. Three of them [(RelBAb/RelEAb)2 and two versions of HigBAb/HigAAb in opposite directions] form a single cluster, whereas another different HigBAb/HigAAb (GP49Ab/HTHAb) pair and the (HipBAb/HipAAb)2 pair are located further upstream.
In the collection of clinical A. baumannii isolates, two of the most abundant TA systems were the HigBAb/HigAAb and SplTA TA systems, which are solely plasmid borne according to the bioinformatic analysis. These noncanonical TA systems were the most prevalent in the clinical A. baumannii isolates belonging to the ECI and ECII lineages, which are spread worldwide. Furthermore, according to our recent observations, these TA modules are harbored by the newly observed ∼11-kb plasmid pAB120. This small plasmid is prevalent in carbapenem-resistant clinical A. baumannii ECII isolates and carries two copies of the gene for OXA-72 β-lactamase, conferring resistance to carbapenems (24). These observations suggest a probable role of plasmid-borne A. baumannii TAs as plasmid stabilization systems contributing to the evolution of antibiotic resistance in this opportunistic pathogen.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the European Social Fund under National Integrated Programme Biotechnology & Biopharmacy grant VP1-3.1-SMM-08-K01-005.
We thank Julija Armalytė for the critical reading of the manuscript and helpful comments.
Footnotes
Published ahead of print 10 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00237-13.
REFERENCES
- 1. Makarova KS, Wolf YI, Koonin EV. 2009. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol. Direct 4:19. 10.1186/1745-6150-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamaguchi Y, Park JH, Inouye M. 2011. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45:61–79 [DOI] [PubMed] [Google Scholar]
- 3. Mutschler H, Gebhardt M, Shoeman RL, Meinhart A. 2011. A novel mechanism of programmed cell death in bacteria by toxin-antitoxin systems corrupts peptidoglycan synthesis. PLoS Biol. 9:e1001033. 10.1371/journal.pbio.1001033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Unoson C, Wagner EG. 2008. A small SOS-induced toxin is targeted against the inner membrane in Escherichia coli. Mol. Microbiol. 70:258–270 [DOI] [PubMed] [Google Scholar]
- 5. Leplae R, Geeraerts D, Hallez R, Guglielmini J, Drèze P, Van Melderen L. 2011. Diversity of bacterial type II toxin-antitoxin systems: a comprehensive search and functional analysis of novel families. Nucleic Acids Res. 39:5513–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerdes K, Rasmussen PB, Molin S. 1986. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc. Natl. Acad. Sci. U. S. A. 83:3116–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Melderen L. 2010. Toxin-antitoxin systems: why so many, what for? Curr. Opin. Microbiol. 13:781–785 [DOI] [PubMed] [Google Scholar]
- 8. Lehnherr H, Maguin E, Jafri S, Yarmolinsky MB. 1993. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J. Mol. Biol. 233:414–428 [DOI] [PubMed] [Google Scholar]
- 9. Ramage HR, Connolly LE, Cox JS. 2009. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet. 5:e1000767. 10.1371/journal.pgen.1000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamaguchi Y, Inouye M. 2009. mRNA interferases, sequence-specific endoribonucleases from the toxin-antitoxin systems. Prog. Mol. Biol. Transl. Sci. 85:467–500 [DOI] [PubMed] [Google Scholar]
- 11. Van Melderen L. 2012. GhoSTly bacterial persisters. Nat. Chem. Biol. 8:812–813 [DOI] [PubMed] [Google Scholar]
- 12. Schuster CF, Bertram R. 2013. Toxin-antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol. Lett. 340:73–85 [DOI] [PubMed] [Google Scholar]
- 13. Georgiades K, Raoult D. 2011. Genomes of the most dangerous epidemic bacteria have a virulence repertoire characterized by fewer genes but more toxin-antitoxin modules. PLoS One 6:e17962. 10.1371/journal.pone.0017962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33:966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerdes K, Maisonneuve E. 2012. Bacterial persistence and toxin-antitoxin loci. Annu. Rev. Microbiol. 66:103–123 [DOI] [PubMed] [Google Scholar]
- 16. Kolodkin-Gal I, Verdiger R, Shlosberg-Fedida A, Engelberg-Kulka HA. 2009. Differential effect of E. coli toxin-antitoxin systems on cell death in liquid media and biofilm formation. PLoS One 4:e6785. 10.1371/journal.pone.0006785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins PG, Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:233–238 [DOI] [PubMed] [Google Scholar]
- 18. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 19. Snitkin ES, Zelazny AM, Montero CI, Stock F, Mijares L, NISC Comparative Sequence Program, Murray PR, Segre JA. 2011. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc. Natl. Acad. Sci. U. S. A. 108:13758–13763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Towner KJ, Evans B, Villa L, Levi K, Hamouda A, Amyes SG, Carattoli A. 2011. Distribution of intrinsic plasmid replicase genes and their association with carbapenem-hydrolyzing class D beta-lactamase genes in European clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:2154–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roca I, Espinal P, Vila-Farrés X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 3:148. 10.3389/fmicb.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sevin EW, Barloy-Hubler F. 2007. RASTA-Bacteria: a Web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome Biol. 8:R155. 10.1186/gb-2007-8-8-r155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shao Y, Harrison EM, Bi D, Tai C, He X, Ou HY, Rajakumar K, Deng Z. 2011. TADB: a Web-based resource for type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 39:D606–D611. 10.1093/nar/gkq908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Povilonis J, Šeputienė V, Krasauskas R, Juškaitė R, Miškinytė M, Sužiedėlis K, Sužiedėlienė E. 19 December 2012. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J. Antimicrob. Chemother. 10.1093/jac/dks499 [DOI] [PubMed] [Google Scholar]
- 25. Motiejūnaitė R, Armalytė J, Markuckas A, Sužiedėlienė E. 2007. Escherichia coli dinJ-yafQ genes act as a toxin-antitoxin module. FEMS Microbiol. Lett. 268:112–119 [DOI] [PubMed] [Google Scholar]
- 26. Armalytė J, Jurėnaitė M, Beinoravičiūtė G, Teišerskas J, Sužiedėlienė E. 2012Characterization of Escherichia coli dinJ-yafQ toxin-antitoxin system using insights from mutagenesis data. J. Bacteriol. 194:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan WT, Moreno-Córdoba I, Yeo CC, Espinosa M. 2012. Toxin-antitoxin genes of the Gram-positive pathogen Streptococcus pneumoniae: so few and yet so many. Microbiol. Mol. Biol. Rev. 76:773–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dziewit L, Jazurek M, Drewniak L, Baj J, Bartosik D. 2007. The SXT conjugative element and linear prophage N15 encode toxin antitoxin-stabilizing systems homologous to the tad-ata module of the Paracoccus aminophilus plasmid pAMI2. J. Bacteriol. 189:1983–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christensen-Dalsgaard M, Jørgensen MG, Gerdes K. 2010. Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol. Microbiol. 75:333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haebel PW, Gutmann S, Ban N. 2004. Dial tm for rescue: tmRNA engages ribosomes stalled on defective mRNAs. Curr. Opin. Struct. Biol. 14:58–65 [DOI] [PubMed] [Google Scholar]
- 31. Hayes F, Van Melderen L. 2011. Toxins-antitoxins: diversity, evolution and function. Crit. Rev. Biochem. Mol. Biol. 46:386–408 [DOI] [PubMed] [Google Scholar]
- 32. Hallez R, Geeraerts D, Sterckx Y, Mine N, Loris R, Van Melderen L. 2010. New toxins homologous to ParE belonging to three-component toxin-antitoxin systems in Escherichia coli O157:H7. Mol. Microbiol. 76:719–732 [DOI] [PubMed] [Google Scholar]
- 33. Khoo SK, Loll B, Chan WT, Shoeman RL, Ngoo L. 2007. Molecular and structural characterization of the PezAT chromosomal toxin-antitoxin system of the human pathogen Streptococcus pneumoniae. J. Biol. Chem. 282:19606–19618 [DOI] [PubMed] [Google Scholar]
- 34. Vetting MW, de Carvalho LPS, Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS. 2005. Structure and functions of the GNAT super-family of acetyltransferases. Arch. Biochem. Biophys. 433:212–226 [DOI] [PubMed] [Google Scholar]
- 35. Vetting MW, Magnet S, Nieves E, Roderick SL, Blanchard JS. 2004. A bacterial acetyltransferase capable of regioselective N-acetylation of antibiotics and histones. Chem. Biol. 11:565–573 [DOI] [PubMed] [Google Scholar]
- 36. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bujard H, Gentz R, Lanzer M, Stueber D, Mueller M, Ibrahimi I, Haeuptle MT, Dobberstein B. 1987. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 155:416–433 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.