Abstract
YdiV, a degenerate EAL domain protein, represses motility by interacting with FlhD to abolish FlhDC interaction with DNA. Here, we demonstrate that deletion of ydiV dysregulates coordinate control of motility and adherence by increasing adherence of Escherichia coli CFT073 to a bladder epithelial cell line by specifically increasing production of P fimbriae. Interestingly, only one of the two P fimbrial operons, pap_2, present in the genome of E. coli CFT073 was upregulated. This derepression of the pap_2 operon is abolished following deletion of either cya or crp, demonstrating cyclic AMP (cAMP)-dependent activation of the P fimbrial operon. However, the absence of YdiV does not affect the gene expression of cya and crp, and loss of SdiA in the ydiV mutant does not affect the derepression of the pap_2 operon, suggesting that YdiV control of adherence acts in response to cAMP levels. Deletion of ydiV increases motility by increasing expression of fliA, suggesting that in E. coli CFT073, YdiV regulates motility by the same mechanism as that described previously for commensal E. coli strains. Furthermore, analysis of site-directed mutations found two putative Mg2+-binding residues of four conserved YdiV residues (E29 and Q219) that were involved in regulation of motility and FliC production, while two conserved c-di-GMP-binding residues (D156 and D165) only affected motility. None of the four conserved YdiV residues appeared to affect regulation of adherence. Therefore, we propose a model in which a degenerate EAL, YdiV, utilizes different domains to regulate motility through interaction with FlhD and adherence to epithelial cells through cAMP-dependent effects on the pap_2 promoter.
INTRODUCTION
Uropathogenic Escherichia coli (UPEC) is the most common etiological agent of uncomplicated urinary tract infections (UTIs). This heterogeneous group of bacteria utilizes a variety of virulence factors to colonize and ascend the urinary tract, including fimbriae and flagella. Flagella are transiently utilized to ascend the urethra to the bladder and again when the bacteria ascend the ureters to the kidneys (1). Without expression of FliC, the main subunit of the flagellum, UPEC are nonmotile and cannot ascend the urinary tract (2). On the other hand, fimbriae are utilized to adhere to epithelial cells in the bladder and kidneys, allowing UPEC to colonize these tissues and withstand the shear force of urination. E. coli CFT073, a prototypical pyelonephritis isolate, carries 12 fimbrial operons in its genome (3). Two of these operons encode P fimbriae, which bind to Gal (α1-4) Gal-terminal globoceramide receptors on kidney epithelium (reviewed in reference 4). While P fimbriae are thought to be necessary for colonization of the kidneys (5), these fimbriae can also bind to exfoliated human bladder epithelial cells (6).
P fimbriae are regulated by an epigenetic switch controlled by two methylation sites (GATCprox and GATCdist) in its promoter region found within the two sets of binding sites (promoter proximal sites 1, 2, and 3 and promoter distal sites 4, 5, and 6) for the leucine-responsive regulatory protein, Lrp (7). When the promoter is in the “off” state, Lrp is bound to sites 1, 2, and 3, blocking methylation of GATCprox, but in the “on” state, Lrp is bound to the distal sites 4, 5, and 6. PapI, an activator of the pap operon, induces the switch to the on state by increasing the affinity of Lrp for sites 5 and 2. However, shifting the binding of Lrp from sites 1, 2, and 3 to sites 4, 5, and 6 is not sufficient for activation of pap operon transcription. To fully express P fimbriae, the cyclic AMP-catabolite gene activator protein (CAP-cAMP) must bind to the promoter upstream of Lrp site 4 (8). Deletion of either crp (which encodes CAP) or cya (encoding adenylate cyclase, which synthesizes cAMP) abolishes expression of P fimbriae (8). The expression of P fimbriae is enhanced on solid agar medium as opposed to liquid cultures (9). However, the mechanism of preferential expression has not been elucidated.
The reciprocal control of flagellum-mediated motility and fimbria-mediated adherence is controlled in part by the intracellular concentration of cyclic diguanylate monophosphate (c-di-GMP), a second messenger that is regulated in bacteria by diguanylate cyclases that synthesize c-di-GMP and phosphodiesterases that degrade c-di-GMP (10). E. coli CFT073 carries 32 genes that encode diguanylate cyclases, phosphodiesterases, or related proteins that regulate c-di-GMP concentration and thus the balance between motility and adherence (3, 11). Each of these genes was mutated, and motility and adherence phenotypes were assigned (11). One of these proteins, YdiV, has been examined in more detail because of its demonstrated link between fimbriae and flagellar expression (11–15). Here we demonstrate that YdiV, a degenerative c-di-GMP phosphodiesterase known to be a repressor of flagellum-mediated motility within genera of the Enterobacteriaceae (13, 16, 17), represses expression of P fimbriae (specifically the pap_2 operon) in liquid medium, subsequently reducing adherence to epithelial cells through a pathway that involves cAMP. Deletion of ydiV increases the expression of papA_2; however, this derepression is abolished following deletion of either cya or crp.
The mechanism by which YdiV regulates motility is now understood to be the same in Salmonella enterica serovar Typhimurium and in E. coli K-12. YdiV binds to FlhD in the FlhD4C2 complex, inhibiting its interaction with the fliA promoter (15, 18). Specifically, YdiV binds each of the four FlhD subunits of the heterohexamer, forcing the ringlike structure of the FlhD4C2 complex to open. Consequently, the master regulator of the flagellar expression no longer binds DNA (19). A second mechanism by which YdiV inhibits flagellar gene expression has also been suggested, whereby YdiV binds and strips FlhD4C2 from DNA and targets the complex for ClpXP-dependent proteolysis (14). Here, we show that although YdiV regulates motility in E. coli CFT073 by the same mechanism as that observed in E. coli K-12, key residues that are essential for the regulation of motility are not necessary for repression of the expression of P fimbriae. Therefore, we propose a model in which YdiV utilizes different domains to regulate motility through interaction with FlhD and adherence to epithelial cells through cAMP-dependent regulation of the pap_2 promoter.
MATERIALS AND METHODS
Construction of mutants.
Deletion mutants were constructed in E. coli CFT073 using the lambda red recombinase system (20). Primers containing sequences homologous to the 5′ and 3′ ends of the target sequence were designed and used to amplify the resistance cassette from the template plasmid pKD3 (encoding chloramphenicol resistance). Lambda red-mediated recombination was used to replace the genes slyB, sdiA, crp, and cya, individually with these PCR products in both the E. coli CFT073 wild-type background and an unmarked ΔydiV background. Primers homologous to flanking regions of each gene were designed for confirmation of replacement.
Site-directed mutagenesis.
Previously, ydiV was cloned into pGEN-MCS with its endogenous promoter for complementation experiments and designated pYdiV (13). Using QuikChange site-directed mutagenesis kit (Strategene), specific base pair changes were introduced, resulting in alanine substitutions for seven polar conserved residues in YdiV according to the manufacturer's instructions. Mutations were confirmed by sequencing. Plasmids bearing site-directed mutants are pYdiV-S11A, pYdiV-E29A, pYdiV-E55A, pYdiV-E116A, pYdiV-K125A, pYdiV-D156A, pYdiV-D165A, and pYdiV-Q219A.
Motility assays.
Motility was evaluated for each mutant in soft agar plates (1% tryptone, 0.5% NaCl, and 0.25% agar) and compared to that of the parental wild-type strain as described previously (2). Mutants were cultured overnight in LB broth, used to inoculate 5 ml of sterile LB broth, and incubated at 37°C with aeration to an optical density at 600 nm (OD600) of 1.0 to 1.2. Cultures were standardized to an OD600 of 1.0 and used to stab the center of soft agar plates with an inoculating needle. Plates were incubated for 16 h at 30°C, at which time the diameter of motility was measured. Diameters are directly correlated with bacterial motility (21). Motility of the complemented mutants was examined similarly, but the medium and the soft agar contained ampicillin (100 μg/ml) for maintenance of the plasmid. Wild-type E. coli CFT073 and each mutant transformed with the empty plasmid pGEN-MCS were included as controls.
Western blot assays to detect flagella and P fimbriae.
Samples to detect FliC (flagellin), the main subunit of flagella, were prepared as described previously (13) with the following modification. Briefly, cultures were grown in LB medium to an OD600 of 0.3, and 1.5 ml of culture was carefully collected by centrifugation (1,000 × g, 10 min, 4°C) to avoid shearing flagella into the supernatant. Whole cells were resuspended in 100 μl of distilled water (dH2O), and 10 μl was prepared for SDS-polyacrylamide gel electrophoresis. Following SDS-PAGE, samples were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Blots were incubated with a 1:20,000 dilution of rabbit polyclonal antiserum to H1 flagella, followed by a 1:40,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). Blots were developed using chemiluminescence according to the manufacturer's instructions (Amersham ECL Prime; GE Healthcare Life Sciences).
Samples for the detection of P fimbriae were prepared by diluting aerated overnight LB cultures into fresh LB media (1:100) and grown statically or inoculated onto agar plates at 37°C for 24 h. Bacterial cultures were standardized to an OD600 of 1.0, and 1.0 ml of the standardized suspensions was centrifuged (6,000 × g, 10 min, 4°C) to pellet bacteria. Bacteria were lysed by resuspension in 100 μl dH2O and 6× SDS sample buffer (20 μl) and boiled for 10 min. Sample lysates (20 to 30 μl) were electrophoresed on a 12% denaturing SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Blots were incubated with a 1:10,000 dilution of rabbit polyclonal antiserum to PapA, followed by a 1:40,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). Blots were developed using chemiluminescence according to the manufacturer's instructions (Amersham ECL Prime; GE Healthcare Life Sciences).
Shear preparation of fimbriae.
Overnight static cultures (50 ml) of wild-type E. coli CFT073 and the ΔydiV mutant were shaken horizontally for 2 min to shear fimbriae from the bacterial cell surface. Cultures were centrifuged (8,000 × g, 20 min, 4°C) to pellet intact bacterial cells. Culture supernatants containing fimbriae (as confirmed by transmission electron microscopy [TEM]) were ultracentrifuged (40,000 × g, 1 h, 25°C). Supernatant was removed, and pellets were resuspended in 100 μl of dH2O and 20 μl 6× SDS sample buffer. Samples were boiled for 7 min, and a sample (30 μl) was electrophoresed on a 15% denaturing SDS-polyacrylamide gel and stained with Coomassie blue. For type 1 fimbriae, samples were first boiled in acidified water, pH 1.8. Any polypeptide that was differentially expressed was excised and sent to the Proteomics Resource Facility at the University of Michigan for identification by liquid chromatography-tandem mass spectrometry (LC-MS/MS) sequencing.
Adherence assays.
Cell culture and adherence assays were performed as described previously (22) using the immortalized bladder epithelial cell line, UM-UC-3 (ATCC CRL-1749). Adherence was expressed as cell-associated CFU/initial CFU/well, and each mutant was normalized to the wild-type control. All assays were performed in triplicate.
In vivo murine cochallenge model of ascending UTI.
Six- to 8-week-old CBA/J mice were infected transurethrally as previously described (23) with the following modification. Overnight cultures of E. coli CFT073 wild-type and ΔydiV strains were centrifuged (3,500 × g, 30 min, 25°C) to collect bacteria. Bacteria were resuspended in 30 ml phosphate-buffered saline (PBS), quantified in a spectrophotometer at 600 nm, and diluted to an OD600 of 4.0 (∼109 CFU/ml). The ΔydiV mutant was mixed 1:1 with the parental strain, and then 50 μl of this mixture (108 CFU) was transurethrally inoculated into the bladder of each mouse through a sterile 0.28-mm polyethylene catheter attached to an infusion pump (Harvard Apparatus). The inoculum was quantified on LB agar with and without kanamycin to differentiate the resistant mutant from the susceptible wild-type strain. At 48 h postinoculation (hpi), mice were euthanized, target organs were removed and homogenized in 3 ml sterile PBS with a GLH homogenizer (Omni International), and dilutions were spiral plated onto LB agar with and without kanamycin to quantify the bacterial load using an Autoplate 4000 spiral plater (Spiral Biotech). The competitive index (CI) was calculated as mutant(Output/Input)/wild-type(Output/Input). The CI was log10 normalized, and a two-tailed Wilcoxon signed-rank test was conducted, where a P value of <0.05 was considered significant. Animal protocols were approved for use by the University of Michigan UCUCA (approval #08999-3).
RNA isolation, cDNA synthesis, and reverse transcriptase qPCR.
E. coli CFT073, cultured overnight in LB broth with aeration at 37°C, was quantified by spectrophotometry at OD600, and 1-ml samples were diluted to 109 CFU/ml. Bacteria were collected by centrifugation, washed once with PBS, and resuspended in 5 ml fresh LB broth. Bacteria were cultured statically at 37°C, and samples (2 ml) were removed during mid-exponential phase. RNA was stabilized with 4 ml RNAprotect (Qiagen), and total RNA was isolated using the RNeasy Mini system (Qiagen) according to the manufacturer's instructions. Total RNA and cDNA sample concentrations were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). cDNA was synthesized from total RNA using the Superscript Double-Stranded cDNA Synthesis system (Invitrogen) according to the manufacturer's instructions. Reverse transcriptase quantitative PCR (qPCR) was performed in an Mx300P thermal cycler (Stratagene), using 30 ng cDNA template, 0.1 μM primers, and brilliant SYBR green reagents (Stratagene). Data were normalized to gapA and analyzed with MxPro 4.0 software (Stratagene).
Transmission electron microscopy.
Wild-type E. coli CFT073 and the ΔydiV mutant were cultured in LB broth statically overnight at 37°C. Samples were swirled gently to resuspend the cultures, and 10 μl of the culture was dropped onto Formvar carbon support film on TEM specimen grids (Electron Microscopy Sciences). Drops were incubated at room temperature for 5 min, and excess medium was wicked off with filter paper. Grids were washed once with 10 μl of dH2O and stained for 2 min with 10 μl of 1% phosphotungstic acid (pH 6.8). Excess stain was removed, and the grids were washed immediately with dH2O. Grids were dried under a petri dish lid on the benchtop and visualized using a Philips CM-100 transmission electron microscope.
RESULTS
YdiV reduces P fimbria production.
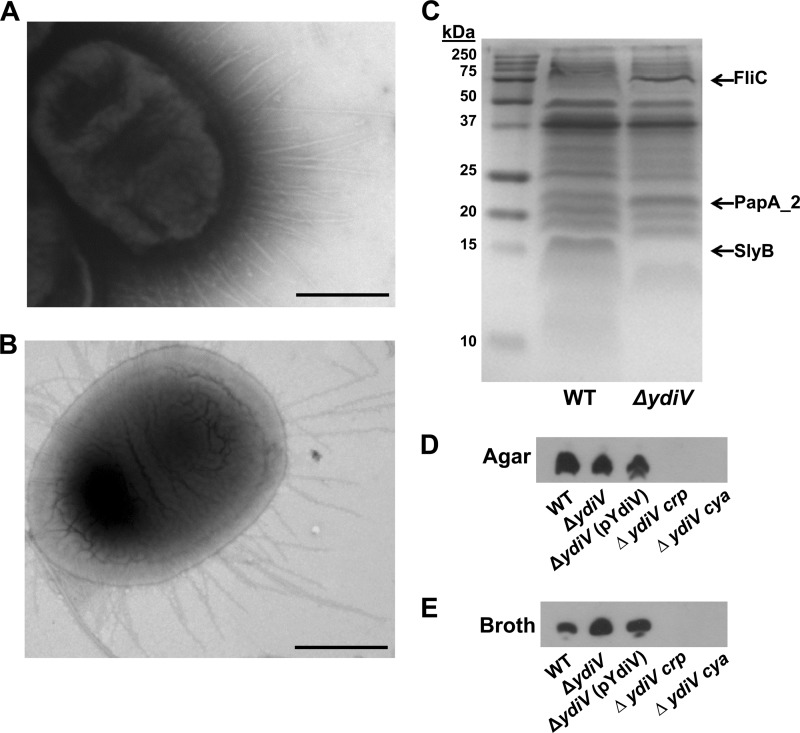
Previously, we reported that the ΔydiV mutant is more adherent than wild-type E. coli CFT073 to the UM-UC-3 immortalized human bladder epithelial cell line (11). Thus, we hypothesized that the ΔydiV mutant expresses more fimbriae than does wild-type E. coli CFT073 or expresses a specific fimbria that is not ordinarily expressed under these conditions. To pursue this possibility, wild-type E. coli CFT073 and the ΔydiV strain were cultured statically overnight in LB broth at 37°C and tested for mannose-resistant hemagglutination (HA) of human red blood cells. CFT073 ΔydiV demonstrated a 4-fold increase in mannose-resistant HA titer (data not shown). In addition, fimbriae were visualized by negative staining and transmission electron microscopy. Interestingly, both the wild-type bacteria (Fig. 1A) and the ΔydiV mutant (Fig. 1B) appeared highly fimbriated. These fimbriae, however, were not type 1 fimbriae, based on a Western blot of whole-cell lysates from wild-type E. coli CFT073 and the ΔydiV mutant cultured statically overnight in LB broth at 37°C, developed with anti-FimA (11). To determine which fimbrial type was overexpressed in the ΔydiV mutant, fimbrial shear preparations from the mutant and wild-type E. coli CFT073 were prepared, denatured, and separated on a 15% denaturing gel by SDS-PAGE and stained with Coomassie blue (Fig. 1C). Two bands were found at higher density in the ΔydiV mutant than in the wild type (apparent molecular sizes, 75 kDa and 23 kDa). The overexpressed bands were excised and subjected to mass spectrometry. The ∼75-kDa protein corresponded to FliC (actual molecular size, 65.5 kDa), and the ∼23-kDa protein was PapA_2 (actual molecular size, 21.8 kDa). The latter result was consistent with the increase in mannose-resistant hemagglutination titer. One band, absent in the ΔydiV strain but present in the wild type (apparent molecular size, 15 kDa) was identified as SlyB, a putative lipoprotein (actual molecular size, 17.0 kDa) (Fig. 1C).
Fig 1.
The ΔydiV mutant overexpresses P fimbriae under static culture conditions. (A, B) Transmission electron micrographs of negatively stained wild-type E. coli CFT073 (A) and ΔydiV (B) strains. Images are taken at a magnification of ×36,000 from static overnight LB broth cultures incubated at 37°C. Bar, 500 nm. (C) Comparison of proteins (black arrows) found in fimbrial shear preps from wild-type and ΔydiV strains demonstrates that FliC and PapA_2 are overexpressed and SlyB is not expressed in ΔydiV. (D) When cultured on agar plates, there is no difference in PapA expression between the mutant, wild-type E. coli CFT073, or the complemented mutant. Deletion of crp or cya abrogates P-fimbrial production in the ΔydiV mutant. (E) Western blotting confirmed that PapA is overexpressed in the ΔydiV mutant only when cultured statically in broth compared to wild-type E. coli CFT073 and the complemented mutant.
Western blot assays were conducted to confirm that the ΔydiV mutant overexpresses PapA, the main structural subunit of P fimbria (Fig. 1D). When cultured overnight in LB broth, the ΔydiV strain demonstrated increased PapA expression compared to both wild-type E. coli CFT073 and the complemented mutant E. coli CFT073 ΔydiV(pYdiV). However, when cultured overnight on LB agar, no difference in PapA expression was observed. Therefore, when statically cultured in liquid medium, which is generally nonpermissive for the production of P fimbriae, the ΔydiV mutant overexpresses P fimbriae.
YdiV represses activation of the pap_2 operon through catabolite-activating protein and adenylate cyclase.
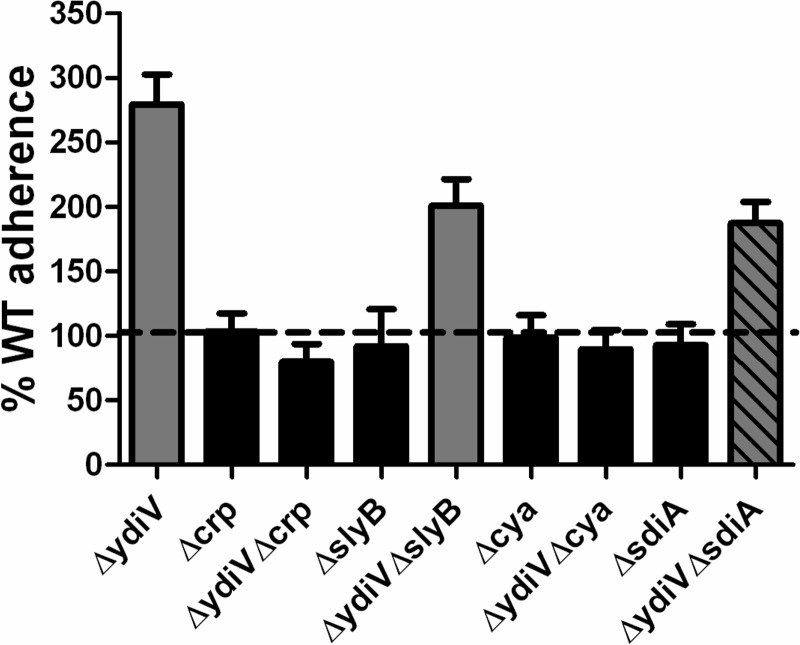
Since the PapA_2 protein is overexpressed in the ΔydiV strain, we hypothesized that YdiV must ordinarily repress the expression of the pap_2 operon, and since CAP-cAMP is known to activate the expression of the pap operon (8), we determined whether deletion of either crp (encoding CAP) or cya (encoding adenylate cyclase) affected the adherence of the ΔydiV mutant to bladder epithelial cells. Mutation of crp or cya in the ΔydiV strain eliminated PapA production as determined by Western blotting (Fig. 1E). Similarly, deletion of either crp or cya from wild-type E. coli CFT073 had no significant effect on adherence to bladder epithelial cells (Fig. 2). However, when either crp or cya was also deleted in a ΔydiV background, adherence was restored to wild-type levels compared to those of the ΔydiV strain (Fig. 2). The results from the adherence assay indicate that the wild-type baseline adherence was independent from the production of P fimbriae, and in the absence of YdiV, CAP-cAMP activates the expression of P fimbriae, which resulted in an increase in adherence to the bladder cell line.
Fig 2.
Deletion of crp or cya in a ΔydiV background restores wild-type adherence to cultured immortalized bladder epithelial cells. Data represent the adherence (CFU adherent bacteria/CFU inoculum) of each mutant strain to cultured UM-UC-3 epithelial cells normalized to wild-type E. coli CFT073. Data are the averages of three assays conducted in triplicate and are expressed as percent wild-type adherence. Error bars indicate standard errors of the means. Gray bars have a P value of <0.05 compared to the wild type as assessed by Student's t test. The gray bar with diagonal stripes is significantly different from both the wild-type (P = 0.0129) and the ΔydiV (P = 0.0233) strains.
YdiV has previously been linked to the production of cAMP, as a double deletion of ydiV and sdiA reduces the intracellular cAMP concentration in E. coli K-12 (24). Therefore, the effect of deletion of sdiA, which encodes a quorum-sensing transcriptional regulator thought to activate transcription of ydiV (24), on adherence in both wild-type and ΔydiV backgrounds was assessed. As observed with Δcrp and Δcya strains, ΔsdiA had no effect on adherence compared to wild-type E. coli CFT073 (92.7% ± 16.2% of wild-type adherence, P = 0.683) (Fig. 2). However, a double mutant in ΔydiV and ΔsdiA remained more adherent than the wild type to the bladder epithelial cell line (187.3% ± 16.4%, P = 0.013).
Since SlyB appeared to be absent in the fimbrial preparation of the ΔydiV mutant, a single deletion (ΔslyB) mutant and a double mutant with ΔydiV were constructed to determine if any of the phenotypes observed were due to the absence of SlyB. Deletion of slyB (ΔslyB), however, had no effect on adherence to bladder epithelial cells, demonstrating that the effect of ΔydiV on adherence is not an indirect effect due to loss of SlyB (Fig. 2). Likewise, the double deletion (ΔydiV ΔslyB) strain remained more adherent than the wild type, again demonstrating that the increased adherence to the bladder cell line results solely from the absence of ydiV.
ΔydiV, ΔydiV Δcrp, and ΔydiV Δcya specifically affect expression of papA_2 but not papA.
Since there are two pap operons in the genome of E. coli CFT073 and the mass spectrometry data demonstrated that PapA_2 was overexpressed in the ΔydiV mutant, the effect of each deletion on gene expression of each pap operon was determined. Briefly, RNA was extracted from overnight static LB cultures, and gene expression was assessed by qPCR using operon-specific primers. Compared to wild-type E. coli CFT073, there were no differences in papA expression for ΔydiV, Δcrp, Δcya, ΔydiV Δcrp, and ΔydiV Δcya mutants (data not shown). However, papA_2 was 2.36-fold higher in the ΔydiV strain (P = 0.0027) than in wild-type E. coli CFT073, and as was observed in the adherence assays, deletion of either crp or cya restored papA_2 expression to wild-type levels. Together, finding that the increased expression of Pap_2 and the increased adherence, in the absence of YdiV, can be restored to wild-type levels by mutation of either crp or cya suggests that the YdiV effect on adherence may result from a role in controlling or responding to cAMP levels in the cell.
Neither sdiA, cya, nor crp affects ydiV expression.
A previous study in E. coli K-12 found that SdiA activates expression of ydiV (24). Therefore, we determined by qPCR if ydiV expression was altered in the ΔsdiA mutant compared to wild-type E. coli CFT073. ydiV expression, however, was not significantly different in the ΔsdiA strain ([0.867 ± 0.158]-fold wild-type levels, P = 0.487). Likewise, deletion of cya or crp had no effect on ydiV expression (Δcya strain, [0.906 ± 0.222]-fold wild-type levels, P = 0.712; and Δcrp strain, [0.986 ± 0.209]-fold, P = 0.954). Therefore, in E. coli CFT073, the expression of ydiV is not affected by CAP-cAMP or SdiA. Thus, it is likely that YdiV acts to affect cAMP or cAMP-CRP levels rather than cAMP or cAMP-CRP acting to control ydiV expression.
Deletion of both ydiV and sdiA decreases crp and cya expression.
To determine if YdiV affects transcription of the pap_2 operon by affecting the expression of crp or cya, expression of these genes was measured by qPCR in CFT073 ΔydiV, ΔsdiA, and ΔydiV ΔsdiA. Expression of crp was also analyzed in CFT073 Δcya and ΔydiV Δcya, whereas cya expression was also analyzed in CFT073 Δcrp and ΔydiV Δcrp. Expression in CFT073 ΔydiV of crp ([0.733 ± 0.165]-fold, P = 0.25) or cya ([0.782 ± 0.066]-fold, P = 0.08) was not significantly different from wild-type expression. Likewise, no single gene deletion tested significantly modulated crp or cya expression. However, the double deletion ΔydiV ΔsdiA reduced expression of crp to (0.554 ± 0.011)-fold wild-type levels (P = 0.0006), and cya to (0.580 ± 0.082)-fold wild-type levels (P = 0.036). This result is consistent with the observation by Zhou et al. (24) that ΔydiV ΔsdiA reduces intracellular cAMP concentration about 2-fold in E. coli K-12.
YdiV affects class II flagellar gene expression.
Several reports of the effect of YdiV on motility have demonstrated that YdiV is a repressor of flagellar biosynthesis in Salmonella and is a cryptic repressor of flagellar biosynthesis in E. coli K-12 (15, 18, 19). We recently reported, as previously observed in Salmonella, that deletion of ydiV increases motility (11). In S. Typhimurium, YdiV affects class II flagellar gene expression by binding to FlhD, inhibiting the master regulatory complex FlhD4C2 from activating the expression of FliA (15). In E. coli CFT073, YdiV reduces FliC expression at the protein level (Fig. 3B). To determine if YdiV affects flagellar biosynthesis by controlling transcript levels, we conducted qPCR of flhD (class I), fliA (class II), and fliC (class III) in CFT073 ΔydiV, ΔydiV(pYdiV), ΔydiV(pBAD-ydiV) induced with 5 mM arabinose, and wild-type E. coli CFT073. There were no differences in expression of flhD between the strains; however, the ΔydiV strain expressed 83.4-fold more fliA transcript than did the wild type (P = 0.0009), and when ydiV was overexpressed, fliA expression was reduced to 0.023-fold wild-type levels (P = 0.0003). Consistent with the Western blot and motility data, expression of fliC was increased in the ΔydiV mutant (7.0-fold, P = 0.0455) and reduced both in CFT073 ΔydiV(pYdiV) (0.2-fold, P = 0.0021) and when YdiV was overexpressed (0.022-fold, P < 0.0001). Thus, YdiV inhibits motility by reducing expression of the sigma factor FliA, thereby reducing fliC expression independent from transcription of the class I flhD promoter.
Fig 3.
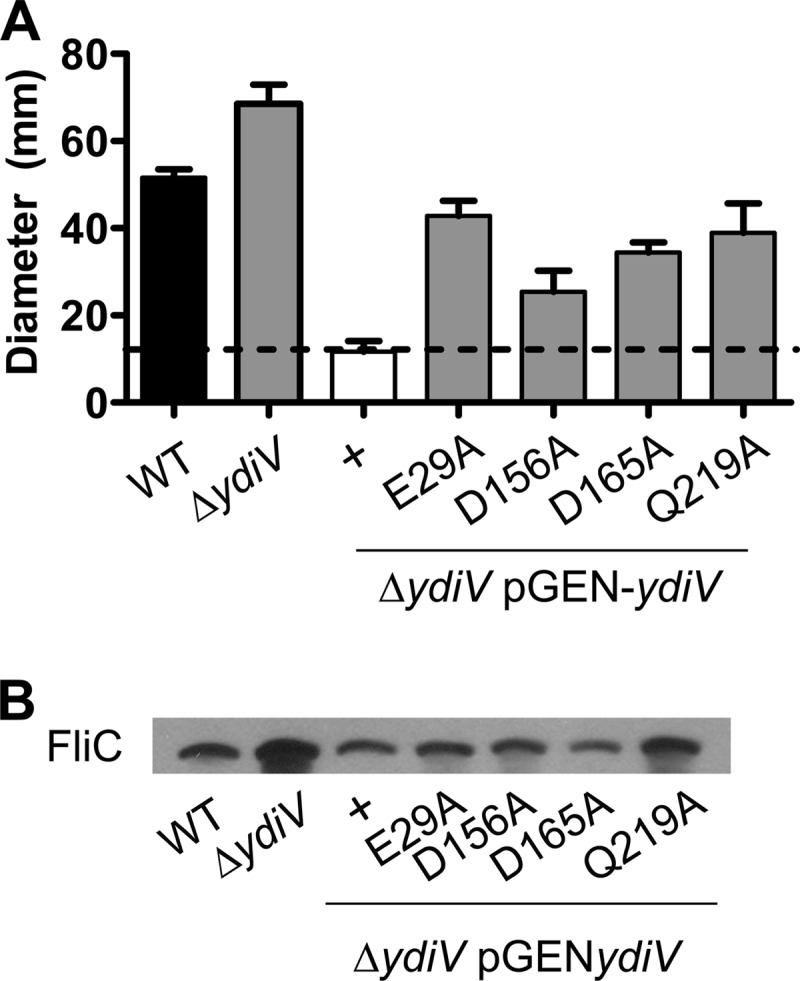
Analysis of effects of conserved YdiV residues E29, D156, D165, and Q219 on motility and flagellin production. (A) Swimming motility diameter of wild-type E. coli CFT073, the ΔydiV mutant, the ΔydiV mutant complemented with wild-type protein (pYdiV), and site-directed mutants (E29A, D156A, D165A, and Q219A) cultured at 30°C in soft LB agar plates for 16 h. Data are averages of three independent experiments performed in triplicate. Error bars indicate standard errors of the means. For all data shown, P values are <0.05 as assessed by Student's t test. (B) Western blot of flagellin (FliC) from E. coli CFT073 wild-type (WT), ΔydiV, and ΔydiV complemented with wild-type protein (pYdiV), or site-directed mutants (E29A, D156A, D165A, and Q219A). Whole-cell lysates were subjected to SDS-PAGE and Western blotting using rabbit polyclonal antiserum to H1 flagella. FliC indicates the predicted electrophoretic mobility of flagellin. All cultures were diluted to an OD600 of 0.30 before the samples were boiled to ensure equal loading of protein.
Four site-directed mutants of YdiV affect motility.
To determine residues that may be involved in the inhibition of motility by YdiV, site-directed mutagenesis of the conserved Mg2+ binding residues E29A (the E in the ELI motif), E116A, and Q219A, and the conserved c-di-GMP-binding residue D165A were constructed, along with three 100% conserved polar residues (S11, K125, and D156) from an alignment of YdiV with 20 homologs from other members of the Enterobacteriaceae (see Fig. S1 in the supplemental material). As a control, E55, a residue that is not conserved, was also replaced with alanine. These constructs were used to complement the ΔydiV mutant, and motility assays were conducted (Fig. 3A). The entire set of site-directed mutants was less motile than either wild-type (pGEN) or the CFT073 ΔydiV(pGEN) controls, suggesting that these residues partially contribute to the function of the protein. In addition, compared to CFT073 ΔydiV(pYdiV) (+), the mutants complemented with pYdiV-E29A, pYdiV-D156A, pYdiV-D165A, or pYdiV-Q219A were significantly more motile (2.17- to 3.66-fold more motile; P < 0.05) (Fig. 3A), while complementation of CFT073 ΔydiV with four other mutants, pYdiV-S11A, pYdiV-E55A, pYdiV-E116A, or pYdiV-K125A, had no effect on motility (data not shown).
Western blots using antisera specific for H1 flagellin (FliC) demonstrated that the mutants complemented with pYdiV-E29A or -Q219A both produced more flagellin than CFT073 ΔydiV(pYdiV) (Fig. 3B). These two site-directed mutations with the largest effects on motility and FliC production, E29A and Q219A, were tested for dominant negative phenotypes in a wild-type background. The diameter of swimming motility in the wild-type background carrying cloned YdiV [E. coli CFT073(pYdiV)] was 5.2 ± 0.4 mm and was increased to 6.5 ± 1.4 mm by pYdiV-Q219A and 9.5 ± 1.0 mm by pYdiV-E29A. Thus, the E29A mutation and, to a lesser extent, the Q219A mutation are dominant negative to YdiV, as the mutant proteins affect the ability of YdiV to inhibit motility because wild-type E. coli CFT073 bearing either pYdiV-E29A (P < 0.0001) or pYdiV-Q219A (P = 0.0464) was significantly more motile than wild-type E. coli CFT073 bearing pYdiV. Interestingly, only mutations of the known Mg2+-binding residues within YdiV (E29 and Q219) affected the proteins' ability to inhibit motility and reduce FliC production, while mutation of c-di-GMP binding residues in YdiV did not affect FliC production.
The residues of YdiV important for motility have no effect on adherence.
Complementation of the ΔydiV mutant with pYdiV restored adherence to bladder epithelial cells (135% ± 21% of wild-type, P = 0.123) to wild-type levels. We therefore examined the effect of the site-directed mutants of YdiV on adherence to the bladder epithelial cell line UM-UC-3 to determine if the residues found to be involved in regulation of motility are also necessary to regulate P-fimbrial adherence to host cells. Surprisingly, all of the site-directed mutants of YdiV (S11A, E29A, E55A, E116A, K125A, D156A, D165A, and Q219A) complemented adherence (data not shown). Thus, YdiV may function through different mechanisms to affect adherence and motility.
Motility assays in soft Dulbecco's modified Eagle medium (DMEM) agar were conducted to determine whether the medium accounted for the differential effect of the site-directed mutants on motility and adherence to epithelial cells. In soft DMEM agar, wild-type E. coli CFT073 displayed reduced motility (12.9 ± 2.8 mm). The ΔydiV mutant had significantly increased motility compared to wild-type E. coli CFT073 in soft DMEM agar, to 23.7 ± 2.0 mm (P < 0.0001), and CFT073 ΔydiV(pYdiV) had reduced motility, further than the wild-type strain (2.9 ± 0.6 mm, P = 0.0019). As observed in regular motility agar, substitution of E29 (P = 0.0163), D156 (P = 0.0202), D165 (P = 0.0162), and Q219 (P = 0.0154) with alanine significantly increased motility. Thus, the change in medium does not account for the differential effects of the site-directed mutants of YdiV on sessility and motility. Intriguingly, the Mg2+-binding residues appeared to have a greater effect than the c-di-GMP residues, as also suggested by Western blotting results and soft agar motility assays (Fig. 3A).
The effect of YdiV on motility does not occur via SlyB, CAP-cAMP, or SdiA.
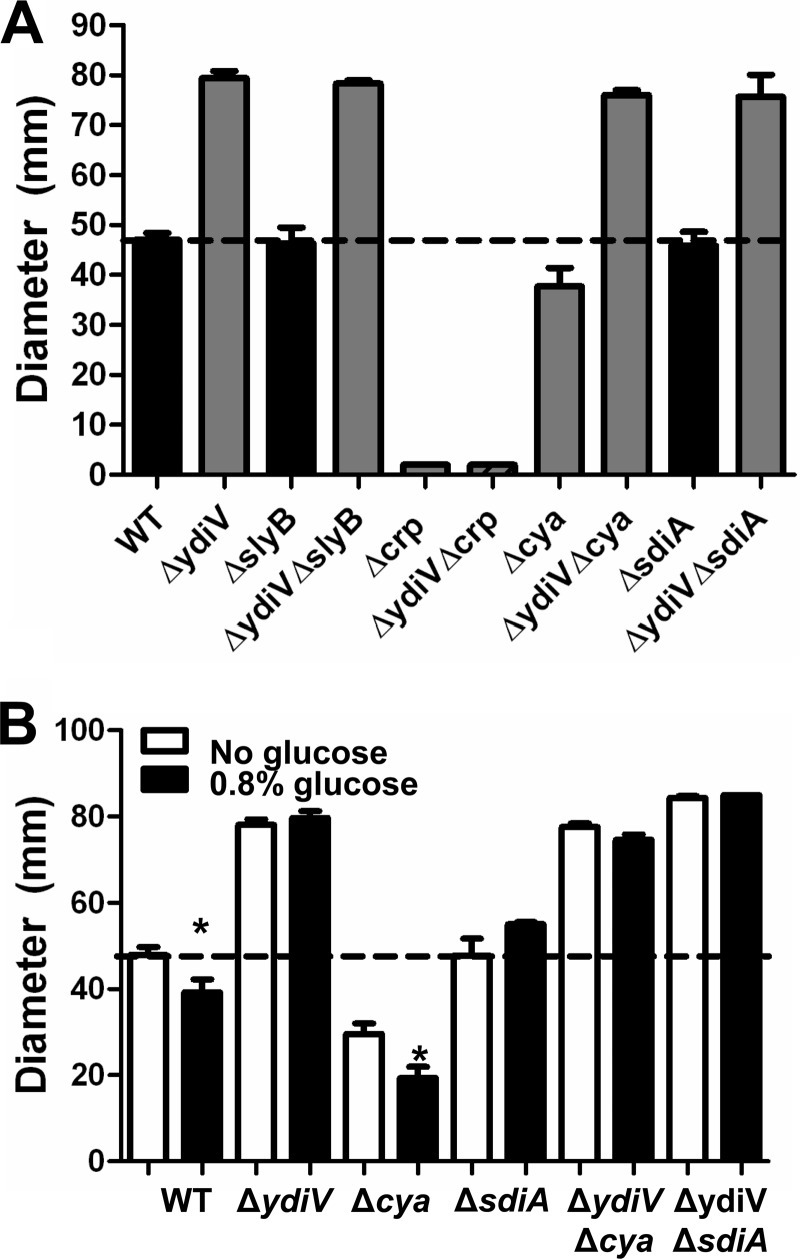
Since CAP-cAMP has been implicated in the regulation of motility by activation of the flhD operon (25), we determined if either CAP or Cya is involved in the effect of YdiV on motility. Motility is abolished in the Δcrp strain, as previously observed (25), due to the absence of FlhD, in both the wild-type E. coli CFT073 and the ΔydiV backgrounds (Fig. 4A). While not exhibiting as severe of a phenotype, the Δcya mutant (37.7 ± 3.7 mm) was less motile than the wild type (47.0 ± 1.4 mm, P = 0.0094) but had no significant effect in the ΔydiV background (ΔydiV strain, 79.4 ± 1.4 mm; ΔydiV Δcya strain, 76.0 ± 1.0 mm; P = 0.071 [Fig. 4A]). Finding that the hypermotility caused by the absence of YdiV is dependent on crp but is independent of adenylate cyclase suggests that cAMP is not required for CAP activation of flhD or that cya is dispensable for cAMP production in the ydiV mutant bacteria.
Fig 4.
YdiV affects motility independent of glucose at a step in flagellar biosynthesis downstream from CAP activation of flhDC. (A) Swimming motility diameter of wild-type E. coli CFT073 and the ΔydiV, ΔslyB, Δcrp, Δcya, ΔsdiA, ΔydiV ΔslyB, ΔydiV Δcrp, ΔydiV Δcya, and ΔydiV ΔsdiA mutants, cultured at 30°C in soft LB agar plates for 16 h. Data are averages of three independent experiments performed in triplicate. Error bars indicate standard errors of the means. Gray bars have a P value of <0.05 compared to the wild type as assessed by Student's t test. (B) Addition of excess glucose (0.8%) to motility agar decreases swimming motility in wild-type E. coli CFT073 and the Δcya mutant, but the ΔydiV, ΔsdiA, ΔydiV Δcya, and ΔydiV ΔsdiA strains are glucose insensitive. Data are averages of three independent experiments performed in triplicate. Error bars indicate standard errors of the means. *, P value < 0.05 as assessed by Student's t test.
SlyB is encoded by an operon downstream of slyA. In a previous study, the ΔslyA mutation was found to confer increased motility in E. coli CFT073, similar to what was observed with ΔydiV (13). Therefore, we hypothesized that the ΔslyB mutant would be more motile than the wild type. However, ΔslyB had no effect on motility. Furthermore, the ΔydiV ΔslyB strain is not significantly different from the ΔydiV strain, demonstrating that SlyB is not involved in the regulation of motility.
Deletion of sdiA was shown to increase motility in enterohemorrhagic E. coli O157:H7 (26). However, in E. coli CFT073, the ΔsdiA mutant had no motility defect, and the ΔydiV ΔsdiA mutant was not significantly different in motility from the ΔydiV mutant, demonstrating that SdiA is not involved in the regulation of motility in E. coli CFT073.
YdiV affects motility downstream of glucose import and adenylate cyclase.
Since glucose was reported to reduce ydiV expression (24) and we found that crp but not cya is required for hypermotility in ydiV mutant bacteria, we hypothesized that addition of 0.8% glucose to motility medium would reduce the expression of ydiV in wild-type E. coli CFT073, thus increasing motility as observed in the ΔydiV mutant. As expected, addition of glucose, which would decrease cAMP levels by decreasing adenylate cyclase activity, had no effect on the motility of the ΔydiV mutant, which swam with a diameter of 78.1 ± 1.3 mm in the absence and 79.7 ± 1.5 mm in the presence of excess glucose (P = 0.442) (Fig. 4B). As expected, motility was significantly reduced in the wild type, by 8.6 ± 3.5 mm (P = 0.026), since glucose would limit CAP-cAMP activation of the flhD promoter. Furthermore, while the Δcya mutant responds to addition of glucose similarly to wild-type E. coli CFT073, the ΔydiV Δcya mutant is glucose insensitive. Interestingly, ΔsdiA, which has no effect on motility in regular soft agar, also confers glucose insensitivity. Similar to what is observed in regular soft agar plates, motility in the ΔydiV ΔsdiA strain is not significantly different from that in the ΔydiV strain and is also glucose insensitive (Fig. 4B). Together, these results support the hypothesis that cya is dispensable for cAMP production in the ydiV mutant bacteria and demonstrate that cAMP is required for CAP activation of flhD when YdiV is present.
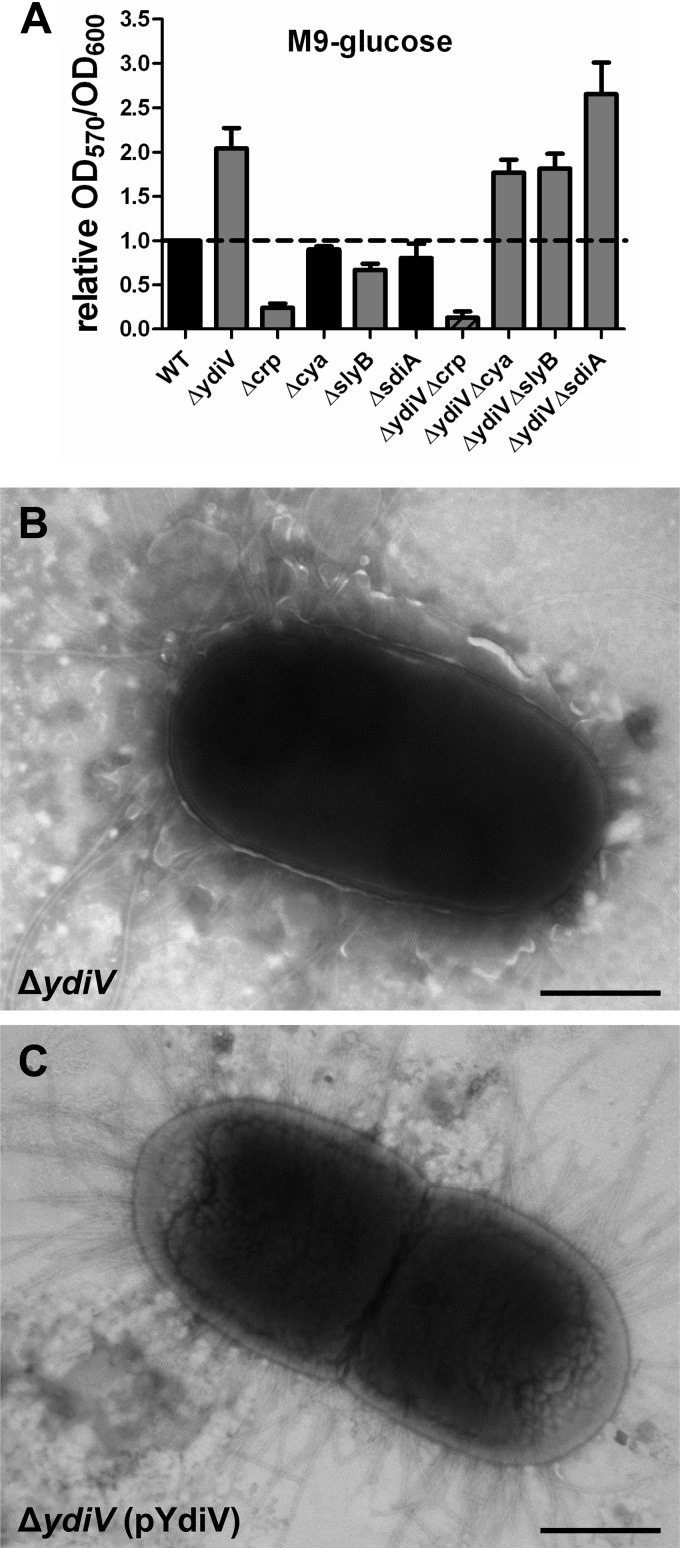
The ΔydiV mutant overproduces an extracellular matrix under both aerated and static culture conditions.
Similarly, mutation of ydiV increases biofilm formation in CFT073 (Fig. 5A). As seen with motility, this increase in biofilm is insensitive to glucose, suggesting that YdiV acts downstream from cAMP-CRP. In the absence of CAP (Δcrp), biofilm formation is abrogated in wild-type bacteria and in the ydiV mutant (Fig. 5A). Mutation of adenylate cyclase (Δcya) did not affect biofilm formation in CFT073 or in the ydiV mutant. Interestingly, mutation of sdiA in the ydiV mutant increased biofilm formation above what was observed with the ydiV single mutant (Fig. 5A). In both aerated mid-exponential-phase cultures and overnight static cultures, the ΔydiV mutant was observed under transmission electron microscopy to produce an extracellular matrix (a representative micrograph is shown in Fig. 5B) not observed in the complemented mutant (a representative micrograph is shown in Fig. 5C). This matrix is not curli or cellulose, as the ΔydiV mutant does not express these polymers (11).
Fig 5.
The ΔydiV mutant produces an extracellular matrix. (A) Biofilm formation in M9 minimal medium with glucose as the sole carbon source. Transmission electron microscopy of negatively stained CFT073 ΔydiV (B) and ΔydiV (pYdiV) (C). Bacteria were cultured for 3 h with aeration at 37°C in LB. Bar, 1.0 μm.
Deletion of ydiV reduces colonization of the upper urinary tract.
Since ydiV is involved in regulating several phenotypes related to virulence, we determined the effect of deletion of this gene on uropathogenesis in vivo in the mouse model of ascending UTI (n = 10 mice). When in direct competition with wild-type CFT073, the ΔydiV mutant was less fit in the kidneys of mice (8.7-fold, P = 0.0166). There was no significant difference from wild-type levels in bladder colonization (P = 0.8469). Therefore, YdiV contributes to successful in vivo colonization of the upper urinary tract.
DISCUSSION
YdiV, a versatile protein involved in repression of both motility and sessility, contributes to the control of motility and P fimbria-mediated adherence in E. coli CFT073. Here, we demonstrate that in E. coli CFT073, a prototypical pyelonephritis isolate, YdiV uniquely inhibits adherence to uroepithelial cells by suppressing expression of P fimbriae (Fig. 6). Furthermore, we demonstrate that site-directed mutations in YdiV that affect the inhibition of motility do not affect the inhibition of adherence phenotypes, suggesting that different domains of the protein are utilized in regulation of these competing phenomena.
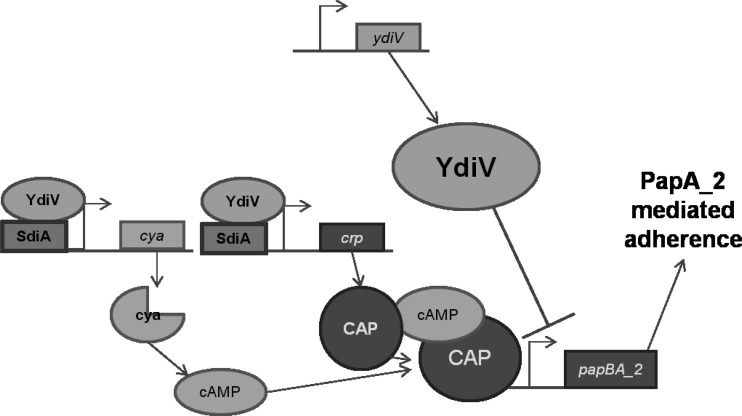
Fig 6.
Model for the cAMP-dependent YdiV adherence regulatory network. YdiV inhibits expression of the pap_2 operon. Together with SdiA, YdiV activates transcription of cya and crp. Cya produces cAMP, which binds to CAP (the product of crp), and CAP-cAMP activates transcription of the pap_2 operon.
The mechanism by which YdiV inhibits motility in S. Typhimurium and E. coli K-12 has been well established; YdiV binds to FlhD, causing a decrease in fliA transcription (15, 16, 18). In E. coli CFT073, YdiV similarly inhibits transcription of fliA and fliC without affecting flhD expression, causing a reduction in motility by reducing expression of flagella. These findings are consistent with other models, suggesting that YdiV inhibits motility by the same mechanism elucidated for E. coli K-12 and Salmonella. However, there are strain differences between E. coli K-12 and CFT073. In one study of E. coli K-12, deletion of ydiV does not affect motility, since this strain does not naturally express ydiV (18), whereas in E. coli CFT073, the ΔydiV mutant is significantly more motile than the wild-type strain (references 11 and 13 and this study). In E. coli K-12, one study has proposed that ydiV expression is inhibited posttranscriptionally by the translational start site being occluded due to the formation of a secondary structure in the mRNA (18). However, by alignment of the intergenic region between ydiV and the closest gene upstream in the E. coli K-12 and CFT073 genomes, we found only one base pair difference between the two strains.
Although predicted to be a degenerate phosphodiesterase, three of the four site-directed mutations in YdiV that affected motility and FliC production to the greatest extent in E. coli CFT073 were in residues conserved within the EAL domain that are putatively required for Mg2+ binding (E29 and Q219) (27), while c-di-GMP-binding residues (D156 and D165) affected FliC production and motility to a lesser extent. However, D156 and D165 both flank the hydrophobic region necessary for interaction with FlhD (19) and thus may be involved in direct interaction or stabilization of the interacting α-helix. E29 and Q219 appear to be located in the β-sheets that make up the TIM-barrel-like central core of YdiV (19), and therefore, substitution of these residues with alanine possibly changes the structural integrity of the protein, causing it to be less able to bind FlhD. However, the same residues are not important for inhibiting adherence to bladder epithelial cells, demonstrating that a different domain of YdiV from that required to modulate motility is involved in this phenotype.
YdiV affects adherence to bladder epithelial cells by repressing fimbriae expressed on the cell surface in liquid culture. Specifically, YdiV represses the expression of one of the two P fimbrial operons present in the CFT073 genome (28), as the ΔydiV mutant has increased levels of papA_2 gene expression and PapA_2 protein compared to wild-type E. coli CFT073. Furthermore, deletion of either crp or cya restores the ΔydiV mutant to wild-type levels of both adherence and papA_2 gene expression, and since CAP-cAMP is known to be a direct activator of the pap operon, these data confirm that YdiV represses P fimbrial expression (Fig. 6). While in E. coli K-12 YdiV has not been implicated in regulation of adhesins, in S. Typhimurium, YdiV has been suggested to regulate CsgD and therefore curli biosynthesis (17), again demonstrating that pathogenic Enterobacteriaceae have evolved to utilize a common protein, YdiV, for regulation of virulence factors.
Although SdiA has previously been described as an activator of ydiV gene expression in E. coli K-12 (24), deletion of sdiA had no effect on ydiV transcript levels. Similarly, deletion of crp and cya does not affect ydiV gene expression. However, the double deletion ΔydiV ΔsdiA significantly reduced the expression of cya, the gene encoding adenylate cyclase, and crp, the gene encoding CAP, 2-fold. Thus, YdiV and SdiA are involved in activation of gene expression of CAP and the enzyme that synthesizes cAMP (Fig. 6), which is consistent with the observation by Zhou et al. (24) that ΔydiV ΔsdiA reduced intracellular cAMP concentration about 2-fold in E. coli K-12. Furthermore, the reduction in crp and cya expression is consistent with the observation that the ΔydiV ΔsdiA mutant, while hyperadherent compared to wild-type E. coli CFT073, is less adherent than the ΔydiV mutant, just as though the level of CAP and cAMP was reduced.
YdiV, a protein encoded by all E. coli strains tested thus far, is a repressor of flagellar motility and P fimbrial expression in the uropathogenic strain E. coli CFT073 (Fig. 6). While present in both pathogenic and commensal strains of E. coli, this protein has apparently taken on additional regulatory roles in the pathogenic strain, as the regulation of P fimbriae demonstrates. Future studies must be conducted to determine if YdiV interacts directly with the pap operon promoter or, as in the case of the regulation of motility, YdiV acts as an anti-transcription factor binding to regulatory proteins necessary for the expression of P fimbriae. Alternatively or in addition, our findings suggest that degenerate EAL domain proteins like YdiV may specifically function through an effect on cAMP levels rather than from c-di-GMP.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Public Health Service grants AI059722 and DK094777 from the National Institutes of Health.
Footnotes
Published ahead of print 10 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02254-12.
REFERENCES
- 1. Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. U. S. A. 104:16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane MC, Lockatell V, Monterosso G, Lamphier D, Weinert J, Hebel JR, Johnson DE, Mobley HL. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73:7644–7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Welch RA, Burland V, Plunkett G, III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane MC, Mobley HL. 2007. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 72:19–25 [DOI] [PubMed] [Google Scholar]
- 5. Pecha B, Low D, O'Hanley P. 1989. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J. Clin. Invest. 83:2102–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johanson I, Lindstedt R, Svanborg C. 1992. Roles of the pap- and prs-encoded adhesins in Escherichia coli adherence to human uroepithelial cells. Infect. Immun. 60:3416–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braaten BA, Platko JV, van der Woude MW, Simons BH, de Graaf FK, Calvo JM, Low DA. 1992. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 89:4250–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weyand NJ, Braaten BA, van der Woude M, Tucker J, Low DA. 2001. The essential role of the promoter-proximal subunit of CAP in pap phase variation: Lrp- and helical phase-dependent activation of papBA transcription by CAP from −215. Mol. Microbiol. 39:1504–1522 [DOI] [PubMed] [Google Scholar]
- 9. Hoschutzky H, Lottspeich F, Jann K. 1989. Isolation and characterization of the alpha-galactosyl-1,4-beta-galactosyl-specific adhesin (P adhesin) from fimbriated Escherichia coli. Infect. Immun. 57:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simm R, Morr M, Kader A, Nimtz M, Romling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 11. Spurbeck RR, Tarrien RJ, Mobley HL. 2012. Enzymatically active and inactive phosphodiesterases and diguanylate cyclases are involved in regulation of motility or sessility in Escherichia coli CFT073. mBio 3(5):e00307–12. 10.1128/mBio.00307-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lane MC, Simms AN, Mobley HL. 2007. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J. Bacteriol. 189:5523–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simms AN, Mobley HL. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J. Bacteriol. 190:3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takaya A, Erhardt M, Karata K, Winterberg K, Yamamoto T, Hughes KT. 2012. YdiV: a dual function protein that targets FlhDC for ClpXP-dependent degradation by promoting release of DNA-bound FlhDC complex. Mol. Microbiol. 83:1268–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wada T, Morizane T, Abo T, Tominaga A, Inoue-Tanaka K, Kutsukake K. 2011. EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:1600–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wozniak CE, Lee C, Hughes KT. 2009. T-POP array identifies EcnR and PefI-SrgD as novel regulators of flagellar gene expression. J. Bacteriol. 191:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simm R, Remminghorst U, Ahmad I, Zakikhany K, Romling U. 2009. A role for the EAL-like protein STM1344 in regulation of CsgD expression and motility in Salmonella enterica serovar Typhimurium. J. Bacteriol. 191:3928–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wada T, Hatamoto Y, Kutsukake K. 2012. Functional and expressional analyses of the anti-FlhD4C2 factor gene ydiV in Escherichia coli. Microbiology 158:1533–1542 [DOI] [PubMed] [Google Scholar]
- 19. Li B, Li N, Wang F, Guo L, Huang Y, Liu X, Wei T, Zhu D, Liu C, Pan H, Xu S, Wang HW, Gu L. 2012. Structural insight of a concentration-dependent mechanism by which YdiV inhibits Escherichia coli flagellum biogenesis and motility. Nucleic Acids Res. 40:11073–11085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper LA, Simmons LA, Mobley HL. 2012. Involvement of mismatch repair in the reciprocal control of motility and adherence of uropathogenic Escherichia coli. Infect. Immun. 80:1969–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. 2011. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect. Immun. 79:4753–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson DE, Lockatell CV, Hall-Craigs M, Mobley HL, Warren JW. 1987. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J. Urol. 138:632–635 [DOI] [PubMed] [Google Scholar]
- 24. Zhou X, Meng X, Sun B. 2008. An EAL domain protein and cyclic AMP contribute to the interaction between the two quorum sensing systems in Escherichia coli. Cell Res. 18:937–948 [DOI] [PubMed] [Google Scholar]
- 25. Helmann JD. 1991. Alternative sigma factors and the regulation of flagellar gene expression. Mol. Microbiol. 5:2875–2882 [DOI] [PubMed] [Google Scholar]
- 26. Sharma VK, Bearson SM, Bearson BL. 2010. Evaluation of the effects of sdiA, a luxR homologue, on adherence and motility of Escherichia coli O157:H7. Microbiology 156:1303–1312 [DOI] [PubMed] [Google Scholar]
- 27. Rao F, Yang Y, Qi Y, Liang ZX. 2008. Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: a study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J. Bacteriol. 190:3622–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mobley HL, Jarvis KG, Elwood JP, Whittle DI, Lockatell CV, Russell RG, Johnson DE, Donnenberg MS, Warren JW. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.