Abstract
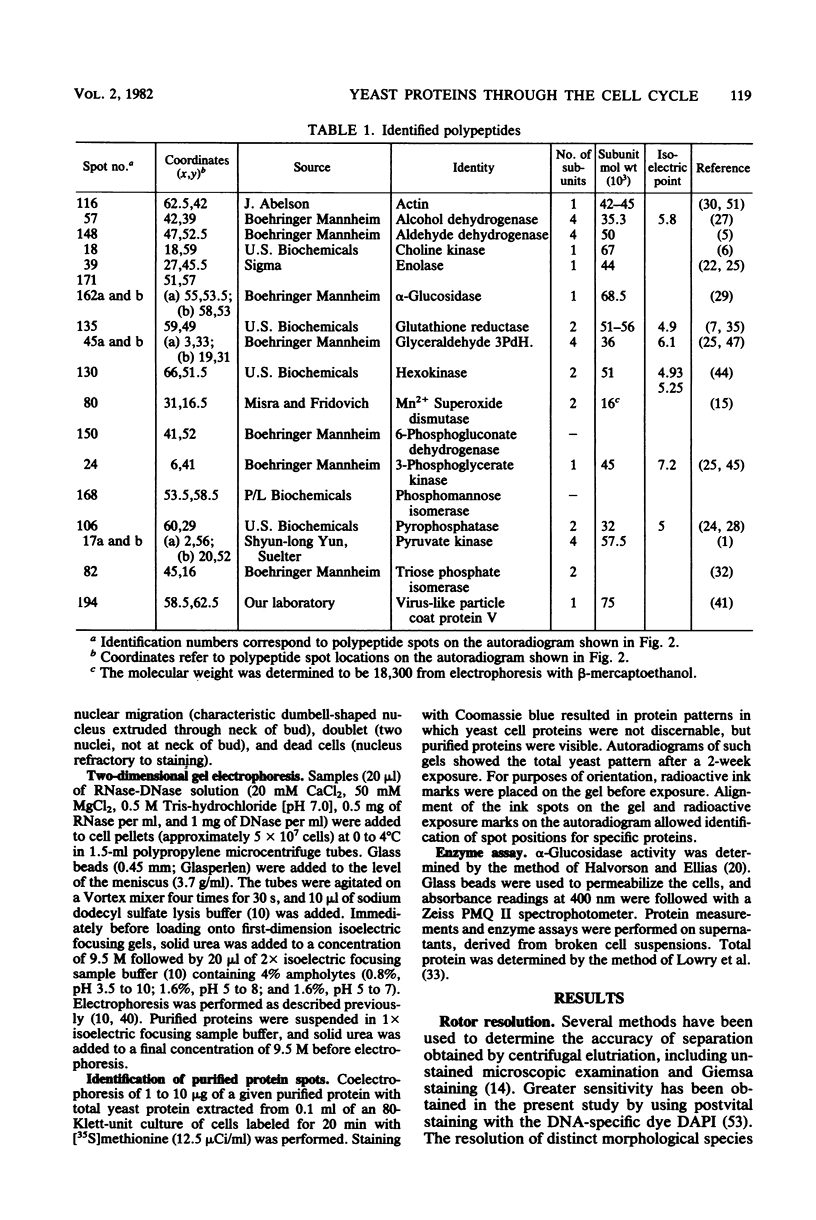
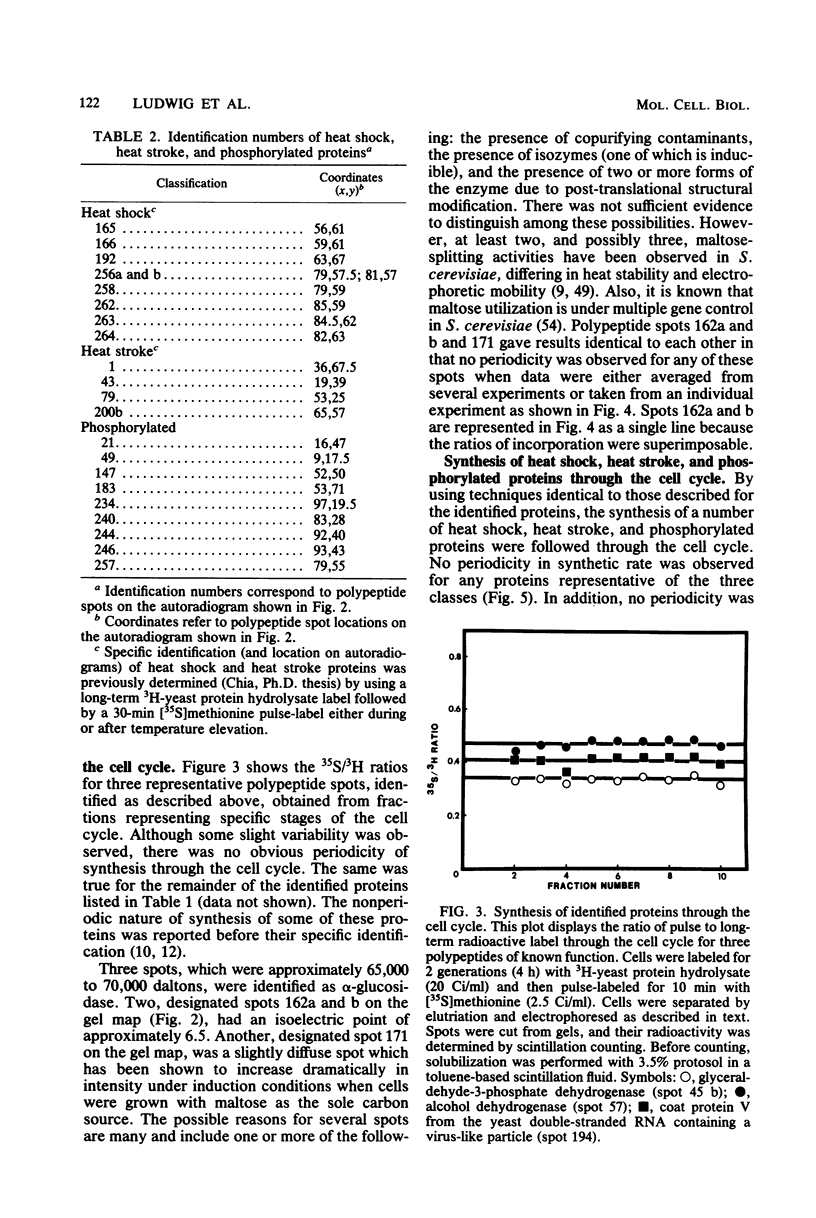
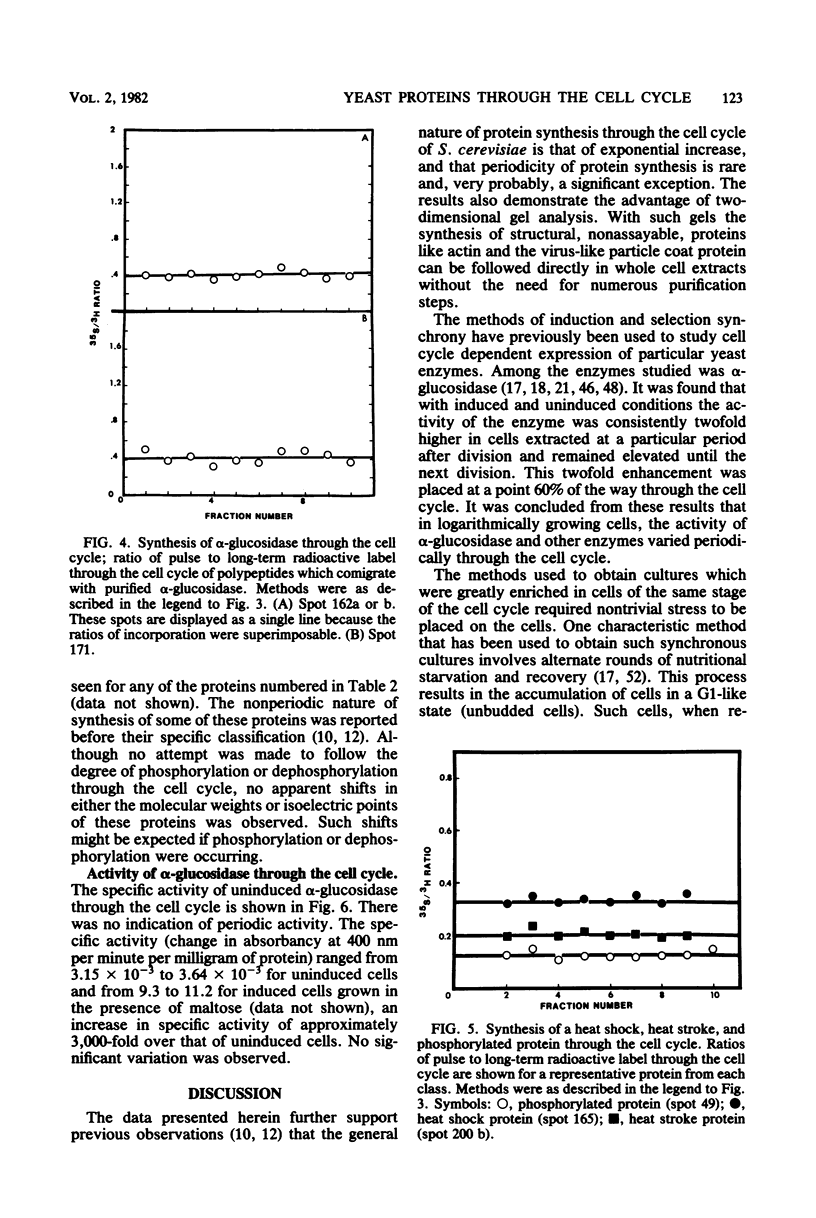
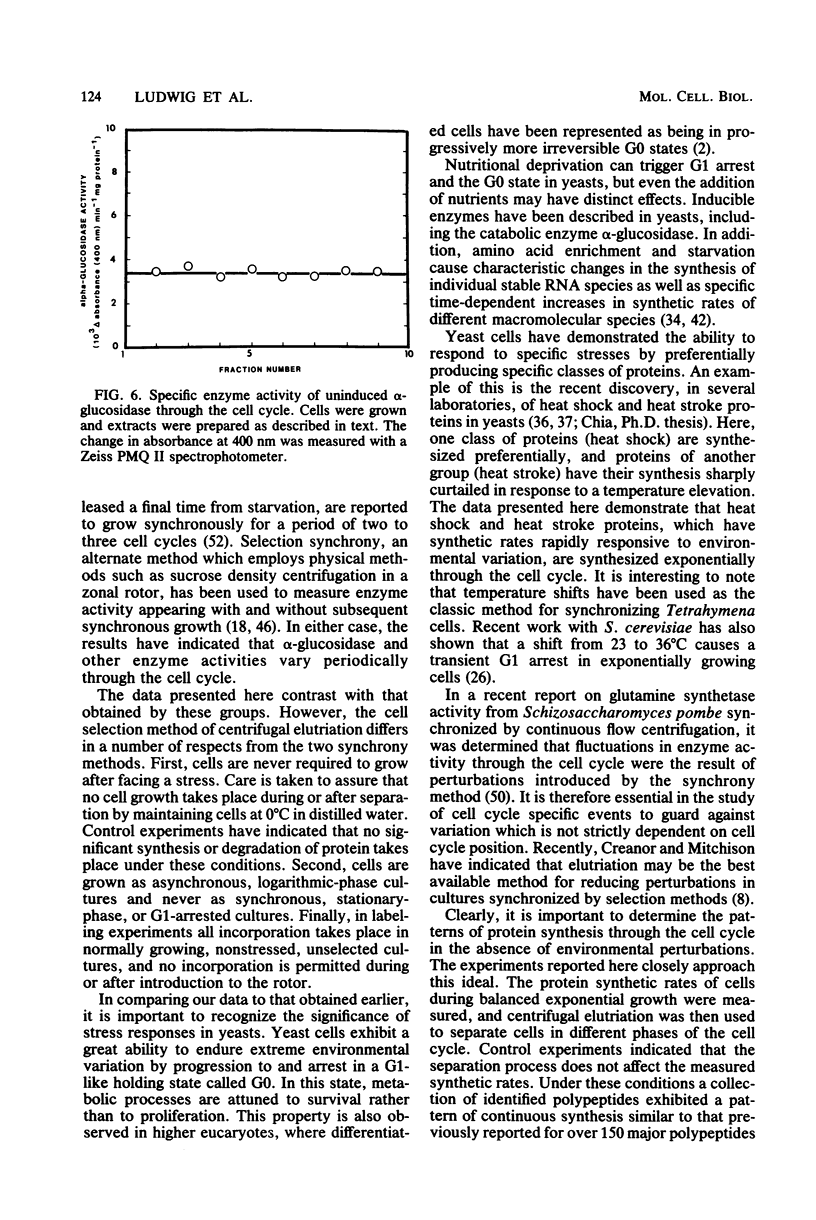
The methods of centrifugal elutriation, two-dimensional gel electrophoresis, and dual isotopic labeling were applied to the study and identification of a number of purified yeast proteins. The location of polypeptide spots corresponding to specific proteins was determined on two-dimensional gels. A dual-label method was used to determine the rates of synthesis through the cell cycle of the identified proteins as well as to confirm the results of previous studies from our laboratory on unidentified proteins. The identified proteins, and the more generally defined phosphorylated, heat shock, and heat stroke proteins were found to follow the general pattern of exponential increase in rate of synthesis through the cell cycle. In addition, colorimetric enzyme activity assays were used to examine the catabolic enzyme alpha-glucosidase (EC 3.2.1.20). Both the activity and synthesis of alpha-glucosidase were found to be nonperiodic with respect to the cell cycle. These data contrast with earlier reports of periodicity, which employed induction and selection synchrony to study enzyme expression through the yeast cell cycle.
Full text
PDF

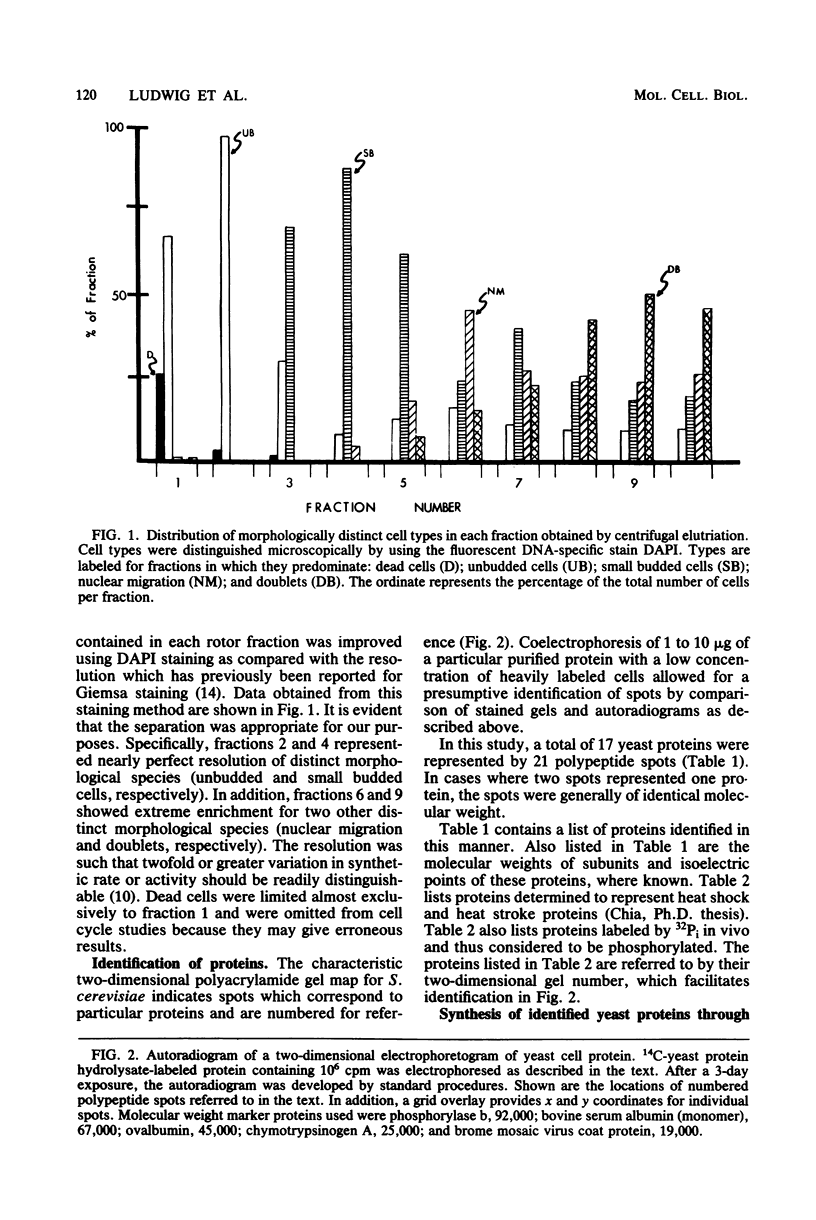


Images in this article
Selected References
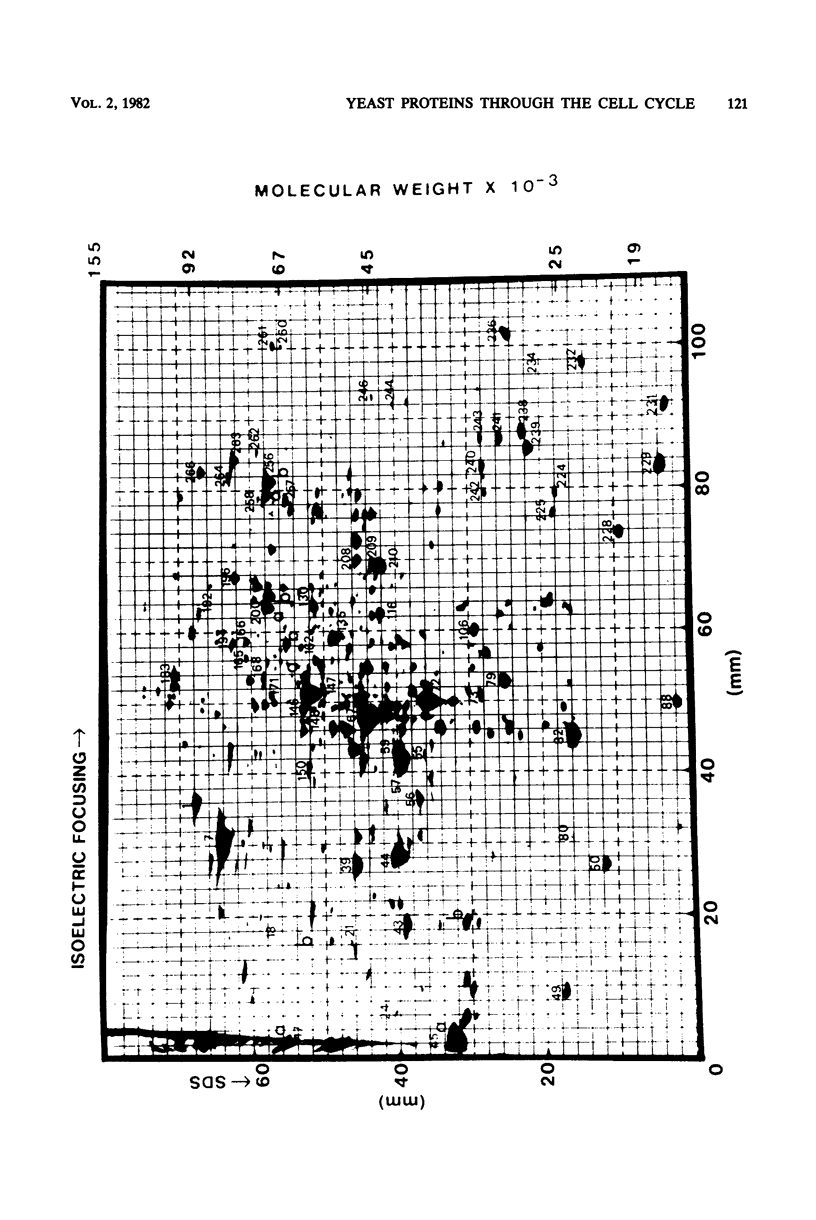
These references are in PubMed. This may not be the complete list of references from this article.
- Aust A. E., Suelter C. H. Homogeneous pyruvate kinase isolated from yeast by two different methods is indistinguishable from pyruvate kinase in cell-free extract. J Biol Chem. 1978 Oct 25;253(20):7508–7512. [PubMed] [Google Scholar]
- Baserga R. Resting cells and the G1 phase of the cell cycle. J Cell Physiol. 1978 Jun;95(3):377–382. doi: 10.1002/jcp.1040950316. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Valenzuela P., Rutter W. J. Phosphorylation of yeast RNA polymerases. Nature. 1976 Jun 3;261(5559):429–431. doi: 10.1038/261429a0. [DOI] [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury S. L., Clark J. F., Steinman C. R., Jakoby W. B. Aldehyde dehydrogenase from baker's yeast. Methods Enzymol. 1975;41:354–360. doi: 10.1016/s0076-6879(75)41079-5. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Browning E. T. Choline kinase from brewers' yeast. Partial purification, properties, and kinetic mechanism. J Biol Chem. 1973 Apr 10;248(7):2364–2371. [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Purification by affinity chromatography of yeast glutathione reductase, the enzyme responsible for the NADPH-dependent reduction of the mixed disulfide of coenzyme A and glutathione. Biochim Biophys Acta. 1977 Oct 13;484(2):268–274. doi: 10.1016/0005-2744(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Eaton N. R., Zimmermann F. K. Thermal inactivation of maltase and its application to temperature-sensitive mutants of yeast. Mol Gen Genet. 1976 Oct 18;148(2):199–204. doi: 10.1007/BF00268385. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Regulation of RNA synthesis in yeast. III. Synthesis during the cell cycle. Mol Gen Genet. 1979 Feb 1;169(3):237–243. doi: 10.1007/BF00382269. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Synthesis and modification of proteins during the cell cycle of the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Mar;137(3):1185–1190. doi: 10.1128/jb.137.3.1185-1190.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. G., Warner J. R., McLaughlin C. S. Synthesis of ribosomal proteins during the cell cycle of the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Feb;137(2):1048–1050. doi: 10.1128/jb.137.2.1048-1050.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. N., Elliott S. C. Fractionation of Saccharomyces cerevisiae cell populations by centrifugal elutriation. J Bacteriol. 1977 Jan;129(1):97–100. doi: 10.1128/jb.129.1.97-100.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goscin S. A., Fridovich I. The purification and properties of superoxide dismutase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Dec 7;289(2):276–283. doi: 10.1016/0005-2744(72)90078-2. [DOI] [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- HALVORSON H., GORMAN J., TAURO P., EPSTEIN R., LABERGE M. CONTROL OF ENZYME SYNTHESIS IN SYNCHRONOUS CULTURES OF YEAST. Fed Proc. 1964 Sep-Oct;23:1002–1008. [PubMed] [Google Scholar]
- Haddox M. K., Magun B. E., Russell D. H. Differential expression of type I and type II cyclic AMP-dependent protein kinases during cell cycle and cyclic AMP-induced growth arrest. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3445–3449. doi: 10.1073/pnas.77.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O., Carter B. L., Tauro P. Synthesis of enzymes during the cell cycle. Adv Microb Physiol. 1971;6(0):47–106. [PubMed] [Google Scholar]
- Hargrave P. A., Wold F. Studies on yeast enolase. Quantitative end group analyses and the effect of exopeptidase digestion. J Biol Chem. 1971 May 10;246(9):2904–2909. [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Sterner R., Noyes C., Cooperman B. S., Bruckmann R. H. On the subunit structure of yeast inorganic pyrophosphatase. J Biol Chem. 1973 Apr 10;248(7):2521–2528. [PubMed] [Google Scholar]
- Holland M. J., Holland J. P. Isolation and identification of yeast messenger ribonucleic acids coding for enolase, glyceraldehyde-3-phosphate dehydrogenase, and phosphoglycerate kinase. Biochemistry. 1978 Nov 14;17(23):4900–4907. doi: 10.1021/bi00616a007. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. Ribosomal precursor RNA metabolism and cell division in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1980;178(2):357–360. doi: 10.1007/BF00270484. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Kasho V. N., Avaeva S. M. Isolation of the inorganic pyrophosphatase from brewer's yeast and studies on the localization of this enzyme in brewer's and baker's yeast. Int J Biochem. 1978;9(1):51–56. doi: 10.1016/0020-711x(78)90138-6. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Purification and characterization of maltase and alpha-methyl glucosidase from yeast. Biochim Biophys Acta. 1967 Sep 12;146(1):173–180. doi: 10.1016/0005-2744(67)90084-8. [DOI] [PubMed] [Google Scholar]
- Koteliansky V. E., Glukhova M. A., Bejanian M. V., Surguchov A. P., Smirnov V. N. Isolation and characterization of actin-like protein from yeast Saccharomyces cerevisiae. FEBS Lett. 1979 Jun 1;102(1):55–58. doi: 10.1016/0014-5793(79)80927-8. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K. Triosephosphate isomerase from yeast. Methods Enzymol. 1975;41:434–438. doi: 10.1016/s0076-6879(75)41094-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Oliver S. G., McLaughlin C. S. The regulation of RNA synthesis in yeast II: Amino acids shift-up experiments. Mol Gen Genet. 1977 Dec 30;158(2):117–122. doi: 10.1007/BF00268303. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Stellwagen E. Purification and subunit structure of glutathione reductase from bakers' yeast. J Biol Chem. 1968 Feb 25;243(4):809–814. [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McLaughlin C. S. The regulation of RNA synthesis in yeast. I: Starvation experiments. Mol Gen Genet. 1977 Jul 20;154(2):145–153. doi: 10.1007/BF00330830. [DOI] [PubMed] [Google Scholar]
- Phillips T. A., Bloch P. L., Neidhardt F. C. Protein identifications on O'Farrell two-dimensional gels: locations of 55 additional Escherichia coli proteins. J Bacteriol. 1980 Dec;144(3):1024–1033. doi: 10.1128/jb.144.3.1024-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Colowick S. P. Chemistry and subunit structure of yeast hexokinase isoenzymes. Arch Biochem Biophys. 1973 Oct;158(2):458–470. doi: 10.1016/0003-9861(73)90537-7. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. 3-phosphoglycerate kinase of baker's yeast. Methods Enzymol. 1975;42:134–138. doi: 10.1016/0076-6879(75)42106-1. [DOI] [PubMed] [Google Scholar]
- Sebastian J., Carter B. L., Halvorson H. O. Use of yeast populations fractionated by zonal centrifugation to study the cell cycle. J Bacteriol. 1971 Dec;108(3):1045–1050. doi: 10.1128/jb.108.3.1045-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Mockrin S. C., Koshland D. E., Jr A rapid purification procedure for glyceraldehyde 3-phosphate dehydrogenase from Bakers' yeast. J Biol Chem. 1972 Oct 10;247(19):6277–6279. [PubMed] [Google Scholar]
- Tauro P., Halvorson H. O. Effect of gene position on the timing of enzyme synthesis in synchronous cultures of yeast. J Bacteriol. 1966 Sep;92(3):652–661. doi: 10.1128/jb.92.3.652-661.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., SCOPES A. W. The behaviour of nucleic acids in synchronously dividing cultures of Saccharomyces cerevisiae. Exp Cell Res. 1960 Aug;20:338–349. doi: 10.1016/0014-4827(60)90162-2. [DOI] [PubMed] [Google Scholar]
- WINGE O., ROBERTS C. Identification of the maltase genes in some American haploid and European diploid yeasts. C R Trav Lab Carlsberg Chim. 1955;25(12):331–340. [PubMed] [Google Scholar]
- Water R. D., Pringle J. R., Kleinsmith L. J. Identification of an actin-like protein and of its messenger ribonucleic acid in Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1143–1151. doi: 10.1128/jb.144.3.1143-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]