Abstract
The intracellular bacterial agent of Q fever, Coxiella burnetii, translocates effector proteins into its host cell cytosol via a Dot/Icm type IV secretion system (T4SS). The T4SS is essential for parasitophorous vacuole formation, intracellular replication, and inhibition of host cell death, but the effectors mediating these events remain largely undefined. Six Dot/Icm substrate-encoding genes were recently discovered on the C. burnetii cryptic QpH1 plasmid, three of which are conserved among all C. burnetii isolates, suggesting that they are critical for conserved pathogen functions. However, the remaining hypothetical proteins encoded by plasmid genes have not been assessed for their potential as T4SS substrates. In the current study, we further defined the T4SS effector repertoire encoded by the C. burnetii QpH1, QpRS, and QpDG plasmids that were originally isolated from acute-disease, chronic-disease, and severely attenuated isolates, respectively. Hypothetical proteins, including those specific to QpRS or QpDG, were screened for translocation using the well-established Legionella pneumophila T4SS secretion model. In total, six novel plasmid-encoded proteins were translocated into macrophage-like cells by the Dot/Icm T4SS. Four newly identified effectors are encoded by genes present only on the QpDG plasmid from severely attenuated Dugway isolates, suggesting that the presence of specific effectors correlates with decreased virulence. These results further support the idea of a critical role for extrachromosomal elements in C. burnetii pathogenesis.
INTRODUCTION
Coxiella burnetii is an intracellular bacterial pathogen of humans that causes Q fever. Humans are exposed to infectious C. burnetii by inhalation of contaminated aerosols and typically present with acute symptoms, including high fever and pneumonia (1). Persistent infection can lead to chronic disease commonly presenting as endocarditis, a condition that is difficult to treat with current antibiotics (2). The low numbers of reported Q fever cases have risen in the United States since the disease became notifiable in 1999 (3), and a large outbreak occurred in the Netherlands from 2007 to 2010, highlighting our limited understanding of basic C. burnetii biology and pathogenesis (4, 5). Numerous C. burnetii isolates have been collected from a wide variety of geographic areas, disease scenarios, and hosts, including humans, rodents, and ticks. Isolates are characterized by the type of lipopolysaccharide (LPS) present on the bacterial surface, with organisms in phase I producing full-length LPS and organisms in phase II producing truncated LPS that results in attenuation (6). Isolates from differing sources also possess distinct genome signatures placing them in genomic groups, with members of individual groups causing similar types of disease (7, 8). Additionally, early studies suggested that pathotype differences are linked to the plasmid content of individual isolates (9, 10).
Five C. burnetii plasmids of various sizes and compositions have been reported, and the gene content of three, QpH1, QpRS, and QpDG, is well characterized. The Nine Mile reference isolate represents an acute-disease isolate and contains QpH1, the first C. burnetii plasmid identified (10). Following identification of QpH1, QpRS was isolated from an endocarditis isolate (9) and QpDG was discovered in severely attenuated isolates collected in Dugway, UT, in the 1950s (11, 12). QpDG is ∼54 kbp, QpRS is ∼39 kbp, and QpH1 is ∼37 kbp in length, and each plasmid contains replication and partition genes needed for plasmid propagation. Early work suggested that acute-disease-causing isolates exclusively harbor QpH1 and that chronic-disease isolates contain QpRS (9). However, a later study of 173 isolates from France indicated that this differentiation is not absolute, as QpH1 was not strictly associated with acute-disease isolates (13). Interestingly, two isolates, G and S, were derived from patients with chronic Q fever and lack an extrachromosomal plasmid but have ∼25 kbp of core plasmid gene content integrated into their chromosomes (8, 14). Maintenance of this subset of plasmid genes by G and S supports the hypothesis that some plasmid genes are essential for conserved C. burnetii functions. However, the role of plasmids in C. burnetii biology and host cell parasitism has been a mystery since their discovery 30 years ago.
During growth in host cells, C. burnetii uses a Dot/Icm type IV secretion system (T4SS) to inject effector proteins into the host cytosol, where they direct formation of a parasitophorous vacuole (PV) required for replication and promote host cell survival (15, 16). Shortly following entry into a eukaryotic cell, C. burnetii is encased in a tight-fitting phagosome that ultimately fuses with lysosomes wherein pathogen metabolism is triggered by acidic pH (∼5.0). Doubling in growth every ∼12 h, C. burnetii promotes expansion of the PV via fusion with endosomes, autophagosomes, and lysosomes to accommodate hundreds of replicating organisms (17). In the absence of a functional T4SS, the C. burnetii-containing phagosome transits to a lysosome, but vacuole expansion does not occur and bacteria do not replicate (15, 16). In contrast to other pathogens, C. burnetii is not degraded in the phagolysosome, and when T4SS function is restored, the PV expands and replication ensues (15). Furthermore, T4SS-defective C. burnetii cannot prevent host cell apoptotic death, an event needed for sustaining a prolonged infectious cycle. These observations support a critical role for Dot/Icm substrates in host cell parasitism.
Although the T4SS is essential for intracellular growth, secreted effector function is largely uncharacterized. To date, over 65 Dot/Icm substrates have been identified (16, 18–20), including AnkG and CaeB, effectors that modulate host cell survival (21, 22). Six effectors are encoded by the C. burnetii cryptic plasmid; three are conserved among all C. burnetii isolates, and three are specific to QpH1 (18). However, the possibility of the presence of other effector genes carried by QpRS or QpDG or shared by two plasmids but not conserved among all has not been explored. In the current study, we further examined QpH1, QpRS, and QpDG as representative of acute-disease, chronic-disease, and severely attenuated isolate plasmids, respectively, for genes encoding hypothetical, C. burnetii-specific Dot/Icm substrates. Using the well-established Legionella pneumophila translocation assay (23), we discovered six novel plasmid-specific effectors, four of which are encoded only by attenuated Dugway isolates, implicating distinct T4SS effectors in pathotype virulence.
MATERIALS AND METHODS
Bacterial and eukaryotic cell culture.
C. burnetii Nine Mile phase II, clone 4 (RSA439), and Dugway (7E65-68) organisms were cultured in acidified citrate cysteine medium (ACCM) for 7 days at 37°C, 5% CO2, and 2.5% O2 prior to infecting mammalian cells (24). Organisms were washed three times with sucrose phosphate buffer prior to use. Dugway organisms were handled only in the Centers for Disease Control and Prevention-approved biosafety level 3 facility at the University of Arkansas for Medical Sciences. L. pneumophila strains were cultured on charcoal yeast extract agar plates, and transformant plates contained 10 μg/ml chloramphenicol. For growth of L. pneumophila LELA3118, plates also contained 25 μg/ml kanamycin. L. pneumophila transformations were conducted as previously described (19). Escherichia coli TOP10 cells (Invitrogen) were used for all recombinant DNA procedures. Strain properties are described in Table 1.
Table 1.
Bacteria and plasmids used in this study
| Bacterium or plasmid | Genotype or descriptiona | Reference or source |
|---|---|---|
| C. burnetii strains | ||
| Nine Mile (RSA439) | Phase II, clone 4, Montana, tick, 1936 | 8 |
| Nine Mile (RSA493) | Phase I, Montana, tick, 1936 | 8 |
| K (Q154) | Phase I, Oregon, heart valve, 1976 | 8 |
| Dugway (7E65-68) | Phase I, Utah, rodent, 1958 | 11 |
| L. pneumophila strains | ||
| JR32 | Salt-sensitive isolate of AM511 | 45 |
| LELA3118 | JR32 dotA::Tn903dIIlacZ3118, DotA− Kmr | 45 |
| E. coli strain | ||
| TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pJB2581 | cyaA fusion vector, Cmr | 46 |
| pDHVA0006 | cbua0006 in pJB2581 | 18 |
| pPMA0003 | cbua0003 in pJB2581 | This study |
| pPMA0007 | cbua0007 in pJB2581 | This study |
| pPMA0017 | cbua0017 in pJB2581 | This study |
| pPMA0020 | cbua0020 in pJB2581 | This study |
| pPMA0021 | cbua0021 in pJB2581 | This study |
| pPMA0022 | cbua0022 in pJB2581 | This study |
| pPMA0024 | cbua0024 in pJB2581 | This study |
| pPMA0025 | cbua0025 in pJB2581 | This study |
| pPMA0026 | cbua0026 in pJB2581 | This study |
| pPMA0028 | cbua0028 in pJB2581 | This study |
| pPMA0031 | cbua0031 in pJB2581 | This study |
| pPMA0032 | cbua0032 in pJB2581 | This study |
| pPMA0033 | cbua0033 in pJB2581 | This study |
| pPMA0034 | cbua0034 in pJB2581 | This study |
| pPMKA0014 | cbuka0014 in pJB2581 | This study |
| pPMKA0026 | cbuka0026 in pJB2581 | This study |
| pPMKA0027 | cbuka0027 in pJB2581 | This study |
| pPMKA0028 | cbuka0028 in pJB2581 | This study |
| pPMDA0009 | cbuda0009 in pJB2581 | This study |
| pPMDA0010 | cbuda0010 in pJB2581 | This study |
| pPMDA0011 | cbuda0011 in pJB2581 | This study |
| pPMDA0012 | cbuda0012 in pJB2581 | This study |
| pPMDA0014 | cbuda0014 in pJB2581 | This study |
| pPMDA0020 | cbuda0020 in pJB2581 | This study |
| pPMDA0021 | cbuda0021 in pJB2581 | This study |
| pPMDA0022 | cbuda0022 in pJB2581 | This study |
| pPMDA0023 | cbuda0023 in pJB2581 | This study |
| pPMDA0024 | cbuda0024 in pJB2581 | This study |
| pPMDA0059 | cbuda0059 in pJB2581 | This study |
| pCR2.1-TOPO | TA TOPO vector, Ampr | Invitrogen |
| pENTR-D/TOPO | Gateway entry vector, Kmr | Invitrogen |
| pcDNA6.2/N-EmGFP | N-terminal EmGFP fusion vector, Ampr | Invitrogen |
| GFP-CpeG | EmGFP::cbua0025 | This study |
| GFP-CpeH | EmGFP::cbua0034 | This study |
| GFP-CpeI | EmGFP::cbuda0009 | This study |
| GFP-CpeJ | EmGFP::cbuda0014 | This study |
| GFP-CpeK | EmGFP::cbuda0023 | This study |
| GFP-CpeL | EmGFP::cbuda0024 | This study |
| GFP-CpeLΔSEL1-1 | EmGFP::cbuda0024-SEL1-1 | This study |
| GFP-CpeLΔSEL1-2 | EmGFP::cbuda0024-SEL1-2 | This study |
| GFP-CpeLΔSEL1-1 | EmGFP::cbuda0024-SEL1-3 | This study |
Amp, ampicillin; Cm, chloramphenicol; Km, kanamycin; Str, streptomycin.
THP-1 human monocytic cells (TIB-202; American Type Culture Collection) and HeLa (human epitheloid carcinoma) cells (CCL-2; ATCC) were maintained in RPMI 1640 medium (Invitrogen) containing 10% fetal calf serum (Invitrogen) at 37°C and 5% CO2. THP-1 cells were differentiated into macrophage-like cells by incubation with 200 nM phorbol 12-myristate 13-acetate (PMA; EMD Biosciences) for 18 h. PMA was removed prior to infections.
Plasmid construction.
pJB2581 was used for expression of CyaA fusion proteins in L. pneumophila. C. burnetii genes were amplified from Nine Mile I (RSA493), K (Q154), or Dugway genomic DNA by PCR using Accuprime Taq polymerase (Invitrogen) and gene-specific primers (Integrated DNA Technologies) where the 5′ primer incorporated a BamHI site and the 3′ primer incorporated a SalI or PstI site (see Table S1 in the supplemental material). Products were cloned into pCR2.1-TOPO (Invitrogen), plasmids digested with either BamHI/SalI or BamHI/PstI (New England BioLabs), and gene-containing fragments ligated to similarly digested pJB2581 using Ligate-IT (U.S. Biologicals). For green fluorescent protein (GFP) fusions, C. burnetii genes were amplified by PCR with gene-specific primers with the forward primer containing CACC at the 5′ end for directional cloning and a 5′ Kozac sequence (ATGGGC) for eukaryotic expression. Products were cloned into pENTR-D/TOPO (Invitrogen), and then subcloned into pcDNA6.2/N-GFP using LR Clonase II (Invitrogen). Plasmid constructs were confirmed by sequencing and are listed in Table 1.
Immunoblotting and CyaA translocation assays.
L. pneumophila transformants were incubated with 1 mM 4′,6-diamidino-2-phenylindole (IPTG) (ICN Biomedicals) for 2 h and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting using a mouse monoclonal antibody directed against CyaA (clone 3D1; Santa Cruz Biotechnology). Reacting proteins were detected using anti-mouse IgG antibody conjugated to horseradish peroxidase (Cell Signaling Technology) and enhanced chemiluminescence using ECL Pico reagent (Pierce). L. pneumophila expressing correctly sized fusion proteins was used in CyaA assays performed as previously described (19), and cyclic AMP (cAMP) concentrations were determined using the cAMP Enzymeimmunoassay (GE Healthcare). Positive secretion of fusion proteins was scored as ≥2.5-fold more cytosolic cAMP than that seen with cells infected with L. pneumophila expressing CyaA alone (18, 25). CyaA fused to C. burnetii CpeA was used as a positive control, and Dot/Icm-dependent secretion was confirmed using the L. pneumophila DotA− mutant LELA3118.
Operon analysis of cpeK and cpeL.
Differentiated THP-1 cells were either left uninfected or infected with Dugway for 4 days. Cells were harvested in TRIzol reagent (Invitrogen), and RNA was isolated with an RNeasy kit (Qiagen). RNA was converted to cDNA with a Superscript III first-strand synthesis system (Invitrogen) using random hexamer primers. Genomic DNA isolated with a microbial DNA isolation kit (MoBio Laboratories) according to the protocol of the manufacturer was used as a positive and negative control. Targeted genes, or intergenic regions, were amplified by PCR using sequence-specific primers (see Table S1 in the supplemental material). Amplified products were subjected to agarose gel electrophoresis to confirm the correct size of products.
HeLa cell transfections.
Nine Mile II- or Dugway-infected HeLa cells (multiplicity of infection = ∼10) were transfected with constructs encoding GFP fused to individual effector proteins using Effectene (Qiagen). At 18 h posttransfection, cells were processed for fluorescence microscopy and incubated with DAPI (Invitrogen) to stain DNA. LC3 antibody (Cell Signaling Technology) was used to detect host autophagosomes, FK2 antibody (Enzo Life Sciences) was used to detect labeled ubiquitinated proteins, and a C. burnetii-specific antibody was used to detect bacteria. Fluorescence microscopy was performed using a Nikon Ti-U microscope, and images were acquired with a 60× oil immersion objective and DS-Qi1Mc camera (Nikon). Images were processed using NIS-Elements software (Nikon).
Effector sequence analysis.
Individual Dot/Icm substrates and encoding genes were subjected to in silico analysis using i-TASSER, Database of prOkaryotic OpeRons (DOOR), and SMART prediction programs. DOOR (26) was used to predict operons present on QpDG, SMART (27) predicted SEL1 domains in CpeL, and i-TASSER (28, 29) was used to generate a predicted tertiary structure for CpeL. i-TASSER-generated structures were further analyzed and rendered using iMOL (30), and HcpC was rendered using Protein Data Bank coordinates 1OUV.
RESULTS AND DISCUSSION
C. burnetii contains many plasmid-specific open reading frames (ORFs) that encode hypothetical proteins.
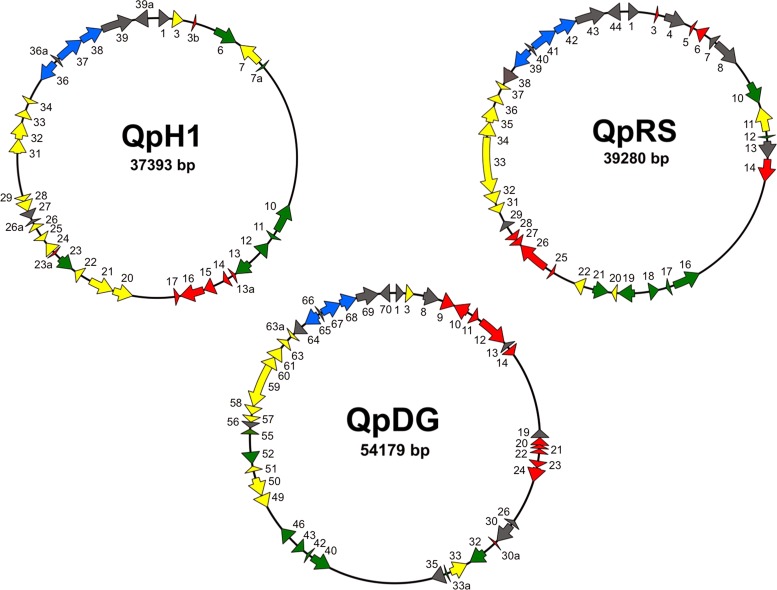
Previously, we discovered two QpH1-specific ORFs (CBUA0014 and CBUA0015) that encode Dot/Icm substrates with eukaryotic motifs/domains (18). Additionally, we found three genes present in all C. burnetii isolates and one QpH1-specific gene that encode hypothetical proteins translocated by the Dot/Icm T4SS. Hypothetical proteins are often critical for host-pathogen interactions specific to a certain bacterium. Thus, we further explored the three best-characterized C. burnetii plasmids to determine the content of genes encoding hypothetical proteins. Ten genes specific to QpDG (CbuDA0009, -10, -11, -12, -14, -20, -21, -22, -23, and -30a) encode hypothetical proteins, the most among the three plasmids, with eight QpRS-specific genes (CbuKA0003, -5, -6, -14, -25, -26, -27, and -28) and seven QpH1-specific genes (CBUA0003b, -13a, -14, -15, -16, -17, and -23a) encoding hypothetical proteins (Fig. 1). The remaining genes encoding hypothetical proteins are shared between two of the plasmids but not all three, demonstrating extensive variability in C. burnetii plasmid content. Additionally, QpDG-specific CBUDA0024 encodes a protein with predicted SEL1 domains, belonging to the tetratricopeptide repeat (TPR) protein family. TPR-containing proteins are often found in eukaryotic organisms and direct a variety of protein-protein interactions (31), making them candidate bacterial effectors that potentially interact with host proteins following delivery to the cytosol.
Fig 1.
C. burnetii plasmid gene content is highly diverse. Maps of QpH1, QpRS, and QpDG show locations of conserved ORFs (green), plasmid-specific ORFs (red), and ORFs present in one or two plasmids but not all three (yellow). Gray ORFs encode proteins not predicted to be involved in host cell manipulation (i.e., phage integrase-related proteins) and not predicted to be effectors. Blue ORFs encode plasmid partition proteins, which are also not effector candidates.
A subset of plasmid-specific C. burnetii proteins are Dot/Icm substrates.
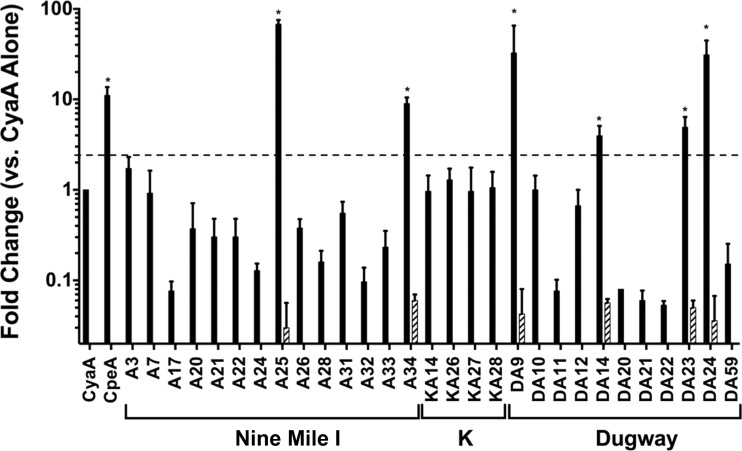
Recent progress in transformation of avirulent C. burnetii confirmed L. pneumophila studies by demonstrating secretion of effector proteins by C. burnetii during infection of macrophage-like cells (9, 15, 18). However, transformation of C. burnetii isolates in phase I, including K and Dugway, has not yet been reported. To efficiently screen many potential Dot/Icm substrates in the current study, we used the well-established L. pneumophila model of Dot/Icm-mediated secretion that correlates entirely with plasmid effector translocation by C. burnetii (18, 23). For this analysis, we screened only proteins composed of at least 50 amino acids that lack a predicted signal sequence (23), as signal peptide-containing proteins are not typically T4SS substrates. Each candidate effector gene was fused in frame to cyaA, the gene encoding adenylate cyclase (CyaA), and expressed in L. pneumophila (data not shown). Following infection of macrophage-like THP-1 cells by L. pneumophila transformants, cells were harvested, cAMP was extracted, and concentrations were determined. As shown in Fig. 2, L. pneumophila expressing CyaA fused to CBUA0025, CBUA0034, CBUDA0009, CBUDA0014, CBUDA0023, and CBUDA0024 triggered substantially increased cAMP production during infection of THP-1 cells similarly to previously identified effectors. When these six fusion proteins were expressed in T4SS-defective L. pneumophila (DotA mutant), cAMP concentrations returned to basal levels (data not shown), indicating that the proteins were Dot/Icm substrates.
Fig 2.
A subset of plasmid-specific proteins are translocated into mammalian cells by the Dot/Icm T4SS. Intracellular cAMP levels were determined following infection of THP-1 cells with L. pneumophila strains producing the indicated CyaA fusion proteins (solid bars) for 30 min. Results are expressed as fold change over cAMP levels triggered by CyaA alone (negative control). CpeA served as the positive control. C. burnetii isolates encoding individual effector candidates are presented below the graph. Asterisks indicate changes in cAMP levels substantially different from those seen with CyaA alone (≥2.5-fold increase) that were negated when the same fusion was expressed in DotA-deficient L. pneumophila (hatched bars). The dashed horizontal line indicates a 2.5-fold cutoff. Increased levels of cAMP were observed after infection by L. pneumophila expressing CBUA0025 (CpeG), CBUA0034 (CpeH), CBUDA0009 (CpeI), CBUDA0014 (CpeJ), CBUDA0023 (CpeK), and CBUDA0024 (CpeL). cAMP levels returned to the basal level when these proteins were expressed in L. pneumophila deficient in type IV secretion.
Dot/Icm substrates identified in this study have been designated Coxiella plasmid effectors G to L (CpeG to -L; Table 2), corresponding to previously identified plasmid-encoded effectors (18). Interestingly, four new effectors (CpeI to -L) are encoded only by the QpDG plasmid found in attenuated C. burnetii isolates (11, 12). This finding suggests that Dugway isolates encode effectors not needed to cause disease, as virulent Nine Mile I C. burnetii causes acute Q fever and G was isolated from a patient with chronic endocarditis, while both isolates lack CpeI to -L. Alternatively, Dugway-specific effectors may allow the organism to remain undetected by the host immune system during in vivo growth without causing overt disease. Indeed, we recently showed that Dugway has the capacity to replicate within primary human alveolar macrophages (32), indicating that the macrophage response to infection is not sufficient to degrade this isolate.
Table 2.
C. burnetii plasmid-specific effectors identified in this study
| Protein designationa | ORFb |
Predicted motif(s)/domain(s) | Molecular mass (kDa) | |||
|---|---|---|---|---|---|---|
| Nine Mile (QpH1) | K (QpRS) | G (IPS) | Dugway (QpDG) | |||
| CpeG | CBUA0025 | Absent | CbuG0074 | Absent | None | 10 |
| CpeH | CBUA0034 | CbuKA0037 | Absent | CbuDA0063a | None | 8 |
| CpeI | Absent | Absent | Absent | CbuDA0009 | None | 34 |
| CpeJ | Absent | Absent | Absent | CbuDA0014 | None | 18 |
| CpeK | Absent | Absent | Absent | CbuDA0023 | None | 15 |
| CpeL | Absent | Absent | Absent | CbuDA0024 | SEL1c | 32 |
New effector designations. Cpe, Coxiella plasmid effector.
Plasmid types are shown in parentheses beside the respective isolate designations. GenBank accession numbers: Nine Mile, AE016828; K, CP001020; G, CP001019; Dugway, CP000733. IPS, integrated plasmid sequences.
SEL1, member of the tetratricopeptide repeat protein family.
Plasmid effector characteristics.
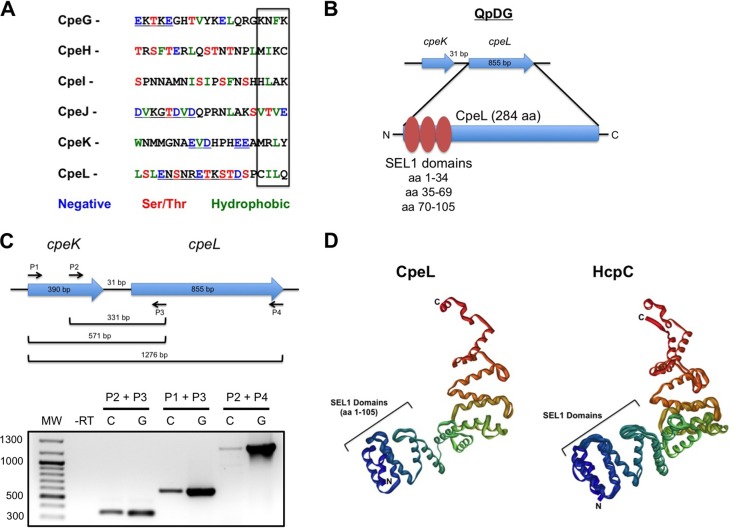
As more C. burnetii effectors have been identified, sequence analysis has shed light on regions of similarity between effectors that contain potential translocation signals. A combination of characteristics is proposed to comprise the Dot/Icm translocation signal. First, most Dot/Icm substrates contain a hydrophobic residue in their C-terminal 3 to 4 residues (33). When CpeG to -L were assessed at the amino acid sequence level (Fig. 3A), all 6 proteins contained one or two hydrophobic residues in this region, agreeing with the hydrophobic-tail hypothesis. Second, many Dot/Icm effectors contain an E-block motif in the C-terminal 20 amino acid residues consisting of 6 to 8 residue regions with 2 to 3 Asp or Glu residues per region (34). Of our six effectors, four (CpeG, -J, -K, and -L) contained a potential E-block motif, further supporting the idea of a role for this signal in Dot/Icm-mediated translocation. Third, a recent study assessed 140 L. pneumophila effectors and found a lack of Asp, Glu, Ser, and Thr residues in the C-terminal 3 to 4 amino acid region, while these residues were more prominent in the C-terminal 5 to 20 amino acids (35). In agreement with this analysis, only one plasmid effector, CpeJ, contained Thr and Glu in the C-terminal 4 amino acids.
Fig 3.
Properties of novel C. burnetii Dot/Icm substrates. (A) The C-terminal 20 amino acid residues were aligned for each effector. The box indicates the last 4 C-terminal residues containing hydrophobic residues that comprise the predicted Dot/Icm translocation signal. Amino acid properties and corresponding colors are shown below the sequences. Underlined residues constitute a potential E-block. Only CpeJ contains a negatively charged residue in the last four-residue region. (B) cpeL lies in a predicted operon with cpeK, with the genes separated by 31 bp. cpeL encodes a 284-amino-acid (aa) protein containing three SEL1 domains. (C) Operon analysis of cpeK and cpeL. cDNA converted from Dugway-infected THP-1 RNA was included in PCRs using the indicated P1 to P4 primers. Each primer condition indicates that cpeK and cpeL are organized in an operon on the QpDG plasmid. (D) i-TASSER structural analysis predicts that CpeL SEL1 domains form three α-helical repeats in the N-terminal 105 amino acids (left) similar to the SEL1 domain arrangement of Helicobacter HcpC (right).
We previously found that QpH1 harbors two effector-encoding genes (cpeD and cpeE) in a predicted operon (18). Because cpeK and cpeL also lie in close proximity on QpDG, we subjected all QpDG genes to operon analysis using the Database of prOkaryotic OpeRons (DOOR) prediction program (26). DOOR analysis predicted that cpeK and cpeL lie in an operon with only 31 bases separating the genes (Fig. 3B). This prediction was confirmed using reverse transcription-PCR (RT-PCR) analysis of cpeK and cpeL transcripts following Dugway infection of THP-1 cells. Using three different primer sets, cDNA synthesized from infected cell RNA contained products corresponding to cpeK and cpeL organized in an operonic fashion (Fig. 3C). CpeL is annotated as a TPR family protein with three predicted SEL1 repeats (Fig. 3B). In silico structural analysis using i-TASSER indicated that CpeL contains three α-helical repeats in the SEL1 repeat region (Fig. 3D). This finding was supported by SMART analysis, which predicted three SEL1 repeats in the N-terminal 105 amino acids of CpeL. SEL1 repeats are a subfamily of TPR proteins found largely in eukaryotic organisms and regulate turnover of endoplasmic reticulum proteins (36). However, bacterial SEL1-containing proteins mediate interactions between the pathogen and the host. i-TASSER analysis predicted CpeL to be structurally most similar to Helicobacter cysteine-rich protein C (HcpC; Fig. 3D), a SEL1 repeat-containing protein that interacts with eukaryotic Nek9, Hsp90, and Hsc71 (37). L. pneumophila produces two SEL1-containing proteins, L. pneumophila Entry (LpnE) and Enhanced Entry protein C (EnhC), involved in bacterial uptake by susceptible host cells (38, 39). EnhC is also required for proper intracellular growth under stress conditions, including tumor necrosis factor alpha (TNF-α) treatment, and dampens Nod1-dependent activation of the host transcription factor NF-κB, effectively downregulating a detrimental immune response (40). C. burnetii likely does not need CpeL for cellular entry, as the pathogen targets naturally phagocytic macrophages and CpeL is present only in Dugway. However, C. burnetii actively regulates host cellular immune responses and effectors such as CpeL may influence Dugway's ability to avoid immune-mediated clearance.
Subcellular localization of novel Dot/Icm substrates.
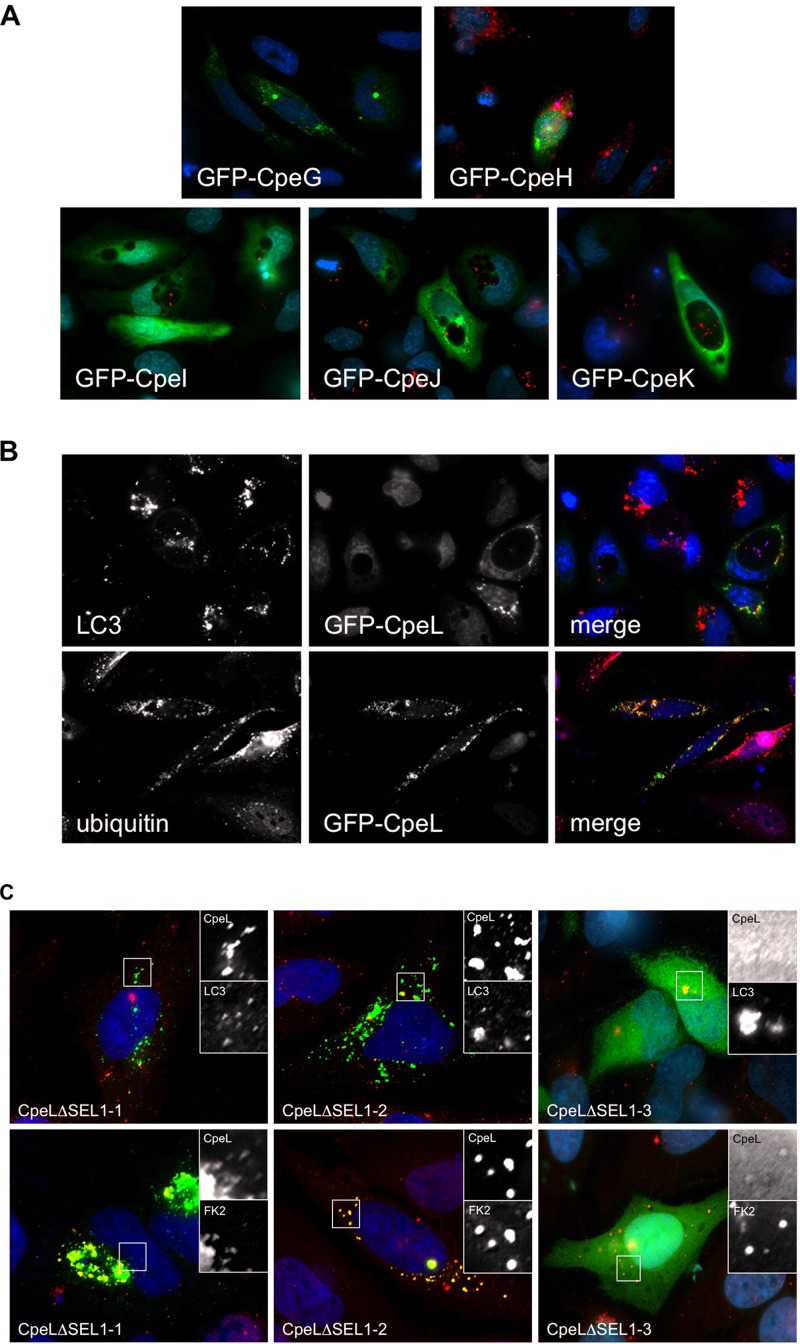
Previous functional studies benefited from analyzing intracellular trafficking of individual effectors ectopically expressed in host cells (18, 41). Therefore, we assessed the subcellular localization of each new C. burnetii Dot/Icm substrate using a GFP fusion approach. HeLa cells were infected with either C. burnetii Nine Mile II (for CpeG and -H) or Dugway (for CpeI to -L) organisms and then transfected with plasmids encoding individual effectors N-terminally fused to GFP. As shown in Fig. 4A, CpeI, CpeJ, and CpeK were dispersed nonspecifically throughout the cytoplasm, and CpeG and CpeH were present in the cytoplasm in punctate structures that did not label specifically with any cellular markers assessed (CD63, LC3, cathepsin D, calnexin, GM130, CoxIV). CpeL partially colocalized with host LC3 (Fig. 4B), indicating that the effector traffics to autophagosomes when present in the host cytosol, as LC3 triggers the final step in sealing of autophagosomes prior to fusion with lysosomes. These findings are similar to those for CpeB, a conserved C. burnetii plasmid effector that colocalizes with LC3 and is present on the PV membrane when ectopically expressed in HeLa cells (18). Together, these studies highlight C. burnetii interaction with host autophagic machinery and further suggest that the pathogen uses T4SS effectors to mediate autophagosome recruitment, a process that requires C. burnetii protein synthesis (42). Additionally, CpeL localized around the PV with ubiquitinated proteins (Fig. 4B) in a manner similar to that seen with the previously identified QpH1-specific CpeC (18), suggesting that Dugway isolates also encode a protein that may regulate modification of host or bacterial proteins by ubiquitination. SEL1 repeats present in CpeL may mediate PV fusion events, as L. pneumophila SEL1-containing LpnE influences replication vacuole maturation and interacts with host obscurin-like protein 1 (38). The requirement of the CpeL SEL1 domains in establishing proper subcellular localization was assessed using CpeL mutants lacking one, two, or all three domains (Fig. 4C). This analysis showed that only CpeL lacking all three SEL1 domains was unable to localize to LC3- or ubiquitin-positive structures in the cytoplasm of eukaryotic cells, while CpeL lacking the first two domains retained proper localization. These results indicate the importance of SEL1 domains, particularly the third domain, in coordinating CpeL trafficking in the cytoplasm.
Fig 4.
Subcellular localization of plasmid-specific Dot/Icm substrates. Each Dot/Icm substrate fused to GFP was ectopically expressed in HeLa cells infected with either C. burnetii Nine Mile phase II (CpeG and -H) or Dugway (CpeI to -L), and cells were processed for fluorescence microscopy. (A and B) Cellular markers are indicated in each panel, and C. burnetii is shown in red (A) or violet (B). CpeI, CpeJ, and CpeK dispersed nonspecifically throughout the host cytosol, and CpeG and CpeH localized to punctate spots in the cytoplasm. (B) CpeL colocalized with LC3 (upper panel; red) and ubiquitinated proteins (lower panel; red) around the PV and in the cytosol. (C) Subcellular localization of CpeL lacking one, two, or all three SEL1 domains. Insets highlight regions of GFP-CpeL mutant expression. When the first two SEL1 domains are removed, CpeL retains trafficking to LC3- and ubiquitin-positive structures. However, CpeL lacking all three SEL1 domains localizes nonspecifically to the cytoplasm.
Concluding remarks.
In the current study, we further refined the effector repertoire of C. burnetii plasmids. Combined with data from our previous study (18), 12 plasmid-encoded Dot/Icm substrates have been identified, with 3 specific to QpH1 and 4 specific to QpDG. Aside from CpeL, which contains SEL1 repeats, all newly identified plasmid effectors are hypothetical proteins found only in C. burnetii, suggesting pathogen-specific requirements for these proteins. Since its discovery 30 years ago (10), a role for the plasmid in C. burnetii pathogenesis has remained elusive, and the discovery of numerous plasmid-encoded T4SS effectors provides a novel function for these extrachromosomal elements in pathogen virulence. It is atypical for a pathogen to maintain effector-encoding genes on a plasmid while harboring secretion system structural genes on the chromosome. However, this scenario provides a logical reason for C. burnetii to maintain the plasmid genes. Finally, it is intriguing that Dugway encodes effectors not found in other C. burnetii isolates. Dugway is severely attenuated in animal models compared to other isolates (43), suggesting that Dugway-specific effectors may influence bacterial pathogenic potential, as isolates lacking these effectors have increased virulence. Alternatively, these effectors may allow Dugway to escape the host immune system and establish infection, as nothing is known about Dugway infection of humans. The importance of plasmid-specific Dot/Icm substrates for successful parasitism of eukaryotic cells and in vivo infection now awaits testing using gene-specific knockout mutants of C. burnetii generated with newly developed genetic techniques (44).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by funding to D.E.V. from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01AI087669).
We thank Laura MacDonald and Caylin Winchell for critical review of the manuscript, P. Scott Hefty for helpful discussions about i-TASSER predictions, and Jon Blevins for helpful discussions about operon analysis.
Footnotes
Published ahead of print 17 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00180-13.
REFERENCES
- 1. Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 5:219–226 [DOI] [PubMed] [Google Scholar]
- 2. Mazokopakis EE, Karefilakis CM, Starakis IK. 2010. Q fever endocarditis. Infect. Disord. Drug Targets 10:27–31 [DOI] [PubMed] [Google Scholar]
- 3. Anderson A, Bijlmer HA, Fournier PE, Graves S, Hartzell JD, Kersh G, Limonard GJ, Marrie T, Massung R, McQuiston J, Nicholson WL, Paddock C, Sexton DJ. 2013. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm. Rep. 62(RR-03):1–30 [PubMed] [Google Scholar]
- 4. Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, Vellema P, Schneeberger PM. 2012. The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol. Med. Microbiol. 64:3–12 [DOI] [PubMed] [Google Scholar]
- 5. Kampschreur LM, Dekker S, Hagenaars JC, Lestrade PJ, Renders NH, de Jager-Leclercq MG, Hermans MH, Groot CA, Groenwold RH, Hoepelman AI, Wever PC, Oosterheert JJ. 2012. Identification of risk factors for chronic Q fever, the Netherlands. Emerg. Infect. Dis. 18:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amano K, Williams JC, Missler SR, Reinhold VN. 1987. Structure and biological relationships of Coxiella burnetii lipopolysaccharides. J. Biol. Chem. 262:4740–4747 [PubMed] [Google Scholar]
- 7. Beare PA, Unsworth N, Andoh M, Voth DE, Omsland A, Gilk SD, Williams KP, Sobral BW, Kupko JJ, 3rd, Porcella SF, Samuel JE, Heinzen RA. 2009. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect. Immun. 77:642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beare PA, Samuel JE, Howe D, Virtaneva K, Porcella SF, Heinzen RA. 2006. Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons. J. Bacteriol. 188:2309–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Samuel JE, Frazier ME, Mallavia LP. 1985. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect. Immun. 49:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samuel JE, Frazier ME, Kahn ML, Thomashow LS, Mallavia LP. 1983. Isolation and characterization of a plasmid from phase I Coxiella burnetii. Infect. Immun. 41:488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stoenner HG, Lackman DB. 1960. The biologic properties of Coxiella burnetii isolated from rodents collected in Utah. Am. J. Hyg. 71:45–51 [DOI] [PubMed] [Google Scholar]
- 12. Stoenner HG, Holdenried R, Lackman D, Orsborn JS., Jr 1959. The occurrence of Coxiella burnetii, Brucella, and other pathogens among fauna of the Great Salt Lake Desert in Utah. Am. J. Trop. Med. Hyg. 8:590–596 [DOI] [PubMed] [Google Scholar]
- 13. Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, Tokarevich N, Kovacava E, Marrie TJ, Raoult D. 2005. Coxiella burnetii genotyping. Emerg. Infect. Dis. 11:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savinelli EA, Mallavia LP. 1990. Comparison of Coxiella burnetii plasmids to homologous chromosomal sequences present in a plasmidless endocarditis-causing isolate. Ann. N. Y. Acad. Sci. 590:523–533 [DOI] [PubMed] [Google Scholar]
- 15. Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, Cockrell DC, Howe D, Voth DE, Heinzen RA. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2:e00175–11. 10.1128/mBio.00175-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carey KL, Newton HJ, Luhrmann A, Roy CR. 2011. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 7:e1002056. 10.1371/journal.ppat.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voth DE, Heinzen RA. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9:829–840 [DOI] [PubMed] [Google Scholar]
- 18. Voth DE, Beare PA, Howe D, Sharma UM, Samoilis G, Cockrell DC, Omsland A, Heinzen RA. 2011. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J. Bacteriol. 193:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, Luo ZQ, Samuel JE. 2010. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 107:21755–21760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lührmann A, Nogueira CV, Carey KL, Roy CR. 2010. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc. Natl. Acad. Sci. U. S. A. 107:18997–19001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klingenbeck L, Eckart RA, Berens C, Luhrmann A. 6 November 2012. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell. Microbiol. [Epub ahead of print.] 10.1111/cmi.12066 [DOI] [PubMed] [Google Scholar]
- 23. Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, Shuman HA, Pupko T, Segal G. 2013. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc. Natl. Acad. Sci. U. S. A. 110:E707–E715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106:4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lockwood S, Voth DE, Brayton KA, Beare PA, Brown WC, Heinzen RA, Broschat SL. 2011. Identification of Anaplasma marginale type IV secretion system effector proteins. PLoS One 6:e27724. 10.1371/journal.pone.0027724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao F, Dam P, Chou J, Olman V, Xu Y. 2009. DOOR: a database for prokaryotic operons. Nucleic Acids Res. 37:D459–D463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P. 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32:D142–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. 10.1186/1471-2105-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rotkiewicz P. 2007. iMol molecular visualization program. http://www.pirx.com/iMol/index.shtml
- 31. D'Andrea LD, Regan L. 2003. TPR proteins: the versatile helix. Trends Biochem. Sci. 28:655–662 [DOI] [PubMed] [Google Scholar]
- 32. Graham JG, Macdonald LJ, Hussain SK, Sharma UM, Kurten RC, Voth DE. 2013. Virulent Coxiella burnetii pathotypes productively infect primary human alveolar macrophages. Cell. Microbiol. 15:1012–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U. S. A. 102:826–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, O'Connor TJ, Chen C, Machner M, Montminy T, Isberg RR. 2011. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell. Microbiol. 13:227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T. 2009. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 5:e1000508. 10.1371/journal.ppat.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mittl PR, Schneider-Brachert W. 2007. SEL1-like repeat proteins in signal transduction. Cell. Signal. 19:20–31 [DOI] [PubMed] [Google Scholar]
- 37. Roschitzki B, Schauer S, Mittl PR. 2011. Recognition of host proteins by Helicobacter cysteine-rich protein C. Curr. Microbiol. 63:239–249 [DOI] [PubMed] [Google Scholar]
- 38. Newton HJ, Sansom FM, Dao J, McAlister AD, Sloan J, Cianciotto NP, Hartland EL. 2007. SEL1 repeat protein LpnE is a Legionella pneumophila virulence determinant that influences vacuolar trafficking. Infect. Immun. 75:5575–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cirillo SL, Lum J, Cirillo JD. 2000. Identification of novel loci involved in entry by Legionella pneumophila. Microbiology 146:1345–1359 [DOI] [PubMed] [Google Scholar]
- 40. Liu M, Haenssler E, Uehara T, Losick VP, Park JT, Isberg RR. 2012. The Legionella pneumophila EnhC protein interferes with immunostimulatory muramyl peptide production to evade innate immunity. Cell Host Microbe 12:166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. 2008. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 4:e1000117. 10.1371/journal.ppat.1000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891–909 [DOI] [PubMed] [Google Scholar]
- 43. Russell-Lodrigue KE, Andoh M, Poels MW, Shive HR, Weeks BR, Zhang GQ, Tersteeg C, Masegi T, Hotta A, Yamaguchi T, Fukushi H, Hirai K, McMurray DN, Samuel JE. 2009. Coxiella burnetii isolates cause genogroup-specific virulence in mouse and guinea pig models of acute Q fever. Infect. Immun. 77:5640–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beare PA, Larson CL, Gilk SD, Heinzen RA. 2012. Two systems for targeted gene deletion in Coxiella burnetii. Appl. Environ. Microbiol. 78:4580–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sadosky AB, Wiater LA, Shuman HA. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bardill JP, Miller JL, Vogel JP. 2005. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56:90–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.