Abstract
In the low-G+C-content Gram-positive bacteria, resistance to antimicrobial peptides is often mediated by so-called resistance modules. These consist of a two-component system and an ATP-binding cassette transporter and are characterized by an unusual mode of signal transduction where the transporter acts as a sensor of antimicrobial peptides, because the histidine kinase alone cannot detect the substrates directly. Thus, the transporters fulfill a dual function as sensors and detoxification systems to confer resistance, but the mechanistic details of these processes are unknown. The paradigm and best-understood example for this is the BceRS-BceAB module of Bacillus subtilis, which mediates resistance to bacitracin, mersacidin, and actagardine. Using a random mutagenesis approach, we here show that mutations that affect specific functions of the transporter BceAB are primarily found in the C-terminal region of the permease, BceB, particularly in the eighth transmembrane helix. Further, we show that while signaling and resistance are functionally interconnected, several mutations could be identified that strongly affected one activity of the transporter but had only minor effects on the other. Thus, a partial genetic separation of the two properties could be achieved by single amino acid replacements, providing first insights into the signaling mechanism of these unusual modules.
INTRODUCTION
Many bacteria produce antibiotics to gain a competitive advantage over other microorganisms inhabiting the same ecological niche. Among the low-G+C-content Gram-positive bacteria, the Firmicutes, production of antimicrobial peptides is widely distributed. These compounds generally act by an inhibition of the lipid II cycle of cell wall synthesis (1, 2). For example, lantibiotics such as nisin or mersacidin bind to lipid II directly (1–3), whereas the non-ribosomally synthesized bacitracin prevents the dephosphorylation and recycling of the lipid carrier undecaprenyl-pyrophosphate (4). For self-protection, producer strains of antimicrobial peptides express so-called immunity proteins, summarily referred to as LanI, as well as ATP-binding cassette (ABC) transporters of the LanFEG or BcrAB type (5–8).
In order to compete successfully with such strains, nonproducing Firmicutes bacteria have also developed resistance mechanisms, the most efficient of which are again ABC transporters. However, these transporters differ in sequence and domain architecture from those of the producer strains and are collectively referred to as BceAB-type transporters (8, 9). They consist of an ATPase (BceA) and a permease (BceB) with 10 transmembrane helices and a characteristic, large extracellular domain of approximately 200 amino acids between helices VII and VIII. The eponymous and best-characterized example is BceAB of Bacillus subtilis, which confers resistance to bacitracin, mersacidin, and actagardine (10, 11). BceAB-type transporters are found in a conserved genomic arrangement with two-component systems (referred to as BceRS-like) whose histidine kinases possess two transmembrane helices but lack any extracellular sensory domains (9, 12, 13). Together, the transporter and the two-component system constitute peptide antibiotic resistance modules. Their most striking property is the unusual mode of signal transduction: the histidine kinases alone cannot detect the presence of antimicrobial peptides but instead rely on the transporters for stimulus perception. In the presence of a substrate peptide, the transporter somehow communicates with the sensor kinase, which leads to activation of the cognate response regulator and, subsequently, an induction of transporter gene expression. Importantly, ATP hydrolysis by the transporter and therefore active transport are required for the signaling process (10, 14). Experimental evidence from a number of homologous systems from B. subtilis, Staphylococcus aureus, Streptococcus mutans, and Lactobacillus casei confirms this signaling pathway as a general characteristic of the Bce-type modules (10, 11, 14–17). Based on the coevolution of the proteins involved and supported by bacterial two-hybrid analyses of the VraFG transporter and GraRS two-component system of S. aureus, it is thought that communication within these modules involves direct protein-protein contacts between the transporter and histidine kinase (9, 15, 18). However, no information is available regarding the mechanism of signaling.
In this study, we performed a random mutagenesis of the transport permease BceB of B. subtilis with the aim to identify regions or residues within the transporter that are involved in signaling and/or resistance. A central question was whether amino acid replacements could be identified that affected only one but not the other function of BceB. In other words, are the two traits genetically separable in a dual-function transporter such as BceAB? We show that a partial separation of signaling and resistance could be obtained from single point mutations and further identify the C-terminal region of the transport permease as functionally important.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. subtilis and Escherichia coli were routinely grown in Luria-Bertani (LB) medium at 37°C with agitation (200 rpm). During cloning of bacterial two-hybrid constructs, all media for E. coli were supplemented with 0.4% (wt/vol) glucose. Transformations of B. subtilis were carried out as described previously (19). All strains used in this study are listed in Table 1. Selective media contained kanamycin (10 μg ml−1 for B. subtilis, 50 μg ml−1 for E. coli), spectinomycin (100 μg ml−1), chloramphenicol (5 μg ml−1), or ampicillin (100 μg ml−1). Solid media contained 1.5% (wt/vol) agar.
Table 1.
Plasmids and strains used in this study
| Plasmid or strain | Descriptiona | Reference or source |
|---|---|---|
| Plasmids | ||
| pAC6 | Vector for transcriptional promoter fusions to lacZ; integrates at amyE; Cmr | 20 |
| pAH328 | Vector for transcriptional promoter fusions to luxABCDE (luciferase); integrates in sacA; Cmr | 21 |
| pKT25 | Translational fusion of cyaA T25 fragment to N terminus of insert polypeptide; lac promoter; Kanr | 22 |
| pUT18 | Translational fusion of cyaA T18 fragment to C terminus of insert polypeptide; lac promoter; Ampr | 22 |
| pXT | Vector for xylose-inducible gene expression; integrates in thrC; Spcr | 23 |
| pAS1803 | pUT18-bceS | This study |
| pAS1804 | pUT18-bceA | This study |
| pAS2505 | pKT25-bceB | This study |
| pER603 | pAC6-PbceA-lacZ | 10 |
| pER703 | pXT-bceB | 10 |
| pFK727 | pXT-bceAB-FLAG3 | This study |
| pSDlux101 | pAH328-PbceA-luxABCDE | This study |
| pSG704 | pXT-bceAB | This study |
| E. coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′::Tn10 proAB lacIq Δ(lacZ)M15] | Stratagene |
| BTH101 | F− cya-99 araD139 galE15 galK16 rpsL1 hsdR2 mcrA1 mcrB1 | Euromedex |
| B. subtilis strains | ||
| W168 | Wild type, trpC2 | Laboratory stock |
| SGB079 | W168 bceAB::Kan sacA::pSDlux101 | This study |
| SGB082 | W168 bceAB::Kan sacA::pSDlux101 thrC::pSG704 | This study |
| SGB170 | W168 bceAB::Kan thrC::pSG704 | This study |
| SGB176 | W168 bceAB::Kan thrC::pFK727 | This study |
| TMB035 | W168 bceAB::Kan | 10 |
| TMB301 | W168 bceB::Kan amyE::pER603 | 10 |
| TMB378 | W168 bceB::Kan amyE::pER603 thrC::pER703 | 10 |
Only constructs with WT sequence inserts and derived strains are listed; mutated constructs and strains are named according to their amino acid exchanges throughout the article. Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Kanr, kanamycin resistance; Spcr, spectinomycin resistance.
Plasmid construction.
All plasmids created during this study are listed in Table 1; all primer sequences are given in Table 2. Only plasmids and derived strains harboring constructs with the wild-type sequence of bceB are listed; all other strains are referred to as their wild-type counterparts, with the amino acid exchange given in parentheses.
Table 2.
Primers used in this study
| Primer name | Sequence (5′–3′) |
|---|---|
| Oligonucleotides for cloninga | |
| 0011 | GAATGGATCCGATTATCCAATATAAAGGAGACTGCG |
| 0554 | GATCGAATTCGAACATGTCATAAGCGTGTGACG |
| 1355 | GTCATCTAGAGATGATTAAAGCATTCCTTATCG |
| 1356 | GTCAGGTACCTGCACGCTTATGACATGTTC |
| 1357 | GTCATCTAGAGATGGTGATTTTAGAAGCG |
| 1358 | GTCAGGTACCTGATGTTCATGCTGCACC |
| 1359 | GTCATCTAGAGATGAACATTAATCAGCTCATCC |
| 1360 | GTCAGGTACCTGCAACGACGATTTAATGACC |
| 1524 | AATTGGATCCTTTTCTGTTTCACAACGACG |
| 1706 | TTAAGGATCCTCACTTGTCGTCATCGTCTTTGTAGTCGATATCATGATCCTTATAATCACCATCATGATCCTTATAATCCAACGACGATTTAATGACC |
| 2241 | AATTGCGGCCGCTATCGATGCCCTTCAGCACTTCC |
| Oligonucleotides for site-directed mutagenesisb | |
| R9Q-fwd | CATTAATCAGCTCATCCTGCAAAATTTGAAAAAGAATCTCCGG |
| R9Q-rev | CCGGAGATTCTTTTTCAAATTTTGCAGGATGAGCTGATTAATG |
| V22 M-fwd | CCGGAATTACTATTTGTATATGTTTGCGCTCATCTTTAGCG |
| V22 M-rev | CGCTAAAGATGAGCGCAAACATATACAAATAGTAATTCCGG |
| A75T-fwd | GTGGCTGTAGTGGCGATCTTCATTTTATATACCAATACGATTTTT |
| A75T-rev | AAAAATCGTATTGGTATATAAAATGAAGATCGCCACTACAGCCAC |
| R83W-fwd | CAATACGATTTTTATTAAGAGATGGAGTAAAGAAATCGGGCTG |
| R83W-rev | CAGCCCGATTTCTTTACTCCATCTCTTAATAAAAATCGTATTG |
| A117T-fwd | GTATTTCGGTTCATTAACGATCGGGGTAGCGGC |
| A117T-rev | GCCGCTACCCCGATCGTTAATGAACCGAAATAC |
| V130I-fwd | CGGGTTTTCGATATCGAAGCTTATCTTGATGATTCTGTTC |
| V130I-rev | GAACAGAATCATCAAGATAAGCTTCGATATCGAAAACCCG |
| G215R-fwd | GCATCGTGTTGATTTTGACAAGATACTATGTGTCTTCTGAG |
| G215R-rev | CTCAGAAGACACATAGTATCTTGTCAAAATCAACACGATGC |
| S219F-fwd | GACAGGATACTATGTGTTTTCTGAGCTGTTTGGCG |
| S219F-rev | CGCCAAACAGCTCAGAAAACACATAGTATCCTGTC |
| S219C-fwd | GACAGGATACTATGTGTGTTCTGAGCTGTTTGGCGG |
| S219C-rev | CCGCCAAACAGCTCAGAACACACATAGTATCCTGTC |
| G247R-fwd | CTGGGGAGCGTCATTATCAGGACGTTTTTGTTTTATAAAG |
| G247R-rev | CTTTATAAAACAAAAACGTCCTGATAATGACGCTCCCCAG |
| A301T-fwd | CAACCGTTTCAACGCTCGCCATCGGGC |
| A301T-rev | GCCCGATGGCGAGCGTTGAAACGGTTG |
| S316L-fwd | GCTTACATCTCGTATTACTTGTCGGAAAAGACCGC |
| S316L-rev | GCGGTCTTTTCCGACAAGTAATACGAGATGTAAGC |
| G381D-fwd | GCAGGGCGATCCCGACAATATGCAGCTTG |
| G381D-rev | CAAGCTGCATATTGTCGGGATCGCCCTGC |
| V426I-fwd | GGACTCTGGTGTTATTAAAATAAAAAGCAAGCACGAGACG |
| V426I-rev | CGTCTCGTGCTTGCTTTTTATTTTAATAACACCAGAGTCC |
| H430Y-fwd | GGTGTTATTAAAGTAAAAAGCAAGTACGAGACGCAGCCATT |
| H430Y-rev | AATGGCTGCGTCTCGTACTTGCTTTTTACTTTAATAACACC |
| G525D-fwd | GCGCAAAAATCACTGTTTGATATGGTGATGTTCATCGTC |
| G525D-rev | GACGATGAACATCACCATATCAAACAGTGATTTTTGCGC |
| G535E-fwd | ATCGTCGGCTTCTTAGAGTTAACGTTCCTGATTACATC |
| G535E-rev | GATGTAATCAGGAACGTTAACTCTAAGAAGCCGACGAT |
| S542L-fwd | GGGTTAACGTTCCTGATTACATTAGGTTGTATCCTTTATTTTAAAC |
| S542L-rev | GTTTAAAATAAAGGATACAACCTAATGTAATCAGGAACGTTAACCC |
| C544Y-fwd | CGTTCCTGATTACATCAGGTTATATCCTTTATTTTAAACAAATGGG |
| C544Y-rev | CCCATTTGTTTAAAATAAAGGATATAACCTGATGTAATCAGGAACG |
| M551I-fwd | CCTTTATTTTAAACAAATAGGTGAAAGTGAAGATGAAAAACCGAGC |
| M551I-rev | GCTCGGTTTTTCATCTTCACTTTCACCTATTTGTTTAAAATAAAGG |
| L567P-fwd | TACAATTTTAAGAAAACCCGGCTTTACGCAGGGCG |
| L567P-rev | CGCCCTGCGTAAAGCCGGGTTTTCTTAAAATTGTA |
| G593D-fwd | CATTCCGCTCGTTGTCGACCTCTTCCACAGTTAC |
| G593D-rev | GTAACTGTGGAAGAGGTCGACAACGAGCGGAATG |
| G593C-fwd | CATTCCGCTCGTTGTCTGCCTCTTCCACAGTTAC |
| G593C-rev | GTAACTGTGGAAGAGGCAGACAACGAGCGGAATG |
| G609R-fwd | CGGCTGGTTCTTATTCAGATCAGAGGTATGGGC |
| G609R-rev | GCCCATACCTCTGATCTGAATAAGAACCAGCCG |
| M616I-fwd | GAGGTATGGGCGCCTATAATTATGGTGATGGTG |
| M616I-rev | CACCATCACCATAATTATAGGCGCCCATACCTC |
| V619 M-fwd | GGGCGCCTATGATTATGATGATGGTGTTATATACTGC |
| V619 M-rev | GCAGTATATAACACCATCATCATAATCATAGGCGCCC |
| T624I-fwd | GGTGATGGTGTTATATATTGCGCTCTACTCCATTTTTGG |
| T624I-rev | CCAAAAATGGAGTAGAGCGCAATATATAACACCATCACC |
| S628P-fwd | GTTATATACTGCGCTCTACCCCATTTTTGGTTTTCTGTC |
| S628P-rev | GACAGAAAACCAAAAATGGGGTAGAGCGCAGTATATAAC |
Restriction sites are underlined; nucleotides encoding the triple FLAG tag are shown in italics.
The introduced mutation is underlined.
To create a plasmid for xylose-inducible expression of bceAB in B. subtilis (pSG704), a 2.8-kb fragment containing the entire operon, including the ribosome binding site, was PCR amplified using primers 0011 and 1524. The resulting product was cloned as a BamHI fragment into vector pXT (23), placing the genes under the control of the vector's xylA promoter. A similar construct expressing bceAB with a triple FLAG tag fused to the C terminus of BceB (pFK727) was created using primers 0011 and 1706 followed by cloning into the BamHI site of pXT.
To create a transcriptional fusion of the bceA promoter, PbceA, to a promoterless luciferase operon (luxABCDE), a 207-bp fragment encompassing the promoter region from 127 bp upstream to 80 bp downstream of the bceA start codon was PCR amplified with primers 0554 and 2241. The resulting product was cloned into the EcoRI and NotI sites of vector pAH328 (21), creating plasmid pSDlux101.
For bacterial two-hybrid analyses (22), a 1.2-kb DNA fragment containing bceS was amplified via PCR using primers 1355 and 1356 and cloned into the XbaI and KpnI restriction sites of vector pUT18, resulting in a translational fusion of the T18 fragment of Bordetella pertussis CyaA to the C terminus of BceS (pAS1803). The same strategy was used to create a translational fusion of the T18 fragment to the C terminus of BceA in pAS1804 (760-bp fragment amplified with primers 1357 and 1358). Furthermore, a 1.9-kb fragment containing bceB was amplified using primers 1359 and 1360 and cloned via the XbaI and KpnI sites into vector pKT25, resulting in a translational fusion of the CyaA T25 fragment to the N terminus of BceB (pAS2505).
Random chemical mutagenesis.
To generate random mutations in bceB, 10 μg of plasmid DNA of pER703 (10) was mixed with 10 vol of HA solution (1 M hydroxylamine hydrochloride, 100 mM NaCl, 50 mM sodium phosphate [pH 6], 2 mM EDTA [pH 8]) and incubated at 75°C for 15 min followed by purification with a HighYield PCR-clean up kit (Süd-Laborbedarf, Gauting, Germany). Mutagenized plasmid DNA was then linearized with ScaI, and 2 μg of this was used to transform B. subtilis strain TMB301 with selection for resistance to kanamycin and spectinomycin. The resulting transformants were replica stamped with velvet pads onto indicator plates containing xylose (0.2% [wt/vol]), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (200 μg ml−1), and Zn2+-bacitracin (Sigma; 0.5 μg ml−1). All white colonies were again replica plated onto indicator plates and selective media (kanamycin, spectinomycin, chloramphenicol) to confirm the phenotype. Positive clones (i.e., those showing white colonies and resistance to all antibiotics) were then tested for threonine auxotrophy to confirm correct integration of the pXT construct into thrC. For this, each clone was inoculated into MNGE medium [100 mM glucose, 80 mM K2HPO4, 45 mM KH2PO4, 10 mM potassium glutamate, 4 mM sodium acetate, 3 mM MgSO4, 50 μg ml−1 tryptophan, and 40 μM Fe(III)-ammonium citrate], with and without addition of 50 μg ml−1 threonine. From each clone that was unable to grow in the absence of threonine, the introduced bceB gene was reamplified by colony PCR using pXT-specific primers, and the resulting product was sequenced to identify any introduced mutations.
Site-directed mutagenesis.
For characterization of the mutations, each amino acid exchange identified by the random mutagenesis approach was reconstructed by site-directed mutagenesis of pSG704. Selected amino acid exchanges were also introduced into pFK727 and pAS2505. Primer design and mutagenesis were performed according to the manufacturer's instructions for the QuikChange II site-directed mutagenesis kit (Agilent Technologies). Where this procedure was not successful, mutations were introduced using the PCR-overlap extension method (24), followed by cloning into the desired vector as described for plasmid construction above. All plasmids were sequenced to confirm introduction of the desired mutation.
Reporter gene assays.
Luciferase activities of strains harboring pSDlux101 were assayed using a Synergy2 multimode microplate reader from BioTek. The reader was controlled using the software Gen5. LB medium was inoculated at 1:1,000 from overnight cultures of reporter strains, and each strain was grown in a 100-μl volume in four wells of a 96-well plate. Cultures were incubated at 37°C with agitation (intensity, medium) and the optical density at 600 nm (OD600) was monitored every 10 min. At an OD600 of 0.02 (4 to 5 population doublings since inoculation, corresponding to an OD600 of 0.1 in cuvettes of 1-cm-light-path length), Zn2+-bacitracin was added to final concentrations of 3, 10, or 30 μg ml−1, with one well left uninduced. Cultures were further incubated for 1 h, and the OD600 and luminescence (end point reads; 1-s integration time; sensitivity, 200) were monitored every 5 min. OD600 values were corrected using wells containing 100 μl LB medium as blanks. Raw luminescence output (measured in relative luminescence units [RLU]) was normalized to cell density by dividing each data point by its corresponding corrected OD600 value (RLU/OD).
Bacitracin sensitivity assays.
The sensitivity of B. subtilis strains to bacitracin was determined as the MIC. For this, serial 2-fold dilutions of Zn2+-bacitracin from 32 μg ml−1 to 2 μg ml−1 were prepared in Mueller-Hinton medium containing 0.2% (wt/vol) xylose. As a control, Mueller-Hinton medium without bacitracin was used. For each concentration, 2 ml of media were inoculated at 1:500 from overnight cultures grown in Mueller-Hinton medium with xylose and selective antibiotics. Each culture was scored for growth after 20 to 24 h of incubation at 37°C with agitation. The MIC was determined as the lowest bacitracin concentration where no growth was detected.
Western blot analyses.
To compare production levels of BceB and its derivatives in membranes of B. subtilis, strain SGB176 carrying plasmid pFK727 (BceB-FLAG3) was used. Cells were grown at 37°C in LB medium supplemented with 0.2% of xylose to an OD600 of 1.0, collected by centrifugation, and resuspended in lysis buffer (100 mM Tris-HCl [pH 8.0], 150 mM NaCl, 10% [wt/vol] glycerol, and 5 mM 2-mercaptoethanol). Cells were lysed by sonication, and debris was removed by centrifugation at 5,400 × g for 15 min at 4°C. Membranes were pelleted by centrifugation at 150,000 × g for 45 min at 4°C and resuspended in lysis buffer. The protein concentration of the membrane fractions was determined with a BCA reagent kit (Pierce) according to the manufacturer's protocol. Membrane fractions (30 μg protein) were separated on denaturing 12% polyacrylamide gels according to the method of Laemmli (25), blotted onto an Immobilon-FL polyvinylidene difluoride (PVDF) membrane (Millipore), and probed with rabbit anti-FLAG antibodies (Sigma) (1:2,000). Alexa Fluor 680-conjugated goat anti-rabbit antibodies (Li-COR) (1:25,000) were used as the secondary antibody. Hybridization of both antibodies was carried out in 2.5% (wt/vol) skim milk powder–1× Tris-buffered saline (TBS)–0.2% (wt/vol) Tween 20. Infrared fluorescence signals were quantified with an Odyssey infrared scanning system (Li-COR).
Bacterial two-hybrid assays.
To test protein-protein interactions between BceB and BceS or between BceB and BceA, E. coli BTH101 was cotransformed with pAS2505 or derived mutant constructs and either pAS1803 or pAS1804. Of each transformation mixture, 10 μl was spotted onto LB agar plates containing 0.5 mM isopropyl β-d-1 thiogalactopyranoside (IPTG) and 40 μg ml−1 X-Gal, with selection for ampicillin and kanamycin resistance. Plates were incubated at 30°C for 48 h. Formation of blue colonies was scored as a positive interaction result.
RESULTS
Identification of signaling-defective derivatives of BceB.
In order to identify mutations that led to a loss of the signaling activity of the BceAB transporter, a plasmid (pER703) containing only the permease gene (bceB) under the control of the xylose-inducible promoter Pxyl was chosen. In a previous study, this construct had been described as leading to poor complementation of signaling in a bceB-deleted strain of B. subtilis (10). However, a subsequent more detailed characterization showed that the signaling activity in this strain was in fact high at very low bacitracin concentrations: it showed a maximal induction of the target promoter, PbceA, between 0.1 and 0.5 μg ml−1 bacitracin (data not shown), whereas previous assays had been performed at 50 μg ml−1 bacitracin, which is lethal to this strain and therefore led to the earlier observation of low signaling output (10). We attribute this phenotype to very weak expression of bceB due to the poor ribosome binding site of the gene, resulting in low levels of the transporter in the cell and thus in increased bacitracin stress and associated signaling at lower concentrations. This construct was ideally suited for the identification of loss-of-function mutations, because screening could be carried out at concentrations that are sublethal even for strains with defects in bceAB (MIC = 4 μg ml−1; see below and Table 3).
Table 3.
Summary of mutations analyzed in this study
| Amino acid exchangea | MIC (μg/ml)b | Signaling phenotypec | Interaction with BceSd |
|---|---|---|---|
| A75T | 8–16 | Altered sensitivity | + |
| R83W | 4–8 | Altered sensitivity | + |
| G215R | 8–16 | Low signal output | − |
| S219F | 4–8 | Wild type like | + |
| S219C | 4 | No activity | ND |
| G247R | 4 | No activity | ND |
| A301T | 8–16 | Low signal output | + |
| S316L | 4 | Low signal output | + |
| G525D | 4–8 | No activity | + |
| G535E | 4–8 | Altered sensitivity | + |
| S542L | 8–16 | Altered sensitivity | + |
| L567P | 32 | Altered sensitivity | ND |
| C544Y | 8 | No activity | + |
| M551I | 16 | Low signal output | + |
| G609R | 4–8 | Altered sensitivity | ND |
| M616I | 8–16 | Wild type like | + |
| V619M | 8–16 | Low signal output | + |
The following mutations showed wild-type behavior: R9Q, V22M, A117T, V130I, G381D, V426I, H430Y, G593D, T624I, and S628P.
Data represent MIC of bacitracin. MICs between two values showed variable results between biological triplicates; the MIC for a strain carrying the wild-type construct is 32 μg/ml and for TMB035 (bceAB::Kan) is 4 μg/ml.
Categories of phenotypes as detailed in the text and shown in Fig. 2.
As determined by bacterial two-hybrid assays (Fig. 4). +, interaction; −, no interaction; ND, not determined.
Chemically mutagenized plasmid DNA of pER703 was used to transform strain TMB301, which carries a deletion of bceB and a PbceA-lacZ reporter construct (10). After growth on indicator plates containing 0.5 μg ml−1 bacitracin and X-Gal, colonies containing functional copies of bceB were dark blue, while those carrying defective constructs were white. We screened approximately 20,000 colonies, leading to the identification of 33 clones with an amino acid exchange causing a loss of signaling activity. Most constructs carried only a single point mutation, and some exchanges were obtained in multiple independent clones (three clones with A301T, two with M551I, and two with a mutation of S219 to F or P, respectively), indicating nearly complete saturation of the screen.
In total, 28 different amino acid exchanges were identified. A striking first observation was an accumulation of mutations in the C-terminal region (from position 523) of BceB, particularly in the eighth and tenth transmembrane helices (Fig. 1). To further characterize the obtained mutations, 26 amino acid exchanges were reconstructed individually using site-directed mutagenesis of plasmid pSG704. This construct contains the entire transporter operon bceAB under the control of Pxyl and shows much stronger complementation of both resistance and signaling activities in a bceAB-deleted strain than pER703 (see below). Importantly, the xylose-inducible promoter ensured equal bceAB expression levels for all of the mutants, irrespective of their signaling activity. Two mutations (T409I and S512L) could not be reconstructed despite several attempts and were therefore not analyzed further. Additionally, the serine residue at position 219 was replaced not only by Phe as in the original mutation but also by Cys to introduce a more conserved change (-OH by -SH group; see below). All 26 exchanges are summarized in Table 3.
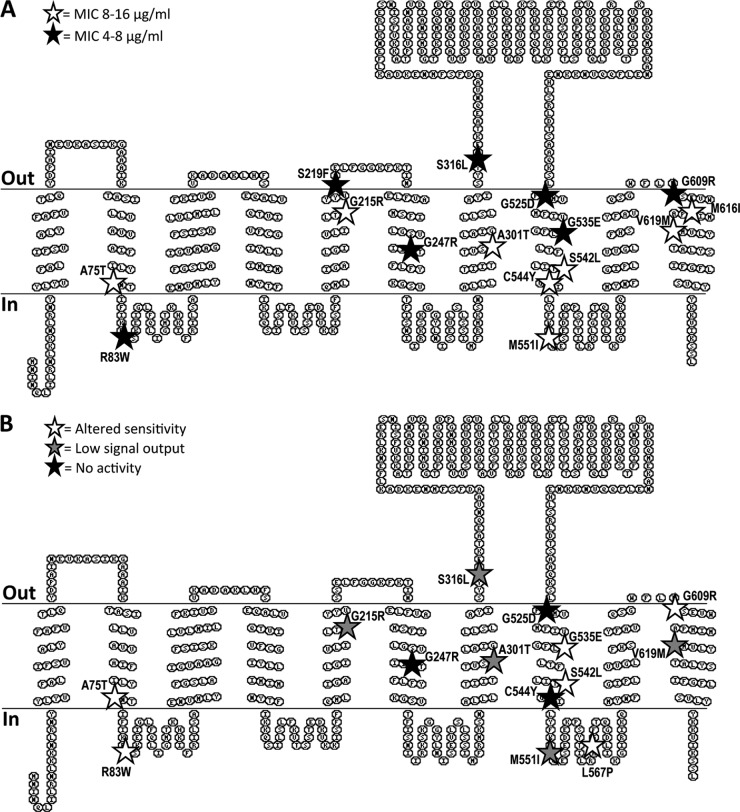
Fig 1.
Distribution of amino acid exchanges in BceB by effects on resistance and signaling. The position of each amino acid exchange in the predicted transmembrane topology of BceB is marked by a star, and the exact exchange is given. Shading of stars is according to the effects of each mutation on bacitracin detoxification (A) or activation of signal transduction (B). Exchanges resulting in wild-type behavior are not shown. Details on the observed phenotypes are given in the text and Table 3. Topology prediction was carried out by MemBrain (30), and the results are displayed using TOPO2 (http://www.sacs.ucsf.edu/). The cytoplasmic membrane is indicated by horizontal lines, and the orientation of the schematic is given on the left.
Resistance behavior of mutant derivatives.
Because the identification of mutations was based on their loss of signaling activity, it was first tested whether they were still able to confer bacitracin resistance or whether both functions of the transporter were affected. For this, the wild-type construct pSG704 and all derived mutant constructs were introduced into strain TMB035 (bceAB::Kan). The MIC of TMB035 was determined as 4 μg ml−1 bacitracin and that of SGB170 (bceAB::Kan transformed with wild-type pSG704) as 32 μg ml−1. It should be noted that the parental strain B. subtilis W168 has an MIC for bacitracin of 128 μg ml−1 and that pSG704 does not fully complement the deletion of bceAB. For the purpose of discussing the obtained mutations, the reduced MIC of strain SGB170 is referred to as the “wild-type” resistance level throughout this article.
The strains carrying mutated complementation constructs fell into three groups of resistance phenotypes (Table 3). Seven mutations caused a (nearly) complete loss of resistance, as seen by MIC values of 4 or of 4 to 8 μg ml−1. Eight mutations retained a partial ability to confer resistance, with the resulting strains displaying MICs between 8 and 16 μg ml−1, and the remaining 11 mutations did not appear to affect resistance (MIC = 32 μg ml−1). Mapping these different categories of phenotypes onto the predicted transmembrane topology of BceB did not reveal any obvious correlation between the position in the protein and the severity of the mutation's effect, apart from the general clustering of mutations in the C-terminal half of BceB as mentioned above (Fig. 1A).
Signaling behavior of mutant derivatives.
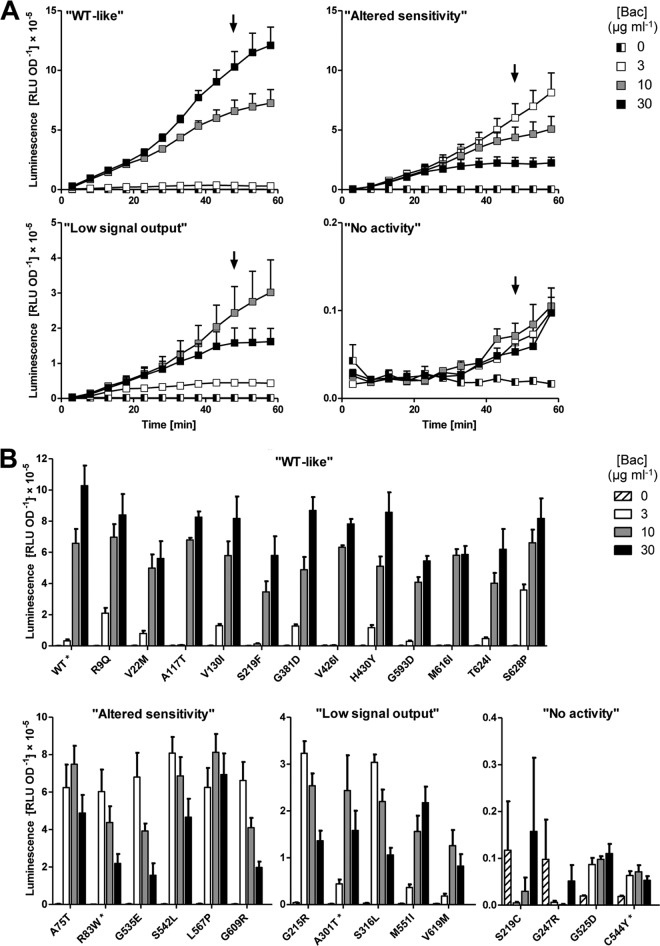
For a detailed analysis of the signaling defects caused by the identified amino acid exchanges, the wild-type plasmid pSG704 and mutated derivatives were introduced into strain SGB079 that carries a deletion of bceAB and a reporter construct of the target promoter PbceA fused to luciferase. The strain harboring the wild-type construct showed only low basal expression of the promoter in the absence of bacitracin (ca. 2 × 103 RLU OD−1), which was induced 10-fold in the presence of 3 μg ml−1 of bacitracin. Addition of 10 or 30 μg ml−1 bacitracin led to a strong upregulation of promoter activity, reaching ca. 6 × 105 and 1 × 106 RLU OD−1, respectively, within 45 min postinduction (Fig. 2A, panel “WT-like”).
Fig 2.
Signal transduction activities. (A) Time courses of promoter induction after addition of Zn2+-bacitracin (Bac; concentrations are given in the key) in strains harboring the PbceA-luxABCDE reporter construct pSDlux101. Bacitracin was added to exponentially growing cultures at time point 0 min, and luminescence (relative luminescence units, RLU) and cell growth (optical density at 600 nm, OD) were measured in 5-min intervals. Luminescence was normalized to cell density and is expressed as RLU OD−1. Example graphs for strains displaying the four observed categories of signaling phenotypes are shown [“WT-like,” strain SGB082 carrying the wild-type sequence construct pSG704; “Altered sensitivity,” strain carrying pSG704(R83W); “Low signal output,” strain carrying pSG704(A301T); “No activity,” strain carrying pSG704(C544Y)]. The arrows indicate the time (48 min postinduction) used to compare all mutated strains in panel B. (B) Dose-response behavior of all analyzed strains at 48 min postinduction. The example strains also shown in panel A are indicated by asterisks. Phenotype categories and labeling of symbols are as described for panel A. All data shown are the means ± standard errors of the means of the results determined with four to six biological replicates.
Analysis of the mutated constructs showed that their signaling phenotypes could be classified into four different categories. About half of the derived strains displayed behavior similar to that of the wild type, with maximum induction occurring after addition of 30 μg ml−1 bacitracin and reaching between 60% and 90% of the maximal activity compared to the wild type (Fig. 2B, panel “WT-like”). Six mutants also reached between 60% and 80% of the maximal activity, and yet they responded most strongly to the lowest concentration of 3 μg ml−1 bacitracin and had reduced activities at 10 or 30 μg ml−1 (Fig. 2, panels “Altered sensitivity”). Most of these strains also had low MIC values, and we speculate that this reduced ability to detoxify bacitracin may be the actual cause of the signaling “defects”: poor efficiency in removing the antibiotic would cause increased bacitracin stress at low concentrations, leading to an increase in signaling compared to the wild-type level. At concentrations above the MIC (i.e., 10 or 30 μg ml−1), growth inhibition and cell death would then cause an apparent reduction in promoter activation. Apart from the changed dose-response behavior, the overall output of this group of BceB derivatives was high (ca. 80% of the wild-type level; Fig. 2), suggesting that the signaling pathway itself was intact in these proteins. The third category of mutants displayed overall low levels of promoter induction, reaching only 20% to 30% of the wild-type level. Their dose-response behavior differed between strains (Fig. 2, panels “Low signal output”). The final group was comprised of four strains that were unable to induce expression of the reporter construct (Fig. 2, panels “No activity”). The apparent slight increase between 40 and 60 min postinduction is likely an experimental artifact derived from normalizing luminescence values to OD600 values near zero, due to addition of lethal bacitracin concentrations. A comparison of signaling phenotypes to bacitracin resistance for each mutant is presented in Table 3.
To determine if there was a correlation between the position of the mutated amino acid in the protein and the observed phenotype, all mutations with an effect on signaling were mapped onto the predicted transmembrane topology of BceB (Fig. 1B). Those mutations with a strong effect (categories “no activity” or “low signal output”; black and gray stars in Fig. 1B) appeared to predominantly lie in the second half of the protein, particularly in the transmembrane helices flanking the extracellular domain of BceB.
Of the 26 amino acid exchanges constructed, 10 displayed wild-type behavior for both signaling and resistance activities. These were either false-positive hits from the screen or had been identified in plasmids containing multiple point mutations and were not further characterized.
Comparison of BceB production levels.
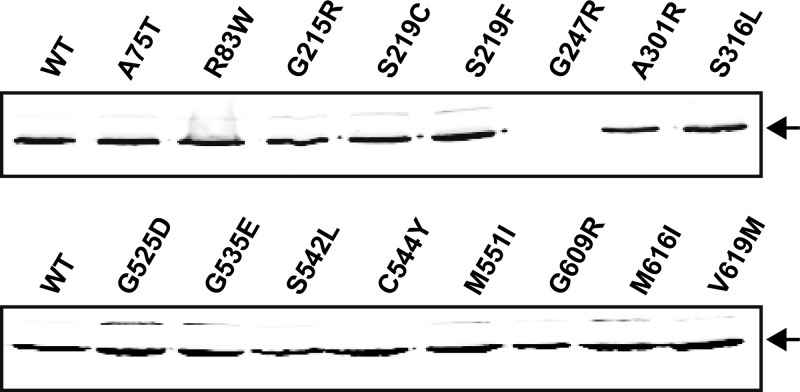
To enable interpretation of the observed effects caused by the mutations, it was first important to test whether there were any differences in production levels of the derived proteins. For this, the plasmid construct used for the characterizations described above was modified to contain a triple FLAG tag on the C terminus of BceB (pFK727), which did not affect the activity of the protein (not shown). After introduction of this plasmid into TMB035 (bceAB::Kan), a single band could be detected in membrane fractions by anti-FLAG Western blot analysis (Fig. 3). This band migrated at ca. 60 kDa, which corresponds to the migration of BceB after expression and purification from E. coli membranes (our own unpublished results). All mutations that caused a defect in at least one of the activities tested as described above were then introduced into pFK727 and expression levels compared to the wild-type construct. With the exception of BceB carrying the G247R substitution, which was not detectable by Western blotting, all derivatives of BceB were produced at the same level as the wild-type protein (Fig. 3). Therefore, the observed phenotypes were direct effects of the amino acid exchanges and cannot be explained by altered protein expression.
Fig 3.
Production and membrane localization of BceB and its derivatives. Single point mutations were generated in pFK727 and transformed into TMB035. Membrane fractions (30 μg) of strains carrying the wild-type sequence construct (WT) or derived mutated constructs were separated on a 12% denaturing polyacrylamide gel and analyzed by Western blotting using an anti-FLAG antibody as described in Materials and Methods. The only detected band in each lane migrated at a size of ca. 60 kDa, indicated by the arrows.
Protein-protein interactions between BceB and BceA or BceS.
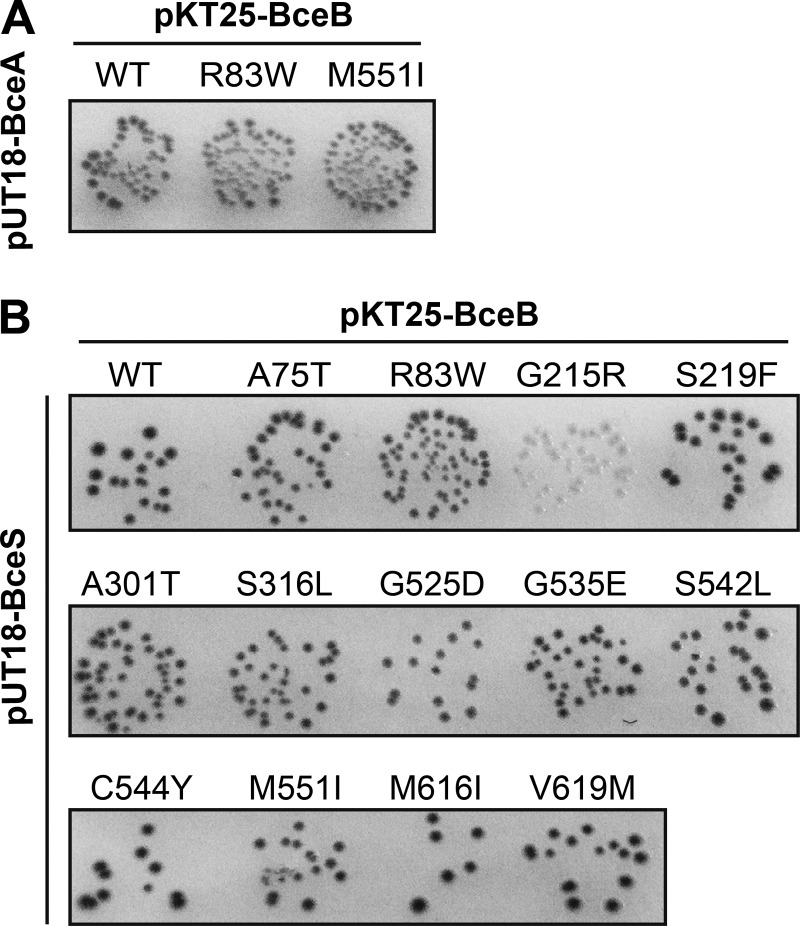
Because the signaling pathway within Bce-like modules involves both the transporter and the histidine kinase (10, 26) and because the permeases and histidine kinases were shown to have coevolved (9), direct interactions between the two proteins have been proposed. The first experimental evidence for this was obtained for a Bce-like system from S. aureus, using a bacterial two-hybrid assay (15). In order to test these interactions within the Bce module of B. subtilis, we fused BceB, BceS, and BceA to the T18 and T25 domains of bacterial adenylate cyclase (22) and tested these constructs for interaction. The optimal combination for interactions between the permease and ATPase components of the transporter was found to be BceA-T18 (pUT18-BceA) with T25-BceB (pKT25-BceB) (Fig. 4A and data not shown). Interaction between BceB and BceS could also be shown, with the strongest results obtained for T25-BceB paired with BceS-T18 (pUT18-BceS) (Fig. 4B and data not shown).
Fig 4.
Protein-protein interactions of BceB and its derivatives. BceB and derivatives were tested for interaction with BceA (A) and BceS (B). Single point mutations were generated in pKT25-BceB and cotransformed with either pUT18-BceA or pUT18-BceS as indicated into E. coli BTH101. Cells were spotted onto LB plates containing X-Gal (40 μg ml−1), IPTG (0.5 mM), and antibiotics for selection. Pictures were taken after 48 h of incubation at 30°C. The blue colonies indicating positive results for interaction are depicted as dark gray in the gray-scale image. The different intensities of colony coloration within individual spots were a common observation that differed between experiments and were most likely due to different levels of protein expression in individual transformants.
Two replacements, R83W and M551I, that both affected signaling and resistance are located in cytoplasmic loops of BceB (Fig. 1). It was therefore conceivable that they affected the interaction between permease and ATPase of the transporter. While there is no conservation in sequence or even location of the interface between permease and ATPase among the different types of ABC transporters, in all cases the interaction involves a small cytoplasmic alpha-helix of the permease (27, 28). Because the two cytoplasmic loops harboring the amino acids 83 and 551, respectively, possess predicted alpha-helical structures and are thus candidate regions for interaction, we introduced these two mutations into the two-hybrid plasmid pKT25-BceB and tested the constructs against BceA-T18. No differences compared to the wild-type constructs were observed (Fig. 4A), showing that the physical interaction between BceB and BceA was not affected. However, this does not exclude potential defects in the coupling of ATP hydrolysis to transport.
We next introduced mutations that caused a signaling defect into pKT25-BceB and tested for interaction with BceS. Again, most constructs showed the same blue coloration of colonies as seen with wild-type BceB, with the exception of G215R, which produced white colonies and was thus no longer able to interact with BceS (Fig. 4B). These results show that the majority of mutations did not abolish the physical interaction between the transporter and histidine kinase but rather affected signaling by more subtle changes.
Genetic separability of signaling and resistance.
Many of the mutations identified in this study affect signaling and resistance to similar degrees; however, several amino acid replacements caused drastic defects in only one of the two functions of the transporter, pointing toward a genetic separability of the two traits.
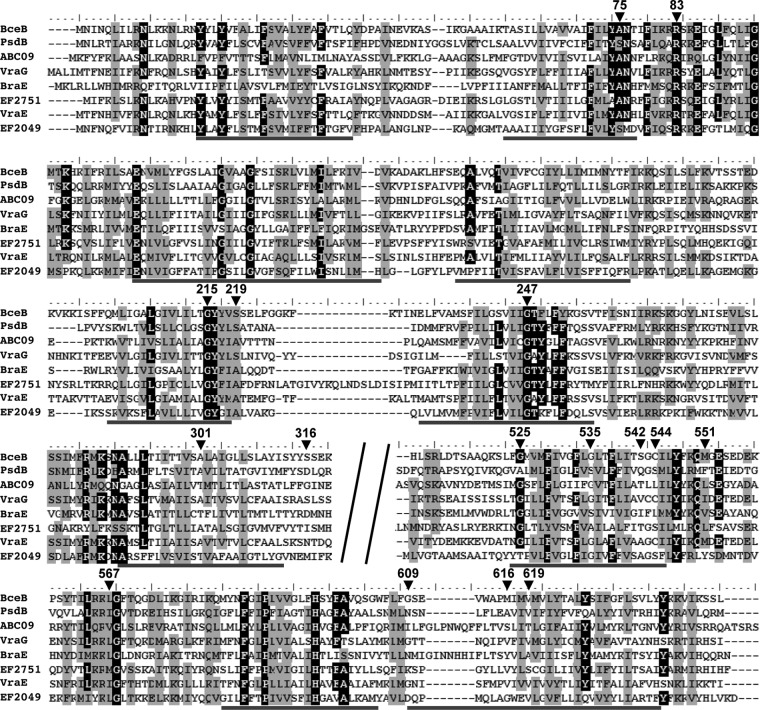
The first group of such mutations includes A301T, M551I, and V619M. In all three cases, signaling was reduced to ca. 10% to 20% of the wild-type activity (Fig. 2B), and yet resistance was still maintained at a significant level (MIC = 16 μg ml−1; Table 3). The latter result shows that transport itself was affected only weakly by the mutation and yet communication with the histidine kinase was severely impaired. The mutations A301T and M551I were isolated in three and two independent clones, respectively, during our initial screen. A multiple sequence alignment of eight BceB homologues shows that the position corresponding to A301 of BceB is occupied by Ala or Thr in other transporters (Fig. 5). At the position corresponding to M551, homologous transporters possess other hydrophobic residues, such as Leu, Val, and Ile, while position 619 contains small residues such as Val, Ala, and Ser (Fig. 5). In all three of these strains, the amino acid exchanges are therefore fairly conservative and thus are unlikely to cause major structural changes within the transporter. This is consistent with the unchanged ability of all three proteins to physically interact with BceS in two-hybrid assays. It is tempting to speculate that these amino acids are important for the communication between transporter and histidine kinase. However, whether they are involved in any direct interactions or are merely required to transmit a signal (e.g., conformational change) within the transporter to the interacting region(s) cannot be answered at this stage.
Fig 5.
Multiple sequence alignment of BceB and homologs. The amino acids of BceB that were changed during mutagenesis are indicated by arrowheads, and their position numbers are given. The alignment was calculated using default settings of ClustalW implemented in BioEdit (31). Predicted transmembrane helices of BceB are marked by gray lines below the alignment. The poorly conserved region of the extracellular domain (residues 320 to 510 [BceB numbering]) was removed, as indicated by the diagonal black bars. Identical residues are shown as white letters on black fields and similar residues as black letters on a gray background. The threshold conservation for shading was 70%. Names of proteins are given on the left. BceB and PsdB are from B. subtilis, ABC09 is from L. casei, VraG, BraE, and VraE are from S. aureus, and EF2751 and EF2049 are from E. faecalis (see the text for references).
The opposite effect is observed with the replacement S219F: while signaling was maintained at ca. 60% of the wild-type level and the dose-response behavior was unaltered, this mutation caused a complete loss of resistance (MIC = 4 μg ml−1; Fig. 2B and Table 3). In a previous study in S. aureus, it was shown that a Bce-like transporter that was involved only in signaling and not in resistance still required ATP hydrolysis and thus active transport for signal transduction (14). This implies that B. subtilis BceB carrying the S219F mutation must still be able to transport bacitracin to facilitate its nearly wild-type signaling behavior, which subsequently gives rise to the question of why it is unable to mediate resistance. A possible explanation may be that the affinity for or transport rate of bacitracin is too low to detoxify the antibiotic but is still sufficient for signaling. The idea of a potential role of S219 in substrate binding or translocation might be supported by its location on the extracellular face of the cytoplasmic membrane (Fig. 1) in the vicinity of the cellular target of bacitracin, undecaprenyl pyrophosphate. Further studies on substrate binding and transport will be required to answer this.
As mentioned above, in a second clone of our initial screen an S219P mutation was identified, emphasizing the importance of this position. The alignment of BceB homologues shows that this position is commonly occupied by the small residue Ser or Ala (Fig. 5), whereas both isolated mutations caused changes to bulky amino acids. We therefore reconstructed the exchange with a replacement by Cys, which merely changes the hyroxyl side chain of Ser to a sulfhydryl group. Surprisingly, this replacement led to a complete loss of function (Table 3), possibly caused by oxidation of the Cys side chain (although BceB does not contain any other extracellular Cys residues that might form disulfide bridges with Cys219), and again supports the idea of the functional importance of position 219 in the transporter.
DISCUSSION
In this study, we chose a random mutagenesis approach to identify residues and regions in the permease of the bacitracin transporter BceAB that are involved in mediating resistance or signaling to the histidine kinase. Fifteen such positions were found. Mapping these amino acids onto the predicted transmembrane topology of BceB showed an accumulation of mutations in the C-terminal half of the protein, with six mutations clustering in transmembrane helix VIII and the adjacent cytoplasmic loop (Fig. 1). This correlation becomes even more pronounced when the degree of conservation of each position is considered: four of the five mutations identified in the N-terminal half of the protein (up to transmembrane helix VI) affect highly conserved positions, i.e., A75, R83, G215, and G247 (Fig. 5). It is therefore likely that these amino acids have a structural role in the transporter and that no or little functional information can be obtained from their characterization. In particular, Gly residues within transmembrane helices, such as G215 and G247, are often important for helix packing in membrane proteins (29) and mutations are unlikely to be tolerated. Consistently, BceB carrying the exchange G247R was no longer produced (Fig. 3), and the mutation G215R abolished physical interaction with BceS (Fig. 4).
In contrast, only one of the six mutations located in transmembrane helix VIII and the adjacent cytoplasmic loop affected a highly conserved position, G525 (Fig. 5), and, again consistent with a structural role of this Gly residue, this mutation led to a complete loss of signaling and resistance activities (Table 3). The remaining five positions, G535, S542, C544, M551, and L567, are conserved only weakly or not at all (Fig. 5), and even drastic mutations such as Gly to Asp or Glu still allowed at least partial activity of the transporter (Table 3). This region of BceB is therefore clearly important for the specific functions rather than the global structure of BceB. Because most of these mutations affect both signaling and resistance, the low degree of conservation at these positions may point toward a role either in substrate specificity or in communication with BceS. It is, however, also conceivable that the function of this transmembrane helix is the transmission of a signal within the transporter. The location of transmembrane helix VIII immediately adjacent to the large extracellular domain, which was shown to constitute the determinant of substrate specificity in BceB-like transporters from S. aureus (14), might well support the latter hypothesis, where extracellular binding of bacitracin may be communicated to the cytoplasmic face of the membrane or even further toward the C-terminal end of the protein. Interestingly, another cluster of mutations was found in the last transmembrane helix, and these also affect positions of low sequence conservation (Fig. 5). However, as these mutations caused differing defects in the transporter (Fig. 1 and Table 3), their functional role remains unclear. There is as yet no clear indication of where the interaction between BceB and BceS occurs. An extracellular interaction appears unlikely, because BceS contains only three predicted extracellular residues. Instead, an interaction either within the membrane or between cytoplasmic domains might be proposed. Such physical contacts could then transmit conformational changes from the transporter to the histidine kinase, causing changes in the autophosphorylation activities. Either potential interaction site could explain the accumulation of mutations in transmembrane helix VIII and the adjacent loop. However, with the current lack of in vitro data, such a model has to remain speculation.
Despite its large size of over 200 amino acids, no mutation could be identified in the extracellular domain of BceB. This is consistent with the very low degree of sequence conservation of this region across all BceB homologues identified to date, even among transporters that share bacitracin as a substrate (9). Our results therefore confirm the previously observed enormous freedom in sequence space of this domain, while it still retains full activity.
Several mutations led to nearly complete loss of both activities. These included all four mutations that were classified as “No activity” in the signaling assays (Fig. 2), which all were also unable to impart bacitracin resistance (MIC of 4 to 8 μg ml−1; Table 3). A similar effect, albeit with 20% to 30% residual signaling activity, was observed for mutations S316L and G215R (Fig. 2 and Table 3). These mutations therefore appear to cause global defects in the transporter. Of these, the mutation G215R presents an interesting situation: the maximal promoter activation obtained in signaling assays reached ca. 30% of wild-type levels (Fig. 2), and yet in bacterial two-hybrid assays this BceB derivative showed no interaction with BceS (Fig. 4). As discussed above, exchange of the highly conserved, membrane-located Gly residue for an Arg most likely introduced structural changes in the transporter, and these appeared to have strong effects on protein-protein interactions within the Bce module. However, the significant signaling activity observed suggests that while these interactions are sufficiently weakened to give negative results in the two-hybrid assay, the signal can still be passed to the histidine kinase. This is in contrast to the remaining mutations that inhibit signaling but all still allow a physical interaction in the two-hybrid analyses (Fig. 4 and Table 3). In these cases, the single amino acid exchange appears to be insufficient to destroy the protein-protein interactions but may rather affect the flow of information between BceB and BceS. Alternatively, the effects of the mutation may have no direct connection to the signaling process itself but may rather affect the transport process or transmission of conformational changes within the transporter as discussed above.
In the course of earlier studies on BceAB-like transporters, several systems could be identified that no longer possess the dual functions for signaling and resistance but have become specialized signaling or detoxification transporters. With the aim of identifying specific residues for one or the other function, examples for all three types of BceAB homologues were chosen for the multiple sequence alignment shown in Fig. 5. BceB and PsdB of B. subtilis as well as ABC09 of L. casei belong to dual-function transporters (10, 11, 17). VraG and BraE of S. aureus are the permeases of transporters that fulfill only a sensory function (14, 15), which is also the case for EF2751 of Enterococcus faecalis (our own unpublished results). As examples for transporters that mediate resistance but no signaling, the permeases VraE of S. aureus (14) and EF2049 of E. faecalis (our own unpublished data) were chosen. Comparison of these sequences and the positions identified as important in our mutagenesis screen did not reveal any patterns of sequence conservation that may be attributed to a particular role in either signaling or resistance.
Nevertheless, we were able to show at least partial genetic separability of the two traits. Three exchanges (A301T, M551I, and V619M) were associated with a strong reduction in signaling activity but retained the ability to mediate significant bacitracin resistance. One BceB derivative (S219F) displayed a complete loss of resistance but was still capable of signaling (Fig. 2 and Table 3). While the maximal signaling output was reduced to ca. 60% of the wild-type level, it should be noted that the bacitracin concentration used (30 μg ml−1), was well above the MIC value of the strain (4 μg ml−1), which may lead to an underestimation of the actual signaling activity of the transporter.
Furthermore, these results, particularly those from the three mutations mentioned above (A301T, M551I, and V619M), help to answer a long-asked question of the transporter's role in signaling. It has been proposed that BceAB might in fact function as an importer rather than an exporter (10, 14). If this were the case, its sole function in signaling might then be to transport bacitracin to the cytoplasm where BceS might detect the antibiotic directly. This hypothesis can now be refuted: BceAB carrying one of these three mutations is still able to transport bacitracin at sufficient rates to impart resistance. Thus, if it actually imports bacitracin, resulting intracellular concentrations similar to those seen with the wild type would have to be expected, and accordingly, if BceS were a sensor of intracellular bacitracin, wild-type-like signaling output should be observed. Yet only very low signaling is observed, demonstrating that not BceS but the transporter itself, irrespective of the direction of transport, is indeed the sensory component of the module. Therefore, our findings not only show that signaling and resistance are not strictly coupled functions of BceB but also provide some valuable insights into the mechanism of these unique resistance modules.
In summary, we here showed that mutations that affect specific functions of BceAB are primarily found in the C-terminal half of the permease, particularly in transmembrane helix VIII. This region will therefore be a primary target for future investigations into the signaling and resistance mechanisms of this unusual transporter. Our results further demonstrate that while signaling and resistance are functionally interconnected, they are not strictly coupled processes, even in a transporter like BceAB that is capable of both activities.
ACKNOWLEDGMENTS
We thank Anna Staroń for cloning of the bacterial two-hybrid constructs, Stefanie Zapf and Ina Lackerbauer for technical assistance, Richard Losick for supplying the vector pAH328, and Thorsten Mascher and Ralf Heermann for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgesellschaft (GE2164/3-1) and the Fonds der Chemischen Industrie.
F.K. performed the site-directed mutagenesis, characterized the mutants, and participated in cloning of constructs; S.D. performed the Western blot and two-hybrid analyses and participated in cloning of constructs and site-directed mutagenesis; R.S. performed the random mutagenesis; and S.G. designed the study, coordinated the experimental work, and wrote the manuscript.
Footnotes
Published ahead of print 17 May 2013
REFERENCES
- 1. Breukink E, de Kruijff B. 2006. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5:321–323 [DOI] [PubMed] [Google Scholar]
- 2. Guder A, Wiedemann I, Sahl HG. 2000. Posttranslationally modified bacteriocins—the lantibiotics. Biopolymers 55:62–73 [DOI] [PubMed] [Google Scholar]
- 3. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 4. Storm DR, Strominger JL. 1973. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J. Biol. Chem. 248:3940–3945 [PubMed] [Google Scholar]
- 5. Draper LA, Ross RP, Hill C, Cotter PD. 2008. Lantibiotic immunity. Curr. Protein Pept. Sci. 9:39–49 [DOI] [PubMed] [Google Scholar]
- 6. Alkhatib Z, Abts A, Mavaro A, Schmitt L, Smits SHJ. 2012. Lantibiotics: how do producers become self-protected? J. Biotechnol. 159:145–154 [DOI] [PubMed] [Google Scholar]
- 7. Neumüller AM, Konz D, Marahiel MA. 2001. The two-component regulatory system BacRS is associated with bacitracin “self-resistance”of Bacillus licheniformis ATCC 10716. Eur. J. Biochem. 268:3180–3189 [DOI] [PubMed] [Google Scholar]
- 8. Gebhard S. 2012. ABC transporters of antimicrobial peptides in Firmicutes bacteria—phylogeny, function and regulation. Mol. Microbiol. 86:1295–1317 [DOI] [PubMed] [Google Scholar]
- 9. Dintner S, Staroń A, Berchtold E, Petri T, Mascher T, Gebhard S. 2011. Coevolution of ABC transporters and two-component regulatory systems as resistance modules against antimicrobial peptides in Firmicutes Bacteria. J. Bacteriol. 193:3851–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rietkötter E, Hoyer D, Mascher T. 2008. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 68:768–785 [DOI] [PubMed] [Google Scholar]
- 11. Staroń A, Finkeisen DE, Mascher T. 2011. Peptide antibiotic sensing and detoxification modules of Bacillus subtilis. Antimicrob. Agents Chemother. 55:515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joseph P, Fichant G, Quentin Y, Denizot F. 2002. Regulatory relationship of two-component and ABC transport systems and clustering of their genes in the Bacillus/Clostridium group, suggest a functional link between them. J. Mol. Microbiol. Biotechnol. 4:503–513 [PubMed] [Google Scholar]
- 13. Mascher T. 2006. Intramembrane-sensing histidine kinases: a new family of cell envelope stress sensors in Firmicutes bacteria. FEMS Microbiol. Lett. 264:133–144 [DOI] [PubMed] [Google Scholar]
- 14. Hiron A, Falord M, Valle J, Débarbouillé M, Msadek T. 2011. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 81:602–622 [DOI] [PubMed] [Google Scholar]
- 15. Falord M, Karimova G, Hiron A, Msadek T. 2012. GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 56:1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouyang J, Tian XL, Versey J, Wishart A, Li YH. 2010. The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob. Agents Chemother. 54:3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revilla-Guarinos A, Gebhard S, Alcántara C, Staroń A, Mascher T, Zúñiga M. 2013. Characterization of a regulatory network of peptide antibiotic detoxification modules in Lactobacillus casei BL23. Appl. Environ. Microbiol. 79:3160–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebhard S, Mascher T. 2011. Antimicrobial peptide sensing and detoxification modules: unravelling the regulatory circuitry of Staphylococcus aureus. Mol. Microbiol. 81:581–587 [DOI] [PubMed] [Google Scholar]
- 19. Harwood CR, Cutting SM. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 20. Stülke J, Martin-Verstraete I, Zagorec M, Rose M, Klier A, Rapoport G. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol. Microbiol. 25:65–78 [DOI] [PubMed] [Google Scholar]
- 21. Schmalisch M, Maiques E, Nikolov L, Camp AH, Chevreux B, Muffler A, Rodriguez S, Perkins J, Losick R. 2010. Small genes under sporulation control in the Bacillus subtilis genome. J. Bacteriol. 192:5402–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karimova G, Ullmann A, Ladant D. 2000. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 328:59–73 [DOI] [PubMed] [Google Scholar]
- 23. Derré I, Rapoport G, Msadek T. 2000. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37 degrees C. Mol. Microbiol. 38:335–347 [DOI] [PubMed] [Google Scholar]
- 24. Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59 [DOI] [PubMed] [Google Scholar]
- 25. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 26. Bernard R, Guiseppi A, Chippaux M, Foglino M, Denizot F. 2007. Resistance to bacitracin in Bacillus subtilis: unexpected requirement of the BceAB ABC transporter in the control of expression of its own structural genes. J. Bacteriol. 189:8636–8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollenstein K, Dawson RJ, Locher KP. 2007. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 17:412–418 [DOI] [PubMed] [Google Scholar]
- 28. Dawson RJP, Hollenstein K, Locher KP. 2007. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 65:250–257 [DOI] [PubMed] [Google Scholar]
- 29. Russ WP, Engelman DM. 2000. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296:911–919 [DOI] [PubMed] [Google Scholar]
- 30. Shen H, Chou JJ. 2008. MemBrain: improving the accuracy of predicting transmembrane helices. PLoS One 3:e2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]