Abstract
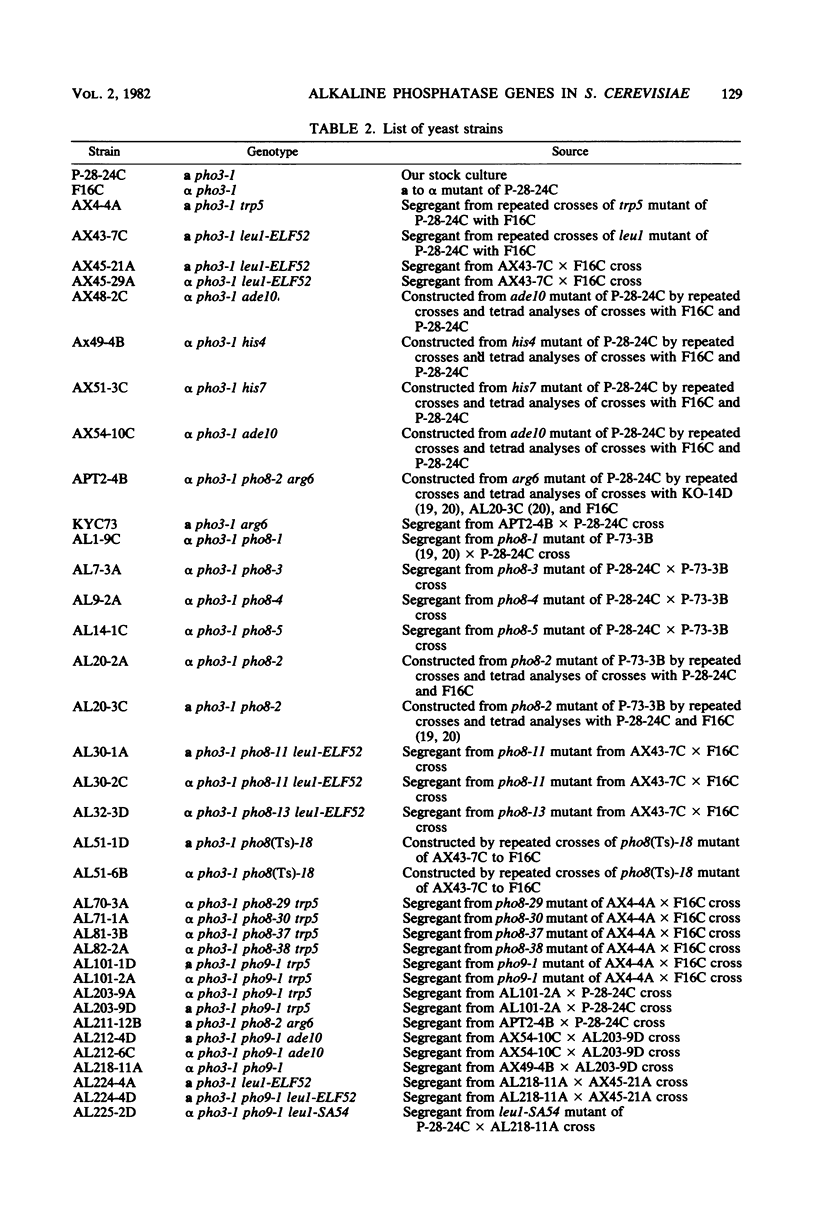
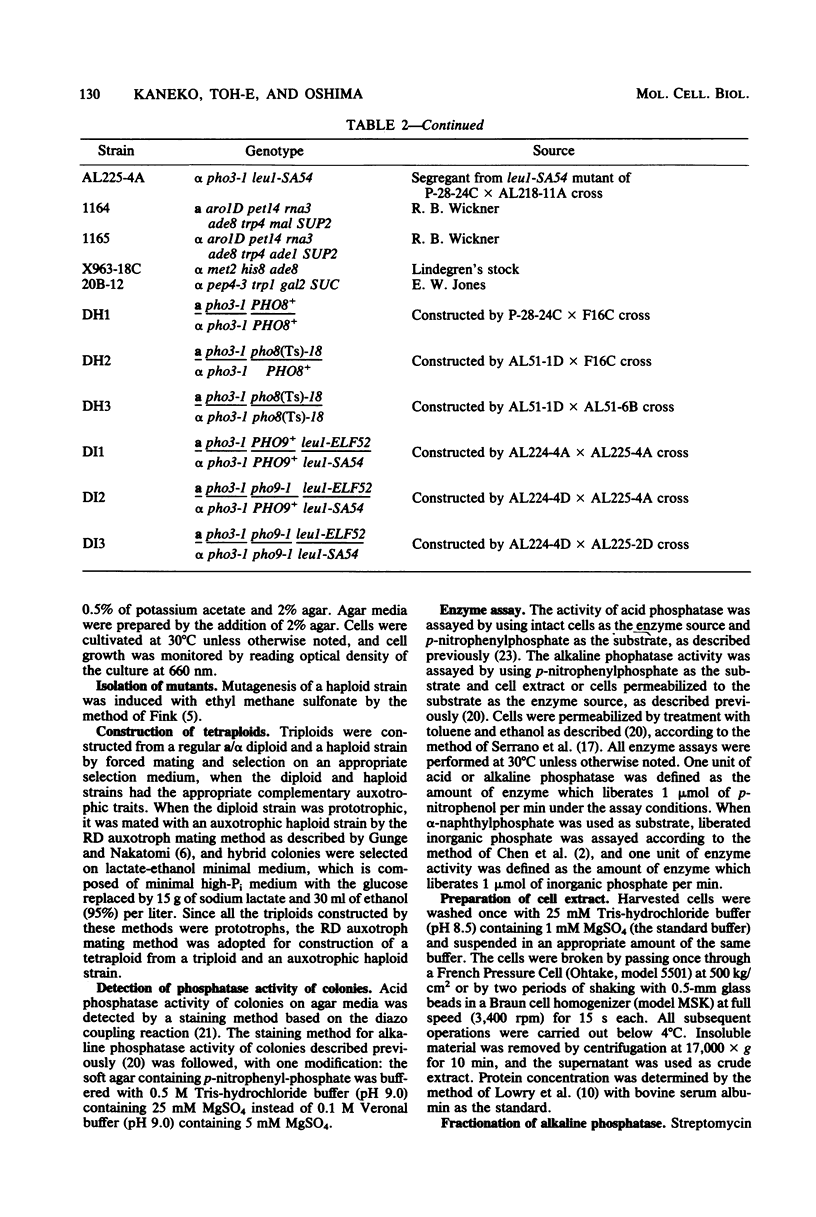
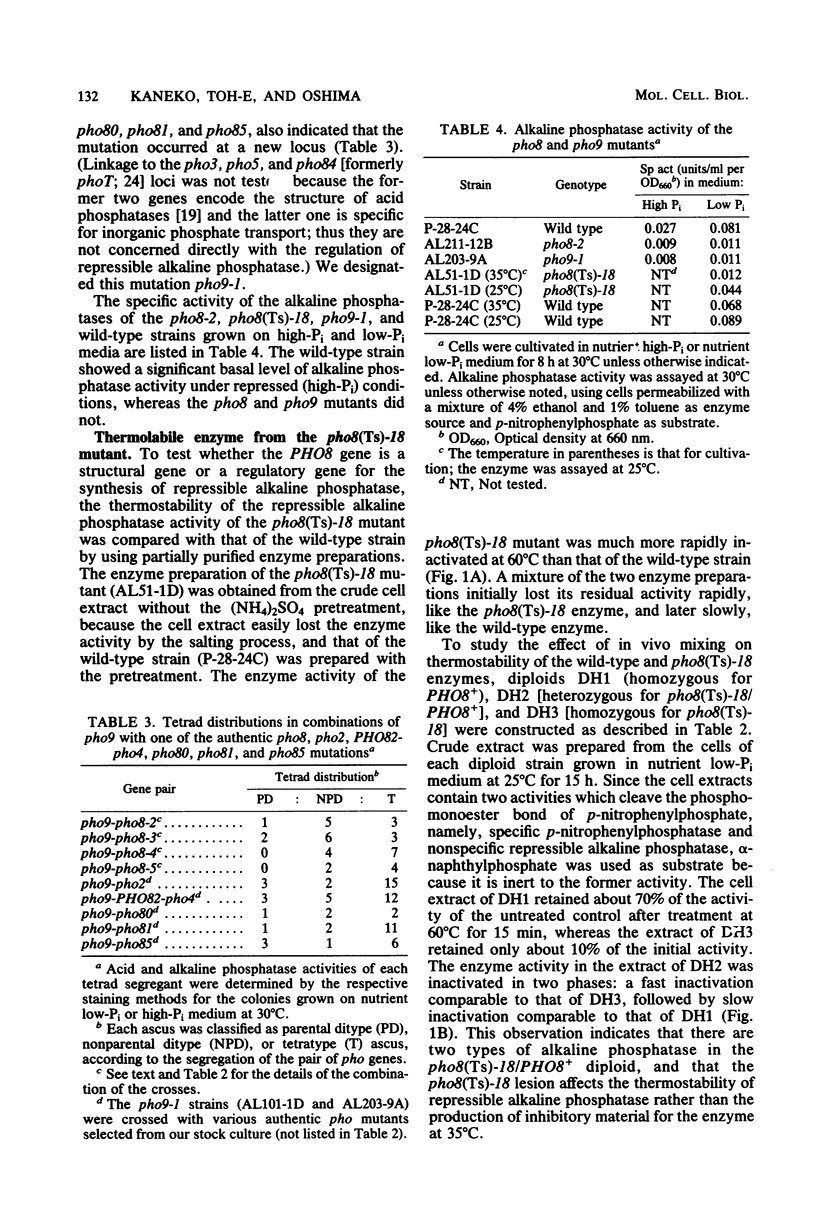
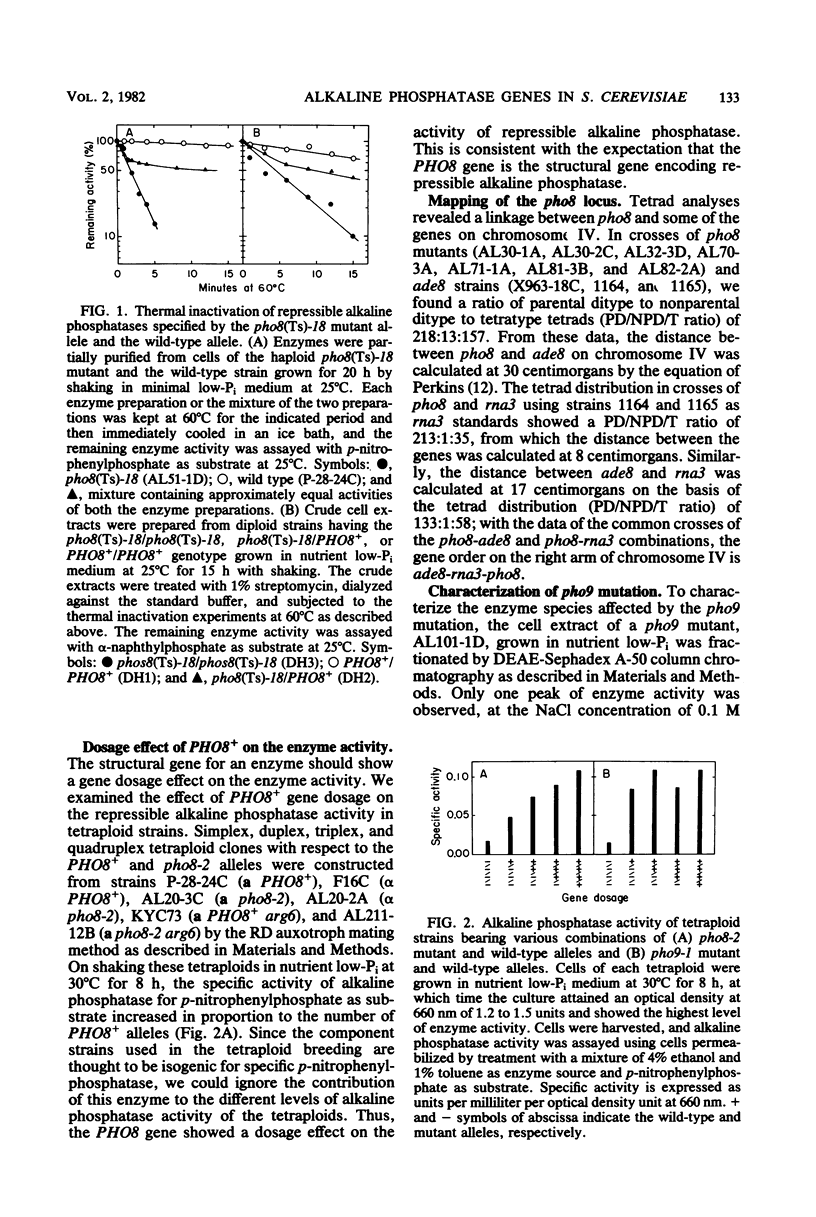
Two lines of evidence showed that the PHO8 gene encodes the structure of repressible, nonspecific alkaline phosphatase in Saccharomyces cerevisiae: (i) the enzyme produced by a temperature-sensitive pho8 mutant at the permissive temperature (25 degrees C) was more thermolabile than that of the wild-type strain, and (ii) the PHO8 gene showed a gene dosage effect on the enzyme activity. The pho8 locus has been mapped on chromosome IV, 8 centimorgans distal to rna3. A new mutant carrying the pho9 gene was isolated which lacks repressible alkaline phosphatase, but has the normal phenotype for the synthesis of repressible acid phosphatase. The pho9 gene segregated independently of all known pho-regulatory genes and did not show the gene dosage effect on repressible alkaline phosphatase activity. The pho9/pho9 diploid hardly sporulated and showed no commitment to intragenic recombination when it was inoculated on sporulation medium. Hence the pho9 mutant has a phenotype similar to the pep4 mutant, which was isolated as a pleiotropic mutant with reduced levels of proteinases A and B and carboxypeptidase Y. An allelism test indicated that pho9 and pep4 are allelic.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostian K. A., Lemire J. M., Cannon L. E., Halvorson H. O. In vitro synthesis of repressible yeast acid phosphatase: identification of multiple mRNAs and products. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4504–4508. doi: 10.1073/pnas.77.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. Genes controlling meiosis and spore formation in yeast. Genetics. 1974 Sep;78(1):215–225. doi: 10.1093/genetics/78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Esposito M. S. Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3172–3176. doi: 10.1073/pnas.71.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Nakatomi Y. Genetic Mechanisms of Rare Matings of the Yeast SACCHAROMYCES CEREVISIAE Heterozygous for Mating Type. Genetics. 1972 Jan;70(1):41–58. doi: 10.1093/genetics/70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Andersen N. Isolation of yeast genes with mRNA levels controlled by phosphate concentration. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6541–6545. doi: 10.1073/pnas.77.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Chromosome replication during meiosis: identification of gene functions required for premeiotic DNA synthesis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3087–3091. doi: 10.1073/pnas.70.11.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. Three forms of the 5.8-S ribosomal RNA species in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jan 3;41(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Nakamura H., Oshima Y. A gene controlling the synthesis of non specific alkaline phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1976 Mar 25;428(1):182–192. doi: 10.1016/0304-4165(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Oshima Y. Characterization of a dominant, constitutive mutation, PHOO, for the repressible acid phosphatase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1974 Nov;120(2):608–617. doi: 10.1128/jb.120.2.608-617.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Inouye S., Oshima Y. Structure and function of the PHO82-pho4 locus controlling the synthesis of repressible acid phosphatase of Saccharomyces cerevisiae. J Bacteriol. 1981 Jan;145(1):221–232. doi: 10.1128/jb.145.1.221-232.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Kakimoto S. Genes coding for the structure of the acid phosphatases in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Dec 30;143(1):65–70. doi: 10.1007/BF00269421. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Oshima Y. A constitutive mutation, phoT, of the repressible acid phosphatase synthesis with inability to transport inorganic phosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1975;136(3):255–259. doi: 10.1007/BF00334020. [DOI] [PubMed] [Google Scholar]
- Ueda Y., To-E A., Oshima Y. Isolation and characterization of recessive, constitutive mutations for repressible acid phosphatase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Jun;122(3):911–922. doi: 10.1128/jb.122.3.911-922.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Jones E. W. Protein degradation, meiosis and sporulation in proteinase-deficient mutants of Saccharomyces cerevisiae. Genetics. 1981 Jan;97(1):45–64. doi: 10.1093/genetics/97.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]



