Abstract
Chronic lower urinary tract symptoms (LUTS), such as urgency and incontinence, are common, especially among the elderly, but their etiology is often obscure. Recent studies of acute urinary tract infections implicated invasion by Escherichia coli into the cytoplasm of urothelial cells, with persistence of long-term bacterial reservoirs, but the role of infection in chronic LUTS is unknown. We conducted a large prospective study with eligible patients with LUTS and controls over a 3-year period, comparing routine urine cultures of planktonic bacteria with cultures of shed urothelial cells concentrated in centrifuged urinary sediments. This comparison revealed large numbers of bacteria undetected by routine cultures. Next, we typed the bacterial species cultured from patient and control sediments under both aerobic and anaerobic conditions, and we found that the two groups had complex but significantly distinct profiles of bacteria associated with their shed bladder epithelial cells. Strikingly, E. coli, the organism most responsible for acute urinary tract infections, was not the only or even the main offending pathogen in this more-chronic condition. Antibiotic protection assays with shed patient cells and in vitro infection studies using patient-derived strains in cell culture suggested that LUTS-associated bacteria are within or extremely closely associated with shed epithelial cells, which explains how routine cultures might fail to detect them. These data have strong implications for the need to rethink our common diagnoses and treatments of chronic urinary tract symptoms.
INTRODUCTION
The diagnosis of urinary tract infections (UTIs) in current clinical practice hinges on the culturing of clean-catch, midstream urine (MSU) samples. Published guidelines for Europe, the United States (1), and the United Kingdom (2) reveal significant discrepancies in the choice of a quantitative threshold to define significant bacteriuria. In the United Kingdom, the diagnosis of UTIs by MSU culture is generally defined by the isolation of ≥105 CFU ml−1 of a single species of a known urinary pathogen to demarcate the border of infection. This criterion, which was described by Kass in 1957 (3), resulted from a study of 74 women with acute pyelonephritis and 444 asymptomatic controls. In a similar study of MSU, Kass sampled women with pyelonephritis, some of whom were pregnant, for the pathological groups (4). Despite the small sample sizes and limited nature of these two studies, more than half a century later the criterion of 105 CFU ml−1 is still in use in many countries for all clinical states of the urinary tract, not just pyelonephritis.
Some of Kass' contemporaries criticized the use of a single threshold for diagnosing UTI in all diseases (5, 6), and recently we and others also have come to question these assumptions (7–9). Biological systems fall along continuous spectra; therefore, a dichotomous judgment of no infection/significant infection using a fixed point, whether this be 105, 104, or 102 CFU ml−1 (9), may be inappropriate, especially for low-grade symptomatic infections. The rejection of mixed growth similarly lacks evidence, as there is no logical reason to assume that infections cannot involve more than one species at a time.
Other common methods of diagnosing UTIs also are problematic. The urinary dipstick test for nitrite and leukocyte esterase, calibrated to the Kass criterion, has been found to be unreliable (10–12). We studied dipsticks, microscopic pyuria, and MSU culture in patients with chronic painless lower urinary tract symptoms (LUTS) and discovered significant sensitivity deficiencies affecting all of these tests (7). Our culture data were very similar to the original findings reported by Stamm et al. in 1982, showing that the Kass threshold is misleading (9). Acute cystitis presenting with frequency and dysuria offers little diagnostic challenge (13), but chronic LUTS are more problematic. Therefore, the discovery of more-sensitive and -reliable diagnostic markers of infection is an important goal.
LUTS are a broad group of signs and symptoms that include the urgency, frequency, and incontinence of overactive bladder, voiding problems such as hesitancy and intermittency, stress incontinence, and pain, all variously overlapping. The prevalence of LUTS increases with age, being reported for 40% of men and 28% of women 70 to 79 years of age (14, 15). Because many of these symptoms overlap those of acute UTIs, the clinician must exclude bacterial infection from the etiology (16). Given the misgivings about the methods that clinicians use to diagnose UTIs, there is no room for complacency over our assumptions about this very important matter.
The situation is further confused by the behavior of uropathogenic microorganisms. In 2003, Anderson et al. (17) reported that uropathogenic Escherichia coli (UPEC) was able to invade the bladder epithelia in a mouse model of acute UTI and to set up intracellular bacterial colonies (IBCs) with biofilm-like characteristics, as well as longer-term quiescent intracellular reservoirs. Thus shielded, the colonies evaded antibiotics and immune surveillance, which left them free to amplify and to emerge at a later time to reinitiate acute infection. Similar findings were described for mouse models (18) and for sloughed urothelial cells from women with acute cystitis (19). In addition, Garofalo et al. took bacteria from patients with various bladder syndromes and showed that they formed IBCs in the murine model (20). These and other studies suggest that E. coli can persist for months in a state of quiescent urothelial parasitization (21) and that the absence of bacterial growth from urine under these conditions might be misleading to the clinician. In the United Kingdom, routine hospital MSU culture usually is performed on media selective for Enterobacteriaceae species under aerobic culture conditions. Anaerobes are not sought, and some aerobes require longer incubation times. Taken together, these observations suggest that current urinalysis methods almost certainly miss many lower-grade or quiescent infections, some of which might be pathogenic, which in turn can lead to the wrong diagnosis and a failure to treat patients properly.
Given these concerns, we set out to reexamine the contribution made by UTIs to the etiology of chronic nondysuric LUTS, including infections with more-fastidious microorganisms. We did this by using alternative culture methods that enrich for a wider range of species and by examining bacteria closely associated with cells shed from patients' urothelia.
MATERIALS AND METHODS
Patients, controls, and sample collection.
Ethical approval to perform this clinical study was obtained from the Moorfields and Whittington Research Ethics Committee (London, United Kingdom). The study was conducted at the LUTS clinic, Whittington Hospital Campus, University College London, between 8 January 2009 and 7 September 2012. Normal volunteers were recruited from among hospital staff members and medical students and were included if they were asymptomatic and without pyuria. Patients ≥18 years of age with chronic LUTS provided informed consent. We recorded demographic data and reported frequency and incontinence; researchers were blind to the clinical data. LUTS were ascertained by questioning the patients using a standard checklist. There are some well-known symptom scales, such as the International Consultation on Incontinence Modular Questionnaire (ICIQ) series (22), that are well validated and can measure changes for a patient, and scores can be normalized for comparative trials. In descriptive studies, adjectival scaling such as “bothersomeness” is affected by variations in interpretations of meaning, causing error (23). To avoid this, we used validated scales that measure symptoms dichotomously as present or absent but introduce scale by counting the number of contexts in which the symptoms are experienced. These are effective measures for cross-sectional observation studies (24–26).
Clean-catch midstream urine (MSU) samples and catheter urine (CSU) samples were collected by standard techniques (27). Volumes of urine varied from patient to patient, but the data were normalized to describe the numbers of bacteria and shed cells per milliliter. To determine pyuria, a fresh 1-μl sample of urine was loaded into a Hawksley DS 748 Neubauer hemocytometer chamber and white blood cells (WBCs) were counted using light microscopy (28). MSU and CSU samples were submitted for routine hospital urine culture as described previously (7); briefly, this involved overnight culture of a 1-μl loop of urine supernatant on chromogenic agar plates under aerobic conditions.
Culture of urinary sediments and initial assessment.
CSU specimens obtained from 55 patients and 26 controls were centrifuged immediately at a relative centrifugal force of 8,000 at room temperature for 5 min. The supernatant was removed, and the sediment was resuspended in normal saline. Serial dilutions to 1 × 10−7 were performed. The resuspended sediment and dilutions were cultured on Columbia blood agar (CBA) plates (E&O Laboratories) and on fastidious anaerobic agar (FAA) plates (E&O Laboratories). The cultured CBA plates were kept in a 5% CO2 incubator at 37°C for 48 h, and the FAA plates were kept at 37°C for 7 days under anaerobic conditions. Following incubation, the different organisms were identified with the aid of standard biochemical tests (API strips; bioMérieux) and quantified. The isolates were cultured separately and stored at −80°C. DNA was extracted from a single bacterial colony isolate using a guanidinium hydrochloride-based DNA extraction kit (Invitrogen), according to the manufacturer's instructions. The purified DNA was used as a template to amplify the 16S rRNA gene using global primers (27f and 1492r; Sigma-Genosys, United Kingdom) via PCR with a Biometra T3000 thermocycler. Cycling conditions were as follows: 5 min at 94°C for initial denaturation and 29 cycles of 1 min at 94°C for denaturation, 1 min at 54°C for annealing, and 1 min at 72°C for extension. After cycling, samples were subjected to a final extension at 72°C for 5 min, followed by cooling to 4°C until the samples were analyzed. DNA was submitted for sequencing at the Cambridge University DNA sequencing facility, for species identification. Sequences were analyzed using the Basic Local Alignment Search Tool (BLAST).
Protocol streamlining and optimization.
In order to expand our survey, we wanted to examine many more patients. After testing samples from the group of 81 individuals described above and analyzing the trends, we optimized the assay as follows. (i) We switched to MSU samples because of patient preference and feasibility in the clinic; this involved giving patients an antiseptic wipe and providing careful instructions on how to collect the sample to avoid contamination (27). (ii) We replaced the aerobic CBA culture medium with chromogenic agar (CPS3; bioMérieux, France), kept at 37°C for 24 h under atmospheric conditions. (iii) We ceased performing anaerobic culture because isolates proved extremely rare. (iv) Sequencing was no longer required since we could gather the same information using CPS3 color identification coupled with the standard biochemical tests described above. We have shown, using a specific antibody raised against the apical epithelial urothelial marker uroplakin III (ProGen), that our meticulous MSU cultures contain mostly urothelial cells, with minimal contamination from the vagina (29).
Antibiotic protection assay.
Resuspended cell sediment (100 μl or one-quarter of the sample) was seeded into one well of a 12-well culture dish containing Eagle's minimal growth medium (Sigma) supplemented with 1% nonessential amino acids (Sigma), 5% fetal bovine serum (Sigma), glutamine to 2 mmol per ml (Sigma), 200 μg per ml of gentamicin (Amdipharm Plc), 200 μg per ml of amoxicillin (Sigma), and 200 μg per ml of linezolid. This antibiotic cocktail was used because it kills a broad spectrum of uropathogens without leading to high intracellular accumulation (see Appendix S1 in the supplemental material), and we knew that our patient urine samples contained multiple bacterial species (see Table S1 in the supplemental material). The plate was incubated at 37°C overnight in 5% CO2. The cells were viewed for adhesion to the culture dish and for the presence of bacteria by using a light microscope (Leica) at ×400 magnification. The supernatant was removed, and the cells were washed three times with saline to remove remaining attached extracellular bacteria. Triton X-100 (0.1%; Sigma) in phosphate-buffered saline (PBS) was added to the well to break down the cell membranes and to release intracellular bacteria; lysates and the preceding wash were cultured to 1 × 103 dilutions on CBA and FAA plates and enumerated as described above.
Invasion assay.
A human urothelial cell line from a transitional cell carcinoma (EJ138) was maintained in Eagle's minimal essential medium supplemented with glutamine to 2 mmol per ml (Sigma), 5% fetal bovine serum (Sigma), and 1% nonessential amino acids (Sigma), in a 5% CO2 atmosphere. Cells were passaged at a ratio of 1:5 twice weekly. Experiments were performed between the 3rd and 15th passages, in triplicate. EJ138 cells were seeded into 5 wells of a 24-well plate (Sigma) at 1 × 105 cells per well and were grown to confluence overnight at 37°C in 5% CO2. Prior to these experiments, all included isolates were shown to be gentamicin sensitive by using dose-response curves generated with antibiotic-impregnated agar and concentrations of bacteria of 1 × 103 CFU/ml, 1 × 106 CFU/ml, and 1 × 108 CFU/ml (30). A bacterial suspension was prepared so that a final multiplicity of infection of 100 bacteria per bladder cell was achieved, and infection was carried out in a final volume of 1 ml. A standard cell line invasion assay was performed (31), using medium, cells, and bacteria-containing wells as controls. Briefly, bacterial isolates were retrieved from frozen storage, and a loop of each isolate was streaked onto a CBA plate and cultured overnight at 37°C in 5% CO2. All samples were processed in triplicate. After experimental infection of the appropriate wells, the cultures were incubated at 37°C in 5% CO2 for 2 h, after which the medium was removed and replaced with medium containing 200 μg/ml gentamicin or with PBS, as a control. Infected cell culture wells were incubated overnight and washed 3 times with PBS, and then Triton X-100 was added to a final concentration of 0.1% for 5 min to lyse the epithelial cells and to release any intracellular bacteria. Various washes and the lysates were plated on CBA plates and incubated overnight at 37°C in 5% CO2 to enumerate bacterial growth.
Statistics.
The analysis examined dominant isolates and the isolate mixture according to quantity and identity. We compared these two dependent variables with the independent factors of age, gender, pyuria, MSU culture result, and control group or LUTS group. The symptoms that patients described were used to characterize the sample and not to study symptom relationships. The sediment colony counts and white blood cell counts (pyuria) were log10 transformed, with zero counts being entered as 10−50. The MSU culture and pyuria results were coded as negative (0) or positive (1), taking MSU culture levels of ≥105 CFU ml−1 for a single species and pyuria levels of ≥10 WBCs μl−1 as thresholds for positivity. The isolated species or genera were coded with ordinal numbers. Linear regression (SPSS, Inc.) was used to analyze the log10 sediment colony and species counts per patient as dependent variables, with independent variables of age, gender (female = 0 or male = 1), MSU and pyuria classes (positive = 0 or negative = 1), and log10 WBC counts. The data were entered into univariate and then multivariate models. Multinomial logistic regression was used to analyze the dependent variables of microbial genera or species. The difference between pathogen and Lactobacillus gasseri counts after lysis was analyzed by t test for independent samples. The demographic data were compared by contingency tables and the χ2 test or linear regression; α was set at 0.05. A priori sample size calculation was not feasible, but retrospective analysis showed that the study was well powered.
RESULTS
Spectrum of LUTS.
To explore whether particular amounts and/or species of bacteria were associated specifically with LUTS and to explore the pathophysiology in more detail, we recruited an initial cohort of 55 female patients (mean age, 48 years [standard deviation {SD}, 16 years]) and 26 female controls (mean age, 43 years [SD, 17]); the age differences were not significant (P = 0.28). As LUTS represent a diverse collection of overlapping symptoms, we first carefully assessed each patient via a questionnaire, according to the International Continence Society definition of LUTS (16). Figure 1A shows a Venn diagram of this patient cohort's distribution of symptoms, which, as expected, consisted of overlapping symptoms typically seen at the LUTS clinic, the most common of which was urgency. Among patients, the average 24-hour frequency of urination was 12 episodes (95% confidence interval [CI], 11 to 13 episodes) and the average number of incontinence episodes in 24 hours was 1.0 episode (95% CI, 0.8 to 1.3 episodes). The average 24-hour frequency among controls was 6.2 episodes (95% CI, 6 to 7 episodes); the controls had no incontinence and no LUTS. Thus, we were confident that our patient group differed significantly from controls and manifested the classic profile of LUTS.
Fig 1.
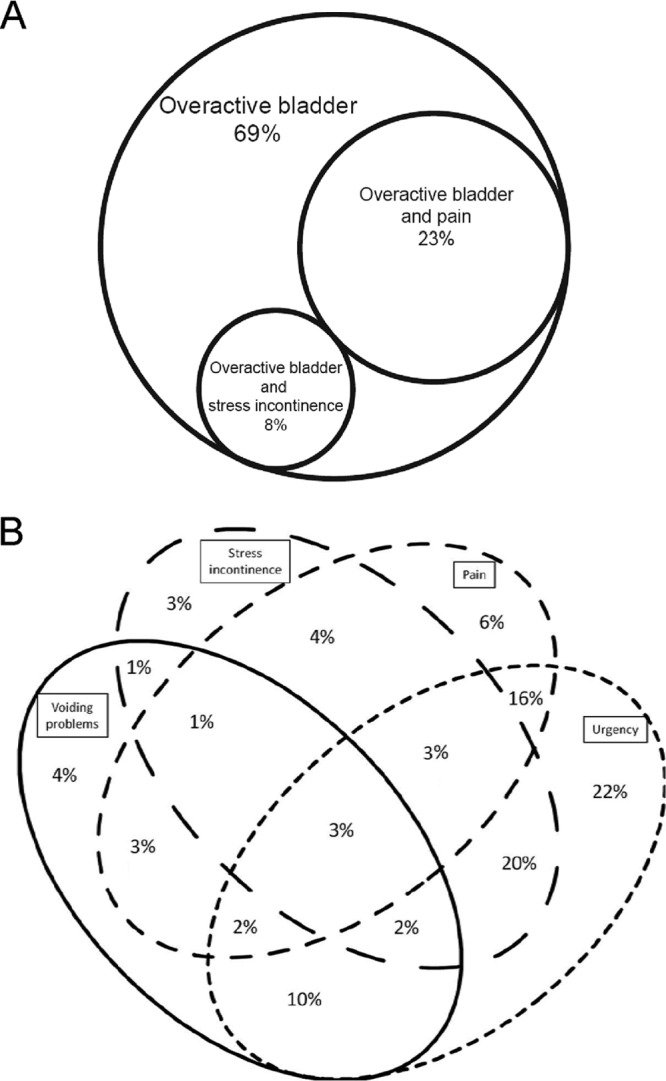
Venn diagrams showing the symptom spectrum for the first female patient group, which provided catheter specimens (A), and for the patient group providing midstream urine specimens (B).
Routine cultures miss underlying infections.
Standard hospital cultures in the United Kingdom assess only planktonic bacteria in a minute amount (typically 1 μl) of sample. Given the highly adherent nature of uropathogens with respect to host tissues, we wanted to determine whether enriching the cultures for apical bladder epithelial (umbrella) cells, which are shed into the urine during UTIs, might reveal a different picture, especially since such cells would settle by gravity at the bottom of any typical urine sample tubes and therefore would be unlikely to be sampled in routine tests of supernatants. Given that routine cultures demonstrating less than 105 CFU ml−1 of a single species of a known urinary pathogen typically would be scored as negative by hospital microbiologists in the United Kingdom and elsewhere, we were also interested in seeing whether this threshold was adequate for our LUTS patients.
We took urine specimens using a catheter (CSU) and submitted each specimen to microscopy to assess pyuria (WBC counts), to routine hospital culture (with the 105 CFU ml−1 threshold), and to sediment culture enriched for shed bladder epithelial cells and tailored to favor a wide variety of species (with no upper threshold imposed). Pyuria was very infrequent overall (22% [n = 12] of LUTS patients versus 8% [n = 2] of controls, which is not statistically different [χ2 = 2.4, P = 0.1]). This infrequency is not surprising, considering how weak this marker is for low-grade infections (7, 32). Routine culture revealed that very few patients in either cohort tested positive by the Kass criterion (7% [n = 4] of LUTS patients and 11% [n = 3] of controls, which is not statistically different [χ2 = 0.4, P = 0.4]). Therefore, routine culture was insufficient to distinguish patients with LUTS from controls.
To expand our initial observations, we next studied a larger group of patients using a streamlined procedure with MSU and chromogenic agar cultured under aerobic conditions. We recruited 47 controls (mean age, 45 years [SD, 12.5 years]; 38 female and 9 male subjects) and 165 patients (mean age, 55 years [SD, 16 years]; 154 female and 11 male subjects); the age difference between groups was significant (mean difference, 12 years [95% CI, 8 to 17 years]; F = 2, P < 0.001). Forty percent (n = 65) of the patients and none (n = 0) of the controls had pyuria; in this larger group, the difference was significant (χ2 = 27, df = 1, P < 0.001). Figure 1B shows the symptom spectrum, which was very similar to that of the first cohort (Fig. 1A). Seventeen percent (n = 28) of patients and 2% (n = 1) of controls were positive with routine hospital MSU culture, with this difference also being significant (χ2 = 7, df = 1, P = 0.004). However, despite the differences between patients and controls in pyuria and hospital culture results, neither was a particularly strong marker correlating with symptoms.
LUTS patients harbor a distinct spectrum of urinary bacterial species.
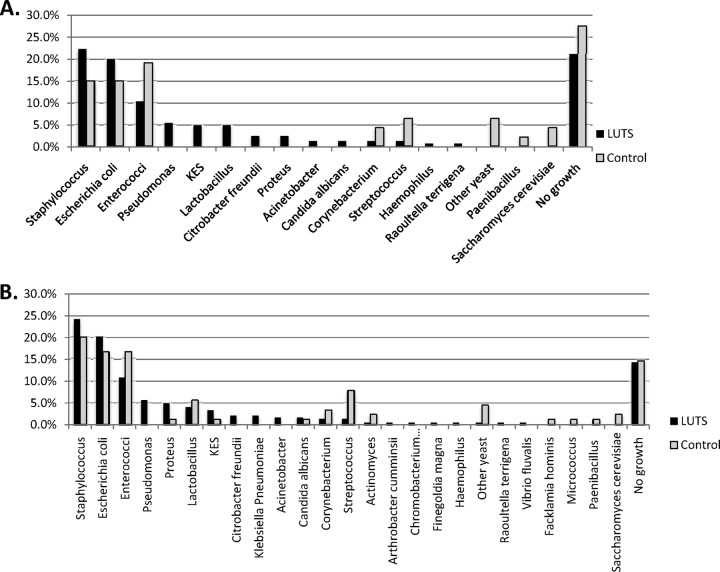
A different picture of bacterial colonization emerged from the sediment cultures; in our first cohort of CSU samples, both patient and control samples demonstrated means of 3.5 different isolates (95% CI, 3 to 4 isolates), without between-group differences (R = 0.05, df = 211, F = 0.189, P = 0.67) in the numbers of different isolates or, perhaps surprisingly, in the mean colony counts for the dominant isolate (102.3 CFU ml−1 [95% CI, 102 to 103 CFU ml−1]) and the mean total colony counts (103.6 CFU ml−1 [95% CI, 103 to 104 CFU ml−1]). There were likewise no bacterial explanatory effects detected for pyuria, MSU results, or age. Strikingly, however, there was a clear between-group difference in the species distributions of the dominant isolates between patients and controls (χ2 = 40, df = 30, Nagelkerke pseudo-R2 = 0.45, P = 0.02). When all isolates were analyzed, this between-group difference was more marked (χ2 = 117, df = 81, Nagelkerke pseudo-R2 = 0.34, P = 0.006). As shown in Fig. 2, the spectrum of species for LUTS patients versus controls for both the dominant isolate (Fig. 2A) and all isolates (Fig. 2B) consisted of both overlapping and nonoverlapping species. Considering the dominant isolates (Fig. 2A), the overlapping species found commonly in both patients and controls included those known to be associated with acute UTIs, including E. coli (implicated in up to 80% of acute UTI episodes [33]), Enterococcus spp., Staphylococcus spp., and Streptococcus spp. In contrast, a number of bacteria were found most frequently only in patients, such as Proteus spp. and Micrococcus spp., or only in controls, such as Actinomyces spp. and Prevotella spp. The complete data for all isolates in all groups, including information on mixed growths, are provided as the supplemental material (see Table S1 in the supplemental material).
Fig 2.
Graphs showing bacterial isolates from catheter specimens from patients and control volunteers. (A) Dominant isolates as percentages of all isolates. (B) Full spectrum of all isolates cultured as percentages of all isolates identified.
We next repeated these analyses with our second, larger cohort of MSU samples. In sediment cultures, the patients and controls produced means of 1.36 different isolates (95% CI, 1.2 to 2.1 isolates), without between-group differences (R = 0.25, df = 1, F = 3.35, P = 0.069). The MSU results and pyuria status had no explanatory effect on outcomes, but increasing age was associated with a reduction in the number of isolates per person (R2 = 0.28, β = 0.012, df = 211, P = 0.015). The slight reduction in the number of different isolates per person in our second cohort, compared with our first, likely is a reflection of the more-restricted culture conditions in our streamlined protocol (see Materials and Methods); nevertheless, a diverse array of species was detected. The mean colony counts for the dominant isolate were 101.4 CFU ml−1 (95% CI, 101 to 102 CFU ml−1) for controls and slightly higher at 102 CFU ml−1 (95% CI, 102 to 102.5 CFU ml−1) for LUTS patients, with the difference being significant (R2 = 0.019, β = 0.714, df = 211, P = 0.044). However, as in our first cohort, the mean total colony counts did not differ (102.2 CFU ml−1 [95% CI, 102 to 102.5 CFU ml−1]; R = 0.09, df = 211, F = 1.6, P = 0.2).
In this larger cohort, positive routine culture results predicted higher colony counts for the dominant genera and all isolates (R2 = 0.059, β = 1.5, df = 211, P < 0.001) (Fig. 3). The occurrence of pyuria showed similar properties (R2 = 0.04, β = 0.96, df = 211, P = 0.003) (Fig. 4). As shown in Fig. 5A, as with the first cohort, there was a strong difference in the distributions of genera that were identified as the dominant isolate in patients versus controls (χ2 = 45, df = 18, Nagelkerke pseudo-R2 = 0.19, P < 0.001). An analysis of all isolates (Fig. 5B) detected similar differences in distributions between the two groups (χ2 = 52, df = 25, Nagelkerke pseudo-R2 = 0.14, P = 0.001). Again, the common acute UTI pathogens E. coli, Enterococcus spp., and Staphylococcus spp. occurred in both the patient and control groups, whereas different species were associated with one group or the other.
Fig 3.
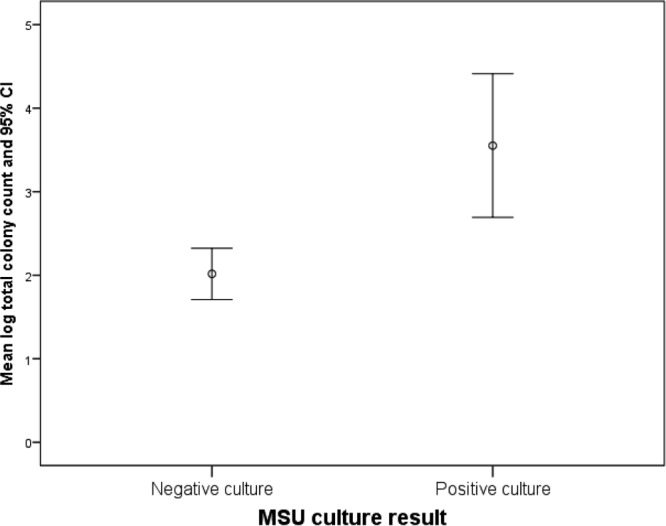
Graph showing the relationship between routine culture results (with a threshold of 105 CFU ml−1) and colony counts for cultures of urinary sediments in a cohort of midstream urine (MSU) specimens from patients with lower urinary tract symptoms. Positive routine culture results were associated with higher bacterial counts.
Fig 4.
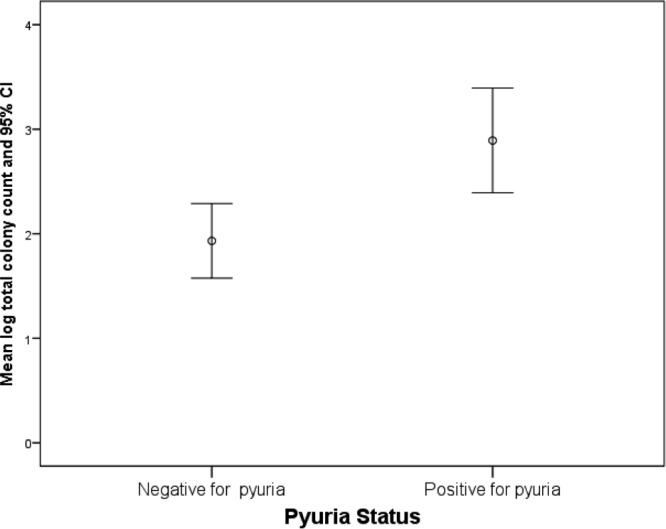
Graph showing the relationship between bacterial counts (CFU ml−1) and microscopic pyuria (>0 WBCs/ml) in the patient cohort for both MSU and CSU samples.
Fig 5.
Graphs showing bacterial isolates from midstream urine (MSU) specimens from patients and control volunteers. (A) Dominant isolates as percentages of all isolates. (B) Full spectrum of all isolates cultured as percentages of all isolates identified.
Taken together, these data from both cohorts support Wolfe et al. (34) in the idea that bacterial colonization of the bladder in healthy people is more common than previously supposed. Moreover, while we found many organisms known to be responsible for acute UTIs, we have shown that other organisms not traditionally associated with acute UTIs may be implicated in chronic LUTS. In particular, E. coli was not the only organism involved. The data also support the growing notion that routine hospital cultures may underestimate the extent of infection.
Cell lysis of shed epithelial cells releases known uropathogens only in patients with LUTS.
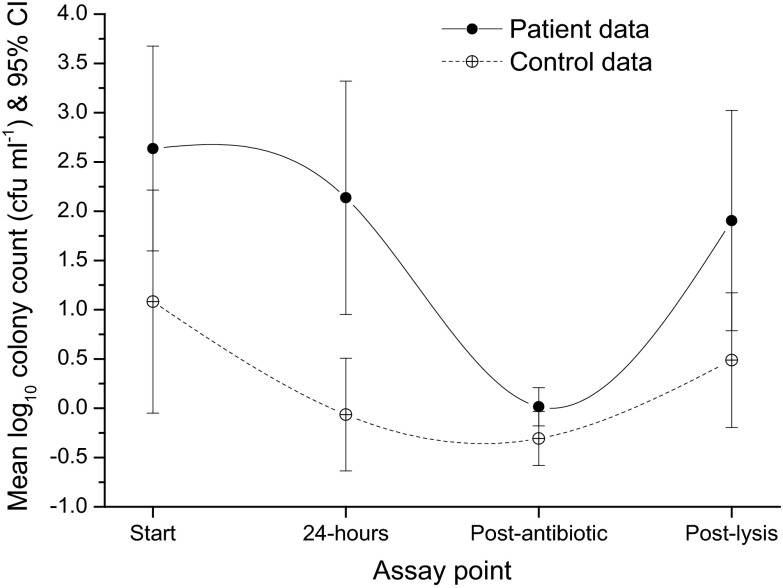
To explore further the pathogen-host interactions in LUTS patients, we sought to determine whether the bacteria in shed urothelial cells were intracellular by performing an antibiotic protection assay. Patient cells were plated in culture medium and treated with a cocktail of antibiotics (known to kill bacteria but not to penetrate the cell membrane) for a set period of time before being washed and lysed to release intracellular bacteria protected from the treatment. The wash supernatant and lysates were plated to yield colony counts. Sixteen female patients with chronic LUTS (mean age, 56 years [SD, 17 years]) were randomly selected from the first CSU cohort (see Fig. 1A). The 16 patients had a mean 24-h frequency of 8 episodes (95% CI, 7 to 12 episodes) and a mean of 2 incontinence episodes in 24 h (95% CI, 1 to 3 episodes) (see Table S2 in the supplemental material). Seven asymptomatic female controls (mean age, 29 years [SD, 12 years]) provided CSU samples. The routine culture results with a threshold of 105 CFU ml−1 were reported as positive for 19% (n = 3) of LUTS patients, none of whom had pyuria; none of the control samples had a positive routine culture result and all were negative for pyuria. As shown in Fig. 6, after lysis 94% (n = 15) of the LUTS samples showed evidence of intracellular bacterial colonization of the urothelium, compared with only 29% (n = 2) of the control specimens (χ2 = 7.6, df = 1, P = 0.006). The median total intracellular bacterial count in the patient group was 101.8 CFU ml−1 (mean, 102 CFU ml−1 [95% CI, 100.7 to 103.1 CFU ml−1]) and that in the control group was 100 CFU ml−1 (mean, 100.5 CFU ml−1 [95% CI, 100 to 101.2 CFU ml−1]). This difference was not statistically significant (difference, 213 CFU ml−1 [95% CI, 100.7 to 103 CFU ml−1]; P = 0.21) but species differences were. Specifically, 16S rRNA ribotyping of intracellular bacteria revealed significant between-group differences in species; the patients manifested colonization with E. coli, Enterococcus faecalis, Streptococcus anginosus, and Proteus mirabilis, whereas the two controls demonstrated commensal Lactobacillus gasseri (χ2 = 15, df = 1, P < 0.0005). The isolates did not show atypical antibiotic sensitivities (data not shown).
Fig 6.
Total bacterial counts at different stages of the antibiotic protection assay for LUTS patients and control volunteers, using the antibiotics gentamicin, amoxicillin, and linezolid at concentrations of 200 μg/ml. Start, total bacterial counts (CFU ml−1) at the beginning of the assay; 24-hours, total bacterial counts after 24 h of incubation; Post-antibiotic, total bacterial counts after incubation with antibiotics plus three washes with saline; Post-lysis, total bacterial counts after the addition of Triton X-100.
These experiments show that LUTS patients manifest a different spectrum of bacterial species in sediment cultures than do controls. Moreover, they have more uropathogens that appear to be intracellular or very closely associated with epithelial cells. Strikingly, the species of bacteria most commonly implicated in UTIs, namely, E coli, was found to be closely associated with cells only in patient samples.
Patient-derived uropathogenic bacteria, but not a control-derived commensal strain, can invade a bladder epithelial cell line.
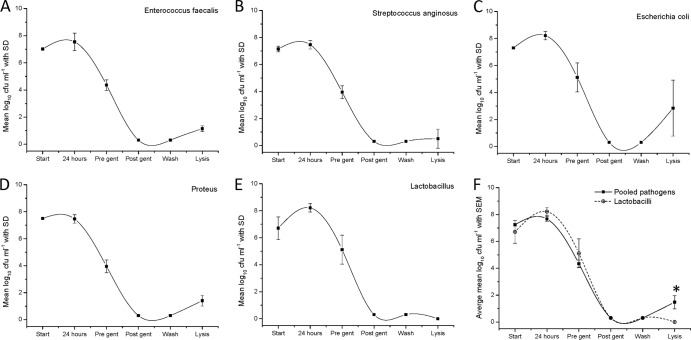
To further investigate the potential invasive properties of various patient-derived uropathogens, we experimentally infected the bladder epithelial cell line EJ138 and determined intracellular counts by lysing the cells following infection in the presence of antibiotics, as described above. We tested eight patient-derived strains (2 E. coli, 2 E. faecalis, 2 S. anginosus, and 2 P. mirabilis) and two control commensal strains (L. gasseri), all derived from patients initially assessed with the antibiotic protection assay described above. The bacterial strains were all tested for sensitivity to gentamicin prior to the experiment and were shown to be sensitive. The viable urothelial cell counts at the end of the assays were between 1.4 × 105 and 2 × 105 cells per ml. Uninfected control wells were negative for bacterial growth. Figure 7 shows the bacterial counts at different stages of the assays. The same bacterial species are grouped together. The graphs demonstrate complete killing of extracellular bacteria after the addition of gentamicin (200 μg/ml) overnight. They also demonstrate the release of bacteria after cell lysis. This occurred in all cases except L. gasseri from control patients, for which no bacterial growth was shown after cell lysis. The average bacterial counts postlysis (intracellular bacterial counts) were E. coli at 101 CFU ml−1, S. anginosus at 103 CFU ml−1, and P. mirabilis at 103 CFU ml−1, with the highest being E. faecalis at 104 CFU ml−1. The difference between the four pathogens and L. gasseri after lysis was significant (mean, 101.5 CFU ml−1; 95% CI, 102.5 to 100.4 CFU ml−1 ; t = 3.4, df = 7, P = 0.012). These experiments showed that all bacterial species shown to reside inside or very closely associated with patient cells in the antibiotic protection assay also seemed competent to invade tissue culture cells de novo.
Fig 7.
(A to E) Graphs showing mean bacterial counts (CFU ml−1) and standard deviations at different stages of the cell line invasion assay for five patient-derived uropathogens (A to D) and one control commensal strain (E). (F) Graph comparing mean bacterial counts (CFU ml−1), with the standard error of the mean (SEM), for pooled uropathogens and the commensal strain. *, statistically significant difference of the means. Start, total bacterial counts at the beginning of the assay; 24 hours, total bacterial counts after 24 h of incubation with the cell line; Pre gent, total bacterial counts after washing with saline and prior to the addition of gentamicin (200 μl/ml); Post gent, total bacterial counts after incubation with gentamicin (200 μg/ml) overnight; Wash, total bacterial counts after washing with saline after incubation with gentamicin; Lysis, total bacterial counts after cell lysis with Triton X-100.
DISCUSSION
Lower urinary tract symptoms are on the rise; as our global population ages, this debilitating disease is projected to increase significantly in the next decade, particularly in the developing world (27). It is therefore imperative to learn more about the potential role of bacterial infections in its pathophysiology. Our study suggests that the diagnostic procedures routinely used by clinicians to rule out bacterial infections in the etiology of LUTS are insufficient. Furthermore, our sensitive methods demonstrate that LUTS patients manifest polymicrobial colonizations of the urothelium that are qualitatively different from those cultured from controls, which may well be significant for diagnosis and treatment.
First, we found that patient and control urinary sediments contained similar amounts of bacteria. Given that CSU samples collected directly from the bladder grew these organisms and that control plates processed simultaneously did not become contaminated, these growths are likely to represent bona fide bladder inhabitants. Although the conventional wisdom holds that urine is sterile, the urethra connects the bladder to the outside environment, so it is not surprising that biological adaptation would enable commensal organisms to reside there. Indeed, reports of bacteriuria in normal controls are found in the literature (8, 34, 35). A reason why these commensal organisms are not readily detected may lie in the insensitivity of routine cultures, as shown here and by others previously (5–7, 9).
Comparing MSU and CSU isolates, it is interesting that Lactobacillus spp., which are common vaginal commensal organisms, were more prevalent in the CSU samples, which supports the idea that such organisms isolated from MSU samples were not contaminants from the vagina. In addition, of the five species most commonly isolated from MSU samples and CSU samples (Fig. 2B versus Fig. 5B), three were the same (Enterococcus sp., E. coli, and Staphylococcus spp.). The fact that the profiles were not identical might imply differences in urethral colonization, which CSU samples largely bypass, but we must be cautious, given the small sample size.
Although patient and control urinary sediments harbored similar numbers of bacteria, the species distributions were qualitatively and significantly different. The bacteria we isolated from our LUTS patients, while known uropathogens, were not limited to species typically found in acute UTIs. Two of the largest groups of bacteria isolated were Streptococcus spp. and Enterococcus spp. These are known vaginal commensal species that can cause urinary infections, and Streptococcus spp. are incriminated in vaginal infections (36). There was overlap between species isolated from controls and patients, including the most cited urinary pathogen, E. coli, again suggesting that a balance exists in the bacterial community, resulting in pathogenesis or not, perhaps due to virulent and nonvirulent strains of organisms, which our study was not able to distinguish. In addition, recent studies have discovered new contributions of the innate defense mechanisms, rendering individuals susceptible to chronic infections. Hannan et al. (37) showed that inflammatory events in the bladder during early uropathogenic E. coli (UPEC) infections in mice predisposed the mice to subsequent susceptibility to recurrent infections. Two genome-wide transcriptomic studies, by Duell et al. (38) and Tan et al. (39), have provided insights into the factors that might contribute to innate resistance to UPEC and have shown that bladder inflammation in response to UPEC is pathogen-specific and that UPEC somehow harnesses complex innate immune responses in the bladder to promote bacterial survival and a predisposition to chronicity.
Routine laboratory cultures in the United Kingdom focus on isolating E. coli and use selective media to do so. Our data suggest that the causative agents of LUTS might be much more diverse than and different from those of acute UTIs. This finding is not surprising, as they are not the same disease.
Our data were also notable for demonstrating mixed growths of bacteria in all groups (40). Traditionally, in many countries including the United Kingdom (2), the culturing of more than one pathogen in a routine culture is grounds for dismissing the result as nonsignificant “contamination” (41). However, there is no logical reason or evidence to dismiss mixed growths, which are wholly biologically plausible; indeed, they were recently shown to be clinically significant by Kline et al. (8). Microbes show marked interdependence and have evolved in association with other microbes, occupying different niches of the same ecosystem, so there is no reason to rule out mixed growths in pathogenicity. More studies will be necessary to determine which species and strains of bacteria, and in what combination, are most positively correlated with LUTS.
Bacterial adhesion to urothelial cells through expression of organelles such as type 1 pili is the first essential step in infection (42). Routine urine cultures use uncentrifuged urine specimens and might overlook bacteria attached to cells, which tend to settle in samples sitting for more than a few minutes. Sediment cultures use concentrated suspensions of cells, which might enhance pathogen detection. This analysis did not adopt a threshold for defining significant infection in terms of CFU per ml. Given the correlation of infection with symptoms, however, we predict that more-sensitive methods of culturing and a lower threshold for colony counts will be needed for accurate diagnosis in cases of chronic LUTS. Our current clinical experiences suggest that 102 CFU/ml might be an appropriate threshold to explore.
We present strong evidence that cellular colonization might be a hallmark of LUTS, just as it appears to be in acute UTIs. A subgroup of LUTS patients, all with overactive bladder, showed microbiological and cytological evidence of cell adhesion with E. coli, E. faecalis, Staphylococcus spp., Pseudomonas spp., Streptococcus spp., and Proteus spp. The same species seem to invade a cultured urothelial cell line in vitro, in contrast to the principal control isolate L. gasseri. Although antibiotic protection assays are used widely to detect intracellular bacteria, the evidence is indirect. Microbes attached to membrane fragments might mimic bacteria released from the cells by lysis. Some bacteria are highly adherent and might not be washed off. In addition, the surface of umbrella cells is folded and bears niches where bacteria might escape from the detergent. Finally, some uropathogens, particularly E. coli and Enterococcus spp., are known to form extracellular biofilms that are resistant to antibiotics; such external biofilms might survive the extracellular antibiotics used in the assay (43). Therefore, in order to prove conclusively the claim of intracellular colonization, careful imaging studies will be required to supplement invasion assays. These techniques are not easy, because shed urothelial cells must be harvested by centrifugation onto slides and the resultant flattening makes three-dimensional imaging difficult.
This is the first report of evidence for close bacterial association with the urothelium in patients with chronic LUTS. In acute UTIs, intracellular invasion is a known mechanism for bacterial evasion of the host immune response, leading to persistence and treatment failure; future studies are needed to determine whether a similar situation exists in LUTS. Regardless, it is evident that a large number of patients with persistent and debilitating symptoms are being let down by the incorrect exclusion of bacterial infection. The development of more-sensitive diagnostic methods for standard clinical practice is vital. Furthermore, if the bacteria associated with LUTS are found to form intracellular reservoirs, this will have major implications for treatment with antibiotics. Specifically, longer-term treatments with cell-permeable antibiotics and careful monitoring may be required to ensure that infections are truly being cleared. A randomized controlled trial of long-term cell-permeable antibiotic therapy in patients with chronic LUTS, in which shed urothelial samples are monitored for bacterial clearance, might be a useful clinical exercise to guide the future management of these patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Age UK and the International Urogynecology Association (IUGA) for funding this work.
We thank Harry Horsley, Jonathan Pratten, and Derren Reddy for technical assistance.
Footnotes
Published ahead of print 17 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03314-12.
REFERENCES
- 1. American College of Obstetricians and Gynecologists 2008. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet. Gynecol. 111:785–794 [DOI] [PubMed] [Google Scholar]
- 2. Scottish Intercollegiate Guidelines Network 2012. Management of suspected bacterial urinary tract infection in adults: a national clinical guideline. Scottish Intercollegiate Guidelines Network, Edinburgh, Scotland [Google Scholar]
- 3. Kass EH. 1957. Bacteriuria and the diagnosis of infection in the urinary tract. Arch. Intern. Med. 100:709–714 [DOI] [PubMed] [Google Scholar]
- 4. Kass EH. 1960. Bacteriuria and pyelonephritis of pregnancy. Arch. Intern. Med. 105:194–198 [DOI] [PubMed] [Google Scholar]
- 5. Bradley GM. 1968. Differentiating epithelial cells from leukocytes in urine. Postgrad. Med. 43:245–248 [DOI] [PubMed] [Google Scholar]
- 6. Wear JB., Jr 1966. Correlation of pyuria, stained urine smear, urine culture and the uroscreen test. J. Urol. 96:808–811 [DOI] [PubMed] [Google Scholar]
- 7. Khasriya R, Khan S, Lunawat R, Bishara S, Bignal J, Malone-Lee M, Ishii H, O'Connor D, Kelsey M, Malone-Lee J. 2010. The inadequacy of urinary dipstick and microscopy as surrogate markers of urinary tract infection in urological outpatients with lower urinary tract symptoms without acute frequency and dysuria. J. Urol. 183:1843–1847 [DOI] [PubMed] [Google Scholar]
- 8. Kline KA, Schwartz DJ, Gilbert NM, Hultgren SJ, Lewis AL. 2012. Immune modulation by group B Streptococcus influences host susceptibility to urinary tract infection by uropathogenic Escherichia coli. Infect. Immun. 80:4186–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. 1982. Diagnosis of coliform infection in acutely dysuric women. N. Engl. J. Med. 307:463–468 [DOI] [PubMed] [Google Scholar]
- 10. Deville WL, Yzermans JC, van Duijn NP, Bezemer PD, van der Windt DA, Bouter LM. 2004. The urine dipstick test useful to rule out infections: a meta-analysis of the accuracy. BMC Urol. 4:4. 10.1186/1471-2490-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hurlbut TA, III, Littenberg B. 1991. The diagnostic accuracy of rapid dipstick tests to predict urinary tract infection. Am. J. Clin. Pathol. 96:582–588 [DOI] [PubMed] [Google Scholar]
- 12. Williams GJ, Macaskill P, Chan SF, Turner RM, Hodson E, Craig JC. 2010. Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta-analysis. Lancet Infect. Dis. 10:240–250 [DOI] [PubMed] [Google Scholar]
- 13. Colgan R, Williams M. 2011. Diagnosis and treatment of acute uncomplicated cystitis. Am. Fam. Physician 84:771–776 [PubMed] [Google Scholar]
- 14. Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, Chapple CR, Kaplan S, Tubaro A, Aiyer LP, Wein AJ. 2009. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 104:352–360 [DOI] [PubMed] [Google Scholar]
- 15. Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P. 2006. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC Study. Eur. Urol. 50:1306–1314 [DOI] [PubMed] [Google Scholar]
- 16. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. 2003. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49 [DOI] [PubMed] [Google Scholar]
- 17. Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107 [DOI] [PubMed] [Google Scholar]
- 18. Mysorekar IU, Hultgren SJ. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 103:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4:e329. 10.1371/journal.pmed.0040329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. 2007. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect. Immun. 75:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson GG, Martin SM, Hultgren SJ. 2004. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 6:1094–1101 [DOI] [PubMed] [Google Scholar]
- 22. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. 2004. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 23:322–330 [DOI] [PubMed] [Google Scholar]
- 23. Heuer RJ. 2006. Psychology of intelligence analysis. Novinka Books, New York, NY [Google Scholar]
- 24. Al-Buheissi S, Khasriya R, Maraj BH, Malone-Lee J. 2008. A simple validated scale to measure urgency. J. Urol. 179:1000–1005 [DOI] [PubMed] [Google Scholar]
- 25. Gill K, Kupelian A, Brackenridge L, Horsley H, Sathiananthamoorthy S, Malone-Lee J. 2011. Surprising symptoms indicating urinary tract infection. Abstr. 41st Annu. Meet. Int. Continence Soc., abstr. 444 [Google Scholar]
- 26. Kupelian A, Chaliha C, Gill K, Brackenridge L, Horsley H, Malone-Lee J. 2009. Pain symptoms as part of the OAB complex. Int. Urogynecol. J. 22:189–190 [Google Scholar]
- 27. Brown J, Meikle J, Webb C. 1991. Collecting midstream specimens of urine—the research base. Nurs. Times 87:49–52 [PubMed] [Google Scholar]
- 28. McGinley M, Wong LL, McBride JH, Rodgerson DO. 1992. Comparison of various methods for the enumeration of blood cells in urine. J. Clin. Lab. Anal. 6:359–361 [DOI] [PubMed] [Google Scholar]
- 29. Horsley H, Tuz M, Collins L, Swamy S, Malone-Lee J, Rohn J. Investigating the origin of epithelial cells found in the urine of LUTS patients using immunofluorescence; contamination or inflammation? Int. Urogynecol. J., in press [Google Scholar]
- 30. Khasriya R. 2011. Occult urine infection in the aetiology of the overactive bladder. Ph.D. thesis University College London, London, United Kingdom [Google Scholar]
- 31. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kupelian A, Horsley H, Khasriya R, Amussah R, Badiani R, Courtney A, Chandhyoke N, Riaz U, Savlani K, Moledina M, Montes S, O'Connor D, Visavadia R, Kelsey M, Rohn J, Malone-Lee J. 2013. Discrediting microscopic pyuria and leucocyte esterase as diagnostic surrogates for infection in patients with lower urinary tract symptoms: results from a clinical and laboratory evaluation. BJU Int. 10.1111/j.1464-410X.2012.11694.x [DOI] [PubMed] [Google Scholar]
- 33. Amna MA, Chazan B, Raz R, Edelstein H, Colodner R. 2013. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection 41:473–477 [DOI] [PubMed] [Google Scholar]
- 34. Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. 2012. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salvador E, Wagenlehner F, Kohler CD, Mellmann A, Hacker J, Svanborg C, Dobrindt U. 2012. Comparison of asymptomatic bacteriuria Escherichia coli isolates from healthy individuals versus those from hospital patients shows that long-term bladder colonization selects for attenuated virulence phenotypes. Infect. Immun. 80:668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73:407–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 6:e1001042. 10.1371/journal.ppat.1001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, Schembri MA, Derrington P, Irving-Rodgers H, Brooks AJ, Cripps AW, Crowley M, Ulett GC. 2012. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 188:781–792 [DOI] [PubMed] [Google Scholar]
- 39. Tan CK, Carey AJ, Cui X, Webb RI, Ipe D, Crowley M, Cripps AW, Benjamin WH, Jr, Ulett KB, Schembri MA, Ulett GC. 2012. Genome-wide mapping of cystitis due to Streptococcus agalactiae and Escherichia coli in mice identifies a unique bladder transcriptome that signifies pathogen-specific antimicrobial defense against urinary tract infection. Infect. Immun. 80:3145–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sathiananthamoorthy S, Swamy S, Kupelian A, Horsley H, Gill K, Collins L, Malone-Lee J. 2012. “Mixed growth of doubtful significance” is extremely significant in patients with lower urinary tract symptoms. Neurourol. Urodyn. 31:736–737 [Google Scholar]
- 41. Bartlett RC, Treiber N. 1984. Clinical significance of mixed bacterial cultures of urine. Am. J. Clin. Pathol. 82:319–322 [DOI] [PubMed] [Google Scholar]
- 42. Hunstad DA, Justice SS. 2010. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64:203–221 [DOI] [PubMed] [Google Scholar]
- 43. Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.