Abstract
The public health impact of the emergence of new norovirus (NoV) strains is uncertain. A biennial pattern of alternating quiescent and epidemic levels of NoV outbreak activity associated with the emergence of new GII.4 variants was observed in Alberta, Canada, between July 2000 and June 2008. In this study, NoV genogroup I (GI) and GII strains isolated from 710 outbreak specimens in Alberta between July 2008 and January 2013 were characterized to update historical data. The seasonality and annual variation in NoV outbreak burden were analyzed over a 10-year period (July 2002 to June 2012). We found that GII.4-2006b had persisted as the predominant variant over three observation periods (July 2006 to June 2009) during which the biennial NoV outbreak pattern continued. The emergence of GII.4-2010 (winter 2009) was not associated with increased outbreak activity, and outbreak activity between July 2009 and June 2012 when GII.4-2010 predominated (67.5 to 97.7%) did not follow a biennial pattern. GII.4-2012 first emerged in Alberta in September 2011 and became predominant in observation period July 2012 to June 2013. NoV GI, relatively rare in past years, had a higher activity level (37.3%) as represented by GI.6 and GI.7 in the winter of 2012 to 2013. A higher proportion of GI outbreaks occurred in non-health care facility settings compared to GII. Our study suggests that factors other than new variants emergence contribute to the levels of NoV outbreak activity in Alberta.
INTRODUCTION
Norovirus (NoV) is the leading cause of gastroenteritis outbreaks worldwide. Based on genetic variability, NoV strains are classified into genogroups, genotypes, and genotype variants. The viral RNA genome has three open reading fames (ORFs), and most of the genetic variability resides in the capsid, which is encoded by ORF2 and ORF3. Specifically, ORF2 contains the major capsid gene, VP1, which contains the hypervariable region and receptor binding site (1).
Five NoV genogroups (GI to GV) have been identified: GI, GII, and GIV infect humans, with most infections caused by GI and GII (1, 2). Based on sequence variations in ORF2, 8 GI genotypes and at least 21 GII genotypes have been described using the numeric designation for the various strains (3, 4, 5). Another 14 GI genotypes and at least 29 GII genotypes have been described using numeric or alphabetic designations based on variations in ORF1. The majority of global gastroenteritis outbreaks are caused by GII.4 strains (6, 7). The GII.4 strains have demonstrated faster evolution than other strains (8), and new GII.4 clusters or variants emerge every 2 to 5 years (9).
Our understanding of NoV GI genetic evolution and outbreak activity are limited due to the overall low prevalence of GI outbreaks (4). GI strains appear to be more common in outbreak settings other than health care facilities, and different GI genotypes predominate over time (4, 10).
A unique biennial pattern of NoV outbreak activity was observed in Alberta, Canada between July 2000 and June 2008 (11). Increases in NoV outbreak activity to epidemic levels over this period every 2 years were always associated with the emergence of new GII.4 variants. In this study, we extend our analysis and describe the NoV outbreak activity and characterize the circulating NoV outbreak strains between July 2008 and January 2013. Since February 2002, laboratory investigations of gastroenteritis outbreaks have been standardized across the province and basic demographic data and results of laboratory investigations have been retained in a centralized database within the Provincial Laboratory for Public Health (ProvLab) (11). We performed an updated analysis of the periodic variations of NoV outbreak activity in the province of Alberta over a 10-year period from July 2002 to June 2012.
MATERIALS AND METHODS
Outbreak investigation.
In Alberta, communicable disease outbreaks are reportable to the Medical Officer of Health and investigated by public health officials (e.g., environmental health officers); ProvLab provides laboratory testing for all outbreaks. Laboratory testing of gastroenteritis outbreak specimens may include testing for enteric bacteria, enteric viruses, and/or parasitic agents. Public health officials request the testing of stool specimens for NoV when outbreak illnesses are clinically consistent with this pathogen.
Testing and characterization of norovirus in stool specimens.
Stool specimens submitted to ProvLab for suspected NoV gastroenteritis outbreaks were tested for NoV genogroups I (GI) and II (GII) by multiplex real-time reverse transcription (RT)-PCR (12). For NoV strain characterization, the nucleic acid extract of one positive stool specimen from each NoV outbreak was amplified and sequenced as previously described (11), using primers Mon381 and Mon383 (region E) (13) or G2SKF and G2SKR (region C) (14) for NoV GII, and using primers G1SKF and G1SKR (region C) (14) or CapA, CapB1, and CapB2 (region D) for NoV GI (15). Genotyping was performed using the norovirus genotyping tool (5) (available at http://www.rivm.nl/mpf/norovirus/typingtool) and in-house phylogenetic analysis. Specimen and reference sequences (listed in Table S1 in the supplemental material) were aligned with MAFFT 6, and neighbor-joining trees were constructed in MEGA 5.05 using the Kimura two-parameter model with a branch support of 100 bootstrap replicates.
Amplification and phylogenetic analysis of GII.4-2012 capsid sequences.
The two earliest NoV GII.4-2012 strains, AlbertaEI337 and AlbertaEI63, were further characterized by sequencing the complete ORF2. Nucleic acid extracts were amplified with Taq DNA polymerase (Invitrogen, CA) according to the manufacturer's instructions, using three overlapping sets of primers to span the entire major capsid gene (coding for VP1): (i) NV4611 and Mon381, (ii) Mon383 and NVR7, and (iii) NVF8 and NVR8 (13, 16, 17). Sequencing of PCR products was performed as previously described (11).
The phylogenetic analysis was conducted in MEGA 5.05. The following reference sequences were included in the analysis: X86557.1 for GII.4-1990 (Bristol); AB303923.1, AJ004864.1, and AY038600.3 for GII.4-1996 (Grimsby); AB303933.1, AY502023.1, and JX445152.1 for GII.4-2002 (Farmington Hills); AY883096.1, DQ078794.2, and JX445153.1 for GII.4-2004 (Hunter); EF126963.1, GQ849126.1, and JX445157.1 for GII.4-2006a (Yerseke); EF126966.1, EU078417.1, and JX445159.1 for GII.4-2006b (Den Haag); AB434770.1, AB541321.1, and GQ845369.3 for GII.4-2008a (Osaka); AB445395.1, GU270580.1, and JX445161.1 for GII.4-2008b (Apeldoorn); GU445325.2, JN595867.1, and JX445168.1 for GII.4-2010 (New Orleans); and JX459907.1 and JX459908.1 for GII.4-2012 (Sydney). Specimen and reference sequences were aligned using multiple sequence comparison by log-expectation (MUSCLE). Pairwise distances and maximum likelihood trees were calculated using the best nucleotide and amino acid substitution models based on Bayesian information criterion scores, the Jones-Taylor-Thornton model, and the Kimura two-parameter model, respectively, with gamma-distributed rate variation across sites and 1,000 bootstrap replicates.
Data inclusion and statistical analysis.
All NoV-positive outbreaks, defined by the detection of NoV in one or more of the outbreak stool specimens submitted for testing, and NoV-negative outbreaks, defined by all outbreak stool specimens testing negative for NoV, were included in this study. Outbreaks in community-based and hospital-based long-term care facilities, supportive living facilities, and group homes were grouped as “LTC/SL/GH” for the analysis of outbreak settings. Outbreaks in LTC/SL/GH and acute care hospitals were further combined as outbreaks in health care facilities compared to non-health care facility outbreaks, which included outbreaks in day care centers, schools, camps, dormitories, community functions, conferences, and other settings. The chi-square test was used to compare the proportion of health care facility and non-health care facility outbreaks for GI and GII. NoV strains causing outbreaks between July 2008 and January 2013 in Alberta were sequenced and characterized as described.
NoV outbreak activity was analyzed using annual observational periods from 1 July to 30 June of the following year because of the winter seasonality of NoV gastroenteritis. Temporal variation in the number of NoV-positive outbreaks in Alberta between July 2002 and June 2012 was analyzed using the Edwards test (18), correlogram (19), and periodogram (20). The Edwards test considers the null hypothesis of no seasonality versus the alternative that the time series is a sinusoidal curve with one peak and one trough. Autocorrelation functions are plotted against the time in the correlogram. The periodogram is a sample estimate of spectral density function used in the frequency-domain time series methods. The seasonality in the proportion of positive NoV was analyzed using the Walter and Elwood test, which allows for the adjustment of varied numbers of the total tests done for each year (21, 22).
RESULTS
Norovirus outbreaks by GII and GI.
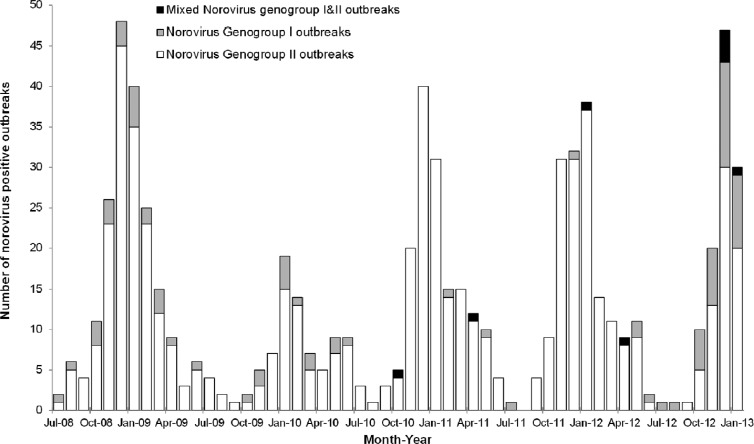
Between July 2008 and January 2013, 1,594 gastroenteritis outbreak investigations were initiated at ProvLab by public health officials. NoV was listed as a suspect agent for 1,410 outbreaks, and specimens were received for NoV testing for 964 (68.4%) of these outbreaks. NoV testing was also performed for 54 of 75 (72%) outbreaks where no suspect agent was indicated in the ProvLab database and 20 of 109 (18.3%) outbreaks where NoV was not initially listed as a suspected agent. Of the 1,038 outbreaks with laboratory testing for NoV, 710 (68.4%) were NoV positive: 622 (87.6%) were GII, 79 (11.1%) were GI, and 9 (1.3%) were mixed GI and GII outbreaks (Table 1). Examination of the monthly NoV genogroup-specific outbreaks revealed an increase in GI activity since June 2012 (Fig. 1). GI caused 37.3% of all NoV outbreaks during the first 7 months of observation period July 2012 to June 2013 compared to 4.3% from July 2011 to June 2012, 2.5% from July 2010 to June 2011, 15.5% from July 2009 to June 2010, and 11.8% from July 2008 to June 2009.
Table 1.
Number of norovirus outbreaks by genogroups and genotypes, from 1 July 2008 to 31 January 2013
| Genotype or genogroup | No. (%) of norovirus outbreaks for observation period: |
|||||
|---|---|---|---|---|---|---|
| July 2008–June 2009 | July 2009–June 2010 | July 2010–June 2011 | July 2011–June 2012 | July 2012–January 2013a | Total | |
| GI | 23 | 13 | 2 | 5 | 36 | 79 |
| Mixed GI and GII | 0 | 0 | 2 | 2 | 5 | 9 |
| GII | 172 | 71 | 155 | 155 | 69 | 622 |
| GII sequencedb | 152 (88.4) | 62 (87.3) | 142 (90.4) | 150 (95.5) | 50 (67.6) | 556 (88.1) |
| GII.1 | 0 | 0 | 0 | 13 | 0 | 13 |
| GII.2 | 0 | 0 | 1 | 2 | 0 | 3 |
| GII.3 | 0 | 10 | 1 | 1 | 0 | 12 |
| GII.4 | 141 | 40 | 131 | 130 | 50 | 492 |
| GII.4-2006b | 134 | 13 | 10 | 1 | 2 | 160 |
| GII.4-2008a | 2 | 0 | 0 | 0 | 0 | 2 |
| GII.4-2008b | 4 | 0 | 0 | 0 | 0 | 4 |
| GII.4-2010 | 1 | 27 | 120 | 127 | 3 | 278 |
| GII.4-2012 | 0 | 0 | 0 | 2 | 44 | 46 |
| GII.4 unidentified variants | 0 | 0 | 1 | 0 | 1 | 2 |
| GII.6 | 0 | 0 | 4 | 0 | 0 | 4 |
| GII.7 | 1 | 0 | 0 | 0 | 0 | 1 |
| GII.12 | 1 | 11 | 4 | 0 | 0 | 16 |
| GII.13 | 5 | 0 | 1 | 1 | 0 | 7 |
| GII.14 | 3 | 1 | 0 | 1 | 0 | 5 |
| GII.15 | 1 | 0 | 0 | 2 | 0 | 3 |
| GI sequencedb | 21 (91.3) | 11 (84.6) | 3 (75) | 4 (57.1) | 29 (70.7) | 68 (77.3) |
| GI.1 | 0 | 0 | 1 | 2 | 0 | 3 |
| GI.3 | 20 | 1 | 1 | 0 | 0 | 22 |
| GI.4 | 1 | 2 | 1 | 1 | 0 | 5 |
| GI.6 | 0 | 7 | 0 | 1 | 16 | 24 |
| GI.7 | 0 | 1 | 0 | 0 | 13 | 14 |
Data were collected up to 31 January 2013 for the observation period July 2012 to June 2013.
Includes sequences from mixed GI and GII outbreaks. Genotype results were obtained from 6 of the 9 mixed GI and GII outbreaks, with both GI and GII genotyped for only one outbreak (GI.3 and GII.4-2010), two outbreaks of GII.4-2012 and one each of GII.4-2010, GI.1, and GI.7 were identified in another five mixed outbreaks.
Fig 1.
Monthly distribution of norovirus outbreaks by genogroups in Alberta, Canada, 1 July 2008 to 31 January 2013.
The three most common settings for GI outbreaks were LTC/SL/GH (74.7% [59/79]), followed by acute care hospitals (6.3% [5/79]) and day care centers (6.3% [5/79]). GII outbreaks had a similar pattern, with most of the outbreaks in LTC/SL/GH (78.0% [485/622]), followed by acute care hospitals (13.8% [86/622]) and day care centers (2.6% [16/622]). Six of the nine mixed GI and GII outbreaks occurred in LTC/SL/GH, two in a geographic community, and one in an acute care hospital. Excluding the mixed GI and GII outbreaks, 19.0% (15/79) of GI outbreaks occurred in non-health care facility settings versus 8.2% (51/622) for GII (P < 0.005, chi-square test).
Norovirus GII diversity.
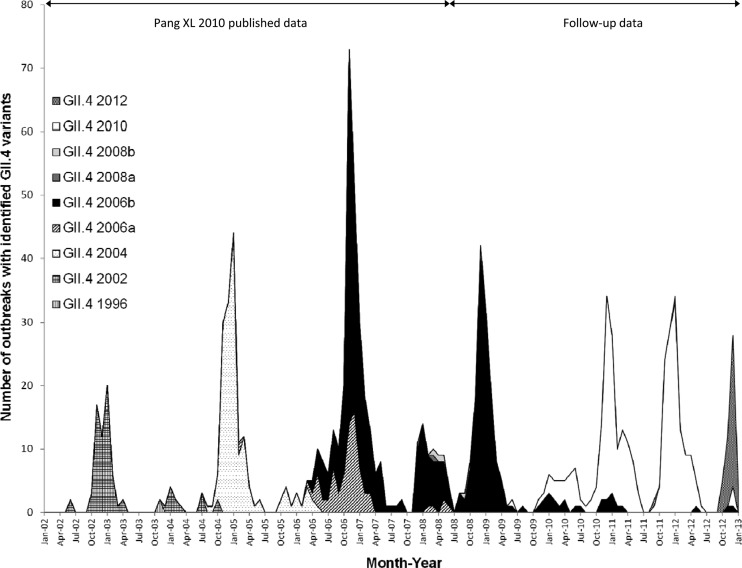
Sequences were obtained for 556 (88.1%) of the 631 outbreaks with NoV GII. Ten different GII genotypes were identified, and GII.4 (88.5%) was the most prevalent, followed by GII.12 (2.9%) and GII.1 (2.3%) (Table 1). The emergence and circulation of the various GII.4 variants since 2002 are shown in Fig. 2. Two of the five GII.4 variants, GII.4-2008a and GII.4-2008b, have caused only 11 outbreaks since their emergence in the winter of 2007. GII.4-2006b was the predominant strain in the observation period July 2008 to June 2009, causing 95.0% of all GII.4 outbreaks. GII.4-2010 first emerged in June 2009 and became predominant in observation period July 2009 to June 2010, causing 67.5% of GII.4 outbreaks with GII.4-2006b at 32.5% during the same period. From July 2010 to June 2011, GII.4-2010 and GII.4-2006b variants were identified in 91.7% and 7.6% of GII.4 outbreaks, respectively. GII.4-2010 remained the most prevalent variant from July 2011 to June 2012, causing 97.7% of GII.4 outbreaks. A new variant, GII.4-2012, first appeared in September 2011 and was identified in only 2 outbreaks from July 2011 to June 2012. However, GII.4-2012 became the predominant variant in the first 7 months of observation period July 2012 to June 2013, causing 88% of GII.4 outbreaks during this time.
Fig 2.
GII.4 variants identified in norovirus outbreaks in Alberta, Canada, 1 July 2002 to 31 January 2013.
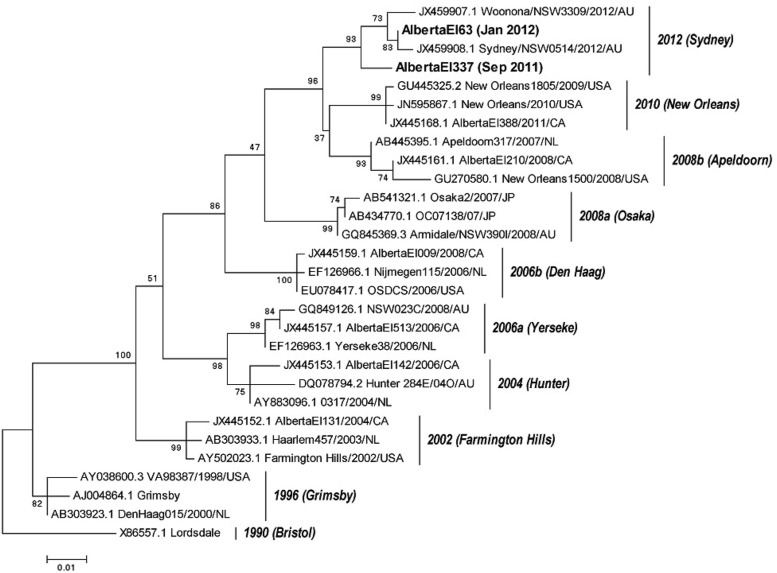
Phylogenetic analysis based on complete VP1 sequences of the first two GII.4-2012 strains (Fig. 3), showed that AlbertaEI337 circulated during September 2011 had a 2.9% nucleotide and 2.0% amino acid difference compared with Sydney 2012 (JX459908.1), whereas AlbertaEI63, which circulated during January 2011, had a 0.5% nucleotide and 0.4% amino acid difference.
Fig 3.
Phylogenetic analysis of complete VP1 amino acid sequences of GII.4 variants identified in Alberta. The names used by the Norovirus Genotyping Tool are shown in parentheses. The scale bars represent the number of substitutions per site.
Norovirus GI diversity.
Sixty-eight of the 88 (77.3%) outbreaks with NoV GI were successfully sequenced, and five GI genotypes were identified (Table 1). The three most common genotypes were GI.6, causing 35.3% of sequenced GI outbreaks, followed by GI.3 (32.4%) and GI.7 (20.6%). Among these three GI genotypes, GI.3 was predominant in observation period July 2008 to June 2009, causing 95.2% of GI outbreaks, GI.6 was predominant from July 2009 to June 2010 (63.6%), and GI.7 (44.8%) caused a similar proportion of outbreaks to GI.6 (55.2%) during the first 7 months of observation period July 2012 to June 2013.
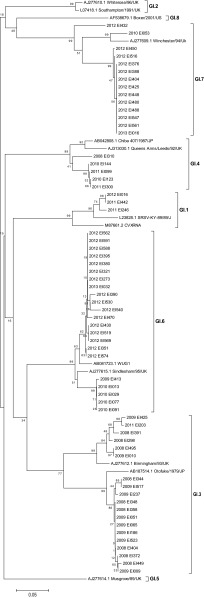
Phylogenetic analysis based on region C (320 nucleotides) in the capsid gene for the GI genotypes (n = 63) is shown in Fig. 4. The latest GI.7 sequences (n = 13), circulated in 2012 and 2013, clustered separately from the earlier GI.7 strain in 2010. A distinct group, formed by 12 of the 13 latest GI.7 sequences, showed a 12% nucleotide difference from the older strain. Similarly, the most recent GI.6 strains, circulated in 2012 and 2013 (n = 5), clustered separately from the earlier strains circulated in 2009 and 2010 (n = 17), with a 5.5% nucleotide difference between the two groups. Two clusters were also observed within GI.3 strains, but there was no grouping by time of circulation.
Fig 4.
Neighbor-joining tree of NoV GI sequences (region C) from samples identified in Alberta. The phylogenetic analysis was performed using the Kimura two-parameter model with gamma distribution of rate variation among sites and 1,000 bootstrap replicates. The scale bars represent the number of substitutions per site.
The seasonality of norovirus outbreaks.
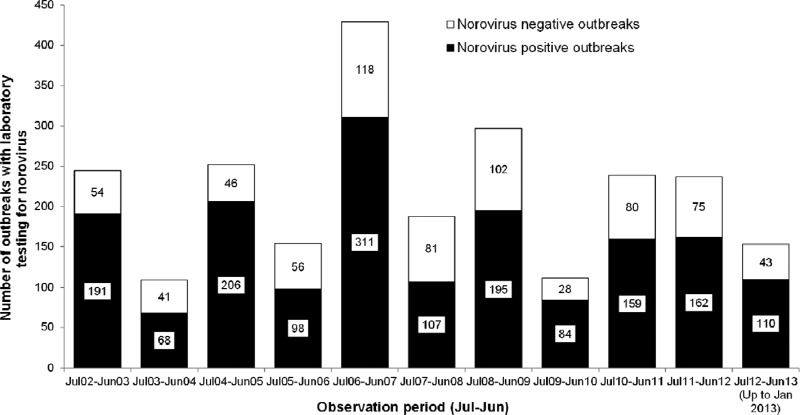
Significant seasonality (P ≤ 0.01, Edwards test) with a temporal pattern consisting of a periodicity of 2 years and highest peaks at 2 years was observed when the number of NoV-positive outbreaks for ten observation periods from July 2002 to June 2012 was analyzed using correlogram and periodogram analyses (Fig. 5). A clear change from the biennial pattern was observed for two observation periods, July 2010 to June 2011 and July 2011 to June 2012, as these two intervals had similar numbers of NoV-positive outbreaks—159 and 162, respectively (Fig. 5). When the data were analyzed in terms of the proportion of NoV-positive outbreaks for the 10 observation periods, no significant seasonality (P = 0.23, Walter and Elwood test) and no biennial pattern were detected.
Fig 5.
Numbers of norovirus-positive and -negative outbreaks in Alberta, Canada, by observation period: 1 July 2002 to 31 January 2013.
DISCUSSION
In our present study, we observed that the levels of NoV outbreak activity in two observation periods, July 2010 to June 2011 and July 2011 to June 2012, deviated from the historical biennial pattern of alternative years of quiescent and epidemic activity levels previously described (11). Several novel observations were made when the data from July 2008 to January 2013 were added to the previous data. NoV GII.4-2006b persisted and predominated over three consecutive observation periods from July 2006 to June 2009, while other new variants such as GII.4-2008a (Osaka) and -2008b (Apeldoorn) never became predominant. The persistence of a biennial pattern for NoV outbreaks despite the predominance of a single variant, GII.4-2006, for 3 years demonstrates that changes in the level of NoV outbreak activity can occur in the absence of new variants. We postulate that the high levels of outbreak activity caused by GII.4-2006b in observation period July 2008 to June 2009 were related to the waning of short-lived immunity from infection with the same variant in observation period July 2006 to June 2007 interplaying with other factors, such as virulence of GII.4-2006b, circulation of NoV in the environment and in human reservoirs, and exposure of susceptible populations.
In contrast to previous observations, the emergence and predominance of GII.4-2010 were not associated with high levels of NoV outbreak activity from July 2009 to June 2010. Similar observations related to this variant were made in the United States during the 2009-2010 winter season (23). The low outbreak activity related to GII.4-2010 may be due to multiple factors. Pandemic H1N1 influenza was rampant in Alberta between May 2009 and December 2009, limiting laboratory-based gastroenteritis outbreak investigations during the winter of 2009 because of resource constraints (24), possibly reducing the number of NoV outbreaks documented during this period. On the other hand, the number of suspected NoV outbreaks reported to the Medical Officers of Health in Alberta during this period was also lower than anticipated (Alberta Health Services, unpublished data). We postulate that the widespread and aggressive public hand hygiene and infection control educational campaigns targeting pandemic influenza might have decreased the transmission of GII.4-2010 within the population at risk for NoV outbreaks in observation period July 2009 to June 2010.
Similar levels of NoV outbreak activity were observed for the second and third observation periods during which GII.4-2010 remained predominant (July 2010 to June 2011 and July 2011 to June 2012). This was different from the biennial pattern observed for GII.4-2006 and other variants. Lower than normal levels of herd immunity resulting from less transmission within the general population in observation period July 2009 to June 2010, combined with short-lived or incomplete immunity in those exposed, may have resulted in increased susceptibility to NoV infection in the Alberta population during the two following observation periods, July 2010 to June 2011 and July 2011 to June 2012.
Differences in the virulence of GII.4 variants may also have contributed to the NoV outbreak burden. Genotyping was based on partial sequences of either the S domain (region E) or the N-terminal/shell (N/S) domain of VP1 (region C). Sequence alterations or antigenic variation occurring in other regions within the capsid, such as the P2 subdomain, were not analyzed. GII.4-2006 was unusual in its predominance over three consecutive observation periods and continued to represent ∼30% of circulating strains after the emergence of GII.4-2010. Complete capsid sequence analysis of GII.4-2006b and GII.4-2010 strains from different observation periods would allow a more comprehensive analysis of genetic variability, drift, and virulence of NoV GII.4 strains.
The newest variant, GII.4-2012, also referred to as Sydney 2012, was first reported in Australia in March 2012, thereafter in Europe and New Zealand (25), and has emerged as the predominant strain causing NoV outbreaks globally (26). In Alberta, the first GII.4-2012-like strain was identified in September 2011 and the second in January 2012. However, this variant did not become predominant until October 2012, almost 1 year after its first appearance. Although it has been suggested that GII.4-2012 variants were the result of recombination of two earlier GII.4 variants (25), the amino acid distance between the first GII.4-2012-like strain and later epidemic ones (Sydney 2012) suggests that the present GII.4-2012 strains might also have evolved by antigenic drift. Analysis of region C sequences of all GII.4-2012 strains identified in Alberta since October 2012 (representing 44 outbreaks; see Fig. S1 in the supplemental material) showed base changes in two nucleotide positions (nt 5327 and 5339 relative to JX459908.1) compared to the initially identified GII.4-2012 strain. The first GII.4-2012-like strain in Alberta seemed to be a “pre-Sydney 2012 variant” with unique intermediate sequence features. This implies that newly emergent NoV GII.4 variants could be monitored and detected by phylogenic analysis as transitional strains before they evolve into new variants with higher virulence or which escape herd immunity.
There are few published studies of GI genotypes in NoV outbreaks. The updated Alberta NoV outbreak data demonstrated GI prevalence similar to that of other countries. The median percentage of NoV outbreaks over a decade of investigation (from July 2002 to June 2012) caused by GI in Alberta was 7.8% (range, 2.1% to 29.4%) (11), comparable to those recently reported by Australia (4.3% in 2002 to 2010) (4) and New Zealand (8.7% in 2002 to 2009) (27). However, we have observed episodic high levels of GI outbreak activity with peaks from July 2003 to June 2004 (29.4%) (11) and the first 7 months of the observation period July 2012 to June 2013 (37.3%). Data from Noronet, an international NoV surveillance network that collects outbreak data from several European and Asian countries, showed that GI.4, GI.6, and GI.3 (in decreasing order) were the ORF2-based GI genotypes most commonly reported between 2008 and 2011 (7). Australia reported similar data for the period 2002 to 2010 (4). In Alberta, GI.6 and GI.3 were also the predominant GI genotypes observed between July 2008 and June 2012; however, GI.4 was seen very infrequently, and GI.7 has increased in prevalence only during the recent 2012-2013 winter season. The reasons for these recent changes in GI genotype diversity and prevalence are unknown; although differences in nucleotide sequences were observed in the latest and more predominant GI.6 and GI.7 strains, additional research is required to determine if antigenic drift, route of transmission, and type of outbreak setting are factors. Our recent observations related to GI outbreak activity will need to be interpreted in the context of ongoing global NoV surveillance.
In Alberta, GI and GII outbreaks occurred mostly in long-term care, supportive living facilities, and health care facilities, as described in Europe (28). The higher proportion of GI in non-health care facility settings was similar in Australia (4). There were some variations in the predominant outbreak setting for GI over different observation periods (data not shown), but the number of outbreaks per category was too small for trending.
In conclusion, our updated NoV outbreak activity data (2008 to 2012) demonstrated a recent deviation from the historical biennial pattern of NoV outbreak activity previously reported in Alberta (2002 to 2008). Increased NoV outbreak activity was not always associated with new GII.4 variants. The recent divergence from the biennial pattern may be due to multiple factors which need further study, such as host susceptibility, levels of herd immunity, the virulence of specific NoV strains, and public health interventions that might impact transmission.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Provincial Laboratory for Public Health, Alberta, Canada, and Alberta Health, Canada (RES0015904).
We thank Michael Janke for assistance with norovirus sequencing and Bart Hazes for expert opinion regarding sequence analysis. We also thank the Provincial Laboratory for Public Health (ProvLab) staff, especially Marie Louie and Greg Tyrrell, the collaborative teams participating in outbreak investigations in Alberta, Northwest Territories, Yukon, and Nunavut, and the members of the Public Health (ProvLab) Outbreak Investigation Committee (OINC).
Footnotes
Published ahead of print 1 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00663-13.
REFERENCES
- 1. Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. 2010. Viral shape-shifting: norovirus evasion of the human immune system. Nat. Rev. Microbiol. 8:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. 2013. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J. Clin. Virol. 56:185–193 [DOI] [PubMed] [Google Scholar]
- 4. Bruggink LD, Oluwatoyin O, Sameer R, Witlox KJ, Marshall JA. 2012. Molecular and epidemiological features of gastroenteritis outbreaks involving genogroup I norovirus in Victoria, Australia, 2002–2010. J. Med. Virol. 84:1437–1448 [DOI] [PubMed] [Google Scholar]
- 5. Kroneman A, Vennema H, Deforche K, v d Avoort H, Penaranda S, Oberste MS, Vinje J, Koopmans M. 2011. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 51:121–125 [DOI] [PubMed] [Google Scholar]
- 6. Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, Vinje J, Baric RS. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5:e31. 10.1371/journal.pmed.0050031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Beek J, Baas D, Kroneman A, Vennema H, Koopmans M. 27 April 2012. Noronet update, April 2012. National Institute of Public Health and the Environment (RIVM) http://www.rivm.nl/en/Inside_the_Library?kwobject=rivmp:78542&contenttype=issue Accessed 12 February 2013 [Google Scholar]
- 8. Bull RA, Eden JS, Rawlinson WD, White PA. 2010. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 6:e1000831. 10.1371/journal.ppat.1000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng DP, Widdowson MA, Glass RI, Vinje J. 2010. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J. Clin. Microbiol. 48:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention 2011. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recommend. Rep. 60:1–18 [PubMed] [Google Scholar]
- 11. Pang XL, Preiksaitis JK, Wong S, Li V, Lee BE. 2010. Influence of novel norovirus GII.4 variants on gastroenteritis outbreak dynamics in Alberta and the Northern Territories, Canada between 2000 and 2008. PLoS One 5:e11599. 10.1371/journal.pone.0011599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang XL, Preiksaitis JK, Lee B. 2005. Multiplex real time RT-PCR for the detection and quantitation of norovirus genogroups I and II in patients with acute gastroenteritis. J. Clin. Virol. 33:168–171 [DOI] [PubMed] [Google Scholar]
- 13. Noel JS, Ando T, Leite JP, Green KY, Dingle KE, Estes MK, Seto Y, Monroe SS, Glass RI. 1997. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J. Med. Virol. 53:372–383 [DOI] [PubMed] [Google Scholar]
- 14. Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 100:107–114 [DOI] [PubMed] [Google Scholar]
- 15. Vinje J, Hamidjaja RA, Sobsey MD. 2004. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J. Virol. Methods 116:109–117 [DOI] [PubMed] [Google Scholar]
- 16. Yuen LK, Catton MG, Cox BJ, Wright PJ, Marshall JA. 2001. Heminested multiplex reverse transcription-PCR for detection and differentiation of Norwalk-like virus genogroups 1 and 2 in fecal samples. J. Clin. Microbiol. 39:2690–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chhabra P, Walimbe AM, Chitambar SD. 2010. Complete genome characterization of genogroup II norovirus strains from India: evidence of recombination in ORF2/3 overlap. Infect. Genet. Evol. 10:1101–1109 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Duran JJ, Gregorio-Dominguez MM. 17 August 2011. Testing for seasonality using circular distributions based on non-negative trigonometric sums as alternative hypotheses. Stat. Methods Med. Res. [Epub ahead of print.] 10.1177/0962280211411531 [DOI] [PubMed] [Google Scholar]
- 19. Cleophas TJ, Zwinderman AH. 7 July 2012. Assessing seasonality in clinical research. Clin. Chem. Lab. Med. [Epub ahead of print.] 10.1515/cclm-2012-0295 [DOI] [PubMed] [Google Scholar]
- 20. Zeger SL, Irizarry R, Peng RD. 2006. On time series analysis of public health and biomedical data. Annu. Rev. Public Health 27:57–79 [DOI] [PubMed] [Google Scholar]
- 21. Walter SD, Elwood JM. 1975. A test for seasonality of events with a variable population at risk. Br. J. Prev. Soc. Med. 29:18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siffel C, Alverson CJ, Correa A. 2005. Analysis of seasonal variation of birth defects in Atlanta. Birth Defects Res. A Clin. Mol. Teratol. 73:655–662 [DOI] [PubMed] [Google Scholar]
- 23. Yen C, Wikswo ME, Lopman BA, Vinje J, Parashar UD, Hall AJ. 2011. Impact of an emergent norovirus variant in 2009 on norovirus outbreak activity in the United States. Clin. Infect. Dis. 53:568–571 [DOI] [PubMed] [Google Scholar]
- 24. Lee BE, Mukhi SN, May-Hadford J, Plitt S, Louie M, Drews SJ. 2011. Determination of the relative economic impact of different molecular-based laboratory algorithms for respiratory viral pathogen detection, including pandemic (H1N1), using a secure web based platform. Virol. J. 8:277. 10.1186/1743-422X-8-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinje J, White PA, Koopmans M, NoroNet 2013. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II. 4, late 2012. Euro Surveill. 18:8–9 [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention 2013. Notes from the Field: emergence of new norovirus strain GII.4 Sydney—United States, 2012. MMWR Morb. Mortal. Wkly. Rep. 62:55. [PMC free article] [PubMed] [Google Scholar]
- 27. Greening GE, Hewitt J, Rivera-Aban M, Croucher D. 2012. Molecular epidemiology of norovirus gastroenteritis outbreaks in New Zealand from 2002–2009. J. Med. Virol. 84:1449–1458 [DOI] [PubMed] [Google Scholar]
- 28. Koopmans M. 2009. Noroviruses in healthcare settings: a challenging problem. J. Hosp. Infect. 73:331–337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.