Abstract
We developed an enzyme-linked immunosorbent assay (ELISA) using eukaryotically expressed E protein as the antigen (termed E-ELISA) to detect antibodies to tembusu virus (TMUV) in ducks. The E-ELISA did not react with antisera to other known pathogens, indicating the E protein is specific for recognizing anti-TMUV antibodies. Compared to the serum neutralization test, the specificity and sensitivity of the E-ELISA was 93.2 and 97.8%, respectively. Therefore, this E-ELISA is a sensitive and rapid method for detecting antibodies against TMUV in ducks.
TEXT
Duck tembusu virus (DTMUV), a member of the Flavivirus genus in the family Flaviviridae, consists of single-stranded, positive-sense viral RNA that encodes three structural proteins and seven nonstructural proteins (10, 11). Since 2010, a novel DTMUV has caused a substantial drop in egg production in infected birds in southeast China (12, 20). Surveillance showed that this virus has continuously caused outbreaks and has spread to northeast China. The transporting of virus-infected birds may be one mechanism by which this virus has been introduced into new and unaffected regions. The considerable morbidity caused by DTMUV demands a rapid and simple identification test, so that appropriate curing or stamp-out procedures can be implemented to prevent the expansion of this disease to other unaffected regions in China and other countries.
The assay of choice for the detection of flavivirus infection is enzyme-linked immunosorbent assay (ELISA) (5, 8, 14). ELISA-based antibody detection tests using recombinant antigens offer higher levels of reproducibility, are easy to standardize, and are less labor-intensive than the use of chemically inactivated viral antigens. More importantly, the production of the noninfectious recombinant antigen used in the assay does not require the cultivation of infectious viruses, reducing the biohazardous conditions (1, 2, 13, 22, 23). Several ELISAs have been developed by using recombinant PrM and E proteins as the antigens for detecting antibodies against flavivirus (4, 6, 7, 21). Recent studies reported that the E protein of West Nile virus induces a strong immune response and provides protection against West Nile virus infection (3, 17), suggesting that the E protein without prM could be used as an antigen to detect antibodies against the virus. An ELISA that uses a recombinant protein as the coating antigen for the detection of antibodies against DTMUV has never been investigated. In this study, we developed an E protein ELISA (E-ELISA) by using Spodoptera fugiperda (Sf9) insect cell-expressed recombinant E protein as the coating antigen to detect antibodies against tembusu virus in ducks.
Specific-pathogen-free (SPF) duck embryonated eggs free of DTMUV were used for virus propagation (11). The E-encoding gene was reverse transcribed to cDNA as described previously (11). The cDNA clone was amplified by PCR with concurrent introduction of a C-terminal His6 tag at the reverse primers. Cloning sites BamHI and XhoI were introduced into the forward primer 5′-CGCGGATCCTTCAGCTGTCTGGGGATGCAG-3′ and the reverse primer 5′-ATCTCGAGCTA gtg atg gtg atg gtg atg GGCATTGACATTTACTGCC-3′ (the cloning site is underlined and the His6 codons are in lowercase letters), respectively. The amplified PCR product was sequenced, resulting in the expected size of 1,503 bp. After sequence verification, the BamHI- and XhoI-digested insert was cloned into a pFastBac1 vector (Novagen, Madison, WI). Isolated recombinant bacmid DNA and pFastBac DNA (as a control) were used to transfect Sf9 cells according to the manufacturer's instructions.
The E fusion proteins in cell debris and supernatant were purified by using a nickel-nitrilotriacetic acid (Ni-NTA) kit (Qiagen, Valencia, CA) and then were analyzed by SDS-PAGE and Western blotting. Nitrocellulose (NC) membranes were probed with DTMUV-positive sera (diluted 1:100) and phosphatase-labeled goat anti-duck IgG (L and H) conjugates (1:500 dilution) (KPL, MD) (12). SDS-PAGE showed the E fusion protein to have an approximate molecular mass of 65 kDa (Fig. 1A), which was 5 kDa higher than expected (54-kDa E protein plus 6-kDa His tag), suggesting that the E fusion protein is glycosylated. We found that there are two potential N-linked glycosylated sites: 154NYS156 and 314NPT316. The amount of expressed E protein in the supernatants was lower than that in the pellets (data now shown). Western blotting showed that DTMUV-positive sera reacted specifically against a purified 65-kDa E fusion protein (Fig. 1B). No other proteins were detected from the pFastBac E-transformed Sf9 cells (data not shown).
Fig 1.
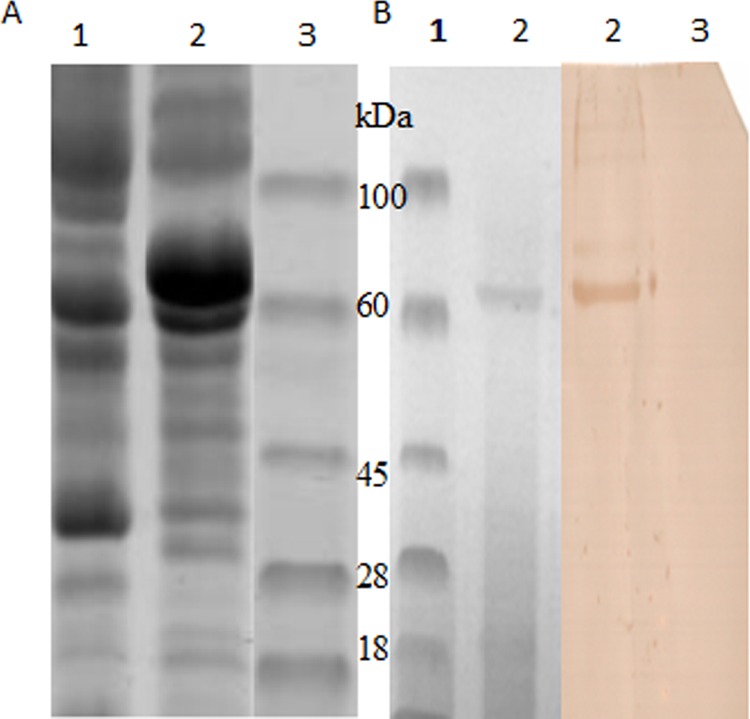
(A) Identification of E protein from transformed cells by SDS-PAGE. Lane 1, Sf9 expressing pFastBac-E; lane 2, Sf9 expressing pFastBac; lane 3, molecular mass marker. (B) Purified His-E protein analyzed by SDS-PAGE and detected by Western blotting with duck anti-tembusu virus sera. Lane 1, molecular mass marker; lane 2, purified His-E protein; lane 3, protein from pFastBac-transformed Sf9 cells.
DTMUV-positive sera were prepared as follows. Thirty SPF ducks were immunized with purified inactivated DTMUV TA strain in complete Freund's adjuvant and boosted twice in incomplete Freund's adjuvant at 2-week intervals. (Approval for this research was obtained from the Harbin Veterinary Research Institute Animal Center.) Sera were collected 2 weeks after the final boost; 30 DTMUV-positive and -negative sera (collected from uninfected SPF ducks as a control) were used to evaluate the E-ELISA and to compare it to serum neutralization (SN) tests. Sera against H5N1 influenza virus (AIV), Newcastle disease virus (NDV), duck plague virus (DPV), duck hepatitis type 1 virus (DHV-1), duck reovirus (DRV), egg drop syndrome virus 76 (EDS-76), and Japanese encephalitis virus (JEV) all were collected at the Harbin Veterinary Research Institute. In addition, 469 clinical serum samples were collected from adult meat-type and egg-laying breeder ducks suffering from egg drop disease at various commercial farms between 2010 and 2012.
As the gold standard method, the SN test was carried out in the 96-well format using DEF cells as described previously, with minor modifications (18). Briefly, 100 μl of heat-inactivated sera diluted in Dulbecco's modified Eagle medium (DMEM; initial dilution, 1:10; 2-fold dilution to 1,280) was incubated with 100 50% tissue culture infectious doses (TCID50) of the TA strain for 1 h at 37°C. The virus-serum mixture (100 μl) was then transferred onto a monolayer of DEF cells in a 96-well plate (triplicate wells). DTMUV-positive and -negative sera, phosphate-buffered saline (PBS), and uninfected DEF cells served as controls. The cytopathic effects (CPE) were observed daily for 5 days. Neutralization titers of sera were calculated by the Reed-Muench method (15). SN titers of <1.5 (1:40) were considered negative, and titers of 1.5 or greater were considered positive. The 30 DTMUV-positive animals showed a neutralizing antibody titer of 1:640, but the 30 DTMUV-negative sera and the sera against the other duck pathogens showed no cross-reaction to DTMUV (<1.5).
To standardize the E-ELISA, the DTMUV-positive or -negative sera and conjugate dilution (from 40× to 1,600×) were used to optimize the detection system. To determine the optimal concentrations, a checkerboard titration was carried out with different amounts of E protein (ranging from 500 to 0.5 ng per well). By using the DTMUV-positive or -negative sera, we found the optimal dilution of the test sera to be 1:100. The optimum E protein concentration was found to be 5 μg/ml. The optimal dilution of 1:400 for the conjugate was determined previously (13). Thus, our standardized E-ELISA procedure is 100 μl of 5 μg/ml purified E protein coated onto the wells of an ELISA plate (BIOFIL; Canada JET Biochemicals Inc.). After washing, 1:100 diluted DTMUV-positive and -negative sera are added. An aliquot (100 μl/well) of the 1:400 diluted conjugate is added after washing. Reactions are stopped by adding 3 M NaOH, and the plate is read on a microplate reader (Bio-Rad, Japan) at 405 nm.
Using this standardized procedure, a good positive/negative (P/N) ratio was obtained by dividing the positive and negative optical density (OD) values (≥2.1). DTMUV-negative and -positive sera (n = 30) were examined in both the E-ELISA and SN tests. The average OD of the DTMUV-negative sera in the E-ELISA were <0.2 ± 0.017 (means ± standard deviations [SD]). The 1:100-diluted serum specimens with OD of <0.25 or ≥0.25 were interpreted as negative or positive, respectively. Based on this criterion, 30 uninfected SPF duck sera all were negative and 30 DTMUV-positive sera all were positive in the E-ELISA.
The detection threshold of the E-ELISA compared to that of the SN test was determined by using serial dilutions of the DTMUV-positive sera. The sensitivities were a dilution of 1:320 sera tested at >0.25 absorbance units for the E-ELISA and a dilution of 1:640 sera for SN titers of >1.5 (1:40). The negative-control sera showed no detectable E- or virus-specific antibodies in the E-ELISA or the SN test at any dilution. Antisera specific for other known duck pathogens and JEV yielded <0.25 OD values, indicating that no cross-reaction was detected by antisera specific for other known duck pathogens or JEV in the E-ELISA.
The sensitivity and specificity of the E-ELISA were also compared to those of the SN test by using the 469 clinical farm serum samples (Table 1). The SN test determined that 366 and 103 samples were DTMUV-positive and -negative, respectively. The E-ELISA result showed that 365 and 104 serum samples were E-antibody positive and negative, respectively. Together, 358 serum samples were positive and 96 serum samples were judged to be negative by both methods (Table 1). Using the SN test as a reference, the specificity and sensitivity of the E-ELISA were calculated to be 93.2 and 97.8%, respectively. The concordance between the two methods was 96.8%.
Table 1.
Comparison between SN tests and our E-ELISA for detection of DTMUV-related antibodies
| Test result | SN positive | SN negative | Total |
|---|---|---|---|
| E-ELISA positive | 358 | 7 | 365 |
| E-ELISA negative | 8 | 96 | 104 |
| Total | 366 | 103 | 469 |
Dot blotting assays were also compared to the SN test by using 454 clinical serum samples (that were in agreement for both the SN test and the E-ELISA). Briefly, 5 μg/ml E fusion protein was spotted onto NC membrane. The membranes were probed with 454 (1:100) clinical sera as described for Western blotting. The sensitivities and specificities for the SN test and dot blotting assays were then analyzed. The specificity and sensitivity were found to be 84.3 and 91.6%, respectively. The results of the SN test and the dot blotting assays were in agreement for 409 samples (Table 2). However, 30 dot blotting serum-negative samples were positive in the SN test, and 15 SN serum-negative samples were positive in the dot blotting assay. The results indicate that the dot blotting assay is less sensitive and less specific than the E-ELISA.
Table 2.
Comparison between SN tests and dot blotting in the detection of DTMUV-related antibodies
| Test result | SN positive | SN negative | Total |
|---|---|---|---|
| Dot blotting positive | 328 | 15 | 343 |
| Dot blotting negative | 30 | 81 | 111 |
| Total | 358 | 96 | 454 |
Sf9-expressed E protein of ∼5 kDa larger than the expected size and the presence of two potential N-linked glycosylated sites suggested that DTMUV E protein is glycosylated. A previous report demonstrated that baculovirus-derived antigen was more sensitive than the bacterial recombinant antigen for detecting virus-specific antibodies in polyclonal sera (1). In this study, the 5 ng/well of recombinant E protein chosen for the E-ELISA allowed the specific and sensitive detection of E antibodies to DTMUV in ducks. The results of the E-ELISA were highly correlated (96.9%) with those of the SN tests. Regarding sensitivity, specificity, and this correlation, this recombinant E protein alone seems to be a suitable antigen to detect specific antibodies to tembusu virus in ducks in an E-ELISA.
Until now, only a limited number of flaviviruses have been reported in ducks (21), and serological evidence of other flavivirus infections in birds in China has not been reported. The E-ELISA showed no cross-reactivity against other known duck pathogens; however, cross-reactions due to unknown flaviviruses cannot be completely ruled out, because the status of other flaviviruses in ducks in China is not known. Because the sensitivity of ELISAs for cross-reactive antibodies is insufficient to permit the use of a single ELISA to screen for antibodies to different flaviviruses (16, 19), the most reliable approach is to continue to use homologous antigens in ELISAs.
Using SN tests as the gold standard, dot blots are less sensitive and less specific than the E-ELISA. SN tests and blocking ELISAs have the advantage that they can be used for multiple species, but they require the propagation and purification of large quantities of flavivirus for use as antigens (9). Moreover, live antigens have the potential danger of spreading to farms when employed for diagnosis. While species-specific secondary antibodies are needed in E-ELISAs, our E-ELISA reduces labor and reagent costs without the risk of pathogenic contamination. Regarding sensitivity and specificity, this E-ELISA seems to be quick and suitable for screening large serum sets for E antibodies against DTMUV.
ACKNOWLEDGMENTS
This study was supported by a grant from the China Agriculture Research System (CARS-43-10) and the Chinese Special Fund for Agro-Scientific Research in the Public Interest (201003012).
Footnotes
Published ahead of print 24 April 2013
REFERENCES
- 1. Barber GN, Ciegg JCS, Lloyd G. 1990. Expression of the Lassa virus nucleocapsid protein in insect cells infected with a recombinant baculovirus: application to diagnostic assays for Lassa virus infection. J. Gen. Virol. 71:19–28 [DOI] [PubMed] [Google Scholar]
- 2. Chan CM, Woo PCY, Leung ASP, Lau SKP, Che XY, Cao L, Yuen KY. 2002. Detection of specific antibodies to an antigenic cell wall galactomanno protein for serodiagnosis of Aspergillus fumigatus aspergillosis. J. Clin. Microbiol. 40:2041–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chu JH, Chiang CC, Ng ML. 2007. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J. Immunol. 178:2699–2705 [DOI] [PubMed] [Google Scholar]
- 4. Davis BS, Chang GJJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowe R, Bunning ML. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme linked immunosorbent assays. J. Virol. 75:4040–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frazier CL, Shope RE. 1979. Detection of antibodies to alphaviruses by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 10:583–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikawa-Yoshida A, Yoshii K, Kuwahara K, Obara M, Kariwa H, Takashima I. 2011. Development of an ELISA system for tick-borne encephalitis virus infection in rodents. Microbiol. Immunol. 55:100–107 [DOI] [PubMed] [Google Scholar]
- 7. Jääskeläinen A, Han X, Niedrig M, Vaheri A, Vapalahti O. 2003. Diagnosis of tick-borne encephalitis by a μ-capture immunoglobulin M-enzyme immunoassay based on secreted recombinant antigen produced in insect cells. J. Clin. Microbiol. 41:4336–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:1827–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li XS, Li GX, Teng QY, Yu L, Wu XG, Li ZJ. 2012. Development of a blocking ELISA for detection of serum neutralizing antibodies against newly emerged duck tembusu virus. PLoS One 7(12):e53026. 10.1371/journal.pone.0053026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindenbach BD, Thiel HJ, Rice CM. 2007. Flaviviridea: the viruses and their replication, p 1101–1152 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 11. Liu M, Liu CG, Li G, Li XJ, Yin XC, Chen YH, Zhang Y. 2012. Complete genomic sequence of duck flavivirus from China. J. Virol. 86:3398–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu M, Chen SY, Chen YH, Liu CG, Chen SL, Yin XC, Li G, Zhang Y. 2012. Adapted tembusu-like virus in chickens and geese in China. J. Clin. Microbiol. 50:2807–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu M, Zhang TT, Zhang Y, Meng FY, Li XJ, Hou ZZ, Feng XC, Kong XG. 2010. Development and evaluation of a VP1-ELISA for detection of antibodies to duck hepatitis type 1 virus. J. Virol. Methods 169:66–69 [DOI] [PubMed] [Google Scholar]
- 14. Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Rochrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed LJ, Muench H. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. (London) 27:493–497 [Google Scholar]
- 16. Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. 2001. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann. N. Y. Acad. Sci. 951:286–297 [DOI] [PubMed] [Google Scholar]
- 17. Schneeweiss A, Chabierski S, Salomo M, Delaroque N, Al-Robaiy S, Grunwald T, Bürki K, Liebert UG, Ulbert S. 2011. A DNA vaccine encoding the E protein of West Nile virus is protective and can be boosted by recombinant domain DIII. Vaccine 29(37):6352–6357 [DOI] [PubMed] [Google Scholar]
- 18. Su JJ, Li S, Hu XD, Yu XL, Wang YY, Liu PP, Lu XS, Zhang GZ, Hu XY, Liu D, Li XX, Su WL, Lu H, Mok NS, Wang PY, Wang M, Tian KG, Gao GF. 2011. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106. 10.1371/journal.pone.0018106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection, J. Clin. Microbiol. 38:2232–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J. Clin. Microbiol. 42:4641–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wojnarowicz C, Olkowski A, Schwean-Lardner K. 2007. First Canadian outbreak of West Nile virus disease in farmed domestic ducks in Saskatchewan. Can. Vet. J. 48:1270–1271 [PMC free article] [PubMed] [Google Scholar]
- 22. Woo PCY, Lau SKP, Wong BHL, Tsoi H, Fung AMY, Kao RYT, Chan KH, Peiris JS, Malik YK. 2005. Differential sensitivities of severe acute respiratory syndrome (SARS) coronavirus spike polypeptide enzyme-linked immunosorbent assay (ELISA) and SARS coronavirus nucleocapsid protein ELISA for serodiagnosis of SARS coronavirus pneumonia. J. Clin. Microbiol. 43:3054–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woo PCY, Lau SKP, Wong BHL, Tsoi HW, Fung AMY, Chan KH, Tam VKP, Peiris JSM, Yuen KY. 2004. Detection of specific antibodies to SARS coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia. J. Clin. Microbiol. 42:2306–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]


