Abstract
The Mycobacterium tuberculosis pandemic is a major health problem, further complicated by an increasing incidence of drug-resistant isolates and the existence of highly transmissible strains, such as those in the Beijing family. Streptomycin (STR)-resistant M. tuberculosis clinical isolates have been analyzed to look for mutations in the rpsL, rrs, and gidB genes. In addition, the Rv1258c gene, which encodes Tap, an efflux pump that transports STR, has been sequenced. Mutations affecting codons 43 and 88 of the rpsL gene were found in 44.4% of the strains, and 16.7% of the strains carried mutations in the rrs gene, both of which probably contribute to STR resistance. Many strains presented with mutations in the gidB gene, but the implication of those mutations in STR resistance remains unclear. Interestingly, a cytosine nucleotide insertion between positions 580 and 581 (denominated Tap580) in the Rv1258c gene has been found in all Beijing isolates included in this study, suggesting that it might be a novel polymorphism specific to the Beijing family of M. tuberculosis. A simple and fast restriction fragment length polymorphism (RFLP)-PCR method for detecting the Tap580 insertion has been developed and used to screen a collection of 220 DNA samples obtained from cultures of M. tuberculosis isolates and 30 respiratory specimens. In all cases, the Beijing and non-Beijing representative samples were identified correctly. Tap580 is a novel polymorphism specific to the highly transmissible Beijing family, which allows for fast detection of these strains even at the very early stages of infection.
INTRODUCTION
Mycobacterium tuberculosis is the cause of millions of incidences of tuberculosis infections and deaths worldwide (1), many of them among HIV-positive patients. This pandemic is further complicated by the increased incidence of drug-resistant strains (1), which generally have accumulated mutations in the genes that encode drug target or drug-activating enzymes. Nevertheless, there are resistant strains in which no mutations linked to drug resistance have been identified, hence suggesting unidentified drug-target proteins or novel mechanisms of drug resistance. A paradigm is the case of streptomycin (STR), an antituberculosis drug that is increasingly used due to the rising incidence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains. Mutations in the genes rpsL, rrs, and gidB (encoding the S12 ribosomal protein, 16S rRNA, and ribosome methyltransferase, respectively) have been found in ca. 70% of M. tuberculosis isolates that are resistant to STR; in the rest of STR-resistant M. tuberculosis strains, the cause of drug resistance remains unknown.
Molecular techniques have allowed for the differentiation of several lineages and families among M. tuberculosis strains, which differ not only in their global geographical distributions, but also in terms of transmissibility, drug resistance, treatment outcome, and other factors. The isolates of the Beijing family of M. tuberculosis are characterized by their increased ability to spread and cause disease and their increased association with drug resistance in comparison with the levels for non-Beijing strains (2). Because of this, many methods have been developed that are aimed at identifying and differentiating M. tuberculosis Beijing isolates (3–6). There are several methods for identifying Beijing isolates, including spoligotyping, restriction fragment length polymorphism (RFLP) using IS6110 as a probe, or the presence of a copy of IS6110 in the dnaA region (7). Other methods are based on the detection of specific genomic deletions, such as region of difference 105 (RD105) or an intact pks15/1 gene (8). Recently, the detection of a single-nucleotide polymorphism (SNP) in the Rv2629 gene by using real-time PCR followed by high-resolution melting has also been described (3).
In this work, we present an analysis of target mutations in clinical isolates of M. tuberculosis that are resistant to STR. In addition, the nucleotide sequence of the Rv1258c gene has been investigated as a potential STR-resistance determinant, since this gene encodes the drug efflux pump Tap in M. tuberculosis, which transports STR and other antibiotics (9–11). A nucleotide insertion in the coding sequence of the Rv1258c gene has been identified, which does not seem to be associated with resistance to this drug. Interestingly, this insertion is exclusive to the Beijing family of M. tuberculosis isolates and has led to the development of a method for screening M. tuberculosis strains (using either purified DNA or directly on respiratory samples) and reliably identifying those belonging to the Beijing family of strains.
MATERIALS AND METHODS
M. tuberculosis DNA samples.
First, DNA samples of 18 M. tuberculosis clinical isolates that are resistant to STR and additional drugs (including several MDR isolates), of the Universidad de Zaragoza culture collection, along with the control strain H37Rv (which is fully drug susceptible) were used as a template to amplify and sequence the target genes (rpsL, rrs, gidB, and Rv1258c). This group included 4 strains that had a spoligotype that is consistent with being in the Beijing family.
Next, 220 DNA samples from M. tuberculosis complex clinical isolates were analyzed, some of them collected recently from hospitals in Huesca, Zaragoza, Madrid, and Barcelona (Spain), and some others from a Spanish national survey on MDR M. tuberculosis that was done between 1998 and 2009. This set included 49 DNA samples having a spoligotype that is consistent with those of the Beijing family, 158 that were representative of other M. tuberculosis distinct lineages (Latin American-Mediterranean [LAM], T, X, S, Haarlem, central Asian [CAS], east African-Indian [EAI], and others) including 5 isolates of the MTZ strain, a highly transmissible M. tuberculosis strain that has caused major outbreaks in Zaragoza (12), and 13 that were Mycobacterium africanum (Table 1). Overall, this collection included samples that were representative of most of the M. tuberculosis genetic lineages that have been described (13). In these samples, the presence of the novel nucleotide insertion in the Rv1258c gene that is described in this work was investigated. In a selection of 30 DNA samples (18 from Hospital Germans Trías i Pujol, Badalona, and 12 from Hospital General Universitario Gregorio Marañón, Madrid) that originated from STR-resistant isolates (five of them belong to the Beijing family), the Rv1258c gene was completely sequenced in order to verify the presence of the nucleotide insertion in the Rv1258c gene that is described in this work, and to identify other potential mutations.
Table 1.
Number of strains belonging to different lineages used in Tap580 screening
| Lineage/speciesa | No. of strains |
|---|---|
| Beijing | 49 |
| T | 27 |
| LAM | 25 |
| Haarlem | 18 |
| U | 16 |
| CAS | 12 |
| EAI | 9 |
| S | 2 |
| X | 1 |
| Not assigned | 48 |
| M. africanum | 13 |
| Total | 220 |
LAM, Latin American-Mediterranean lineage; CAS, central Asian lineage; EAI, east African-Indian lineage.
PCR amplification and sequencing.
The primers used for amplifying and sequencing the genes related to STR resistance, the annealing temperature used in the PCR, and the size of the product are listed in Table 2. PCRs were performed in a final volume of 50 μl using 200 μM (each) deoxynucleoside triphosphate (dNTP), 5 μl of buffer (10× PCR buffer; Applied Biosystems), 1.25 U of AmpliTaq Gold polymerase (Applied Biosystems), 0.25 μM (each) primer, 5 μl of dimethyl sulfoxide, and 2 μl of DNA (20 to 100 ng/μl). Amplifications consisted of an initial step at 94°C for 10 min, followed by 40 cycles of 1 min at 94°C, annealing for 2 min at the temperatures indicated in Table 2, and extension for 2 min at 72°C, with a final extension step of 10 min at 72°C. All genes were amplified in a single product, except for the Rv1258c gene, which was amplified in two overlapping products, one containing the promoter and the first half of the coding sequence, and the second containing the rest of the gene.
Table 2.
Primers used for amplification in this study
| Target | PCR product (bp) | Taa (°C) | Primer | Sequence (5′ → 3′) | Reference |
|---|---|---|---|---|---|
| For amplifying and sequencing | |||||
| rpsL | 501 | 52 | S13 | GGCCGACAAACAGAACGT | 30 |
| S16 | GTTCACCAACTGGGTGAC | 30 | |||
| rrs | 1,037 | 58 | 264 | TGCACACAGGCCACAAGGGA | 31 |
| 285 | GAGAGTTTGATCCTGGCTCAG | 31 | |||
| gidB | 977 | 56 | gidB3 | GAACGGAAGATCGTCCAC | This work |
| gidB4 | CGATAGTTGAAGCCTGGC | This work | |||
| Rv1258cb | 830 | 58 | ctap1 | CAATGTGGATTACCGCGAC | This work |
| ctap2 | GTCTTGCCGGTAGCCGTC | This work | |||
| Rv1258cc | 902 | 58 | ctap3 | CGCAGGTTCCAGACGAAG | This work |
| ctap4 | GATCAGCGCGTTGAGTTC | This work | |||
| For detection of nucleotide insertion | |||||
| Rv1258c | 1,052 | 65 | ctap9 | GTTGTTCGCCACGCTGGTCG | This work |
| ctap10 | CCAGATCCAGTTCGCGCAG | This work |
Ta, annealing temperature.
3′ region of the Rv1258c gene.
Promoter and 5′ region of the Rv1258c gene.
PCR products were purified using ExoSAP-IT (Affymetrix) and sequenced using the same primers used for the amplification. Sequences were then analyzed by comparison with that of the reference H37Rv strain (Cole et al. [14]; TubercuList database [http://tuberculist.epfl.ch/]).
Screening of nucleotide insertion in Rv1258c gene.
First, an internal fragment of the Rv1258c gene containing the location of the cytosine nucleotide insertion between positions 580 and 581 of the coding sequence of the Rv1258c gene (denominated Tap580) was amplified using the primers ctap9 and ctap10 (Table 2). DNA samples were processed for 9 min at 94°C, followed by 35 cycles of 30 s at 94°C, annealing for 30 s at 65°C, extension at 72°C for 90 s, and final extension at 72°C for 10 min. A product of 1,052 bp was obtained, which was digested for 1 h at 37°C with XhoI restriction enzyme, followed by electrophoresis in an 0.8% agarose gel in Tris-borate-EDTA (TBE) buffer. As a control of endonuclease digestion, PCR products were digested for 1 h at 37°C with PvuII restriction enzyme, which produces two fragments of 610 and 442 nucleotides for both Beijing and non-Beijing isolates.
Bioinformatic analysis.
Databases of bacterial genomes were analyzed using the program BLAST at NCBI (National Center for Biotechnology Information, http://blast.ncbi.nlm.nih.gov/) to search for a Tap580 insertion in the Rv1258c gene among the sequenced genomes of the M. tuberculosis complex.
Screening of Tap580 insertion in Rv1258c gene directly in clinical samples.
A blind panel of 30 respiratory samples was assembled by two hospitals (Servicio de Microbiología, Hospital Universitario Lozano Blesa, Zaragoza, Spain, and Servicio de Microbiología Clínica y Enfermedades Infecciosas, Hospital General Universitario Gregorio Marañón, Madrid, Spain). This panel included 29 smear-positive and culture-positive samples from patients diagnosed with tuberculosis caused by Beijing (2 samples) and non-Beijing (27 samples) strains, and one further sputum sample that contained Mycobacterium fortuitum as a negative control. Of the samples containing M. tuberculosis, nine contained between 2 and 10 acid-fast bacilli/field, five samples contained between 10 and 100 acid-fast bacilli/field, and 15 samples contained >100 acid-fast bacilli/field. All samples were decontaminated by N-acetyl-l-cysteine–NaOH, and a fraction was incubated for 20 min at 80°C to kill the live bacilli and extract DNA using the GenoLyse kit (Hain Lifescience). The DNA samples were processed according to the method described above for detecting the Tap580 insertion in the Rv1258c gene.
RESULTS AND DISCUSSION
Mutations in rpsL, rrs, and gidB genes.
In order to find the mutations responsible for STR resistance in 18 M. tuberculosis clinical strains, the genes rpsL, rrs, and gidB, which are known to carry mutations related to resistance to STR, were sequenced and compared with the sequence of the STR-susceptible H37Rv laboratory strain available in the TubercuList database (http://tuberculist.epfl.ch/). All strains analyzed in our study, including the reference H37Rv strain, had the mutations A363G in rpsL and C299T in gidB, which have been reported as sequencing errors in the sequence of H37Rv included in both the TubercuList database and GenBank accession no. AL123456.2 (15, 16).
The significant mutations found in the strains analyzed in our study are summarized in Table 3.
Table 3.
Mutations found in the rpsL, rrs, gidB, and Rv1258c genes in strains of the Universidad de Zaragoza culture collection
| M. tuberculosis STR-resistant strain | Mutation found in: |
||||||
|---|---|---|---|---|---|---|---|
|
rpsL |
rrs DNA |
gidB |
Rv1258c |
||||
| DNA | Protein | DNA | Protein | DNAc | Protein | ||
| Z-07044 | C340T | ||||||
| Z-07047a | A128G | K43R | A276Cb | E92D | InsC 580–581 | 194 frameshift | |
| A615Gb | Silent | ||||||
| HMS-1838 | C413Tb | A138V | |||||
| HCU-2879 | A263G | K88R | |||||
| HCU-2934 | A128G | K43R | T47Gb | L16R | |||
| HCU-2830a | A128G | K43R | A276Cb | E92D | InsC 580–581 | 194 frameshift | |
| A615Gb | Silent | ||||||
| HMS-1695 | A128G | K43R | C492T | T47Gb | L16R | ||
| HMS-1691 | A263G | K88R | A13del | 5 frameshift | |||
| HMS-1781 | G490C | G164R | |||||
| VEN-4145a | A128G | K43R | A276Cb | E92D | InsC 580–581 | 194 frameshift | |
| A615Gb | Silent | ||||||
| VEN-1714 | T47Gb | L16R | |||||
| C409Gb | R137G | ||||||
| VEN-5292 | A324G | T47Gb | L16R | ||||
| T149C | L50P | ||||||
| VEN-2457 | T47Gb | L16R | |||||
| VEN-1667 | T47Gb | L16R | |||||
| G248C | R83P | ||||||
| VEN-2543 | |||||||
| VEN-314 | C159T | Silent | |||||
| VEN-4237 | C462T | C159T | Silent | ||||
| A736G | |||||||
| VEN-3748a | A128G | K43R | A276Cb | E92D | InsC 580–581 | 194 frameshift | |
| A615Gb | Silent | ||||||
Eight of the strains tested (44.4%) carried mutations in the rpsL gene, A128G being the mutation that was found in six of them. The four Beijing strains included in this group carried this mutation, which is the most frequent mutation found in STR-resistant M. tuberculosis Beijing strains (17, 18). Two other strains (11.1%) had the mutation A263G.
Four strains (22.2%) harbored mutations in the rrs gene. Three strains carried the C340T, A324G, or A736G mutation; to our knowledge, this is the first report on mutations in this region of the rrs gene. The strain carrying the mutation A736G also carried the mutation C462T according to TubercuList numbering, a mutation that has been described as C461T in a previous publication (19). Finally, one strain carried the mutation C492T close to the 530 loop of the secondary structure of M. tuberculosis 16S rRNA; this strain is discussed further below.
The role of the GidB protein in conferring high-level STR resistance in M. tuberculosis has been fully characterized (20), although the contribution of certain point mutations in STR resistance is still controversial; it has been speculated that some mutations in the gidB gene might promote the acquisition of mutations in either rpsL or rrs (21). A large number of mutations (including many silent mutations) were found in the gidB gene; in fact, only four strains had a gidB gene identical to that of H37Rv. Many missense mutations have been described in STR-susceptible strains, such as the mutation T47G (the most frequent mutation found in our study), which has been reported as being specific to the Latin American-Mediterranean (LAM) family strains (22); in fact, the 6 strains carrying this mutation in our study belong to the LAM family of strains (data not shown). The four Beijing strains carried two mutations: A276C (E92D), which has been described as an SNP specific to this family (22), and A615G; both mutations can be found in STR-susceptible strains (20). In the Beijing strains, these gidB mutations occur simultaneously with the mutation A128G in the rpsL gene, as mentioned above.
Among the non-Beijing strains, the gidB C413T mutation has been found in both STR-resistant and -susceptible M. tuberculosis isolates (20, 21, 23). One STR-resistant isolate carried the mutation G248C, indicating that it might lead to resistance to this drug; however, other mutations in this position (G248T) have been found in STR-susceptible strains (21). Finally, the only gidB mutation potentially related to resistance to STR is the missense mutation G490C, since this one was detected in a strain lacking mutations in the rpsL and rrs genes.
One strain carried mutations in all three genes; these were rpsL A128G, rrs C492T, and gidB T47G. In this strain, STR resistance is due to the mutation rpsL A128G, since the other two mutations have been found in both STR-resistant and -susceptible isolates. The mutation rrs C492T, also reported elsewhere as C491T, has been associated with the LAM3 genetic lineage of M. tuberculosis (24, 25). This very same genetic lineage of M. tuberculosis also carries the mutation T47G, as mentioned above (22).
Finally, one of the STR-resistant strains lacked mutations in any of the three genes rpsL, rrs, and gidB. In three other strains, only mutations in gidB were found, although these either were silent or have been described also in STR-susceptible strains, hence making it unlikely that they make a major contribution to resistance to this drug. This confirms that other mechanisms must contribute to STR resistance in M. tuberculosis.
Mutations in Rv1258c gene.
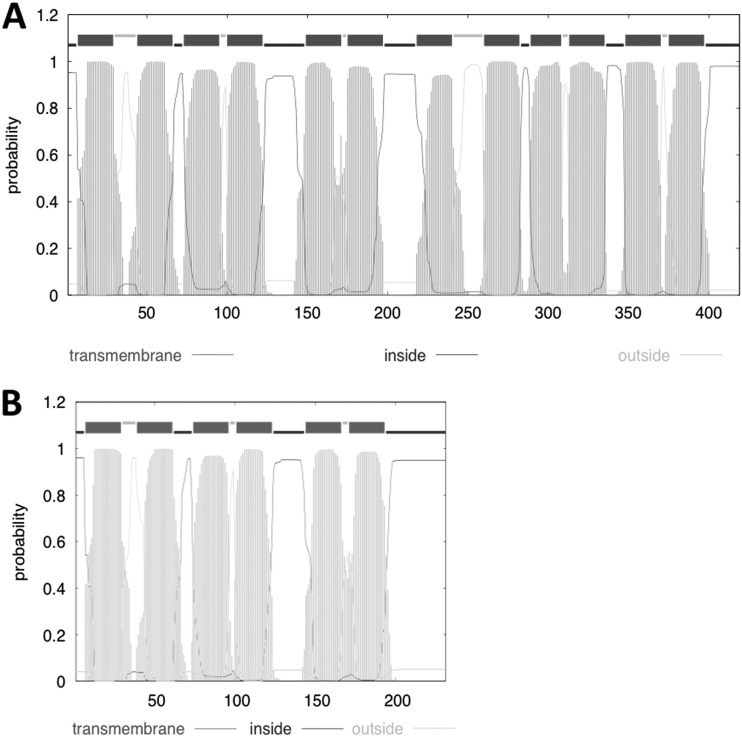
Since STR is a substrate of the Rv1258c efflux pump (11), we hypothesize that mutations affecting the expression levels of the Rv1258c gene or that change the kinetic properties of the efflux pump might contribute to STR resistance. To investigate this further, the Rv1258c gene was amplified and sequenced in the 18 samples of M. tuberculosis STR-resistant clinical strains in which the rpsL, rrs, and gidB genes had been sequenced. Two different mutations in the Rv1258c gene were found. One strain has a deletion of the adenine nucleotide in position 13 (Table 3), producing a frameshift; as a result, a TGA stop codon ends translation of a peptide of only 10 amino acids. Four strains have an insertion of a cytosine nucleotide between positions 580 and 581 (Tap580) (Table 3); this insertion causes a frameshift mutation from codon 194 onwards, resulting in a shorter protein (231 amino acids). This protein probably would not constitute a functional membrane transporter, since it contains only 6 transmembrane segments (TMS) compared with the 12 TMS of the full-length protein (419 amino acids) (Fig. 1). Bacterial drug efflux pumps of the major facilitator superfamily have 12 or 14 TMS, which are required for transport activity.
Fig 1.
Hydrophobicity profile and transmembrane prediction of the Rv1258c proteins of M. tuberculosis H37Rv (A) and Beijing isolates (B) made by using the Hidden Markov model (TMHMM Server v2.0; Center for Biological Sequence Analysis, Technical University of Denmark [http://www.cbs.dtu.dk/services/TMHMM/]).
These five strains carrying mutations in the Rv1258c gene also carried the A263G (K88R) or A128G (K43R) mutation in the rpsL gene, which is probably responsible for the high-level STR resistance of these strains; then, it is difficult to analyze the contribution of Rv1258c mutations in resistance to this drug.
Remarkably, the four strains carrying the Tap580 insertion had been typed as belonging to the Beijing lineage by RFLP and/or spoligotyping (data not shown). We hypothesized that this might represent a novel polymorphism specific to the Beijing family. To test this, the presence of the Tap580 insertion was inspected in 15 genomes of M. tuberculosis available in public databases (NCBI), including clinical isolates, laboratory strains, such as H37Rv and H37Ra, and two strains of the Beijing family. Interestingly, only CCDC5079 and CCDC5081, which belong to the Beijing family (26), had the Tap580 insertion (Table 4), further supporting that Tap580 is an insertion that is specific to the Beijing family isolates. Other species of the M. tuberculosis complex, such as four substrains of Mycobacterium bovis BCG and M. bovis AF2122/97, did not show the Tap580 polymorphism and had complete identity with the Rv1258c gene of H37Rv (Table 4).
Table 4.
Search for Tap580 insertion by bioinformatics analysis of sequenced genomes of the M. tuberculosis complex
| Strain | Beijing lineage | Tap580 insertion |
|---|---|---|
| Mycobacterium tuberculosis | ||
| H37Ra | No | No |
| H37Rv | No | No |
| CCDC5079 | Yes | Yes |
| CCDC5180 | Yes | Yes |
| CDC1551 | No | No |
| CTRI-2 | No | No |
| F11 | No | No |
| KZN 605 | No | No |
| KZN 1435 | No | No |
| KZN 4207 | No | No |
| RGTB327 | No | No |
| RGTB423 | No | No |
| Strain Erdman = ATCC 35801 DNA | No | No |
| UT205 | No | No |
| 7199-99 | No | No |
| Mycobacterium bovis | ||
| BCG Pasteur 1173P2 | No | |
| BCG Mexico | No | |
| BCG Moreau RDJ | No | |
| BCG Tokyo 172 DNA | No | |
| AF2122/97 | No |
The Tap580 insertion is present in clinical strains of the Beijing family of isolates.
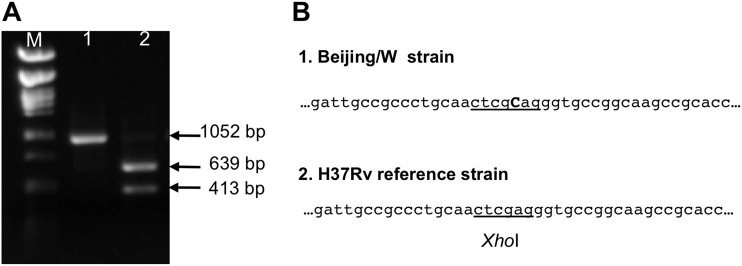
The presence of the Tap580 insertion was further investigated in different subtypes of the Beijing family of isolates, and in isolates of other genetic families. For this, a quick and simple method for detecting the Tap580 insertion was developed. Between positions 577 and 582 of the Rv1258c gene, the sequence CTCGAG is the target for XhoI endonuclease; the insertion of a cytosine nucleotide in the Beijing strains results in CTCGCAG, which is not recognized by this endonuclease (Fig. 2). We designed two primers for amplifying a 1,052-bp fragment of the Rv1258c gene, from nucleotides 165 to 1216, which includes the position of the Tap580 insertion. Digestion of PCR products with XhoI endonuclease resulted in two DNA fragments of 413 and 639 bp in the case of non-Beijing strains, whereas PCR products from Beijing strains remained unaffected (Fig. 2).
Fig 2.
Screening of the Tap580 insertion in Beijing strains. (A) DNA gel electrophoresis showing PCR product with primers ctap9 and ctap10 digested with XhoI. M, size marker, lambda DNA digested with PstI; 1, M. tuberculosis GC1237 (Beijing); 2, M. tuberculosis H37Rv (non-Beijing). (B) Sequence of the region of the Rv1258c gene in a Beijing strain (panel 1) and M. tuberculosis H37Rv strain (panel 2) (GenBank accession no. AL123456.2).
Next, a collection of 220 clinical isolates of M. tuberculosis complex were screened, which included 49 isolates having a spoligotype that is consistent with that of the Beijing family. DNA samples were given random numbers and were blind tested using our RFLP-PCR method described above. The Tap580 insertion was present in all Beijing strains and absent in all strains belonging to other lineages of M. tuberculosis; the Tap580 insertion was also absent from M. africanum isolates (Table 1).
Two controls were included in our assays. First, in each experiment, samples of DNA from the laboratory strain M. tuberculosis H37Rv (which does not belong to the Beijing family of isolates) and from the M. tuberculosis GC1237 strain (a Beijing isolate recently characterized [27]) were included. The PCR product from M. tuberculosis H37Rv was cut by XhoI, whereas that of M. tuberculosis GC1237 was not cut, confirming that all steps in the identification process were carried out satisfactorily. Second, all PCR products were digested with PvuII restriction enzyme, which cuts the amplification products of the Rv1258c gene obtained from both Beijing and non-Beijing samples, demonstrating that the amplification step is specific to the Rv1258c gene and ruling out the possibility of unspecific amplifications.
Finally, out of the 220 samples, the Rv1258c gene was sequenced in the 30 M. tuberculosis STR-resistant clinical isolates from Hospital Gregorio Marañón (Madrid, Spain) and Hospital Germans Trias i Pujol (Badalona, Spain). The Tap580 insertion was consistently found in the 5 Beijing strains and was absent in all the non-Beijing strains, hence validating the method for screening the Tap580 insertion. In addition, a deletion of 4 nucleotides spanning positions 835 and 838 of the Rv1258c gene was found in one of the Beijing samples, which is located downstream of the in-frame stop codon in positions 694 to 696 produced by the Tap580 insertion.
Detection of the Rv1258c nucleotide insertion in clinical samples.
In order to assess the usefulness of this technique for detecting Beijing strains directly in clinical samples, a blind panel of 30 respiratory specimens was assembled by the participating hospitals. This panel included 29 respiratory samples with diverse bacillary loads and that were culture-positive for M. tuberculosis, and one sample containing the nontuberculous mycobacterial species M. fortuitum. After isolating DNA from the sputum samples, the 1,052-bp PCR product was successfully amplified from 28 samples and digested with XhoI endonuclease. Out of these 28 samples, 26 were identified as containing non-Beijing M. tuberculosis strains, and two samples were identified as containing M. tuberculosis strains of the Beijing family, one of which was detected in a specimen with low bacillary load. The genetic lineage identification of the strains included in the panel was confirmed by the two hospitals that had designed the blind panel. The 1,052-bp PCR product could not be amplified from two sputum samples; one contained M. fortuitum, hence demonstrating the specificity of this method for detecting M. tuberculosis, and the second sample had a low bacillary load. Since eight of the nine samples with low bacillary load could be processed by this protocol, we assume that the failure to obtain the PCR amplification product with this sample might be due to other reasons.
The precise effect of this nucleotide insertion in the physiology of M. tuberculosis Beijing isolates remains to be fully elucidated. Recently, the role of the Rv1258c efflux pump in drug tolerance in M. tuberculosis has been reported (28). Since the identified single-nucleotide insertion in the Rv1258c gene in Beijing isolates results in a truncated, and most probably inactive, protein, this suggests that isolates carrying this nucleotide insertion might have a disadvantage in terms of drug resistance and drug tolerance compared with others carrying a fully functional Rv1258c transporter. However, since Beijing isolates are frequently associated with a higher propensity to acquire drug resistance, it is conceivable that other mutations actually compensate for the loss of Rv1258c in this family of M. tuberculosis isolates. Examples of compensatory mutations occurring following the acquisition of drug resistance-associated mutations have been described for M. tuberculosis (29).
In summary, a new genetic polymorphism of M. tuberculosis Beijing strains has been used to develop a simple and reliable technique for identifying isolates of this family. Given the higher transmissibility rate of this family of strains, which in addition are more prone to developing drug resistance, methods for rapid identification constitute a very important tool for the control of outbreaks caused by isolates of the Beijing family. This technique is fast, since it can be performed directly on clinical specimens and there is no need to culture strains; this makes it an ideal method for being implemented in those settings where routine culture of clinical samples cannot be done. In addition, this technique is easy to carry out and does not require sophisticated equipment, as only a thermocycler and DNA electrophoresis system are needed. In addition, samples can be processed individually or in groups of any size, and this can easily accommodate the workflow of a clinical laboratory without the need to process samples in groups of a fixed number. All this, along with the specificity for both the detection of M. tuberculosis and the identification of the Beijing family of strains, makes it ideal for implementation in clinical laboratories, especially in those settings with a high incidence of tuberculosis infections caused by this family of strains.
ACKNOWLEDGMENTS
This work was funded by CIBER de Enfermedades Respiratorias (CIBERES), Spain, and by research grants from the Spanish Ministry of Science (BIO-2009-09405 to J.A.A.) and the Fondo de Investigaciones Sanitarias (PI S09/02205, PI12/02080 to D.G.D.V.). C.V. is a recipient of a PhD fellowship from the Spanish Ministry of Science (FPU, reference AP2008-04730). J.D. is a researcher funded by the “Miguel Servet” program of the Instituto de Salud Carlos III (Spain).
We acknowledge Carlos Lampreave and Carmen Lafoz for their technical assistance, the members of the Grupo de Estudio de la Tuberculosis en Aragón for providing M. tuberculosis strains and Miguel Martínez Lirola (member of INDAL-TB group) from Complejo Hospitalario Torrecárdenas (Almería, Spain) for providing respiratory samples for the blind panel. We thank Patricia Gavín, Henar Alonso, Carlos Martín, and Dessislava Marinova for their helpful discussions and critical reading of the manuscript.
Footnotes
Published ahead of print 24 April 2013
REFERENCES
- 1. WHO 2012. Global tuberculosis control: WHO report 2012. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. Hanekom M, Gey van Pittius NC, McEvoy C, Victor TC, Van Helden PD, Warren RM. 2011. Mycobacterium tuberculosis Beijing genotype: a template for success. Tuberculosis (Edinb.) 91:510–523 [DOI] [PubMed] [Google Scholar]
- 3. Alonso M, Navarro Y, Barletta F, Martínez Lirola M, Gotuzzo E, Bouza E, García de Viedma D. 2011. A novel method for the rapid and prospective identification of Beijing Mycobacterium tuberculosis strains by high-resolution melting analysis. Clin. Microbiol. Infect. 17:349–357 [DOI] [PubMed] [Google Scholar]
- 4. Luo T, Yang C, Gagneux S, Gicquel B, Mei J, Gao Q. 2012. Combination of single nucleotide polymorphism and variable-number tandem repeats for genotyping a homogenous population of Mycobacterium tuberculosis Beijing strains in China. J. Clin. Microbiol. 50:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Millán-Lou MI, Alonso H, Gavin P, Hernández-Febles M, Campos-Herrero MI, Copado R, Cañas F, Kremer K, Caminero JA, Martín C, Samper S. 2012. Rapid test for identification of a highly transmissible Mycobacterium tuberculosis Beijing strain of sub-Saharan origin. J. Clin. Microbiol. 50:516–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schürch AC, Kremer K, Hendriks AC, Freyee B, McEvoy CR, van Crevel R, Boeree MJ, van Helden P, Warren RM, Siezen RJ, van Soolingen D. 2011. SNP/RD typing of Mycobacterium tuberculosis Beijing strains reveals local and worldwide disseminated clonal complexes. PLoS One 6:e28365. 10.1371/journal.pone.0028365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kremer K, Glynn JR, Lillebaek T, Niemann S, Kurepina NE, Kreiswirth BN, Bifani PJ, van Soolingen D. 2004. Definition of the Beijing/W lineage of Mycobacterium tuberculosis on the basis of genetic markers. J. Clin. Microbiol. 42:4040–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsolaki AG, Gagneux S, Pym AS, Goguet de la Salmoniere YO, Kreiswirth BN, Van Soolingen D, Small PM. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aínsa JA, Blokpoel MC, Otal I, Young DB, De Smet KA, Martín C. 1998. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. J. Bacteriol. 180:5836–5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Rossi E, Arrigo P, Bellinzoni M, Silva PA, Martín C, Aínsa JA, Guglierame P, Riccardi G. 2002. The multidrug transporters belonging to major facilitator superfamily in Mycobacterium tuberculosis. Mol. Med. 8:714–724 [PMC free article] [PubMed] [Google Scholar]
- 11. Ramón-García S, Mick V, Dainese E, Martín C, Thompson CJ, De Rossi E, Manganelli R, Aínsa JA. 2012. Functional and genetic characterization of the tap efflux pump in Mycobacterium bovis BCG. Antimicrob. Agents Chemother. 56:2074–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-Calleja AI, Gavín P, Lezcano MA, Vitoria MA, Iglesias MJ, Guimbao J, Lázaro MA, Rastogi N, Revillo MJ, Martín C, Samper S. 2009. Unsuspected and extensive transmission of a drug-susceptible Mycobacterium tuberculosis strain. BMC Pulm. Med. 9:3. 10.1186/1471-2466-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544 [DOI] [PubMed] [Google Scholar]
- 15. Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, Jacobs WR, Jr, Mizrahi V, Parish T, Rubin E, Sassetti C, Sacchettini JC. 2010. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J. Bacteriol. 192:3645–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu G, Shi W, Zhang L, Wang H, Wang S, Zhao G, Zhang Y. 2008. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One 3:e2375. 10.1371/journal.pone.0002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nhu NT, Lan NT, Phuong NT, Chau Nv, Farrar J, Caws M. 2012. Association of streptomycin resistance mutations with level of drug resistance and Mycobacterium tuberculosis genotypes. Int. J. Tuberc. Lung Dis. 16:527–531 [DOI] [PubMed] [Google Scholar]
- 18. Sun YJ, Luo JT, Wong SY, Lee AS. 2010. Analysis of rpsL and rrs mutations in Beijing and non-Beijing streptomycin-resistant Mycobacterium tuberculosis isolates from Singapore. Clin. Microbiol. Infect. 16:287–289 [DOI] [PubMed] [Google Scholar]
- 19. Brzostek A, Sajduda A, Sliwiński T, Augustynowicz-Kopeć E, Jaworski A, Zwolska Z, Dziadek J. 2004. Molecular characterisation of streptomycin-resistant Mycobacterium tuberculosis strains isolated in Poland. Int. J. Tuberc. Lung Dis. 8:1032–1035 [PubMed] [Google Scholar]
- 20. Wong SY, Lee JS, Kwak HK, Via LE, Boshoff HI, Barry CE., III 2011. Mutations in gidB confer low-level streptomycin resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55:2515–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okamoto S, Tamaru A, Nakajima C, Nishimura K, Tanaka Y, Tokuyama S, Suzuki Y, Ochi K. 2007. Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria. Mol. Microbiol. 63:1096–1106 [DOI] [PubMed] [Google Scholar]
- 22. Spies FS, Ribeiro AW, Ramos DF, Ribeiro MO, Martin A, Palomino JC, Rossetti ML, da Silva PE, Zaha A. 2011. Streptomycin resistance and lineage-specific polymorphisms in Mycobacterium tuberculosis gidB gene. J. Clin. Microbiol. 49:2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Via LE, Cho SN, Hwang S, Bang H, Park SK, Kang HS, Jeon D, Min SY, Oh T, Kim Y, Kim YM, Rajan V, Wong SY, Shamputa IC, Carroll M, Goldfeder L, Lee SA, Holland SM, Eum S, Lee H, Barry CE., III 2010. Polymorphisms associated with resistance and cross-resistance to aminoglycosides and capreomycin in Mycobacterium tuberculosis isolates from South Korean patients with drug-resistant tuberculosis. J. Clin. Microbiol. 48:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tudó G, Rey E, Borrell S, Alcaide F, Codina G, Coll P, Martín-Casabona N, Montemayor M, Moure R, Orcau A, Salvado M, Vicente E, González-Martiín J. 2010. Characterization of mutations in streptomycin-resistant Mycobacterium tuberculosis clinical isolates in the area of Barcelona. J. Antimicrob. Chemother. 65:2341–2346 [DOI] [PubMed] [Google Scholar]
- 25. Victor TC, van Rie A, Jordaan AM, Richardson M, van der Spuy GD, Beyers N, van Helden PD, Warren R. 2001. Sequence polymorphism in the rrs gene of Mycobacterium tuberculosis is deeply rooted within an evolutionary clade and is not associated with streptomycin resistance. J. Clin. Microbiol. 39:4184–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Chen C, Liu J, Deng H, Pan A, Zhang L, Zhao X, Huang M, Lu B, Dong H, Du P, Chen W, Wan K. 2011. Complete genome sequences of Mycobacterium tuberculosis strains CCDC5079 and CCDC5080, which belong to the Beijing family. J. Bacteriol. 193:5591–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso H, Aguilo JI, Samper S, Caminero JA, Campos-Herrero MI, Gicquel B, Brosch R, Martín C, Otal I. 2011. Deciphering the role of IS6110 in a highly transmissible Mycobacterium tuberculosis Beijing strain, GC1237. Tuberculosis (Edinb.) 91:117–126 [DOI] [PubMed] [Google Scholar]
- 28. Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Comas I, Borrell S, Roetzer A, Rose G, Malla B, Kato-Maeda M, Galagan J, Niemann S, Gagneux S. 2012. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat. Genet. 44:106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meier A, Kirschner P, Bange FC, Vogel U, Böttger EC. 1994. Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: mapping of mutations conferring resistance. Antimicrob. Agents Chemother. 38:228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Springer B, Stockman L, Teschner K, Roberts GD, Böttger EC. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J. Clin. Microbiol. 34:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]