Abstract
Genotyping of Mycobacterium tuberculosis strains became indispensable for understanding tuberculosis transmission dynamics and designing measures to combat the disease. Unfortunately, typing involves sophisticated laboratory analysis, is expensive, and requires a high level of technical expertise, which limited its use in the resource-poor countries where the majority of tuberculosis cases occur. Spoligotyping is a PCR-based M. tuberculosis complex genotyping method with advantages of technical simplicity, numerical output, and high reproducibility. It is based on the presence or absence of 43 distinct “spacers” separating insertion elements in the direct repeat region of the M. tuberculosis genome. The spoligotyping assay involves reverse hybridization of PCR products to the capture spacers attached to nitrocellulose membranes or to microspheres. Here we report modification of the classic 43-spacer method using the new generation of Luminex multiplexing technology with magnetic microspheres. The method was successfully established and validated on strains with known spoligotypes in our laboratory in Haiti. The distribution of spoligotypes determined in a collection of 758 recent M. tuberculosis isolates was in accordance with previous data for Haitian isolates in the SITWITWEB international database, which were obtained with the traditional membrane-based method. In the present form, spoligotyping may be suitable as a high-throughput, first-line tool for genotyping of Mycobacterium tuberculosis in countries with limited resources.
INTRODUCTION
Molecular epidemiology has become an important tool in determining transmission patterns and preventing Mycobacterium tuberculosis outbreaks (1). Genetic analysis of individual patient M. tuberculosis strains allows investigators to cluster isolates into types. This information complements classic epidemiologic contact tracing and allows investigators to better define transmission dynamics. Patients with genetically identical strains may be infected by a common index case or be part of a larger cluster of cases. This information can in turn be used to implement tuberculosis (TB) control measures to contain outbreaks and design disease prevention programs. Unfortunately, molecular epidemiology relies on sophisticated laboratory analysis, which is not available in most resource-poor countries, where the majority of TB cases occur, and this has limited its use in these poor settings (2).
IS6110 restriction length fragment polymorphism (IS6110-RFLP) is a traditional M. tuberculosis typing method based on comparing patterns of fragments generated by digestion of isolated genomic DNA with restriction enzymes (3). A newer PCR-based method, spoligotyping, has advantages of technical simplicity, numerical output, and superior reproducibility (4). Spoligotyping is based on the presence or absence of the 43 distinct “spacers” separating insertion elements in direct repeat (DR) regions of the M. tuberculosis genome. Spoligotyping is traditionally performed by reverse hybridization of biotinylated PCR products to spacers applied on a nitrocellulose membrane. Spoligotyping was transferred onto the Luminex 200 multiplex platform in 2004 (5). Instead of the nitrocellulose membrane, spacers are attached to 43 sets of microspheres or “beads.” After the 43 individual bead sets carrying their respective capture spacers are combined, the PCR products are detected in a single multiplex assay. Spoligotyping on the Luminex platform increased the reproducibility of the assay and made it less time- and labor-consuming. However, it was more expensive than the membrane-hybridization method (6, 7). MAGPIX, a new generation of Luminex analyzer based on the use of magnetic beads, is now available. It utilizes light-emitting-diode (LED)/charge-coupled-device (CCD) image-based detection in place of the laser-based detection by flow cytometry used in the Luminex 200 system. As a result, the multiplexing method became more affordable and robust without compromise with respect to simplicity and high-throughput capacity.
In this study, we validated the newer Luminex technology for M. tuberculosis spoligotyping in a laboratory in Haiti. We used results obtained with the classic membrane-based method as a reference and compared spoligotypes of 758 recently collected isolates to previous data for Haitian strains in the international database.
MATERIALS AND METHODS
M. tuberculosis strains with known spoligotypes.
Starting from 2009, the TB Laboratory of the Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections (GHESKIO) has been sending selected M. tuberculosis strains to the New York State Department of Health Mycobacteriology Laboratory for quality control of drug susceptibility testing. Spoligotypes of these strains were determined using the traditional membrane-based method in the New York State Laboratory as part of the routine protocol. For validation of the new spoligotyping assay on magnetic microspheres, we have chosen 42 of the strains such that each spacer was represented with multiple positive and negative positions. To obtain positive measurements for spacer 33 (sp33) and sp34, which have been absent in all Haiti strains analyzed so far, we used a DNA extract of a M. bovis BCG strain (spoligotype international type [SIT] 482) provided by New York State Laboratory.
To prepare DNA extracts for validation, frozen strains with known spoligotypes were regrown in the GHESKIO TB Laboratory, and a loopful of bacteria from Lowenstein-Jensen (LJ) slants or 50 μl from a positive mycobacterial growth indicator tube (MGIT) was transferred into a microtube with 300 μl of nuclease-free water. Bacteria were heat killed by incubation for 1 h at 80°C. DNA was released with 15 min of sonication in an ultrasound bath and separated from debris with Costar Spin-X filter tubes (Corning Inc., Lowell, MA) (0.22 μm pore size).
M. tuberculosis strains from the 2008 to 2009 survey.
DNA extracts from M. tuberculosis strains collected in a 2008-to-2009 multidrug-resistant tuberculosis (MDR-TB) survey (8) were also analyzed with spoligotyping. Briefly, sputum samples from first-time TB patients presenting to the 6 largest TB centers in and around Port-au-Prince were transported to the GHESKIO TB Laboratory and cultured on LJ slants. DNA was extracted with repeated freeze-thaw cycles and filtration through Costar Spin-X centrifuge tubes. Extracts were stored at −20°C.
DNA amplification.
DNA extracts (2 μl) prepared by either method were amplified in 35 cycles (1 min at 96°C, 1 min at 55°C, and 30 s at 72°C) using a 50-μl PCR mixture containing 0.2 μM (each) primers DRa and DRb (9), 200 μM deoxynucleoside triphosphates (dNTPs), and 1.25 U of HotStar Taq polymerase (Qiagen, Hilden, Germany) in a reaction buffer supplied with the enzyme. A negative PCR control with H2O in place of the DNA extract was included in each PCR run.
Spoligotyping assay on Luminex MagPlex magnetic beads.
The reagents and consumables and the composition of buffers for coupling and hybridization were adopted from recommendations and protocols available on the Luminex website (http://www.luminexcorp.com/Support/SupportResources/index.htm). However, the recommended 5 M tetramethylammonium chloride (TMAC) solution from Sigma gave high backgrounds in the assay and had to be replaced by 6 M TMAC solution from AppliChem (Darmstadt, Germany) (catalog no. 5456). The composition of the TMAC buffer was adjusted accordingly.
Spacer oligonucleotides from the original publication of Kamerbeek et al. (9) were coupled to 43 sets of 2.5 × 106 MagPlex-C microspheres (Luminex, Austin, TX) as previously described (5). The correspondence of spacers with the microsphere sets is shown in Table 1.
Table 1.
Correspondence of spacers with the microsphere setsa
| Spacer | Oligonucleotide sequence (5′ to 3′) | Microsphere set |
|---|---|---|
| sp1 | ATAGAGGGTCGCCGGTTCTGGATCA | MC10013 |
| sp2 | CCTCATAATTGGGCGACAGCTTTTG | MC10014 |
| sp3 | CCGTGCTTCCAGTGATCGCCTTCTA | MC10015 |
| sp4 | ACGTCATACGCCGACCAATCATCAG | MC10018 |
| sp5 | TTTTCTGACCACTTGTGCGGGATTA | MC10019 |
| sp6 | CGTCGTCATTTCCGGCTTCAATTTC | MC10020 |
| sp7 | GAGGAGAGCGAGTACTCGGGGCTGC | MC10021 |
| sp8 | CGTGAAACCGCCCCCAGCCTCGCCG | MC10022 |
| sp9 | ACTCGGAATCCCATGTGCTGACAGC | MC10025 |
| sp10 | TCGACACCCGCTCTAGTTGACTTCC | MC10026 |
| sp11 | GTGAGCAACGGCGGCGGCAACCTGG | MC10027 |
| sp12 | ATATCTGCTGCCCGCCCGGGGAGAT | MC10073 |
| sp13 | GACCATCATTGCCATTCCCTCTCCC | MC10029 |
| sp14 | GGTGTGATGCGGATGGTCGGCTCGG | MC10030 |
| sp15 | CTTGAATAACGCGCAGTGAATTTCG | MC10074 |
| sp16 | CGAGTTCCCGTCAGCGTCGTAAATC | MC10034 |
| sp17 | GCGCCGGCCCGCGCGGATGACTCCG | MC10035 |
| sp18 | CATGGACCCGGGCGAGCTGCAGATG | MC10036 |
| sp19 | TAACTGGCTTGGCGCTGATCCTGGT | MC10037 |
| sp20 | TTGACCTCGCCAGGAGAGAAGATCA | MC10038 |
| sp21 | TCGATGTCGATGTCCCAATCGTCGA | MC10039 |
| sp22 | ACCGCAGACGGCACGATTGAGACAA | MC10075 |
| sp23 | AGCATCGCTGATGCGGTCCAGCTCG | MC10043 |
| sp24 | CCGCCTGCTGGGTGAGACGTGCTCG | MC10044 |
| sp25 | GATCAGCGACCACCGCACCCTGTCA | MC10045 |
| sp26 | CTTCAGCACCACCATCATCCGGCGC | MC10046 |
| sp27 | GGATTCGTGATCTCTTCCCGCGGAT | MC10047 |
| sp28 | TGCCCCGGCGTTTAGCGATCACAAC | MC10048 |
| sp29 | AAATACAGGCTCCACGACACGACCA | MC10051 |
| sp30 | GGTTGCCCCGCGCCCTTTTCCAGCC | MC10052 |
| sp31 | TCAGACAGGTTCGCGTCGATCAAGT | MC10053 |
| sp32 | GACCAAATAGGTATCGGCGTGTTCA | MC10054 |
| sp33 | GACATGACGGCGGTGCCGCACTTGA | MC10055 |
| sp34 | AAGTCACCTCGCCCACACCGTCGAA | MC10056 |
| sp35 | TCCGTACGCTCGAAACGCTTCCAAC | MC10057 |
| sp36 | CGAAATCCAGCACCACATCCGCAGC | MC10061 |
| sp37 | CGCGAACTCGTCCACAGTCCCCCTT | MC10062 |
| sp38 | CGTGGATGGCGGATGCGTTGTGCGC | MC10063 |
| sp39 | GACGATGGCCAGTAAATCGGCGTGG | MC10064 |
| sp40 | CGCCATCTGTGCCTCATACAGGTCC | MC10065 |
| sp41 | GGAGCTTTCCGGCTTCTATCAGGTA | MC10066 |
| sp42 | ATGGTGGGACATGGACGAGCGCGAC | MC10067 |
| sp43 | CGCAGAATCGCACCGGGTGCGGGAG | MC10072 |
Data represent the pairs of spacer (sp) capture oligonucleotides and Luminex MagPlex-C microsphere regions (designated by their catalog numbers) used in the spoligotyping assay.
Coupled microspheres were resuspended in 50 μl of Tris-EDTA (TE) buffer. A 10-μl volume of each set was used to prepare the stock microsphere mixture. Individual microspheres and the stock microsphere mixture were stored at 4°C in the dark for over a year without impact on the test results. As needed for spoligotyping experiments, a working microsphere mixture was prepared by diluting 60 μl of the stock mixture with 940 μl 1.5× TMAC buffer and 424 μl TE buffer in low-adhesion microtubes (catalog no. 1415-2500; USA Scientific, Ocala, FL). The working bead mix was good for at least 1 month when stored at 4°C in the dark. Information about the shelf life of coupled microspheres is available on the Luminex website (http://www.luminexcorp.com/prod/groups/public/documents/lmnxcorp/oligo-coupled-microsphere.pdf).
For hybridization, 3 μl of the PCR mixture was mixed with 47 μl of the working microsphere mixture (approximately 2,300 of beads from each set) in a 96-well plate. The first well (A1) contained 3 μl of TE buffer in place of PCR. The second well (B1) was reserved for a negative PCR control. The plate was covered with a sealing mat and placed into a ThermoBlock for denaturation (3 min at 95°C) and hybridization (15 min at 52°C). During the hybridization step, the reporter mix was prepared by diluting Streptavidin-R-Phycoerythrin conjugate and acetylated bovine serum albumin (BSA) (catalog no. S-21388 and AM2614; Life Technologies, Carlsbad, CA) in 1× TMAC buffer to final concentrations of 10 μg/ml and 0.1%, respectively. A 25-μl volume of the reporter mix was added to each well and mixed, and the plate was returned to the ThermoBlock for 5 more minutes at 52°C. Immediately after, 30 μl from each well was analyzed on a Luminex MAGPIX instrument with the heater set to 52°C and the internal sample wash function set to “on.” All wells were set to “unknown.” The minimum amount of beads collected per region was set to 50. A maximum of 46 samples were tested each time to avoid possible evaporation of test mix in the analyzer during the run.
Data analysis.
Data for the median fluorescence intensity (MFI) from the output.csv file of the Luminex xPONENT software were copied into an Excel file containing simple formulas for data transformation.
First, the sample-to-noise (S:N) ratio was calculated for every region by dividing the sample MFI by the corresponding MFI of the negative PCR control. Negative-control MFI values were between 50 and 100. Next, S:N values for every sample were transformed into a string of “1” and “0” characters using an S:N cutoff value of 4.0 for all positive spacers (“1”), an S:N cutoff value of 1.5 for negative spacers 1 to 35 and 37 to 43 (“0”), and an S:N cutoff value of 2.5 for negative spacer 36 (“0”). Cutoff values were determined in a validation experiment using 43 strains with known spoligotypes (see Results). Ambiguous positions with an S:N value between the positive and the negative cutoff values were flagged by Excel with a question mark. If a sample contained five or fewer such positions, the test was repeated using same DNA extract.
Finally, the spoligotyping result for each sample was generated in 2 formats: binary (using the “CONCATENATE” function of Excel) and octal (using the “BIN2OCT” function).
Statistical analysis of spoligotype distribution was performed using the Pearson's test of the Mathematica program package.
RESULTS
Validation of the spoligotyping assay using magnetic microspheres and a collection of 43 strains with known spoligotypes.
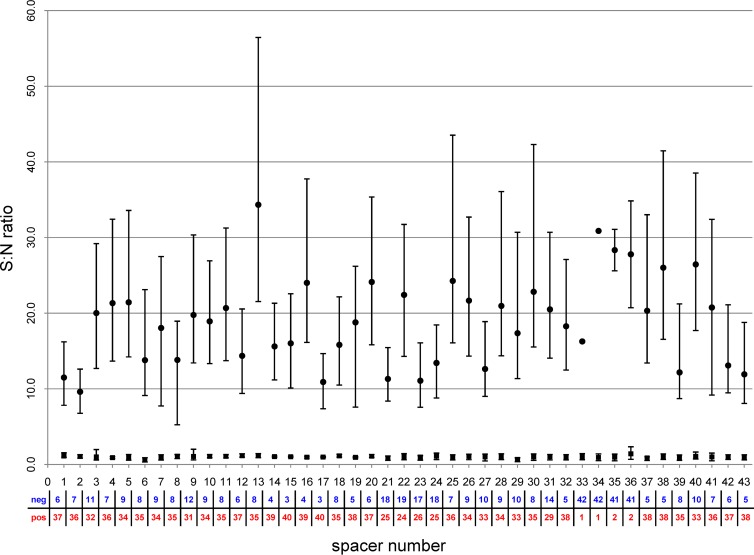
A total of 43 M. tuberculosis complex strains with different spoligotype patterns tested previously in a U.S. reference laboratory with the traditional membrane-based method were used for validation of the new assay on magnetic microspheres. The median fluorescence intensity signal was normalized to obtain S:N data using the MFI value of a negative control, as described in Materials and Methods. For each spacer, positions that were positive as determined by the membrane-based method were well separated from the negative ones (Fig. 1); i.e., the results obtained with the new assay and with the traditional membrane-based method were in agreement.
Fig 1.
Comparison of results generated with spoligotyping on magnetic microspheres in Haiti with those determined with the classic membrane-based method in the New York State reference laboratory. A total of 43 M. tuberculosis complex strains with different spoligotypes were selected to provide multiple negative (neg; total number in blue) and positive (pos; total number in red) positions for each spacer. The median signal-to-noise (S:N) ratios for negative and positive spacers as determined by the membrane-based method are represented by ■ and • symbols, respectively.
Based on the data obtained in the validation experiment, the cutoff S:N value for positive positions was set at 4.0 for all 43 spacers. For negative positions, the cutoff S:N value was set at 1.5 for all spacers, with exception of sp36, which had a higher cutoff value of 2.5.
Spoligotyping of strains collected during the MDR-TB survey conducted from 2008 to 2009.
A total of 764 DNA extracts from M. tuberculosis isolates collected in 2008 to 2009 were subjected to spoligotyping with magnetic microspheres on MAGPIX. A total of 758 samples were successfully analyzed, and 113 distinct patterns were generated; 6 samples failed to generate an interpretable pattern. Patterns or types were compared to those in a public database of spoligotypes—SITVITWEB (http://www.pasteur-guadeloupe.fr:8081/SITVIT_ONLINE/) populated by data from >60,000 samples from 105 countries. Patterns in SITVITWEB have an “orphan” status as long as they are represented by a single sample. Patterns shared by at least 2 samples are assigned a spoligotype international type (SIT) number corresponding to the order of appearance. SIT classification is a convenient and simple way to designate spoligotype patterns.
Among the 113 distinct patterns we found in this study, there were 78 patterns from 707 samples which already had an assigned SIT number in the international SITVITWEB database. Nine more patterns from 20 samples appeared in the database as orphans, and 26 patterns from 31 samples were new and had not been previously reported in SITVITWEB.
SITs 1 (Bejing), 44, 106, 197, 205, 239, 242, 462, 562, 633, 729, 746, 750, 909, 963, 1087, 1205, 1238, 1273, 1624, 1196, 2070, 2161, 2298, 2431, and 2981 were detected in a single sample in our collection of 758 strains from Haiti. The remaining 52 types were represented by clusters of 2 to 79 samples. These types are shown ordered by cluster size in Table 2.
Table 2.
Spoligotyping dataa
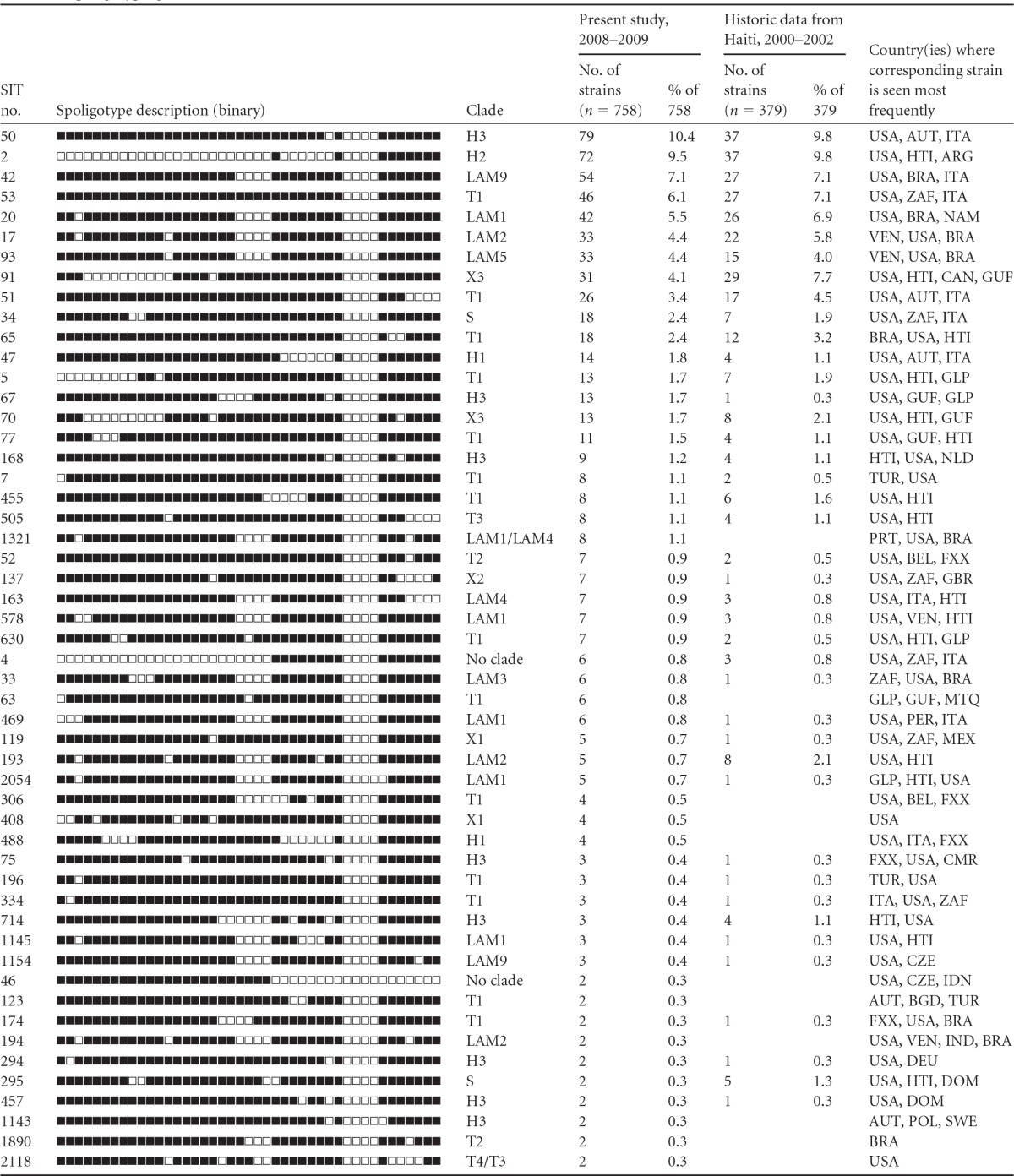
Data are from the SITVIT WEB international database. ARG, Argentina; AUT, Austria; BEL, Belgium; BGD, Bangladesh; BRA, Brazil; CAN, Canada; CMR, Cameroon; CZE, Czech Republic; DEU, Germany; DOM, Dominican Republic; FXX, France; GBR, United Kingdom; GLP, Guadeloupe; GUF, French Guiana; HTI, Haiti; IDN, Indonesia; IND, India; ITA, Italy; MEX, Mexico; MTQ, Martinique; NAM, Namibia; NLD, Netherlands; PER, Peru; POL, Poland; PRT, Portugal; SWE, Sweden; TUR, Turkey; USA, United States; VEN, Venezuela; ZAF, South Africa.
In the course of a collaborative project conducted by GHESKIO and Institute Pasteur Guadeloupe from 2000 to 2002, M. tuberculosis isolates from 378 GHESKIO patients were subjected to spoligotyping with the traditional membrane-based method (10, 11). The results are also shown in Table 2. In both sets of samples, SIT 50 and SIT 2 were the most common types, being responsible together for almost 20% of all TB cases. They were followed by SITs 42, 53, 20, 17, 93, and 91, which combined accounted for 38.6% and 31.5% of cases among the samples collected from 2000 to 2002 and from 2008 to 2009, respectively. We tested the goodness of fit of the two distributions of spoligotypes shown in Table 2 with the Pearson's test. The results strongly support the null hypothesis that the data from 2000 to 2002 follow the same distribution as the data from 2008 to 2009 (probability, 0.99909; chi-square value, 87.045).
As in the study performed from 2000 to 2002, all found spoligotypes were characteristic of M. tuberculosis and belonged mostly to 4 clades: LAM (30.1%), Haarlem (29.8%), T (25.9%), and X (8.6%). These 4 clades are thought to have been introduced to the Caribbean region by Spanish, non-Spanish European, African, and Anglo-Saxon influences, respectively (12). Recent studies suggest that all 4 of those clades might be phylogenetically related, as strains with these spoligotypes appear in the same “Euro-American lineage” according to the results of analysis by the single nucleotide polymorphism and large-sequence polymorphism methods (13, 14). All 4 clades contain patterns found predominantly in Haiti and in countries with a large Haitian population—the Caribbean countries and the United States (Table 2).
Twenty samples from the recent MDR-TB survey generated 9 spoligotypes which were registered in SITVITWEB as orphans, i.e., they were not assigned a SIT number since they were represented in the database by a single prior sample. Interestingly, even though the database contains data from samples collected worldwide, 8 of these orphan types were linked to Haitian patients residing in Haiti (5 types), French Guiana (2 types), or the Dominican Republic (1 type). The ninth type originated from French Guiana.
DISCUSSION
An updated version of the spoligotyping test using a new generation MAGPIX analyzer and magnetic MagPlex microspheres instead of traditional nonmagnetic ones has been successfully established and validated. For 43 control isolates, there was a 100% concordance between results obtained with the traditional membrane method in a U.S. reference laboratory and those produced locally in our laboratory in Haiti with the new technique. The distribution of spoligotype patterns determined with the new assay in a large collection of 758 isolates from 2008 to 2009 had no statistically significant deviations from the distribution of spoligotypes in the samples from Haiti tested with the traditional membrane-based approach in Institute Pasteur de Guadeloupe in 2000 to 2002.
We were unable to type 6 of the total of 764 isolates. Heat-killed extracts may contain PCR-inhibiting chemicals from media as well as contaminating DNA due to the mixed cultures or cross-contamination. In such cases, it may be helpful to prepare a new DNA extract from purified cultures regrown from a single colony.
The implementation of spoligotyping with Luminex magnetic microspheres in resource-poor settings is feasible.
Personnel time and training.
Initial coupling of 43 spacer-specific oligonucleotides to the microspheres and subsequent validation of the test took 2 weeks and produced microspheres sufficient to analyze at least 1,200 individual samples. Subsequent recoupling and testing of new batches of microspheres took 2 days. GHESKIO laboratory personnel are familiar with several PCR-based methods. After a week of training and practice, the Haitian laboratory technician became proficient at spoligotyping with magnetic microspheres. Up to 46 samples (half a plate) were analyzed per run. Five batches, or up to 230 samples, can be easily tested by a single person in the course of a workday. Results were generated automatically from a numerical output of Luminex software by the use of an Excel spreadsheet. For comparison, a very experienced technician is required to test and manually evaluate results for maximum of 40 samples per day with a membrane-based method.
Equipment.
The average price of a Luminex MAGPIX analyzer is $27,500, only half the price of a Luminex 200 analyzer. That cost is higher than the $16,000 needed to set up a membrane-based spoligotyping assay ($12,570 for a Maxi 14 hybridization oven, $2,200 for a MN45 Miniblotter, and approximately $1,300 for foam cushions and film developing equipment) (15).
Reagents.
Since LED image-based detection replaced flow cytometry in MAGPIX, we were able to test samples with only 2,300 beads per set ($4.23) rather than the 5,000 beads required for a previous nonmagnetic version (5, 6). A spoligotyping membrane ($750.00) can be stripped and reused 10 times. The membrane cost per tested sample depends on the number of samples analyzed per run ($1.88 per sample in a 40-sample batch; $7.50 per sample in a 10-sample batch).
The cost efficiency of spoligotyping with Luminex magnetic microspheres compared to that of the traditional membrane-hybridization technique has to be determined in individual laboratories by taking into consideration local labor cost and the volume of processed samples and if the Luminex equipment can be used for multiple diagnostic applications.
Spoligotyping of 758 M. tuberculosis isolates revealed a broad diversity of types circulating in Haiti, which makes it an attractive typing tool for this location. The new technique will allow us to type all isolates in real time in Haiti as opposed to the use of retrospective studies conducted in the United States on exported samples. Results can be used immediately for multiple clinical and public health purposes (to investigate outbreaks, to differentiate between reactivation and reinfection, to uncover strains with increased virulence, to monitor spread of new types, to control for laboratory cross-contamination, etc.).
ACKNOWLEDGMENTS
This project was supported by grants from the Fogarty International Center and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
We are grateful to the Expand TB WHO program for supplying our TB laboratory with materials and reagents, to Foundation Merieux for helping to build and maintain the biosafety level 3 (BSL-3) facility in Haiti, and to the Luminex company for donating the first batch of microspheres. We are very thankful to the staff of the Mycobacteriology Laboratory of the New York State Department of Health for testing our strains and in particular to Michael McGarry, who performed spoligotyping.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, Drucker E, Bloom BR. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710–1716 [DOI] [PubMed] [Google Scholar]
- 2. van der Spuy GD, Warren RM, van Helden PD. 2009. The role of molecular epidemiology in low-income, high-burden countries. Int. J. Tuberc. Lung Dis. 13:419–420 [PubMed] [Google Scholar]
- 3. Van Soolingen D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1–26 [DOI] [PubMed] [Google Scholar]
- 4. Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PW, Martín C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, van Embden JD. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowan LS, Diem L, Brake MC, Crawford JT. 2004. Transfer of a Mycobacterium tuberculosis genotyping method, spoligotyping, from a reverse line-blot hybridization, membrane-based assay to the Luminex multianalyte profiling system. J. Clin. Microbiol. 42:474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Abadia E, Refregier G, Tafaj S, Boschiroli ML, Guillard B, Andremont A, Ruimy R, Sola C. 2010. Mycobacterium tuberculosis complex CRISPR genotyping: improving efficiency, throughput and discriminative power of ‘spoligotyping’ with new spacers and a microbead-based hybridization assay. J. Med. Microbiol. 59:285–294 [DOI] [PubMed] [Google Scholar]
- 7. Abadia E, Zhang J, Ritacco V, Kremer K, Ruimy R, Rigouts L, Gomes HM, Elias AR, Fauville-Dufaux M, Stoffels K, Rasolofo-Razanamparany V, Garcia de Viedma D, Herranz M, Al-Hajoj S, Rastogi N, Garzelli C, Tortoli E, Suffys PN, van Soolingen D, Refrégier G, Sola C. 2011. The use of microbead-based spoligotyping for Mycobacterium tuberculosis complex to evaluate the quality of the conventional method: providing guidelines for quality assurance when working on membranes. BMC Infect. Dis. 11:110. 10.1186/1471-2334-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ocheretina O, Morose W, Gauthier M, Joseph P, D'Meza R, Escuyer VE, Rastogi N, Vernet G, Pape JW, Fitzgerald DW. 2012. Multidrug-resistant tuberculosis in Port-au-Prince, Haiti. Rev. Panam. Salud Publica 31:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferdinand S, Sola C, Verdol B, Legrand E, Goh KS, Berchel M, Aubery A, Timothee M, Joseph P, Pape JW, Rastogi N. 2003. Molecular characterization and drug resistance patterns of strains of Mycobacterium tuberculosis isolated from patients in an AIDS counseling center in Port-au-Prince, Haiti: a 1-year study. J. Clin. Microbiol. 41:694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joseph P, Severe P, Ferdinand S, Goh KS, Sola C, Haas DW, Johnson WD, Rastogi N, Pape JW, Fitzgerald DW. 2006. Multi-drug resistant tuberculosis at an HIV testing center in Haiti. AIDS 20:415–418 [DOI] [PubMed] [Google Scholar]
- 12. Duchêne V, Ferdinand S, Filliol I, Guégan JF, Rastogi N, Sola C. 2004. Phylogenetic reconstruction of Mycobacterium tuberculosis within four settings of the Caribbean region: tree comparative analyse and first appraisal on their phylogeography. Infect. Genet. Evol. 4:5–14 [DOI] [PubMed] [Google Scholar]
- 13. Comas I, Homolka S, Niemann S, Gagneux S. 2009. Genotyping of genetically monomorphic bacteria: DNA sequencing in Mycobacterium tuberculosis highlights the limitations of current methodologies. PLoS One 4:e7815. 10.1371/journal.pone.0007815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato-Maeda M, Gagneux S, Flores LL, Kim EY, Small PM, Desmond EP, Hopewell PC. 2011. Strain classification of Mycobacterium tuberculosis: congruence between large sequence polymorphisms and spoligotypes. Int. J. Tuberc. Lung Dis. 15:131–133 [PMC free article] [PubMed] [Google Scholar]
- 15. Isogen Life Science 2010. ‘Spoligotyping’: a PCR-based method to simultaneously detect and type Mycobacterium tuberculosis complex bacteria (instruction manual). http://www.mycobactoscana.it/micobatteriologia/pdf/alleg12.1.pdf