Abstract
A widely recognized difficulty of presently used methods for cDNA cloning is obtaining cDNA segments that contain the entire nucleotide sequence of the corresponding mRNA. The cloning procedure described here mitigates this shortcoming. Of the 10(5) plasmid-cDNA recombinants obtained per microgram of rabbit reticulocyte mRNA, about 10% contained a complete alpha- of beta-globin mRNA sequence, and at least 30 to 50%, but very likely more, contained the entire globin coding regions. We attribute the high efficiency of cloning full- or nearly full-length cDNA to (i) the fact that the plasmid DNA vector itself serves as the primer for first- and second-strand cDNA synthesis, (ii) the lack of any nuclease treatment of the products, and (iii) the fact that one of the steps in the procedure results in preferential cloning of recombinants with full-length cDNA's over those with truncated cDNA's.
Full text
PDF
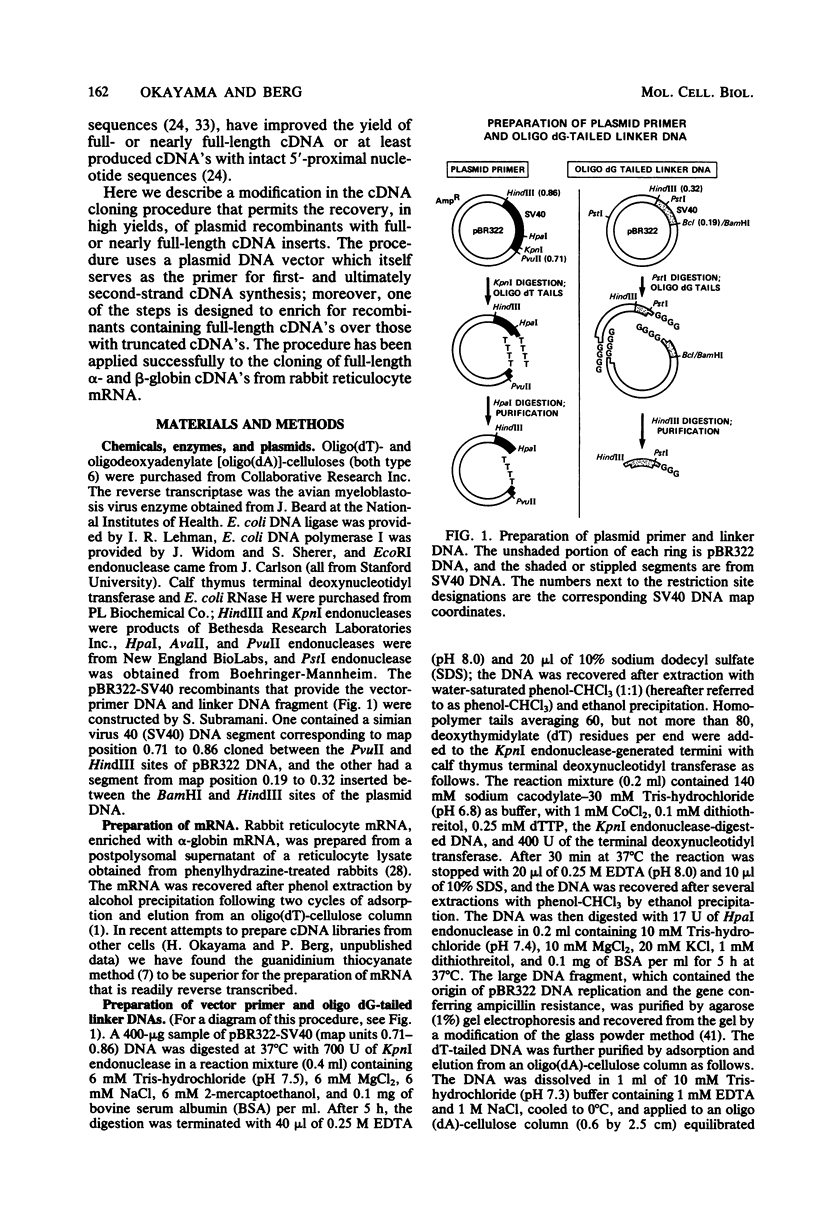



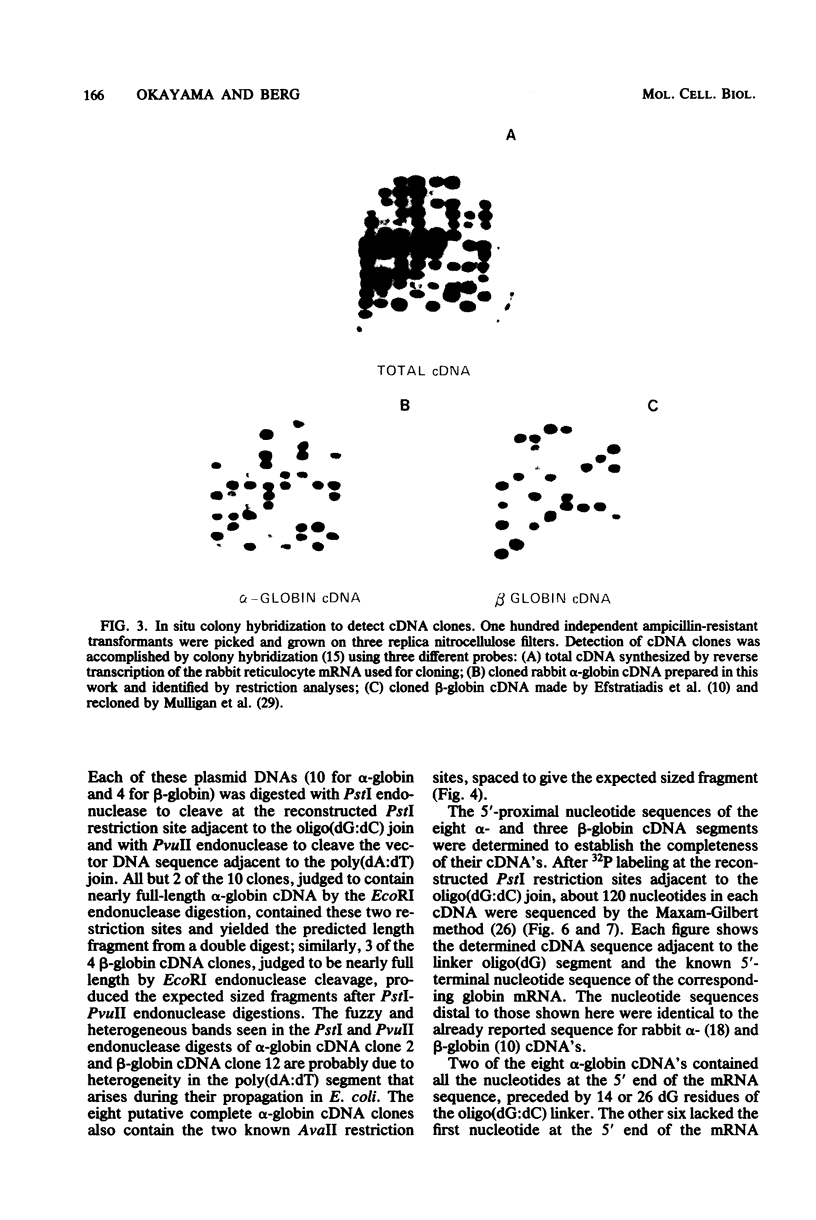
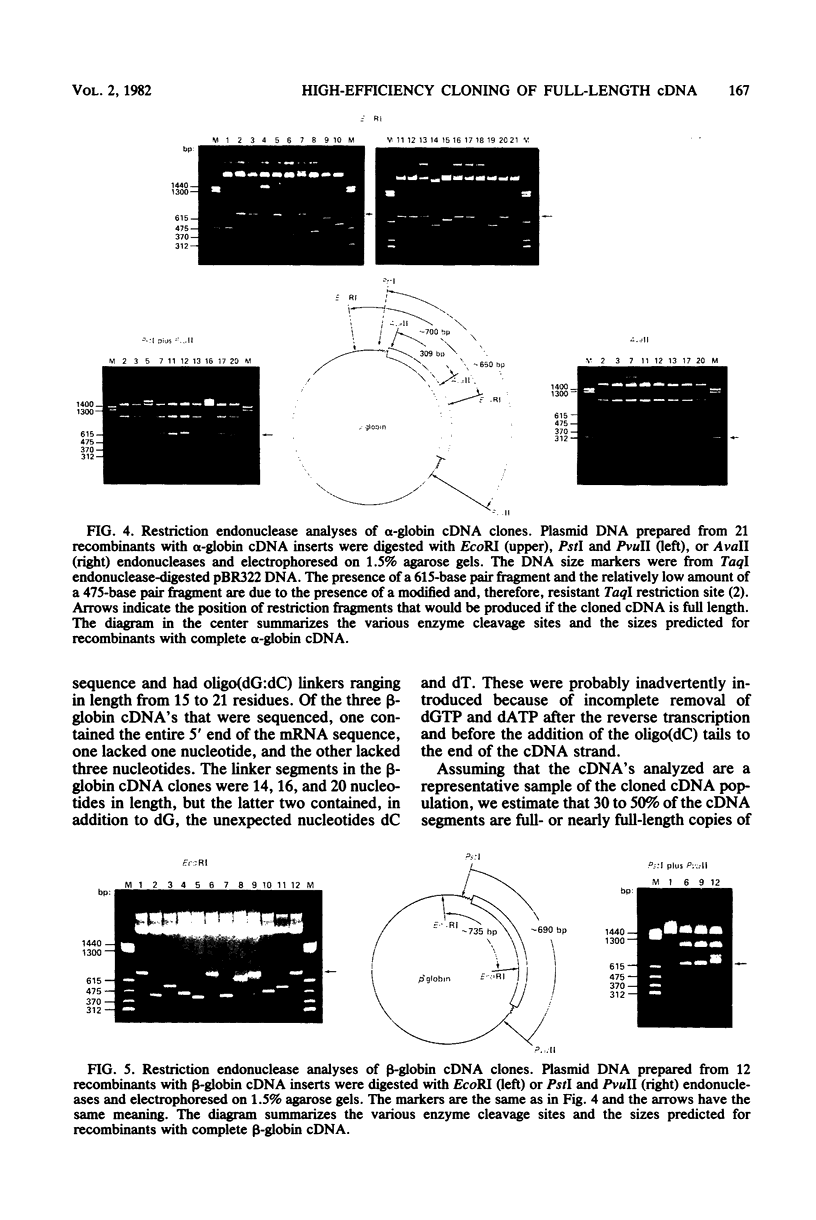



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K. A cautionary note on the use of certain restriction endonucleases with methylated substrates. Gene. 1980 Oct;11(1-2):169–171. doi: 10.1016/0378-1119(80)90097-9. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Stein J. P., Lai E. C., Woo S. L., Dugaiczyk A., Mace M. L., Means A. R., O'Malley B. W. The chick ovomucoid gene contains at least six intervening sequences. Nature. 1979 Mar 22;278(5702):323–327. doi: 10.1038/278323a0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. 8. Hybrids of ribo- and deoxyribonucleotide homopolymers as substrates for polynucleotide ligase of bacteriophage T4. J Biol Chem. 1971 Feb 25;246(4):925–932. [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiland I., Gething M. J. Cloned copy of the haemagglutinin gene codes for human influenza antigenic determinants in E. coli. Nature. 1981 Aug 27;292(5826):851–852. doi: 10.1038/292851a0. [DOI] [PubMed] [Google Scholar]
- Heindell H. C., Liu A., Paddock G. V., Studnicka G. M., Salser W. A. The primary sequence of rabbit alpha-globin mRNA. Cell. 1978 Sep;15(1):43–54. doi: 10.1016/0092-8674(78)90081-8. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Molloy P. L., Symons R. H. Cleavage of DNA.RNA hybrids by type II restriction enzymes. Nucleic Acids Res. 1980 Jul 11;8(13):2939–2946. doi: 10.1093/nar/8.13.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. R., Brinkley S. A., Gorski J., Lingrel J. B. The separation and identification of alpha- and beta-globin messenger ribonucleic acids. J Biol Chem. 1974 Aug 25;249(16):5290–5295. [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Rabbitts T. H. Bacterial cloning of plasmids carrying copies of rabbit globin messenger RNA. Nature. 1976 Mar 18;260(5548):221–225. doi: 10.1038/260221a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Both G. W., Brownlee G. G. The influenza virus haemagglutinin gene: cloning and characterisation of a double-stranded DNA copy. Nucleic Acids Res. 1979 Oct 25;7(4):879–893. doi: 10.1093/nar/7.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. O., Lee J. C. Integration of synthetic globin genes into an E. coli plasmid. Nucleic Acids Res. 1976 Aug;3(8):1961–1971. doi: 10.1093/nar/3.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]






