Abstract
The objective of this study was to evaluate the kinetics of varicella-zoster virus (VZV) loads using quantitative PCR (qPCR) in patients treated for acute retinal necrosis (ARN). Six patients (52 ± 13 years old) with ARN syndrome were consecutively studied. Aqueous humor (AH) was sampled from both eyes of all patients for qPCR evaluation. The patients were treated with intravenous acyclovir and intravitreal injections of antiviral drugs. The mean follow-up time was 17.6 ± 16.4 months. Main outcome measures were the numbers of viral genome copies in the AH, assessed using real-time qPCR with hydrolysis probe technology with a threshold of detection of 200 copies/ml. Two main portions of the viral load curves were observed for each patient: a plateau phase (27.8 ± 24.9 days) and a decrease in the number of viral genome copies. The mean baseline viral load was 3.4 × 107 ± 4.45 × 107 copies/ml (6 × 106 to 1.2 × 108 copies/ml). The viral load decreased according to a logarithmic model, with a 50% reduction obtained in 3 ± 0.7 days. There was a significant viral load (>102 copies/ml) at 50 days after the onset of treatment, despite antiviral drugs. qPCR use demonstrated reproducible VZV DNA kinetics with a two-phase evolution: a plateau followed by a logarithmic decrease. These data suggest that high-dosage antiviral therapy administered for the conventional 10-day duration is insufficient for most patients. This series of patients responded with a similar decrease in viral load once treatment was initiated, and the data from these patients may be used to predict the responses of future patients.
INTRODUCTION
Acute retinal necrosis syndrome (ARN), one of the most serious infectious ocular diseases, is caused by herpesviruses, with varicella-zoster virus (VZV) being the most common (1, 2). Great advances have been made in the diagnosis of viral retinitis with the detection of viral DNA in intraocular fluids (vitreous and aqueous humor [AH]) and accurate quantification of viral genome copies using real-time quantitative PCR (qPCR) (3, 4).
The extent of retinitis, such as anterior chamber and vitreous inflammation, vasculitis, retinal necrosis, and scarring of the involved retina, is currently monitored with physical examination. Vitreous opacities and/or cataract development, however, may preclude visualization of the posterior segment, making clinical monitoring and appraisal of the efficacy of antiviral treatment difficult. At this time, it is not known whether the kinetics of viral DNA during systemic treatment (i.e., acyclovir [5], valacyclovir [6, 7], or famciclovir [6–8] and/or intravitreal therapy [9]) is similar in all patients and can be predicted once the treatment is initiated.
The objective of this case series was to assess the evolution of VZV DNA loads after systemic/intravitreal antiviral treatment in ARN and to evaluate the contribution of qPCR to the monitoring of patients with VZV acute retinal necrosis.
MATERIALS AND METHODS
This retrospective study was conducted in a tertiary center with a consecutive series of six patients with ARN syndrome (10) who were referred to our department during a 5-year period (2005-2009). This study followed the Declaration of Helsinki guidelines for research involving human subjects. The study protocol was approved by the national institutional review board (Ethics Committee of the French Society of Ophthalmology).
All eyes were sampled after two instillations of 5% aqueous povidone iodine solution in the conjunctival sac (1 min). At the beginning of the procedure, after signed informed consent was obtained, aqueous humor (AH) (150 μl, n = 8 to 11 patients) was sampled in a sterile syringe in order to reduce intraocular pressure before intravitreal injection of antivirals and was then used for qPCR evaluation. The patients in this series were treated with both intravenous acyclovir (10 mg/kg/8 h) and intravitreal injections of antivirals (either foscarnet sodium [Foscavir] at 2,400 μg/ml or ganciclovir at 2,000 μg/ml) since the initial findings were judged to be severe (11, 12).
Resolution of retinitis was defined as the absence of active areas of retinitis (13). Onset of healing was defined as the absence of new active areas of retinitis and the onset of the appearance of pigmentary modifications (11).
qPCR technique.
Total DNA from 150 μl ocular fluids was extracted with the QIAmp DNA minikit (Qiagen, Courtaboeuf, France), eluted in 50 μl of elution buffer, and stored at −20°C. Results were expressed as copies of VZV DNA per ml of AH. The extraction efficiency was checked by adding an internal control to the sample, and an external negative control was used to detect possible contamination. qPCRs were run on a LightCycler instrument (Roche, Meylan, France) using the hydrolysis probe technology, the LightCycler DNA master hybridization probes kit (Roche) according to the manufacturer's instructions, and 10 μl of the extracted DNA. The primers (forward primer 5′-CGGCATGGCCCGTCTAT-3′ and reverse primer 5′-TCGCGTGCTGCGGC-3′) and the probe [5′-(6-FAM)-ATTCAGCAATGGAAACACACGACGCC-(BHQ1)-3′] (6-FAM is 6-carboxyfluorescein, and BHQ1 is black hole quencher 1) targeted the DNA polymerase gene and resulted in a 63-bp amplified fragment (14). PCR mixtures also contained primers for the internal control. The multiplex PCR allowed internal controls (phage DNA) to be coamplified and the extraction and amplification processes to be validated. Absolute quantification was performed with external calibration curves constructed from serial 10-fold dilutions of a plasmid containing the DNA polymerase gene as the template. DNA PCR contamination was prevented using dUTP and DNA uracil glycosylase in each run. The linear range of the technique was from 250 to 25 × 106 copies/ml and the threshold was 200 copies/ml. The repeatability and reproducibility of the technique varied by <0.5 log copies/ml over the linear range.
Statistical analysis.
The statistical analysis was performed using the SPSS 12.0 software (SPSS for Windows, Chicago, IL). In order to determine the slope of the viral DNA decrease over time, one segment of the curve illustrating the numbers of VZV genome copies was analyzed for each patient and was defined when a biologically significant change in viral load (0.5 log [15, 16] from baseline) was identified. VZV DNA was modeled using a logarithmic decay curve, expressed by the equation y = y0 e−at, where y is the initial virus load, t the time from the start of the curve, and a the decay constant (17). Data were expressed as means ± standard deviations (SD). Correlation was analyzed using the nonparametric Spearman test, and the exponential model was tested using a linear regression analysis. The statistical analysis was performed using the Statistical Package for the Social Sciences program (SPSS 17.0 for Windows, Chicago, IL). Differences were accepted as significant for P values of <0.05.
RESULTS
Baseline data and clinical course are summarized in Table 1. All patients were HIV negative and ranged in age from 35 to 68 years. Only one patient had myopia (−4 diopters). Patient 3 was considered immunocompromised, having Chester-Erdheim disease treated with alpha-2a interferon (3,000,000 IU, three times/week). Patient 5 received oral steroids (1 mg/kg/day, given for uveitis by his referring ophthalmologist) 12 days before the diagnosis of ARN. The retinitis was considered extensive in all cases since it was ≥180°.
Table 1.
Patients' characteristics, treatment, and prognosis
| Case no./gender | Age (yr) | Ocular lesion scoreb | Initial visual acuity | Time for onset of retinal healing (days) | Duration of plateau/delay of negativation of viral load (days) | Treatmenta |
Anatomic and visual outcomec | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVT | Duration of weekly loading IVT dose/weekly disease-modifying dose (mo) | IV | Duration of IV (mo) | PO | Duration of PO | Pars plana vitrectomy | |||||||
| 1/male | 35 | Tyndall 1+, vitreitis 1+, retinitis 180° | 20/30 | 13 | 29/97 | GCV | 3/1.5 | ACV+ FCV | 3 | VCV + FamCV | VCV 6 mo + FamCV >3 yr | No LP, total cataract, optic nerve atrophy, inferior retinal detachment (day 20); phthisis; FU = 4 yr | |
| 2/male | 47 | Tyndall 1+, vitreitis 3+, lesion 360° | 20/400 | 48; fundus not visible at day 15 | 17/64 | GCV | 1/0.5 | ACV | 1.5 | VCV | 1.5 yr; 1/2 dose >1 yr | Yes, dense vitreous (day 48) | Hand motions; attached retina; FU = 2 yr |
| 3/female | 65 | Tyndall 1+, vitreitis 1+, retinitis 180°, cataract | 20/80 | 17 | 77/113 | FCV | 1/2 | ACV | 4 | Death at 4 mo | 20/125; attached retina; FU = 4 mo | ||
| 4/male | 68 | Tyndall 1+, vitreitis 1+, retinitis 360° | 20/40 | 20 | 20/178 | FCV | 1/0.25 | ACV | 1 | VCV | >7 mo | Yes, retinal detachment (57 days) | 20/60; attached retina after silicone removal and cataract surgery; FU = 14 mo |
| 5/male | 51 | Tyndall 1+, vitreitis 1+, retinitis 360° | Hand motions | 21; fundus not visible at day 12 | 10/69 | FCV | 1/0 | ACV | 1.5 | VCV | >10 mo | Yes, retinal detachment | Hand motions; attached retina; under silicone oil; FU = 10 mo |
| 6/male | 47 | Tyndall 1+, vitreitis 1+, retinitis 360° | 20/30 | 16; fundus not visible at day 44 | 14/ | FCV | 0.5/1.5 | ACV | 1.5 | VCV | >1 mo | Yes, dense vitreous (45 days) + retinal detachment | Attached retina under silicone oil; FU = 6 months |
ACV, acyclovir; FCV, foscarnet sodium; VCV, valaciclovir; GCV, ganciclovir; FamCV, famciclovir (Oravir); IVI, intravitreal injection; PO, per os; IV, intravitreal.
According to the Standardization of Uveitis Nomenclature (SUN) Working Group (17a).
FU, follow-up; LP, light perception.
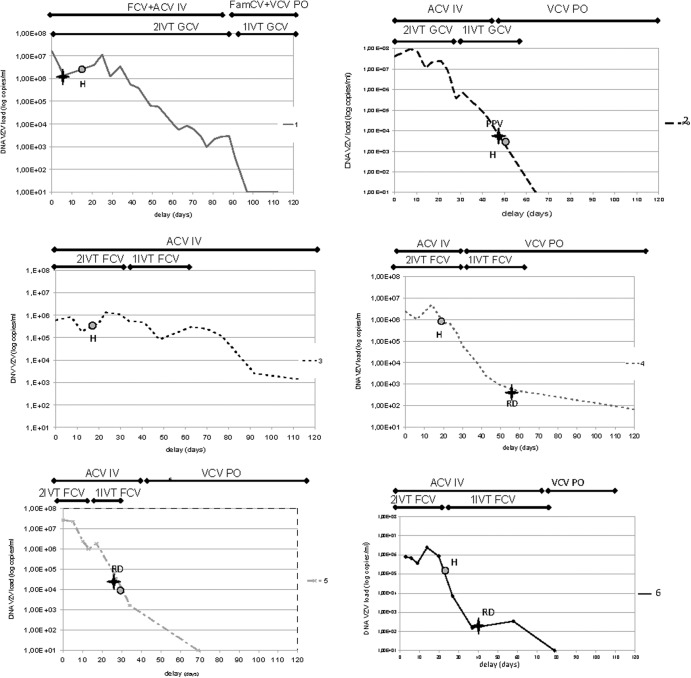
At presentation, all patients were given acyclovir intravenously (10 mg/kg/8 h) with an intravitreal injection of antiviral drugs (Table 1). The duration of each treatment was adapted to the course of inflammation and the patient's drug tolerance. One patient was vitrectomized since dense vitreous opacities did not allow a sufficient view of the fundus, and two other patients were treated with pars plana vitrectomy (PPV) for retinal detachment (RD) (with endolaser and silicone tamponade). Therapeutic strategies for each patient are also summarized in Fig. 1.
Fig 1.
Time course of VZV viral loads in six patients with acute retinal necrosis.  H, healing; ✦ RD, retinal detachment; ■—■ treatment; ACV, aciclovir; VCV, valaciclovir; FCV, foscarnet sodium; GCV, ganciclovir; FamCV, famciclovir; PO, per os treatment; IV, intravitreous treatment; 1IVT, one intravitreal injection per week; 2IVT, two intravitreal injections per week.
H, healing; ✦ RD, retinal detachment; ■—■ treatment; ACV, aciclovir; VCV, valaciclovir; FCV, foscarnet sodium; GCV, ganciclovir; FamCV, famciclovir; PO, per os treatment; IV, intravitreous treatment; 1IVT, one intravitreal injection per week; 2IVT, two intravitreal injections per week.
According to the kinetics defined for each patient (increase, plateau, and decrease), two main portions of the DNA curves were defined for each patient, a plateau phase with a mean duration of 27.8 ± 24.9 days (range, 10 to 77 days), followed by a phase in which the viral DNA amount declined. The mean initial viral load was 3.4 × 107 ± 4.45 × 107 copies/ml (6 × 106 to 1.2 × 108/ml). We found no correlation between the initial viral load and the duration of the plateau (P = 0.3), the onset of efficacy of treatment (P = 0.9), or the age of the patient (P = 0.4). The viral load decreased in accordance with an exponential model, with a similar slope and R2 coefficient (Table 2) (Fig. 2).
Table 2.
Modelization of the reduction in viral loads during treatment (regression analysis)
| Patient no. | Formulaa | Coefficient (R2) | Virus load half-time (days) |
|---|---|---|---|
| y = y0eax | |||
| Log(y) = log(y0) − 0.434 × ax | |||
| 1 | y = 9 × 108 e−0.189x | 0.99 | 3.67 |
| Log(y) = 8.95 − 0.082x | |||
| 2 | y = 4 × 109 e−0.282x | 0.95 | 2.46 |
| Log(y) = 9.60 − 0.122x | |||
| 3 | y = 3 × 1010 e−0.173x | 0.91 | 4.01 |
| Log(y) = 10.48 − 0.075x | |||
| 4 | y = 2 × 108 e−0.268x | 0.84 | 2.59 |
| Log(y) = 8.30 − 0.016x | |||
| 5 | y = 3 × 107 e−0.227x | 0.94 | 3.05 |
| Log(y) = 7.48 − 0.099x | |||
| 6 | y = 3 × 106 e−0.171x | 0.81 | 2.11 |
| Log(y) = 6.47 − 0.07x |
x, time (days); y, viral load.
Fig 2.
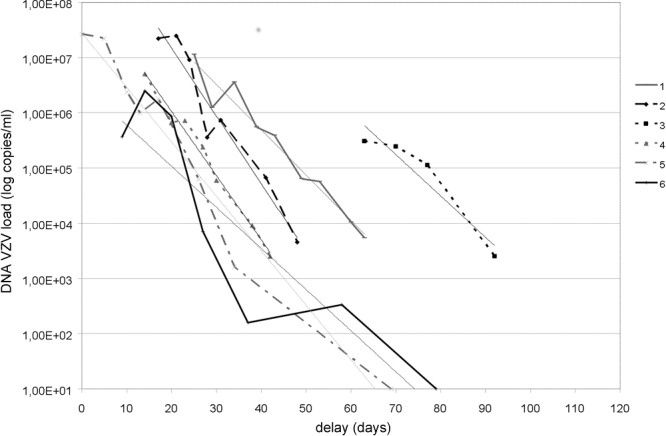
Model of VZV viral loads using a logarithmic decay curve in six patients with acute retinal necrosis. The modelized part of each curve concerns the segment of decreasing DNA load (biological change in viral load defined as 0.5 log).
Clinical evolution was illustrated by the onset of healing and is noted on the curve shown for each patient (Fig. 1, see point H). In two patients, given the clinical course of hyalitis, the examination of the fundus was difficult and the treatment efficacy time was defined approximately. In patient 2, the fundus was not visible on day 15, and retina healing was observed at the time PPV was performed (day 48). In patient 5, since vitreitis precluded the visualization of the retina between day 12 and day 21, the onset of healing was estimated to have occurred on day 21 concurrent with PPV when RD was managed, with areas of healing of the inferior retina. In patient 6, superior peripheral RD associated with dense vitreitis was suspected on B-scan examination. Therefore, PPV, endolaser, and silicone tamponade were performed on day 45. Finally, the onset of healing was calculated as 22.5 ± 12.8 days (range, 13 to 48 days). Three patients had silicone oil in the vitreous cavity 25, 45, and 57 days after the beginning of the medical treatment.
We found no correlation between the duration of the biological plateau and the onset of healing (P = 0.6). As shown in Fig. 1, the onset of the reduction of viral load was delayed from the onset of healing of retinal lesions in patients 1 and 3 (differences of −60 and −16 days, respectively) and advanced in patient 5 (+11 days). Note that given her general health status, patient 3 was not able to have intravitreal injections twice a week for 3 weeks (she had only one injection per week). Despite prolonged intravenous acyclovir treatment, the duration of the plateau was particularly long (77 days). She died 4 months later, secondary to peritonitis and cholecystitis. We found that the clinical course was similar to the biological course in patient 4 (20 days) and patient 5 (21 versus 10 days). In patient 2, the delay in the clinical course was probably overestimated since healing onset was observed concurrent with PPV.
With a mean follow-up of 17.6 ± 16.4 months, in one patient the anatomical outcome was phthisis bulbi, and in five patients visual acuities ranged from no light perception to 20/60 (Table 1).
DISCUSSION
Our results clearly show that the profile of viral DNA in the AH of patients treated for VZV ARN follows a well-defined kinetic pattern of virus load clearance with a plateau phase and then a logarithmic reduction of viral load, resulting in persistence of VZV DNA copies as long as 6 months after the initiation of antiviral therapy.
Quantification of viral DNA was first validated for cytomegalovirus (CMV) in peripheral blood leukocytes (18) and for herpes simplex virus (HSV) in the cerebrospinal fluid (4, 14). The quantitative measurement of viral load using qPCR has recently been described for ocular infections by HSV (19, 20), VZV (20, 21), human herpesvirus type 6 (12, 22), and CMV (12). Real-time PCR has also been used in patients with ARN attributable to VZV (20) and HSV2 (20), but the assay was performed with only two to four samples, which did not allow definition of the kinetics of the viral load over several weeks during and/or after treatment.
The viral load kinetics, as measured by qPCR, were previously well correlated with the clinical course of several types of infection due to herpesviridae, such as HSV in cerebrospinal fluid (23), VZV in bone marrow, (24), Epstein-Barr virus (EBV) in liver after transplantation (25), human herpesvirus type 6 infection (26), and varicella (plasma, within a period of 14 to 30 days after antiviral treatment onset) (24, 27, 28). However, to the best of our knowledge, there is no previous study on virus load kinetics in ARN. From a practical point of view, this approach is feasible in patients with ARN syndrome since aqueous tap is minimally invasive, with very rare adverse events (29), and it can be performed before intravitreal injection in viral retinitis-affected eyes to reduce the risk of increased intraocular pressure (30). An anterior chamber tap is easier than a vitreous biopsy, and one previous study (31) showed that AH and vitreous loads were significantly correlated (data from Table 1, R2 = 0.87, P = 0.004), suggesting that viral load in AH reflects that in the vitreous fluid (32). The correlation between AH or vitreous viral load and that quantified within the retina has never been reported. We did not find a strong correlation between the initial viral load, the duration of the plateau, and the onset of healing. The description of the clinical evolution was, however, limited in three of our patients because dense vitreitis made viewing the fundus impossible. As has been suggested regarding acute anterior uveitis where viral loads correlated with iris damage (22), the fact that four patients out of the six (patients 1, 2, 5, and 6) who had the highest viral load at the time of diagnosis had a poor clinical evolution suggests a relationship between the initial viral load and the final prognosis. These results remain to be confirmed in a larger series of patients. The other limitations of our study were its retrospective nature and the possibility that the results of DNA quantification influenced the therapeutic strategy itself, independently of the clinical course. Moreover, vitrectomy associated with silicone tamponade in three patients may have changed the viral load in AH (with a possible overestimation). However, two out of three patients who underwent vitrectomy had silicone oil in the vitreous cavity, which is apparent in Fig. 1 at the end of the slope of decreasing viral load (patients 4 and 6), and no effects of surgery were attributable to the overall evolution of the viral load. The other patient was operated on when VZV DNA was at the middle part of the slope, and no evident effect of the tamponade was noted on the viral load course after surgery in this case.
We showed that the DNA load covered a large dynamic range (from 102 to 107 copies), as previously reported for other real-time PCR assays. The baseline viral load in AH of the six ARN patients (from 6 × 105 to 4 × 107 copies/ml; mean, 6.88 log ± 0.7 copies/ml) was comparable to that found in other reported cases of HSV ARN (from 3.82 × 105 copies/ml to more than 107 copies/ml, using qPCR) (19, 33) and VZV ARN (9 × 102 and 4.8 × 106 copies/ml, qPCR targeting the VZV glycoprotein B gene) (20) and close to that found in VZV acute anterior uveitis (from 2.2 × 104 to 1.2 × 107 copies/ml, with one patient with 3.8 × 102 copies/ml; mean, 2.04 × 107 ± 3.79 × 107 copies/ml, n = 8, using qPCR targeting the VZV single-stranded DNA-binding protein gene) (22). However, the amplified target genes differed between studies, which precludes direct comparisons between the studies. Finally, the variability of viral load seems to be lower in ARN than in acute anterior uveitis. One possible limitation of qPCR is that detection of viral DNA does not imply the presence of actively infectious viruses, since the amplified sequence of DNA might represent remnants of viruses previously inhibited by the antiviral drug and may also include nonproductive (abortive) particles. This difficulty could have been overcome by quantification of VZV transcripts, but RNA is very sensitive to transportation from the operating room to the lab, and it is very difficult to correlate the amounts of viral RNA with those of infectious particles, since the rate of transcription varies with the level of replication of viral particles. Finally, viral RNA is mostly found in infected cells, i.e., in the retina in ARN, while retinal cells are not present in detectable amounts in AH. Therefore, the major risk of assessing viral RNA would have been misestimation of the extent of the infectious process.
In our case series, the first part of each curve was a plateau (no change within 0.5 log of the viral load), with a variable duration ranging from 10 to 77 days. This antiviral biological response time was not correlated with the initial viral load, but this could have been due to the small number of patients included in the study. The length of this plateau may be related to the antiviral susceptibility of the virus, i.e., the significant interruption of viral replication within the major area of infected retina. In the literature, only Asano et al. (for VZV and HSV2) (20) and Cottet et al. (HSV2) (33) reported consecutive measurements of viral DNA load (three or four times) over a period of 1 to 6 months.
We showed for the first time that when the VZV load begins to decline (after a biologically significant change in viral load of 0.5 log), all DNA curves behave similarly, with comparable slopes. If this viral response model is applicable to all patients with VZV ARN, this suggests that once the viral load decreases, the expected time for a 50% reduction of the initial viral load is about 3 ± 0.7 days.
The kinetics of the VZV load raises the question of dosage and duration of antiviral therapy in VZV ARN. In the literature, intravenous treatment with acyclovir for 10 days (2, 5, 34–36) and intravitreal injections of antiviral drugs for up to 212 days (average course, 59 days) (13, 36) were reported for the largest studies on ARN. Despite variable absorption of antiviral drugs (37), the use of oral antivirals for 5 to 24 weeks (e.g., famciclovir or valacyclovir) (6, 7) has been reported for the treatment of ARN in the acute phase. The duration of maintenance therapy also varied from 2 to 75 months (2, 11, 35, 36, 38). Treatment duration has been mainly based on the data reported by Palay et al., published in 1991 (39), which showed that the risk of bilateral involvement is greatest in the first 14 weeks and that acyclovir significantly reduced this risk. However, for several case series it was reported that viruses may be detected in the eye several months after conventional treatment. For example, in some reported cases, PCR was positive 2 weeks after high-dose acyclovir administration (for VZV; it was then negative at 4 weeks) (40), 20 days after antiviral intravenous acyclovir (for HSV2; viral load, 4.5 × 102 copies/ml) (19), after 36 days of acyclovir treatment (for VZV; 1.2 × 103 at day 43) (20), 2 weeks (HSV2, 1.9 × 104 copies/ml) and 6 months after treatment (acyclovir 14 days intravenously and orally for 3 months, 1.4 × 103 copies/ml), and then negative at 10 months (33). In herpetic keratitis, it is difficult to reduce the viral load quickly despite clinical improvements (41). Also, our data are consistent with the absence of a reduction in HSV1 DNA copy numbers in tears by treatment with 500 mg of valacyclovir daily for 30 days (42), whereas 400 mg of acyclovir can significantly reduce the number of HSV keratic recurrences (31). Biological efficacy is achieved with 70 or 140 mg/kg of valacyclovir in rabbits (43), equivalent to approximately 5 g or 10 g daily for humans, i.e., 10- or 20-fold the usual 500-mg dosage of valacyclovir. Finally, our data add to previously reported clinical data (time for complete resolution of retinitis between 2 and 17 weeks[7, 13, 44], possible recurrence in the same eye despite ongoing antiviral treatment [44]) and biological data (presence of viral genome from 2 weeks to 6 months [20, 33, 40]) and strongly suggest that a more prolonged high-dose therapy (probably up to 50 days) could be considered for patients with ARN, especially those with VZV infection.
Modeling the VZV load in patients with ARN may help the clinician to evaluate the duration of therapy when a decrease of DNA has been initiated. Since AH samples can be collected at the time of intravitreal injections, qPCR may be a useful technique to monitor patients with VZV ARN. An unanswered question is also the viral load threshold at which it can be considered that therapy may be stopped, since low viral load may persist for several months, as demonstrated in this series.
In conclusion, the use of qPCR demonstrated a course of VZV DNA reduction with virus clearance kinetics, including a plateau followed by a logarithmic reduction. These data provide an explanation for the fact that the conventional duration of 10 days of intravenous acyclovir could be insufficient in patients who do not present rapid healing and that therapeutic strategies should be further evaluated. Since all the patients in this series seemed to respond with similar kinetics once the viral load started to decline, modeling the VZV DNA load defined in this study could also be useful to predict the course of viral loads in new patients.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Walters G, James TE. 2001. Viral causes of the acute retinal necrosis syndrome. Curr. Opin. Ophthalmol. 12:191–195 [DOI] [PubMed] [Google Scholar]
- 2. Hillenkamp J, Nolle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. 2009. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology 116:1971–1975 e2. 10.1016/j.ophtha.2009.03.029 [DOI] [PubMed] [Google Scholar]
- 3. Dworkin LL, Gibler TM, Van Gelder RN. 2002. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch. Ophthalmol. 120:1534–1539 [DOI] [PubMed] [Google Scholar]
- 4. Kessler HH, Muhlbauer G, Rinner B, Stelzl E, Berger A, Dorr HW, Santner B, Marth E, Rabenau H. 2000. Detection of herpes simplex virus DNA by real-time PCR. J. Clin. Microbiol. 38:2638–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. 1986. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology 93:296–300 [DOI] [PubMed] [Google Scholar]
- 6. Emerson GG, Smith JR, Wilson DJ, Rosenbaum JT, Flaxel CJ. 2006. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology 113:2259–2261 [DOI] [PubMed] [Google Scholar]
- 7. Aizman A, Johnson MW, Elner SG. 2007. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology 114:307–312 [DOI] [PubMed] [Google Scholar]
- 8. Figueroa MS, Garabito I, Gutierrez C, Fortun J. 1997. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am. J. Ophthalmol. 123:255–257 [DOI] [PubMed] [Google Scholar]
- 9. Luu KK, Scott IU, Chaudhry NA, Verm A, Davis JL. 2000. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am. J. Ophthalmol. 129:811–813 [DOI] [PubMed] [Google Scholar]
- 10. Holland GN. 1994. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am. J. Ophthalmol. 117:663–667 [DOI] [PubMed] [Google Scholar]
- 11. Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. 2010. Treatment of acute retinal necrosis. Ophthalmology 117:818–824 [DOI] [PubMed] [Google Scholar]
- 12. Kawaguchi T, Spencer DB, Mochizuki M. 2008. Therapy for acute retinal necrosis. Semin. Ophthalmol. 23:285–290 [DOI] [PubMed] [Google Scholar]
- 13. Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. 2010. Long-term follow-up of acute retinal necrosis. Retina 30:795–800 [DOI] [PubMed] [Google Scholar]
- 14. Weidmann M, Meyer-Konig U, Hufert FT. 2003. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J. Clin. Microbiol. 41:1565–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gouarin S, Vabret A, Scieux C, Agbalika F, Cherot J, Mengelle C, Deback C, Petitjean J, Dina J, Freymuth F. 2007. Multicentric evaluation of a new commercial cytomegalovirus real-time PCR quantitation assay. J. Virol. Methods 146:147–154 [DOI] [PubMed] [Google Scholar]
- 16. Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK. 2009. Interlaboratory comparison of cytomegalovirus viral load assays. Am. J. Transplant. 9:258–268 [DOI] [PubMed] [Google Scholar]
- 17. Roberts TC, Brennan DC, Buller RS, Gaudreault-Keener M, Schnitzler MA, Sternhell KE, Garlock KA, Singer GG, Storch GA. 1998. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J. Infect. Dis. 178:626–635 [DOI] [PubMed] [Google Scholar]
- 17a. Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group 2005. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 140:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gault E, Michel Y, Dehee A, Belabani C, Nicolas JC, Garbarg-Chenon A. 2001. Quantification of human cytomegalovirus DNA by real-time PCR. J. Clin. Microbiol. 39:772–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arimura E, Deai T, Maruyama K, Uno N, Yamamoto H, Matsumoto C, Shimomura Y. 2005. Herpes simplex virus-2 quantification by real-time polymerase chain reaction in acute retinal necrosis. Jpn. J. Ophthalmol. 49:64–65 [DOI] [PubMed] [Google Scholar]
- 20. Asano S, Yoshikawa T, Kimura H, Enomoto Y, Ohashi M, Terasaki H, Nishiyama Y. 2004. Monitoring herpesvirus DNA in three cases of acute retinal necrosis by real-time PCR. J. Clin. Virol. 29:206–209 [DOI] [PubMed] [Google Scholar]
- 21. Yin PD, Kurup SK, Fischer SH, Rhee HH, Byrnes GA, Levy-Clarke GA, Buggage RR, Nussenblatt RB, Mican JM, Wright ME. 2007. Progressive outer retinal necrosis in the era of highly active antiretroviral therapy: successful management with intravitreal injections and monitoring with quantitative PCR. J. Clin. Virol. 38:254–259 [DOI] [PubMed] [Google Scholar]
- 22. Kido S, Sugita S, Horie S, Miyanaga M, Miyata K, Shimizu N, Morio T, Mochizuki M. 2008. Association of varicella zoster virus load in the aqueous humor with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br. J. Ophthalmol. 92:505–508 [DOI] [PubMed] [Google Scholar]
- 23. Ando Y, Kimura H, Miwata H, Kudo T, Shibata M, Morishima T. 1993. Quantitative analysis of herpes simplex virus DNA in cerebrospinal fluid of children with herpes simplex encephalitis. J. Med. Virol. 41:170–173 [DOI] [PubMed] [Google Scholar]
- 24. Kimura H, Kido S, Ozaki T, Tanaka N, Ito Y, Williams RK, Morishima T. 2000. Comparison of quantitations of viral load in varicella and zoster. J. Clin. Microbiol. 38:2447–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsukura T, Yokoi A, Egawa H, Kudo T, Kawashima M, Hirata Y, Tanaka H, Kagajo K, Wada H, Tanaka K. 2002. Significance of serial real-time PCR monitoring of EBV genome load in living donor liver transplantation. Clin. Transplant. 16:107–112 [DOI] [PubMed] [Google Scholar]
- 26. Yoshikawa T. 2003. Significance of human herpesviruses to transplant recipients. Curr. Opin. Infect. Dis. 16:601–606 [DOI] [PubMed] [Google Scholar]
- 27. Aitken C, Barrett-Muir W, Millar C, Templeton K, Thomas J, Sheridan F, Jeffries D, Yaqoob M, Breuer J. 1999. Use of molecular assays in diagnosis and monitoring of cytomegalovirus disease following renal transplantation. J. Clin. Microbiol. 37:2804–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalpoe JS, Kroes AC, Verkerk S, Claas EC, Barge RM, Beersma MF. 2006. Clinical relevance of quantitative varicella-zoster virus (VZV) DNA detection in plasma after stem cell transplantation. Bone Marrow Transplant. 38:41–46 [DOI] [PubMed] [Google Scholar]
- 29. Van der Lelij A, Rothova A. 1997. Diagnostic anterior chamber paracentesis in uveitis: a safe procedure? Br. J. Ophthalmol. 81:976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morlet N, Young SH. 1993. Prevention of intraocular pressure rise following intravitreal injection. Br. J. Ophthalmol. 77:572–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abe T, Sato M, Tamai M. 1998. Correlation of varicella-zoster virus copies and final visual acuities of acute retinal necrosis syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 236:747–752 [DOI] [PubMed] [Google Scholar]
- 32. Carmichael A. 2012. Cytomegalovirus and the eye. Eye (Lond.) 26:237–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cottet L, Kaiser L, Hirsch HH, Baglivo E. 2009. HSV2 acute retinal necrosis: diagnosis and monitoring with quantitative polymerase chain reaction. Int. Ophthalmol. 29:199–201 [DOI] [PubMed] [Google Scholar]
- 34. Duker JS, Blumenkranz MS. 1991. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv. Ophthalmol. 35:327–343 [DOI] [PubMed] [Google Scholar]
- 35. Lau CH, Missotten T, Salzmann J, Lightman SL. 2007. Acute retinal necrosis features, management, and outcomes. Ophthalmology 114:756–762 [DOI] [PubMed] [Google Scholar]
- 36. Wong R, Pavesio CE, Laidlaw DA, Williamson TH, Graham EM, Stanford MR. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology 117:556–560 [DOI] [PubMed] [Google Scholar]
- 37. Phan DD, Chin-Hong P, Lin ET, Anderle P, Sadee W, Guglielmo BJ. 2003. Intra- and interindividual variabilities of valacyclovir oral bioavailability and effect of coadministration of an hPEPT1 inhibitor. Antimicrob. Agents Chemother. 47:2351–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muthiah MN, Michaelides M, Child CS, Mitchell SM. 2007. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br. J. Ophthalmol. 91:1452–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palay DA, Sternberg P, Jr, Davis J, Lewis H, Holland GN, Mieler WF, Jabs DA, Drews C. 1991. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am. J. Ophthalmol. 112:250–255 [DOI] [PubMed] [Google Scholar]
- 40. Kaneko H, Iida T, Aoki K, Ohno S, Suzutani T. 2005. Sensitive and rapid detection of herpes simplex virus and varicella-zoster virus DNA by loop-mediated isothermal amplification. J. Clin. Microbiol. 43:3290–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hlinomazova Z, Loukotova V, Horackova M, Sery O. 2012. The treatment of HSV1 ocular infections using quantitative real-time PCR results. Acta Ophthalmol. 90:456–460 [DOI] [PubMed] [Google Scholar]
- 42. Kumar M, Hill JM, Clement C, Varnell ED, Thompson HW, Kaufman HE. 2009. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Invest. Ophthalmol. Vis. Sci. 50:5601–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar M, Kaufman HE, Clement C, Bhattacharjee PS, Huq TS, Varnell ED, Thompson H, Hill JM.2010. Effect of high versus low oral doses of valacyclovir on herpes simplex virus-1 DNA shedding into tears of latently infected rabbits. Invest. Ophthalmol. Vis. Sci. 51:4703–4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tran TH, Stanescu D, Caspers-Velu L, Rozenberg F, Liesnard C, Gaudric A, Lehoang P, Bodaghi B. 2004. Clinical characteristics of acute HSV-2 retinal necrosis. Am. J. Ophthalmol. 137:872–879 [DOI] [PubMed] [Google Scholar]