Abstract
Arginase serum levels were increased in human African trypanosomiasis patients and returned to control values after treatment. Arginase hydrolyzes l-arginine to l-ornithine, which is essential for parasite growth. Moreover, l-arginine depletion impairs immune functions. Arginase may be considered as a biomarker for treatment efficacy.
TEXT
Trypanosoma brucei gambiense and T. brucei rhodesiense, the causative agents of human African trypanosomiasis (HAT) or sleeping sickness, are tsetse fly-transmitted protozoan parasites (1). They multiply extracellularly in the bloodstream, lymph, and interstitial fluids during the disease first stage (stage 1). The second stage (stage 2) begins when they cross the blood-brain barrier. Major immune system impairments are observed (2). Medications are toxic and difficult to administer, in particular in stage 2. Diagnosis, treatment, and posttreatment follow-up remain complex due to poorly equipped health facilities and political unrest in most HAT foci; therefore, new biomarkers are needed.
The inducible metabolism of l-arginine plays an important role in infections (3). Arginase (two isoforms) hydrolyzes l-arginine to l-ornithine and urea. l-Arginine is the common substrate of arginases and nitric oxide (NO) synthases (NOS). Its depletion represents a limiting factor of NO production. The balance of inducible NOS/arginase is regulated by the patient's cytokine profile. l-ornithine is the precursor for the synthesis of l-glutamine, l-proline, and polyamines via the ornithine decarboxylase pathway (4). In trypanosomes, polyamines facilitate DNA and trypanothione synthesis, which is essential for maintaining the intracellular redox system and presenting a defense against oxidative stress (5). Moreover, l-arginine depletion, caused by arginase, leads to the loss of T cell receptor-associated CD3 ζ chain and stops T cell proliferation (3). Thus, increased arginase activity is likely to damage the host immune response and favor parasite growth.
Arginase activity and expression were measured in sera and cerebrospinal fluid (CSF) from HAT patients before treatment and 6 months later and from the sera of healthy controls. Patients (n = 32) were recruited from a previously described group (6). The field survey took place in the “Couloir” focus in the Republic of Congo (Brazzaville). The scientific protocol was approved by the WHO Research Ethic Review Committee and authorized by the Congo Ministry of Health. Informed consent was obtained from the patients. Screening tests on whole blood used the serological card agglutination trypanosomiasis test. Parasites were searched in aspirates from cervical lymph glands and/or blood after parasite concentration by capillary tube centrifugation and/or the mini-anion-exchange centrifugation technique (6). Inclusion criteria stipulated that patients were parasitologically confirmed. HAT patients were classified as being in stage 1 (n = 25) or stage 2 (n = 7) according to patient CSF white blood cell counts and/or the absence or presence of CSF trypanosomes. Persons tested negative for HAT were enrolled as controls (n = 23) and matched for age and sex. HIV serology was performed to exclude HIV-positive people.
Two independent measures of arginase were performed. A sandwich enzyme immunoassay was performed to quantify human arginase I (liver type) according to the manufacturer's instructions (BioVendor, Heidelberg, Germany), as described previously (7). The result was confirmed by measurement of the arginase activity (8). Arginase activity indicates a potential role of this enzyme in ornithine production.
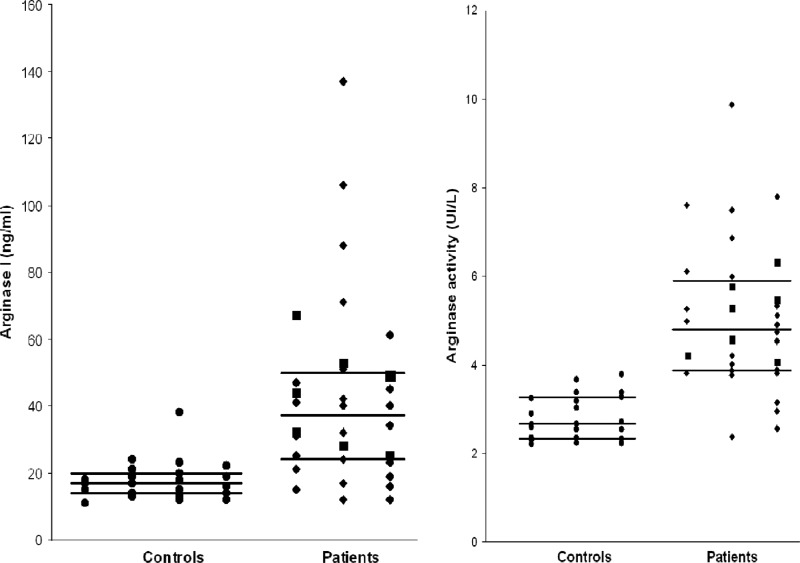
Arginase values are shown in Fig. 1. Intergroup comparisons were made by using a nonparametric test (Mann-Whitney U test). Tests of significance were two tailed. All P values of <0.01 were considered significant. The arginase activity was higher in HAT patients before treatment compared to controls (median values, 4.82 U/liter versus 2.65 U/liter, P < 0.001). The sample of stage 2 HAT patients was not statistically significant, but their arginase activity and expression levels were in the same range as those of stage 1 patients. Higher serum arginase I expression was measured in HAT patients compared to controls (median values, 37 ng/ml versus 17 ng/ml, P < 0.001). A high correlation was found between arginase activity and arginase expression (Spearman coefficient correlation r = 0.84). Arginase I expression and activity were not detected in patient CSF.
Fig 1.
Determination of arginase I expression (left panel) and arginase activity (right panel) in sera from controls (n = 23) and patients with human African trypanosomiasis (n = 32) from the Couloir focus (Republic of Congo). ●, Controls; ◆, stage 1 patients; ■, stage 2 patients. Black lines represent the medians and quartiles for each group. The arginase activity was higher in HAT patients than in controls (median, 4.82 U/liter versus 2.65 U/liter, P < 0.001). Serum arginase I expression was higher in HAT patients than in controls (median, 37 ng/ml versus 17 ng/ml, P < 0.001).
No differences were found between controls and HAT patients in terms of serum markers for hepatic injury and hemolysis. Values, expressed as medians (interquartile ranges), for patients versus controls, respectively, were as follows: alanine amino transferase, 32.1 IU/liter (22.6 to 50.3 IU/liter) versus 31.6 IU/liter (22.5 to 48.1 IU/liter); aspartate amino transferase, 26.7 IU/liter (11.2 to 35.5 IU/liter) versus 25.9 IU/liter (11.4 to 36.2 IU/liter); total bilirubin, 13.3 μmol/liter (8.2 to 17.6 μmol/liter) versus 12.8 μmol/liter (8.3 to 16.4 μmol/liter); and indirect bilirubin, 6.8 μmol/liter (5.4 to 8.5 μmol/liter) versus 6.4 μmol/liter (5.5 to 8.3 μmol/liter). Treatment was performed as described previously (6): stage 1 patients received pentamidine, and stage 2 patients difluoro-methyl ornithine.
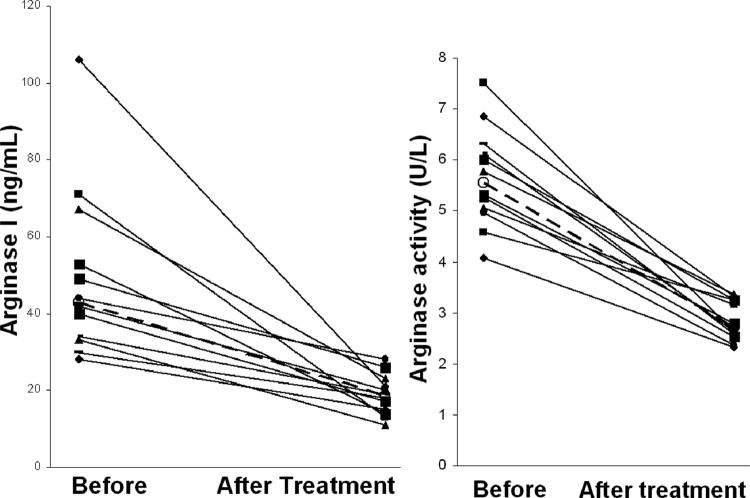
Patient follow-up was performed 6 months later for comparison of pre- and posttreatment sera. Full comparisons were obtained for 14 patients who were present for the arginase follow-up. They were all parasitologically negative after treatment. Two patients maintained normal arginase levels. In the 12 patients with higher pretreatment serum arginase I expression (median, 43 ng/ml), values decreased after treatment (18.5 ng/ml, P < 0.005), to return to healthy control values (17 ng/ml) (Fig. 2). Treatment had a similar effect on arginase activity, with a median of 5.54 U/liter before treatment versus 2.72 U/liter afterward (P < 0.001). Posttreatment values were similar to healthy control values (2.65 U/liter).
Fig 2.
Determination of arginase I expression (left panel) and arginase activity (right panel) in sera from patients with human African trypanosomiasis before treatment and 6 months later (n = 12). Open circles (○) represent the median for each group. Values for each patient are joined by a black line, and medians are joined by a dashed line. Arginase I expression values (median, 43 ng/ml before treatment) decreased after treatment (median, 18.5 ng/ml, P < 0.005) to return to healthy control values (17 ng/ml). Arginase activity (median, 5.54 U/liter before treatment) decreased after treatment (median, 2.72 U/liter, P < 0.001) to return to healthy control values (2.65 U/liter).
High serum arginase levels, confirmed by two independent techniques, were found in HAT patients. Values resumed healthy control levels after treatment. The origin of this increase has not yet been elucidated. The increase in arginase did not reflect hemolysis, since hemolysis serum markers were not found, and did not result from liver injury, since HAT patients did not exhibit any increase in the alanine amino transferase and aspartate amino transferase levels compared to healthy controls, a finding consistent with previous studies (9, 10). In trypanosome-infected mice, the arginase activity increases in macrophages, the main producers of arginase (11). Macrophages from trypanosome-susceptible mice exhibit a greater increase in arginase expression than those from resistant animals (12). In humans, various leukocyte subpopulations synthesizing arginase may be involved (13). High arginase blood levels have been measured in sera from acute hepatitis B, resulting in T cell function suppression, and in various cancers (14, 15, 16). High arginase activity has been measured in cutaneous leishmaniasis lesions (17). High interleukin-10 (IL-10) levels have been measured in HAT (18). Interestingly, a correlation has been reported between increased arginase activity and increased circulating IL-10 levels in trauma (19).
These findings support the hypothesis that arginase plays an essential role in HAT, in terms of parasite proliferation and/or impairment of immune responses. The assessment of NO production and, under suboptimal arginine concentrations due to arginase activity, the generation of various reactive species by inducible NOS represents a next step to investigating this impairment. The best times to perform arginase measurements in the follow-up of stage 1 and stage 2 HAT patients, in addition to standard protocols, deserves further investigations.
ACKNOWLEDGMENTS
This project was funded by WHO/TDR grant A50468, Le Conseil Régional d'Aquitaine, partner universities of Bordeaux, Limoges, and Lyon, and the Association pour le Développement de la Recherche en Parasitologie et Santé Tropicale.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. Van Meirvenne N, Le Ray D. 1985. Diagnosis of African and American trypanosomiasis. Br. Med. Bull. 41:156–161 [DOI] [PubMed] [Google Scholar]
- 2. Vincendeau P, Bouteille B. 2006. Immunology and immunopathology of African trypanosomiasis. Ann. Acad. Bras. Cienc. 78:645–665 [DOI] [PubMed] [Google Scholar]
- 3. Grohmann U, Bronte V. 2010. Control of immune response by amino acid metabolism. Immunol. Rev. 236:243–264 [DOI] [PubMed] [Google Scholar]
- 4. Munder M. 2009. Arginase: an emerging key player in the mammalian immune system. Br. J. Pharmacol. 158:638–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fairlamb AH, Cerami A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695–729 [DOI] [PubMed] [Google Scholar]
- 6. Bouteille B, Mpandzou G, Cespuglio R, Ngampo S, Peeling RW, Vincendeau P, Buguet A. 2010. Cerebrospinal fluid B lymphocyte identification for diagnosis and follow-up in human African trypanosomiasis in the field. Trop. Med. Int. Health 15:454–461 [DOI] [PubMed] [Google Scholar]
- 7. Ikemoto M, Tsunekawa S, Awane M, Fukuda Y, Murayama H, Igarashi M, Ngata A, Kasai Y, Totani M. 2001. A useful ELISA system for human liver-type arginase, and its utility in diagnosis of liver diseases. Clin. Biochem. 34:455–461 [DOI] [PubMed] [Google Scholar]
- 8. Corraliza IM, Campo ML, Soler G, Modolell M. 1994. Determination of arginase activity in macrophages: a micromethod. J. Immunol. Methods 174:231–235 [DOI] [PubMed] [Google Scholar]
- 9. Wellde BT, Chumo DA, Reardon MJ, Mwangi J, Asenti A, Mbwabi D, Abinya A, Wanyama L, Smith DH. 1989. Presenting features of Rhodesian sleeping sickness patients in the Lambwe Valley, Kenya. Ann. Trop. Med. Parasitol. 83(Suppl 1):73–89 [DOI] [PubMed] [Google Scholar]
- 10. Bisser S, Bouteille B, Sarda J, Stanghellini A, Ricard D, Jauberteau MO, Marchan F, Dumas M, Breton JC. 1997. Contribution of biochemical tests in the diagnosis of the nervous phase of human African trypanosomiasis. Bull. Soc. Pathol. Exot. 90:321–326 [PubMed] [Google Scholar]
- 11. Gobert A, Daulouede S, Lepoivre M, Boucher J, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P. 2000. l-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect. Immun. 68:4653–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouède S, Boucher J, Wilson K, Veyret B, Gobert A. 2004. Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J. Immunol. 172:6298–6303 [DOI] [PubMed] [Google Scholar]
- 13. Wood KJ, Bushell A, Hester J. 2012. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12:417–443 [DOI] [PubMed] [Google Scholar]
- 14. Sandalova E, Laccabue D, Boni C, Watanabe T, Tan A, Zong HZ, Ferrari C, Bertoletti A. 2012. Increased levels of arginase in patients with acute hepatitis B suppress antiviral T cells. Gastroenterology 143:78–87 [DOI] [PubMed] [Google Scholar]
- 15. de Boniface J, Mao Y, Schmidt-Mende J, Kiessling R, Poschke I. 2012. Expression patterns of the immunomodulatory enzyme arginase I in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 1:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez G, Olivares IM, Rodriguez MG, Ceballos GM, Garcia Sanchez JR. 2012. Arginase activity in patients with breast cancer: an analysis of plasma, tumors, and its relationship with the presence of the estrogen receptor. Onkologie 35:570–574 [DOI] [PubMed] [Google Scholar]
- 17. Abebe T, Hailu A, Woldeyes M, Mekonen W, Bilcha K, Cloke T, Fry L, Seich Al Basatena NK, Corware K, Modolell M, Munder M, Tacchini-Cottier F, Müller I, Kropf P. 2012. Local increase of arginase activity in lesions of patients with cutaneous leishmaniasis in Ethiopia. PLoS Negl. Trop. Dis. 6:e1684. 10.1371/journal.pntd.0001684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhind SG, Sabiston BH, Shek PN, Buguet A, Muanga G, Stanghellini A, Dumas M, Radomski MW. 1997. The effect of melarsoprol treatment on circulating IL-10 and TNF-α level in human African trypanosomiasis. Clin. Immunol. Immunopathol. 83:185–189 [DOI] [PubMed] [Google Scholar]
- 19. Ochoa JB, Bernard AC, O'Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris SM., Jr 2001. Arginase I expression and activity in human mononuclear cells after injury. Ann. Surg. 233:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]