Abstract
Standard culture methods for diagnosis of Streptococcus pneumoniae pneumonia take at least 24 h. The BinaxNOW urine-based test for S. pneumoniae (BinaxNOW-SP) takes only 15 min to conduct, potentially enabling earlier diagnosis and targeted treatment. This study was conducted to assess whether the use of BinaxNOW-SP at the time of hospital admission would provide adequate sensitivity and specificity for diagnosis of community-acquired pneumonia (CAP) in adult patients. We searched PubMed, EMBASE/OVID, Cochrane Collaboration, Centre for Reviews and Dissemination, INAHTA, and CADTH for diagnostic or etiologic studies of hospitalized predominately adult patients with clinically defined CAP that reported the diagnostic performance of BinaxNOW-SP versus cultures. Two authors independently extracted study details and diagnostic two-by-two tables. We found that 27 studies met our inclusion criteria, and three different reference standards were used between them. A bivariate meta-analysis of 12 studies using a composite of culture tests as the reference standard estimated the sensitivity of BinaxNOW-SP as 68.5% (95% credibility interval [CrI], 62.6% to 74.2%) and specificity as 84.2% (95% CrI, 77.5% to 89.3%). A meta-analysis of all 27 studies, adjusting for the imperfect and variable nature of the reference standard, gave a higher sensitivity of 74.0% (CrI, 66.6% to 82·3%) and specificity of 97.2% (CrI, 92.7% to 99.8%). The analysis showed substantial heterogeneity across studies, which did not decrease with adjustment for covariates. We concluded that the higher pooled sensitivity (compared to culture) and high specificity of BinaxNOW-SP suggest it would be a useful addition to the diagnostic workup for community-acquired pneumonia. More research is needed regarding the impact of BinaxNOW-SP on clinical practice.
INTRODUCTION
Streptococcus pneumoniae pneumonia is believed to be the most common cause of community-acquired pneumonia (CAP) in adults, which in turn is the most common infection-related cause of death in developed countries (1). Diagnosis is usually established by observation of S. pneumoniae in a Gram-stained sputum sample or growth of S. pneumoniae in a culture of blood, sputum, pleural fluid, or other respiratory sample. Although highly specific, culturing is known to be insensitive, with diagnostic yields reported to be <30% for blood culture (2–7) and 57% for sputum culture (the latter in patients who had an etiologic diagnosis established) (8). Cultures also require 24 h or more to produce results. In the absence of a reliable rapid test for pneumonia caused by S. pneumoniae, initial treatment of pneumonia must be empirical, based upon knowledge of local pathogens, patient risk factors and comorbidities, and severity of presentation (7). Empirical therapy is generally effective (7), but there is increasing interest in improved targeting of antibiotics, due to the understanding that this may decrease the community prevalence of antibiotic-resistant bacteria and individual risks of antibiotic-associated Clostridium difficile infection (9–11).
The BinaxNOW Streptococcus pneumoniae test (BinaxNOW-SP; Binax, Inc.) is an immunochromatographic test for the presence of the pneumonococcal C-polysaccharide coat protein in urine. It produces a result within 15 min of a urine sample being obtained, and therefore it can be used as a rapid diagnostic test for S. pneumoniae infection in patients presenting with pneumonia. A number of studies have reported comparisons of BinaxNOW-SP with culture methods. A challenge in reviewing this literature is that a number of these studies were etiologic studies that may have incorporated BinaxNOW-SP in the diagnostic standard, thus artificially overestimating its sensitivity and specificity. Another challenge is that, since culturing has poor sensitivity, an analysis that assumes the reference standard (i.e., the test to which the new test is compared) is perfect may produce an underestimate of the sensitivity and specificity of BinaxNOW-SP (12). The problem of bias is worsened in a meta-analytic setting due to the diversity in reference standards across studies.
We undertook a systematic review and diagnostic meta-analysis of the sensitivity and specificity of BinaxNOW-SP in comparison with established culture methods for the diagnosis of S. pneumoniae infection in patients admitted to the hospital with community-acquired pneumonia. In addition to the standard bivariate model for meta-analysis, we also used a latent class meta-analysis method, which allowed adjustment for an imperfect reference standard and accommodated variable reference standards across studies (13).
MATERIALS AND METHODS
Data sources and searches.
We searched PubMed (inception to 10 July 2012), EMBASE (Ovid; 1996 to 2012 week 27), the Cochrane Collaboration, the Centre for Research and Dissemination, the Canadian Agency for Drugs and Technology in Health (CADTH and a CADTH confederated search across other Canadian health technology assessments), and the Centre for Reviews and Dissemination (DARE) for systematic reviews, health technology assessments, and studies that had assessed sensitivity and specificity for BinaxNOW-SP against any reference standard. The search strategies are detailed in Table S1 in the supplemental material. The search was last updated on 10 July 2012. We also hand-searched the citation lists of articles and review articles retrieved.
Study selection.
Two investigators (A.S. and X.X.) independently screened articles by title and abstract to produce a list of articles for full text review, resolving differences by discussion. We included studies of adult patients admitted to the hospital with suspected CAP that provided data that could be used to construct a two-by-two cross-tabulation for BinaxNOW-SP against a reference test. Studies had to have recruited an identifiable cohort of patients with CAP, defined by clinical signs and symptoms of lower respiratory tract infection or pneumonia, and an X-ray with new abnormalities described as an infiltrate or consolidation or otherwise consistent with pneumonia. We excluded studies that had a case-control design that used patients without CAP as controls or that predominately or exclusively included children, patients with nosocomial pneumonia, or outpatients. The BinaxNOW-SP test performs poorly with children because of their high level of asymptomatic nasal carriage of S. pneumoniae (14). We did not exclude studies with patients with HIV, AIDS, or other forms of immunosuppression, as BinaxNOW-SP measures a bacterial coat protein, the presence of which does not depend upon the patient mounting an immune response.
We included studies that reported BinaxNOW-SP results for nonconcentrated urine that had been collected as part of the initial investigation prior to or at admission or within 48 h of admission. Urine could be frozen prior to assay, provided that storage was not prolonged (greater than 3 years), as the coat protein is considered stable to freezing. If the urine was stored, our risk of bias assessment acknowledged the possibility that the index test results might have been interpreted with knowledge of the reference test. We included studies that reported results for BinaxNOW-SP against a reference standard that consisted of culture of samples from blood alone or from a respiratory site (sputum, pleural fluid, bronchiolar lavage, transthoracic needle aspirate, nasopharyngeal) with or without Gram stain of sputum or pleural fluid. All reference standards had to include blood culture. We excluded studies in which the BinaxNOW test was applied to samples other than urine. We excluded studies that incorporated the results of BinaxNOW-SP in the reference standard and did not provide the data to separate patients diagnosed solely by BinaxNOW-SP.
Data extraction and quality assessment.
Data extracted from each study included the ages of patients, admitting diagnoses, and location of the study, the clinical and X-ray criteria for diagnosis of CAP, and the criteria for diagnosis of S. pneumoniae pneumonia (sites of cultures and other tests conducted). Where definite and probable diagnoses of S. pneumoniae pneumonia were reported separately (usually on the basis of samples from normally sterile sites versus samples from nonsterile sites), we confirmed that the categories were mutually exclusive and combined the results under a single diagnosis of pneumonia. Where BinaxNOW-SP results had been incorporated into the reference standard but sufficient information had been included to separate them, patients who had positive BinaxNOW-SP tests but negative culture results were reclassified as false positives for the purposes of our analysis.
We assessed the risk of bias in each study by using the QUADAS tool (15). Three authors (A.S., X.X., and N.D.) carried out both data extraction and risk of bias assessment independently and discussed any discrepancies to arrive at a consensus assessment.
Data synthesis and analysis.
The reference standards (i.e., comparators for BinaxNOW-SP) in the selected studies were often based on a composite of multiple tests, such that a subject with a positive result for at least one of these tests was classified as reference test positive. We grouped the reference standards into three types according to the etiologic agent identified by each: (i) reference standard type A, a composite of blood culture, sputum (smear or culture), and culture of any other respiratory sample; (ii) reference standard type B, a composite of a blood culture and sputum (smear or culture); (iii) reference standard type C, a blood culture alone. We used hierarchical summary receiver operating characteristic curve (HSROC) meta-analysis models to summarize sensitivity and specificity estimates of BinaxNOW-SP with respect to each reference standard (13, 16, 17). These models assumed that each reference standard had 100% sensitivity and specificity.
As the sensitivity of these reference standards is believed to be poor, we also considered an extension of the meta-analysis model by allowing the reference test to be imperfect via a latent class model and also to be different across studies (13). A latent class model recognizes that the true disease status (i.e., the S. pneumoniae pneumonia status in the current application) is “latent” or not observed. Each cell of the two-by-two table for comparison of BinaxNOW-SP versus the reference standard was assumed to be a mixture of S. pneumoniae pneumonia-positive and S. pneumoniae pneumonia-negative patients. The percentage of patients who were positive or negative in each cell was determined based on the prevalence of S. pneumoniae pneumonia and the sensitivity and specificity of the BinaxNOW-SP test and the reference standard. We used a hierarchical structure that allowed for consideration of between-study variability in determining the sensitivity and specificity of each reference standard.
We estimated the unknown parameters for all models by using a Bayesian approach with noninformative prior distributions that would allow the observed data to dominate the final estimates of sensitivity and specificity. We carried out a meta-regression analysis by extending the latent class meta-analysis model to investigate whether the heterogeneity of sensitivities and specificities of BinaxNOW-SP across individual studies could be explained by study design (retrospective versus prospective), the purpose of the study (diagnostic versus etiologic), or type of hospital (tertiary university-affiliated center versus other). We also studied the impact of adjusting this model for conditional dependence, i.e., a correlation between BinaxNOW-SP and the reference test within the groups of S. pneumoniae-positive and -negative individuals in each study. We considered models with different degrees of correlation (18) and compared them by using the deviance information criterion (19).
From all meta-analysis models, we obtained estimates of the median and 95% credible interval (CrI) of the pooled sensitivity and specificity of BinaxNOW-SP across studies, the predicted and observed specificity in an individual study, and a summary receiver operating characteristic curve. Analyses were carried out using WinBUGS 1.4.3 (20) and R version 2.14.2 (21). The WinBUGS programs used for the meta-analysis are available from the corresponding author.
RESULTS
Search results and patient characteristics.
We identified 27 studies that provided sufficient information on BinaxNOW-SP test performance in patients with CAP to contribute to a meta-analysis (Table 1) (3, 4, 6, 22–45). Detailed results of the literature search, selection, and reasons for exclusion are summarized in the flow chart in Fig. S1 of the supplemental material. Patients in the included studies were predominantly middle-aged or elderly, with the exception of studies that included HIV-positive or AIDS patients. The mean/median age ranged from 43 to 79 years, with the proportion of men from 47% to 79%. Based on the reference standard in individual studies, 4.4% to 38.1% received a diagnosis of S. pneumoniae pneumonia. The proportion with severe disease, as indicated by pneumonia severity index (PSI) class IV or V, ranged from 23 to 61%. One study reported on a cohort of patients admitted to an intensive care unit (ICU) (36). Prior use of antibiotics ranged from 16 to 76%, although some studies assessed antibiotic use postadmission and so may have included in-hospital as well as prior use. Not all studies reported these covariates.
Table 1.
Details of studies of diagnosis of Streptococcus pneumoniae community-acquired pneumonia by using BinaxNOWa
| First author and yr (reference[s])b | Patient characteristics and country |
S. pneumoniae pneumonia case definition |
Mean age (yrs) | % male/% female | % with indicated severity score | % who received prior antibiotics | % immuno suppressed | |
|---|---|---|---|---|---|---|---|---|
| Definite | Probable | |||||||
| Sordé 2011 (24) | Hospitalized adults ≥16 yrs old with CAP, admitted; Spain | Blood+, pleural fluid+, or PCR (pleural fluid)+ | Sputum+ | 64 | 67/33 | 58.2 (PSI IV/V) | 20.3 | |
| Segonds 2010 (26) | Hospitalized adults >18 yrs old with BinaxNOW-SP test; France | Blood+ or pleural fluid+ | Sputum+, BAL+, or BinaxNOW-SP+ | |||||
| Garcia-Suarez 2007 (32) | Adults with serious community-acquired bacterial infection, S. pneumoniae pneumonia subgroup; Spain | Blood+ or pleural fluid+ | Sputum+ or tracheal aspirate+ | 60 | 64/36 | |||
| Lasocki 2006 (36) | Adults with CAP, admitted to ICU; France | Blood+, sputum+, or microbiology+ from respiratory tract | ND | 69 | 66/34 | 46 (SAPS-II; median) | 70 | |
| Tzeng 2006 (37) | Adults with RTI symptoms; Taiwan | Blood+, pleural fluid+, or sputum+ | ND | |||||
| Lauderdale 2005 (38) | Hospitalized adults >16 yrs old with CAP; Taiwan | Blood+, pleural fluid+, or (sputum+ and BinaxNOW-SP+) | Sputum+ or BinaxNOW-SP+ | 56 | 64/36 | 16 | 1.2 | |
| Ishida 2004 (4) | Adults >15 yrs old hospitalized with CAP; Japan | Blood+ or pleural fluid+ | Sputum+ | 65 | 65/35 | 27 (PSI IV/V) | ||
| Róson 2004 (40) | Adults with CAP, admitted to hospital, nonsevere immunosuppression; Spain | Blood+ or sputum+ | ND | 66 | 71/29 | 35 (PSI IV/V) | 18 | |
| Strålin 2004 (41) | Adults with CAP, admitted to hospital; Denmark | Blood+, sputum+, or nasopharynx+ | ND | 71 | 53/47 | 39 (PSI IV/V) | 27 | |
| Butler 2003 (42) | Adults with febrile respiratory illness, subgroup with CAP; US | Blood+ or culture+ from normally sterile body site | Sputum+ and CXR consolidation | 45 | 70/30 | Excluded | ||
| Marcos 2003 (6) | Adults ≥18 yrs old with CAP, admitted to hospital; Spain | Blood+, pleural fluid+, TBAS+, or BAL+ | Sputum+ | 50 | 79/21 | 21 | ||
| Burel 2001 (44) | Adults with CAP, admitted to hospital; France | Blood+, sputum+, BAL+, tracheal aspirate+, pleural fluid+, or latex agglutination+ | ND | |||||
| Shibli 2010 (23) | Adults ≥18 yrs old with CAP, admitted to hospital; Israel | ND | ND | 58 | 58/42 | Excluded | ||
| Charles 2008 (28) | Hospitalized adults >18 yrs old with CAP; Australia | Blood+, sputum+, or BinaxNOW-SP+ | Sputum+ (without Gram stain+) | 65 | 61/39 | 53.5 (PSI IV/V) | 31 | Excluded |
| Weatherall 2008 (30) | Adults >14 yrs old with CAP; Australia | ND | ND | 79 (median) | 56/44 | 40 (PSI IV/V) | 26 | |
| Diaz 2007 (31) | Hospitalized adults ≥16 yrs old with CAP; Chile | Blood+ or BinaxNOW-SP+ | Sputum+ | 66 | 52/48 | 61 (PSI IV/V) | 33 | Excluded |
| Kobashi 2007 (33) | Adults >15 yrs old with CAP, admitted to hospital; Japan | Blood+, pleural fluid+, or sputum+ | ND | 62 | 71/29 | 26 (PSI IV/V) | 45 | 12 |
| Andreo 2006 (34) | Adults ≥16 yrs old with CAP, admitted to hospital; Spain | Blood+, pleural fluid+, transthoracic needle aspirate+, or BinaxNOW-SP+ | Sputum+ | 59 | 70/30 | 26 | Excluded | |
| Ercis 2006 (35) | Adults with CAP, admitted to hospital; Turkey | Blood+ or sputum+ | ND | 18–86 (range) | 64/36 | 7 | ||
| Genne 2006 (3) | Adults >18 yrs old with CAP, admitted to hospital; Switzerland | Blood+ or (sputum+ or microbiology+ from respiratory tract) | ND | 68 | 57/43 | PSI mean score of 106 | ||
| Van der Eerden 2005 (39) | Hospitalized adults ≥18 yrs old with CAP; Denmark | Blood+, pleural fluid+, or pleural fluid antigen+ | Sputum+ or BinaxNOW-SP+ | 64 | 54/46 | 44.3 (PSI IV/V) | 26 | Excluded |
| Farina 2002 (43) | Adults with CAP, hospitalized; Italy | Blood+ or respiratory specimen+ | ND | |||||
| Murdoch 2001 (45) | Adults with CAP, admitted to hospital; New Zealand | Blood+ or sputum+ | ND | 68 (median) | 51/49 | 76 | ||
| Johansson 2010 (22, 53) | Hospitalized adults with CAP; Sweden | Blood+, pleural fluid+, BAL+, or BinaxNOW-SP+ | Sputum+ | 61 | 51/49 | 22 | ||
| Perello 2010 (25) | Hospitalized adults with HIV; Spain | Blood+ | ND | 43 | 65/35 | 48 (Apache-II score of ≥12 | 100 | |
| Smith 2009 (27) | Hospitalized adults with blood+ or blood− CAP; UK | Blood+ | Clinical CAP with specific features | 63 (median of 67) | ||||
| Hohenthal 2008 (29) | Hospitalized adults ≥16 yrs old with CAP; Finland | Blood+ or pleural fluid+ | BinaxNOW-SP+ or sputum+ | 50 | 52/48 | 23 (PSI IV/V) | 29 | Excluded |
Designations: blood+, positive blood culture; sputum+, positive Gram stain and/or sputum culture; pleural fluid+, positive culture from pleural fluid; nasopharynx+, positive culture from the nasopharynx; BAL+, positive culture from bronchioalveolar lavage fluid; respiratory+, positive culture from any respiratory sample; TBAS+, tracheobronchial aspiration; BinaxNOW-SP+, positive urinary BinaxNOW-SP test (to be included in the meta-analysis, studies had to report sufficient detail to separate these results into true and false positives). Abbreviations: CXR, chest X-ray; ND, not defined; RTI, respiratory tract infection; SAPS, simplified acute physiology score; PSI, pneumonia severity index (54).
Studies are ordered by date of publication within reference standard category.
Risk of bias assessment.
A summary of the risk of bias assessment results are shown in Table 2. Items according to QUADAS-1 were grouped into domains according to the approach of the recently published QUADAS-2 (46). No study met the requirement for a perfect reference standard. In a number of studies, the assessment of risk of bias was affected by unclear reporting. Few studies, even those that were primarily diagnostic in design, explicitly declared blinding of the index test and reference test relative to each other, although in some instances the described workflow (e.g., where the urine test was conducted on a fresh sample in the emergency room) implied blinding of the index test. Timing of the two tests relative to each other, and importantly, relative to the start of antibiotic adminstration, was frequently not described. The risk of bias assessment did not identify a subset of higher-quality studies; therefore, we did not attempt to adjust the meta-analysis on the basis of quality.
Table 2.
Summary of risk of bias in studies reporting diagnosis of S. pneumoniae community-acquired pneumonia based on BinaxNOWa
| First author and year (reference) | Representative patient spectrum?b | Low risk of bias in implementing index test?c | Low risk of bias in implementing reference test?d | Low risk of bias in patient flow?e |
|---|---|---|---|---|
| Sordé 2011 (24) | Yes | No | Yes | No |
| Segonds 2010 (26) | Yes | No | No | No |
| Garcia-Suarez 2007 (32) | Yes | No | Yes | Yes |
| Lasocki 2006 (36) | No (all ICU) | No | No | No |
| Tzeng 2006 (37) | Yes | No | No | No |
| Lauderdale 2005 (38) | Yes | No | No | No |
| Ishida 2004 (4) | Yes | Yes | No | Yes |
| Róson 2004 (40) | Yes, minority ambulatory | No | No | No |
| Stralin 2004 (41) | Yes | Yes | Yes | No |
| Butler 2003 (42) | Yes | No | No | No |
| Marcos 2003 (6) | Yes | No | No | Yes |
| Burel 2001 (44) | Yes | No | No | No |
| Shibli 2010 (23) | Yes | No | No | Yes |
| Charles 2008 (28) | Yes | No | No | No |
| Weatherall 2008 (30) | Yes | Yes | No | Yes |
| Diaz 2007 (31) | Yes | No | No | No |
| Kobashi 2007 (33) | Yes | Yes | No | No |
| Andreo 2006 (34) | Yes | No | No | No |
| Ercis 2006 (35) | Yes | No | No | No |
| Genne 2006 (3) | Yes | No | No | No |
| Van der Eerden 2005 (39) | Yes | No | No | Yes |
| Farina 2002 (43) | Yes | No | No | No |
| Murdoch 2001 (45) | Yes | No | No | Yes |
| Johansson 2010 (22) | Yes | No | No | Yes |
| Perello 2010 (25) | No (all HIV) | No | No | No |
| Smith 2009 (27) | Yes | No | No | No |
| Hohenthal 2008 (29) | Yes | No | No | No |
Studies are ordered by date of publication within reference standard. The response under each column heading is reported as “no” if any one of the constituent questions was answered with a “no.”
Was the spectrum of patients representative of the patients who will receive the test in practice?
Were the reference standard results interpreted without knowledge of the results of the index test? Were the index test results interpreted without knowledge of the results of the reference standard? Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? Were uninterpretable/intermediate test results reported?
Was the reference standard likely to classify the target condition correctly? (In all instances, this was no, as the reference standard was known to be imperfect.) Was the reference standard independent of the index test (i.e., the index test did not form part of the reference standard)? (In all instances this was yes, as independence was one of the inclusion criteria for the meta-analysis.) Were the index test results interpreted without knowledge of the results of the reference standard?
Was the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? Did the whole sample, or a random selection of the sample, receive verification using the intended reference standard? Did patients receive the same reference standard irrespective of the index test result? Were withdrawals from the study explained?
Meta-analysis.
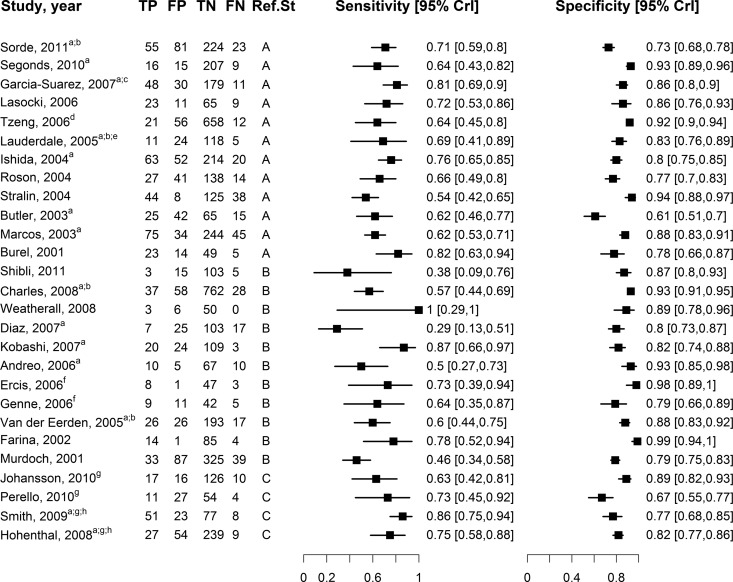
The etiologic agent was identified as S. pneumoniae by either positive sputum Gram stain or positive culture of blood, sputum, or other body fluid in 11 studies (reference standard type A), by either positive sputum Gram stain or positive blood culture in 12 studies (reference standard type B), and by positive blood culture alone in 4 studies (reference standard type C). Sensitivity and specificity estimates with respect to the reference standard in each study ranged from 29% to 100% and 61% to 99%, respectively (Fig. 1). Based on the 12 studies that used reference standard A, sensitivity of BinaxNOW-SP was 68.5% (95% CrI, 62.6% to 74.2%) and specificity was 84.2% (95% CrI, 77.5% to 89.3%). Based on the 11 studies that used reference standard B, sensitivity was 60.3% (95% CrI, 46.4% to 74.4%), and specificity was 89.2% (95% CrI, 82.5% to 94.4%). Finally, based on the four studies with reference standard C, sensitivity was 76.7% (95% CrI, 49.0% to 93·0%) and specificity was 79.6% (95% CrI, 56.3% to 93.1%).
Fig 1.
Forest plot, showing sensitivities and specificites of BinaxNOW-SP with respect to the reference standard in the studies included in our meta-analysis. Studies are ordered by date in descending order and grouped according to reference classes: A (11 studies), B (12 studies), C (4 studies). Footnotes: a, definite and probable S. pneumoniae pneumonia cases were combined into a single category of S. pneumoniae pneumonia; b, authors' definition of S. pneumoniae included a positive BinaxNOW-SP result. and patients diagnosed solely on the basis of a positive BinaxNOW-SP were reclassified as having false-positive results; c, results from the total number of CAP cases derived from the summation of the authors' categories of “pneumococcal infection, pneumonia,” “pneumococcal infection, probable pneumococcal pneumonia,” “nonpneumococcal infections, pneumonia,” and “unknown etiology pneumonia”; d, data included for those patients with lower respiratory tract infections (LRTIs); e, analysis restricted to a subset of patients with complete data; f, data used from those patients with CAP, and data from control patients were omitted; g, complete data to construct a two-by-two table provided only for positive blood culture as a reference standard; h, results for the total number of CAP cases were derived from the summation of the authors' categories of “pneumococcal bacteremia, with pneumonia” and “nonbacteremic pneumonia, combined subtotal.”
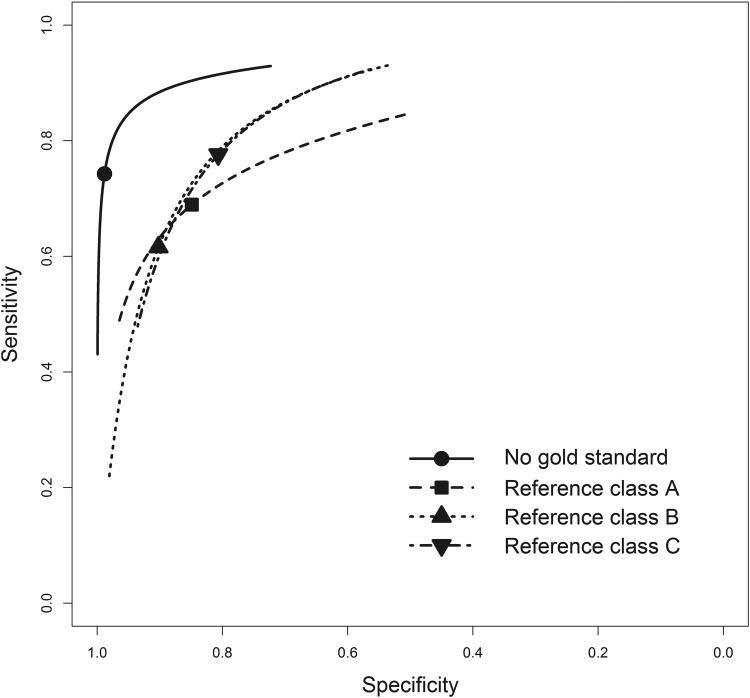
According to our latent class model, based on all 27 studies, the pooled sensitivity of BinaxNOW-SP was 74.0% (95% CrI, 66/6% to 82.3%) and the pooled specificity was 97.2% (95% CrI, 92.5% to 99.8%). Figure 2 provides the summary receiver operating characteristic curve from the different meta-analysis models. As anticipated, assuming the reference standard was perfect resulted in lower estimates of the pooled sensitivity and especially the pooled specificity of BinaxNOW-SP irrespective of the reference standard. The latent class meta-analysis model also provided estimates of the pooled sensitivity and specificity (and 95% credible intervals) of the three reference standards, as follows: (i) reference standard A, sensitivity of 59.4% (43.9% to 76.3%), specificity of 98.6% (95.1% to 99.8%); (ii) reference standard B, sensitivity of 56.2% (35.9% to 80.5%), specificity of 97.4% (93.8% to 99.4%); (iii) reference standard C, sensitivity of 50.3% (24.6% to 78.8%), specificity of 98.3% (91.2% to 99.8%). Figure S2 in the supplemental material shows a forest plot of the latent class model-based estimates of the prevalence of S. pneumoniae and the sensitivities and specificities of BinaxNOW-SP in the studies that were included in the meta-analysis.
Fig 2.
Summary receiver operating curves for BinaxNOW-SP with respect to each reference standard, as well as that based on a latent class model adjusting for the imperfect nature of all three reference standards.
The predicted sensitivity and specificity in a new individual study were 74.3% (95% CrI, 48.8% to 90.9%) and 97.2% (95% CrI, 84.4% to 100.0%), respectively. The 95% credible intervals are much wider than with the pooled estimates, reflecting the heterogeneity among the 27 studies even after adjusting to for differences in the reference standard used (see Fig. S2 in the supplemental material).
Meta-regression analyses that separated diagnostic from etiologic studies, prospective from retrospective studies, and studies within versus outside North America or Europe (on the assumption that seasonal cycles and strains of S. pneumoniae, as well as hospital practice, would be similar within Western institutions), gave similar results and no reduction of heterogeneity. We also considered the effect of institution type, e.g., large urban, tertiary care, or university-associated settings. Although it appeared that there was less heterogeneity in diagnostic (compared to etiologic) studies, in studies using a prospective design and in studies based in a university center there were no statistically significant differences in the pooled sensitivity and specificity between the subgroups. We also examined the effects of prior antibiotic use and average severity of pneumonia in the subgroups of studies that reported these variables, but we did not find any significant effects.
Models adjusting for correlation had a similar fit to the latent class meta-analysis model described above. The best-fitting model adjusting for correlation between BinaxNOW-SP allowed for up to 20% of the maximum correlation. This resulted principally in lowering the pooled sensitivity (67.6% [95% CrI, 58.7% to 77.0%]) but did not affect the pooled specificity (98.1% [95% CrI, 91.8% to 99.9%]).
DISCUSSION
Our systematic review of the medical literature identified 27 studies that reported the sensitivity and specificity of the BinaxNOW test for Streptococcus pneumoniae in hospitalized patients with suspected community-acquired pneumonia. Using a meta-analysis model that adjusted for the lack of a perfect reference test, we estimated the pooled sensitivity of BinaxNOW-SP in the detection of S. pneumoniae infection in patients with CAP to be 74.0% (95% CrI, 66.6% to 82.3%) and the pooled specificity to be 97.2% (95% CrI, 92.5% to 99.8%).
A previous meta-analysis by Boulware et al. (47), which was based on 24 studies and assumed a single perfect reference test, estimated that the pooled sensitivity of BinaxNOW-SP was 74% (95% confidence interval [CI], 72% to 77%) and the pooled specificity was 94% (95% CI, 93% to 95%). Although the pooled results from this earlier meta-analysis may appear numerically similar to the values we estimated from our adjusted model, the two analyses had only 16 studies in common. In addition, Boulware et al. included only those patients in whom etiology had been established, excluding those with an unknown organism, while we included all patients with clinically suspicious CAP, including those with an unknown organism. Finally, the older meta-analysis model used by Boulware et al. did not adjust for the negative correlation between the sensitivity and specificity of BinaxNOW-SP or the imperfect reference standard. This is reflected in the wider credibility intervals around the pooled and predicted sensitivity and specificity obtained with our model.
Compared to more-naive diagnostic meta-analysis models (48), our model allowed for (i) correlations between sensitivity and specificity across studies due to differences in thresholds or diagnostic accuracies, (ii) heterogeneity in BinaxNOW-SP performance between studies due to observed study-level covariates as well as unexplained variation, (iii) the imperfect nature of the reference standard, which would result in higher estimates of both sensitivity and specificity, (iv) three different types of reference standards in individual studies, and (v) heterogeneity in the performance of each type of reference standard across studies.
Based on the input of our expert consultant (our coauthor Marty Teltscher) and a nonsystematic review of the literature (2–4, 6, 7, 49), we determined plausible ranges for the sensitivity and specificity of the three reference standards: reference standard A, sensitivity from 40 to 70%, specificity from 80 to 100%; reference standard B, sensitivity from 30 to 60%, specificity from 80 to 100%; reference standard C, sensitivity from 10 to 40% and specificity from 90 to 100%. These ranges agree well with the estimates of sensitivity and specificity of the reference standard from our latent class model, thus supporting its validity.
Possibility of risk of bias within individual studies.
Recent antibiotic use is known to reduce the diagnostic yield of cultures (7, 9), with S. pneumoniae blood cultures sensitive even to a single dose of antibiotic (7). In the absence of an effect of antibiotics with the BinaxNOW-SP test, this would artificially increase the rate of discordant results for BinaxNOW-SP-positive and culture-negative samples. The effect of prior antibiotic treatment on the sensitivity of the BinaxNOW assay is unclear, with some studies reporting decreased sensitivity (4, 36, 39, 41) while others did not (4, 36, 40, 50).
Some studies differentiated between definite from possible S. pneumoniae, with the former category being restricted to samples from normally sterile sites. For the purposes of this analysis, we combined the two, which potentially increased the rate of discordant culture-positive, BinaxNOW-SP-negative results.
Clinical experience with BinaxNOW-SP.
Two randomized controlled trials (51, 52) and two observational studies (24, 33) have examined the impact of use of BinaxNOW-SP in treatment decision-making on outcomes in hospitalized patients. In the randomized controlled trials, patients were randomized to empirical or targeted therapy, and those receiving targeted therapy who tested positive with BinaxNOW-SP received therapy specific for S. pneumoniae. Neither study showed a difference in important clinical outcomes, although one showed more relapses in the targeted therapy group. Given that patients had to be randomized into the targeted group and test positive via BinaxNOW-SP, the studies were underpowered to detect a difference.
Conclusions.
The higher pooled sensitivity of BinaxNOW-SP compared to culture, and also its high specificity, suggest it could be a useful addition to the workup for diagnosis of community-acquired pneumonia. While this is an important finding, the current work does not address whether rapid diagnosis with BinaxNOW-SP would impact the initial management of CAP patients or changes to the initial management of CAP patients. More research is needed regarding the potential impact of BinaxNOW-SP on clinical practice, particularly in the context of other interventions, such as an antibiotic stewardship program, and taking into account the cost-effectiveness of antibiotics and the longer-term costs of antibiotic resistance and antibiotic-associated Clostridium difficile infection.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the assistance of Ian Schiller, Division of Clinical Epidemiology, McGill University Health Centre Research Institute, Montréal, Quebec, Canada, for assistance with the analyses related to the conditional dependence models and SROC plots.
Nandini Dendukuri holds a salary award from the Fonds de la Recherche en Santé du Québec. This study was originally undertaken in response to a request to the Technology Assessment Unit of the McGill University Health Centre.
We have no conflicts of interest to declare.
Published ahead of print 15 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00137-13.
REFERENCES
- 1. van der Poll T, Opal SM. 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543–1556 [DOI] [PubMed] [Google Scholar]
- 2. Selickman J, Paxos M, File TM, Seltzer R, Bonilla H. 2010. Performance measure of urinary antigen in patients with Streptococcus pneumoniae bacteremia. Diagn. Microbiol. Infect. Dis. 67:129–133 [DOI] [PubMed] [Google Scholar]
- 3. Genne D, Siegrist HH, Lienhard R. 2006. Enhancing the etiologic diagnosis of community-acquired pneumonia in adults using the urinary antigen assay (Binax NOW). J. Infect. Dis. 10:124–128 [DOI] [PubMed] [Google Scholar]
- 4. Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. 2004. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J. Infect. Chemother. 10:359–363 [DOI] [PubMed] [Google Scholar]
- 5. Matta M, Kerneis S, Day N, Lescat M, Buu HA, Varon E, Gutmann L, Mainardi JL. 2010. Do clinicians consider the results of the BinaxNOW Streptococcus pneumoniae urinary antigen test when adapting antibiotic regimens for pneumonia patients? Clin. Microbiol. Infect. 16:1389–1393 [DOI] [PubMed] [Google Scholar]
- 6. Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, Gonzalez J, Martinez E, Garcia E, Mensa J, de Roux A, Torres A. 2003. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur. Respir. J. 21:209–214 [DOI] [PubMed] [Google Scholar]
- 7. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roson B, Carratala J, Verdaguer R, Dorca J, Manresa F, Gudiol F. 2000. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin. Infect. Dis. 31:869–874 [DOI] [PubMed] [Google Scholar]
- 9. Mandell L. 2010. Prospective randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax 65:93–94 [DOI] [PubMed] [Google Scholar]
- 10. Yu VL, Stout JE. 2009. Rapid diagnostic testing for community-acquired pneumonia: can innovative technology for clinical microbiology be exploited? Chest 136:1618–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu VL. 2011. A clinical solution to antimicrobial resistance in community-acquired pneumonia: narrowing the spectrum of antimicrobial therapy. Arch. Intern. Med. 171:172–173 [DOI] [PubMed] [Google Scholar]
- 12. Hadgu A, Dendukuri N, Hilden J. 2005. Evaluation of nucleic acid amplification tests in the absence of a perfect gold-standard test: a review of the statistical and epidemiologic issues. Epidemiology 16:604–612 [DOI] [PubMed] [Google Scholar]
- 13. Dendukuri N, Schiller I, Joseph L, Pai M. 2012. Bayesian meta-analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference. Biometrics 68:1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klugman KP, Madhi SA, Albrich WC. 2008. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin. Infect. Dis. 47(Suppl 3):S202–S206 [DOI] [PubMed] [Google Scholar]
- 15. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. 2003. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutter CM, Gatsonis CA. 2001. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 20:2865–2884 [DOI] [PubMed] [Google Scholar]
- 17. Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. 2010. Analysing and presenting results. In Deeks JJ, Bossuyt PM, Gatsonis C. (ed), Cochrane handbook for systematic reviews of diagnostic test accuracy, version 1.0. The Cochrane Collaboration; http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/Chapter 10 - Version 1.0.pdf [Google Scholar]
- 18. Torrance-Rynard VL, Walter SD. 1997. Effects of dependent errors in the assessment of diagnostic test performance. Stat. Med. 16:2157–2175 [DOI] [PubMed] [Google Scholar]
- 19. Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. 2002. Bayesian measures of model complexity and fit (with discussion). J. R. Stat. Soc. B Stat. Methodol. 64:583–639 [Google Scholar]
- 20. Lunn DJ, Thomas A, Best N, Spiegelhalter D. 2000. WinBUGS. A Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 10:325–337 [Google Scholar]
- 21. Development Core Team R 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 22. Johansson N, Kalin M, Annika Giske T-LCG, Hedlund J. 2010. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibli F, Chazan B, Nitzan O, Flatau E, Edelstein H, Blondheim O, Raz R, Colodner R. 2010. Etiology of community-acquired pneumonia in hospitalized patients in northern Israel. Isr. Med. Assoc. J. 12:477–482 [PubMed] [Google Scholar]
- 24. Sorde R, Falco V, Lowak M, Domingo E, Ferrer A, Burgos J, Puig M, Cabral E, Len O, Pahissa A. 2011. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch. Intern. Med. 171:166–172 [DOI] [PubMed] [Google Scholar]
- 25. Perelló R, Miró O, Marcos MA, Almela M, Bragulat E, Sánchez M, Agustí C, Miro JM, Moreno A. 2010. Predicting bacteremic pneumonia in HIV-1-infected patients consulting the ED. Am. J. Emerg. Med. 28:454–459 [DOI] [PubMed] [Google Scholar]
- 26. Segonds C, Le GA, Chabanon G. 2010. Assessment of the contribution of the immunochromatographic pneumococcal urinary antigen test to the etiological diagnosis of pneumonia in hospitalized adults. Pathol. Biol. (Paris) 58:117–122 (In French.) [DOI] [PubMed] [Google Scholar]
- 27. Smith MD, Sheppard CL, Hogan A, Harrison TG, Dance DA, Derrington P, George RC. 2009. Diagnosis of Streptococcus pneumoniae infections in adults with bacteremia and community-acquired pneumonia: clinical comparison of pneumococcal PCR and urinary antigen detection. J. Clin. Microbiol. 47:1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, Holmes PW, Christiansen KJ, Waterer GW, Pierce RJ, Mayall BC, Armstrong JG, Catton MG, Nimmo GR, Johnson B, Hooy M, Grayson ML. 2008. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin. Infect. Dis. 46:1513–1521 [DOI] [PubMed] [Google Scholar]
- 29. Hohenthal U, Vainionpää R, Meurman O, Vahtera A, Katiskalahti T, Nikoskelainen J, Kotilainen P. 2008. Aetiological diagnosis of community acquired pneumonia: utility of rapid microbiological methods with respect to disease severity. Scand. J. Infect. Dis. 40:131–138 [DOI] [PubMed] [Google Scholar]
- 30. Weatherall C, Paoloni R, Gottlieb T. 2008. Point-of-care urinary pneumococcal antigen test in the emergency department for community acquired pneumonia. Emerg. Med. J. 25:144–148 [DOI] [PubMed] [Google Scholar]
- 31. Díaz A, Barria P, Niederman M, Restrepo MI, Dreyse J, Fuentes G, Couble B, Saldias F. 2007. Etiology of community-acquired pneumonia in hospitalized patients in Chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest 131:779–787 [DOI] [PubMed] [Google Scholar]
- 32. del Mar García-Suárez M, Cima-Cabal MD, Villaverde R, Espinosa E, Falguera M, de Los Toyos JR, Vázquez F, Méndez FJ. 2007. Performance of a pneumolysin enzyme-linked immunosorbent assay for diagnosis of pneumococcal infections. J. Clin. Microbiol. 45:3549–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobashi Y, Yoshida K, Miyashita N, Niki Y, Matsushima T. 2007. Evaluating the use of a Streptococcus pneumoniae urinary antigen detection kit for the management of community-acquired pneumonia in Japan. Respiration 74:387–393 [DOI] [PubMed] [Google Scholar]
- 34. Andreo F, Domínguez J, Ruiz J, Blanco S, Arellano E, Prat C, Morera J, Ausina V. 2006. Impact of rapid urine antigen tests to determine the etiology of community-acquired pneumonia in adults. Respir. Med. 100:884–891 [DOI] [PubMed] [Google Scholar]
- 35. Ercis S, Ergin A, Sahin GO, Hascelik G, Uzun O. 2006. Validation of urinary antigen test for Streptococcus pneumoniae in patients with pneumococcal pneumonia. Jpn. J. Infect. Dis. 59:388–390 [PubMed] [Google Scholar]
- 36. Lasocki S, Scanvic A, Le Turdu F, Restoux A, Mentec H, Bleichner G, Sollet JP. 2006. Evaluation of the Binax NOW Streptococcus pneumoniae urinary antigen assay in intensive care patients hospitalized for pneumonia. Intensive Care Med. 32:1766–1772 [DOI] [PubMed] [Google Scholar]
- 37. Tzeng DH, Lee YL, Lin YH, Tsai CA, Shi ZY. 2006. Diagnostic value of the Binax NOW assay for identifying a pneumococcal etiology in patients with respiratory tract infection. J. Microbiol. Immunol. Infect. 39:39–44 [PubMed] [Google Scholar]
- 38. Lauderdale TL, Chang FY, Ben RJ, Yin HC, Ni YH, Tsai JW, Cheng SH, Wang JT, Liu YC, Cheng YW, Chen ST, Fung CP, Chuang YC, Cheng HP, Lu DC, Liu CJ, Huang IW, Hung CL, Hsiao CF, Ho M. 2005. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir. Med. 99:1079–1086 [DOI] [PubMed] [Google Scholar]
- 39. Van Der Eerden MM, Vlaspolder F, De Graaff CS, Groot T, Jansen HM, Boersma WG. 2005. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 24:241–249 [DOI] [PubMed] [Google Scholar]
- 40. Rosón B, Fernández-Sabé N, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. 2004. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin. Infect. Dis. 38:222–226 [DOI] [PubMed] [Google Scholar]
- 41. Stralin K, Kaltoft MS, Konradsen HB, Olcen P, Holmberg H. 2004. Comparison of two urinary antigen tests for establishment of pneumococcal etiology of adult community-acquired pneumonia. J. Clin. Microbiol. 42:3620–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butler JC, Bosshardt SC, Phelan M, Moroney SM, Tondella ML, Farley MM, Schuchat A, Fields BS. 2003. Classical and latent class analysis evaluation of sputum polymerase chain reaction and urine antigen testing for diagnosis of pneumococcal pneumonia in adults. J. Infect. Dis. 187:1416–1423 [DOI] [PubMed] [Google Scholar]
- 43. Farina C, Arosio M, Vailati F, Moioli F, Goglio A. 2002. Urinary detection of Streptococcus pneumoniae antigen for diagnosis of pneumonia. New Microbiol. 25:259–263 [PubMed] [Google Scholar]
- 44. Burel E, Dufour P, Gauduchon V, Jarraud S, Etienne J. 2001. Evaluation of a rapid immunochromatographic assay for detection of Streptococcus pneumoniae antigen in urine samples. Eur. J. Clin. Microbiol. Infect. Dis. 20:840–841 [DOI] [PubMed] [Google Scholar]
- 45. Murdoch DR, Laing RT, Mills GD, Karalus NC, Town GI, Mirrett S, Reller LB. 2001. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J. Clin. Microbiol. 39:3495–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. 2011. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155:529–536 [DOI] [PubMed] [Google Scholar]
- 47. Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. 2007. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J. Infect. 55:300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gatsonis C, Paliwal P. 2006. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. Am. J. Roentgenol. 187:271–281 [DOI] [PubMed] [Google Scholar]
- 49. Musher DM, Montoya R, Wanahita A. 2004. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 39:165–169 [DOI] [PubMed] [Google Scholar]
- 50. Porcel JM, Ruiz-González A, Falguera M, Nogués A, Galindo C, Carratalá J, Esquerda A. 2007. Contribution of a pleural antigen assay (Binax NOW) to the diagnosis of pneumococcal pneumonia. Chest 131:1442–1447 [DOI] [PubMed] [Google Scholar]
- 51. Falguera M, Ruiz-González A, Schoenenberger JA, Touzón C, Gázquez I, Galindo C, Porcel JM. 2010. Prospective, randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax 65:101–106 [DOI] [PubMed] [Google Scholar]
- 52. van der Eerden MM, Vlaspolder F, de Graaff CS, Groot T, Bronsveld W, Jansen HM, Boersma WG. 2005. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax 60:672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johansson N, Kalin M, Giske CG, Hedlund J. 2008. Quantitative detection of Streptococcus pneumoniae from sputum samples with real-time quantitative polymerase chain reaction for etiologic diagnosis of community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 60:255–261 [DOI] [PubMed] [Google Scholar]
- 54. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243–250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.