Abstract
Staphylococcus aureus capsular polysaccharides (CP) are important virulence factors and represent putative targets for vaccine development. Therefore, the purpose of this study was to develop a high-throughput method to identify and discriminate the clinically important S. aureus capsular serotypes 5, 8, and NT (nontypeable). A comprehensive set of clinical isolates derived from different origins and control strains, representative for each serotype, were used to establish a CP typing system based on Fourier transform infrared (FTIR) spectroscopy and chemometric techniques. By combining FTIR spectroscopy with artificial neuronal network (ANN) analysis, a system was successfully established, allowing a rapid identification and discrimination of all three serotypes. The overall accuracy of the ANN-assisted FTIR spectroscopy CP typing system was 96.7% for the internal validation and 98.2% for the external validation. One isolate in the internal validation and one isolate in the external validation failed in the classification procedure, but none of the isolates was incorrectly classified. The present study demonstrates that ANN-assisted FTIR spectroscopy allows a rapid and reliable discrimination of S. aureus capsular serotypes. It is suitable for diagnostic as well as large-scale epidemiologic surveillance of S. aureus capsule expression and provides useful information with respect to chronicity of infection.
INTRODUCTION
Staphylococcus aureus capsular polysaccharide (CP) expression is known to be an essential cell surface-associated virulence factor that exhibits immunogenic properties. It protects the bacteria from phagocytic clearance and augments microbial virulence (1, 2). Although various S. aureus CP serotypes have been described during the last 3 decades, prototype strains and antisera are available only for serotypes 1 (CP1), 2 (CP2), 5 (CP5), and 8 (CP8) (3, 4). The heavily encapsulated CP1 and CP2 strains are very rare among clinical isolates, and their mucoid colonies are easily recognized on solid medium. Most human S. aureus strains express either a CP5 or CP8 polysaccharide capsule. Therefore, CP5- and CP8-specific antibodies are routinely used for S. aureus capsular serotyping. However, approximately 8% to 30% of human isolates (5–9) and up to 86% of bovine isolates (10) do not express either CP5 or CP8, although almost all of them carry the respective genes coding for CP5 or CP8 (4). S. aureus isolates that do not react with antibodies to serotype CP5 and CP8 and do not produce mucoid colonies on solid agar medium (CP1 and CP2) are subsumed under the term “nontypeable” (NT) (4, 11). Interestingly, almost all NT isolates seem to react with surface antigen 336-specific antibodies (12, 13). Originally, antigen 336 was proposed to be a capsular polysaccharide, but meanwhile, it has been shown that antigen 336 is a poly(ribitol phosphate)-N-acetylglucosamine-containing polymer, resembling cell wall teichoic acid rather than capsule polysaccharides (14, 15). The prevalence of nonencapsulated NT strains was found to be higher in chronic than in acute infections (10, 11, 16, 17). The potential increase of S. aureus infections caused by NT serotypes is therefore of special concern. Continuous monitoring of changes in CP serotype prevalence and loss of CP expression is of utmost importance for epidemiological studies as well as for the development of future vaccines (12, 18).
Currently, the gold standard for S. aureus capsule serotyping is the double immunodiffusion assay, in which capsular extracts react against CP5- or CP8-specific antibodies (19). Alternative methods, such as colony immunoblotting or enzyme-linked immunosorbent assay inhibition, are less sensitive but allow processing of multiple samples in a shorter period of time (20, 21). However, the implementation of these immunoassays is limited to a small number of laboratories. All of them are based on the availability of CP5- and CP8-specific antibodies, which are not commercially available and require access to appropriate S. aureus strains for immunization and animal experiments. In addition, these methods are rather complex to perform and require specifically trained personal. Novel suitable methods for CP serotyping to be implemented in routine diagnostics as well as for epidemiological purposes, requiring high-throughput capacities, are urgently needed.
Metabolic fingerprinting by Fourier transform infrared (FTIR) spectroscopy represents an interesting alternative for a rapid and cost-effective discrimination of S. aureus capsular serotypes. FTIR spectroscopy coupled to chemometric analysis has been widely used for identification, classification, and epidemiological typing of numerous clinic- and food-derived pathogenic microorganisms (22–26). Subspecies differentiation is probably the most promising aspect of FTIR spectroscopy, as it is capable of monitoring conformational, compositional, and quantitative differences of biochemical compounds in microbial cells. The metabolic fingerprints generated by FTIR spectroscopy reflect the balance of all these factors based on inherent strain-specific differences of the genetic and phenotypic background. For instance, FTIR spectroscopy has been successfully employed to differentiate serotypes of Escherichia coli (27), Salmonella enterica (28), Listeria monocytogenes (29), and Yersinia enterocolitica (30). The current study aimed to decipher the potential of FTIR spectroscopy for differential diagnostics of the most clinically relevant S. aureus capsular polysaccharide types, namely, CP5, CP8, and the NT variants.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A panel of 84 S. aureus strains isolated from diverse clinical settings was compiled for this study (Table 1). Fifty-three of the isolates were derived from humans, whereas 31 had animal origin. S. aureus Reynolds prototype strain CP5 and its isogenic mutants Reynolds CP8 and Reynolds CP− (nonencapsulated) (31) were used as controls for geno- and serotyping as well as for FTIR spectroscopic biotyping. S. aureus was propagated on Columbia agar (Difco) supplemented with 2% NaCl for 24 h at 37°C for the preparation of the capsule extract used for the determination of the CP expression by immunoassays. To assess the culture conditions that permit the highest discrimination power between the different S. aureus CP types by means of FTIR spectroscopy, the following conditions were tested: (i) tryptone soy agar (TSA; Oxoid) at 30°C for 24 h, (ii) TSA at 37°C for 24 h, (iii) TSA supplemented with 2% NaCl at 37°C for 24 h, and (iv) Columbia base agar supplemented with 2% NaCl at 37°C for 24 h. NaCl was added because it is known to enhance CP production (32, 33). For all subsequent FTIR spectroscopic biotyping experiments (including the measurement of the capsule extracts) and establishment of the ANN-assisted FTIR spectroscopic typing system, bacterial strains were cultured on TSA plates for 24 h at 30°C.
Table 1.
S. aureus isolates used in this study
| Capsule serotype | No. of isolates: |
||||||
|---|---|---|---|---|---|---|---|
| Total | With each cap allele |
With each origin |
|||||
| cap5 | cap8 | None | Human | Animal | Control | ||
| CP5 | 23 | 23 | 0 | 0 | 18 | 4 | 1 |
| CP8 | 27 | 0 | 27 | 0 | 18 | 8 | 1 |
| NT | 37 | 24 | 11 | 2 | 17 | 19 | 1 |
| Total | 87 | 47 | 38 | 2 | 53 | 31 | 3 |
Capsule extracts.
Capsule extracts from S. aureus isolates were prepared as previously described (34). Briefly, the complete bacterial lawn from one 9-cm-diameter agar plate was harvested using 1 ml of 10 mM phosphate-buffered saline (PBS) (0.15 M NaCl, pH 7.2) and the cell suspensions were subsequently autoclaved for 1 h at 121°C. Bacteria were pelleted by centrifugation at 10,000 × g. The supernatants containing the crude capsule extract were passed through 0.45-μm filters and subjected directly to FTIR spectroscopic analysis, used for CP type-specific antibody production, or utilized for a double immunodiffusion assay.
Immune-based detection of CP expression.
The CP serotype of the isolates was determined by a colony immunoblotting assay using antibodies specific for CP5 and CP8 (34). CP5 and CP8 antibodies were produced by immunization of rabbits with killed encapsulated bacteria as described previously (34), followed by absorption of the sera with unencapsulated mutant strains to render it specific to either CP5 or CP8. Preimmune rabbit serum and absorbed sera were nonreactive by immunodiffusion against bacterial extracts containing CP5 and CP8. Isolates that were nonreactive in the colony immunoblot assay were confirmed to be NT by the double immunodiffusion assay, using the capsule extracts as previously described (35). Briefly, a 1% agarose gel was prepared on a glass slide and wells were punched in a circular fashion around a central hole. Absorbed CP5 and CP8 antiserum was added to the central well, and serial dilutions of bacterial extracts were applied to the outer wells (20). Immunodiffusion was conducted in a moist chamber at room temperature. After 24 h, the precipitin lines formed were visualized by Coomassie brilliant blue staining. CP extracts from Reynolds CP5, Reynolds CP8, and Reynolds CP− strains were used as positive and negative controls.
cap gene determination.
The presence of the cap genes that define the CP5 and CP8 serotypes was determined by PCR amplification using the primers and conditions described elsewhere (15, 17). S. aureus Reynolds CP5, Reynolds CP8, and Becker (CP8) were used as controls.
Sample preparation for FTIR spectroscopy.
For whole-cell FTIR spectroscopy, bacteria were grown as lawn on TSA agar plates as indicated above. One loopful of bacterial biomass was suspended in 100 μl of sterile deionized water (36). An aliquot of 30 μl of the resulting bacterial suspension was spotted on a zinc selenite (ZnSe) optical plate (sample holder) and dried at 40°C for 40 min to yield transparent films, which were used directly for FTIR spectroscopy. For FTIR spectroscopy of capsule extracts, 30 μl of the crude capsule extracts was spotted on the sample holder, dried at 40°C for 40 min, and subsequently measured as described below.
FTIR spectroscopic measurement and spectral preprocessing.
FTIR spectroscopy was conducted with an HTS-XT microplate adapter coupled to a Tensor 27 FTIR spectrometer (Bruker Optics GmbH, Ettlingen, Germany). Infrared spectra were recorded in transmission mode in the spectral range between 4,000 and 500 cm−1. The OPUS software (version 6.5; Bruker Optics GmbH) was used to calculate 2nd derivatives of the original spectra with a 9-point Savitzky-Golay filter to increase spectral resolution and to minimize problems with baseline shifts. Subsequent vector normalization was performed for the whole spectral range to adjust biomass variations among different sample preparations. As a measure of dissimilarity, spectral distance values were calculated in the spectral range of 1,200 to 800 cm−1 in order to determine the best growth conditions for CP discrimination and to obtain the levels of reproducibility among the 10 replicates of each isolate used in the reference strain set (27).
Unsupervised multivariate statistics.
The OPUS software was used for hierarchical cluster analysis (HCA). Only the spectral region that offers the maximum information and discriminatory power for characteristic cellular macromolecules was examined. Normalized 2nd-derivative spectra of the polysaccharide region (1,200 to 900 cm−1) and partially of the “fingerprint” region (900 to 800 cm−1) were selected (22). Dendrograms were generated by using Ward's algorithm with normalization to repro level 30. For principal component analysis (PCA)—disregarding groups—spectral data from these regions were exported to XLSTAT for MS Excel (Addinsoft). By eigenvalue decomposition of a data covariance (n) matrix, a set of correlated variables was reduced to two linear combinations of these variables (principal components PC1 and PC2).
Artificial neuronal network analysis.
To develop and to validate the ANN for discrimination between CP5, CP8, and NT, the software NeuroDeveloper (version 2.5b; SynthonGmbH, Heidelberg, Germany) was used (37). The collection of 87 S. aureus strains was subdivided into two strain sets: 30 strains served as a reference data set (including Reynolds control strains), and 57 strains were used for external validation. The reference data set used to calibrate the model included 10 representative isolates of each serotype, which were measured 10 times at different days to cover the biological and technical variance. Spectra were randomly divided into a training set, a prevalidation set, and a test set for internal validation. The training set comprises eight out of the 10 recorded spectra, and the test set and the validation set comprise one spectrum per strain each. The strains used exclusively for external validation were measured one time, simulating routine diagnostic procedures. Prior to the training process, spectral preprocessing and feature selection in the FeatureDeveloper section of the software were performed according to the following parameters: 2nd-derivative spectral processing (Savitzky-Golay), vector normalization, and feature selection in the spectral windows of 3,000 to 2,800 cm−1 and 1,800 to 500 cm−1 using the COVAR algorithm to identify the 100 most discriminative wavenumbers. In the NeuroSimulator section, the Rprop algorithm was used and the training was stopped when the validation error was at its minimum. By automatically changing the numbers of input and hidden neurons, the ANN training was successively optimized until a correct classification result of >90% of the spectra for the internal validation was achieved. The classification procedure of unknown samples is based on three analysis functions: WTA (winner takes all), the 40-20-40 rule, and a potential extrapolation. To accept classification, at least two of those three criteria have to be achieved (37). Using a trained ANN model, results of measured spectra are finally classified as either (i) correct identification, (ii) incorrect identification, or (iii) unknown classification.
RESULTS AND DISCUSSION
Selection of the strain set and optimization of growth conditions for FTIR spectroscopic capsular biotyping.
A comprehensive set of strains, including human and veterinary clinical isolates, was compiled (Table 1). All strains were initially serotyped by colony immunoblot, and all NT isolates, showing no immunoreactivity against CP5 and CP8 antibodies, were additionally tested by the more-sensitive double immunodiffusion assay. S. aureus Reynolds CP5 and its isogenic mutants Reynolds CP8 and Reynolds CP− were included as control strains for CP expression (31). A reference strain set was defined, comprising a representative selection of 10 isolates of each CP type (including the Reynolds and its isogenic mutants). For all isolates, the genetic background of the cap-specific allele was determined. cap allele determination of NT isolates showing no CP expression revealed that they carry either the cap allele cap5 or cap8 or none or both alleles. From the 37 NT isolates, 65% carried the cap5 allele and 30% carried the cap8-specific allele. The remaining isolates (5%) did not show any amplification products with the cap allele-specific primers (Table 1). NT isolates for the reference strain set were selected in such a way that the different cap genetic backgrounds were well represented.
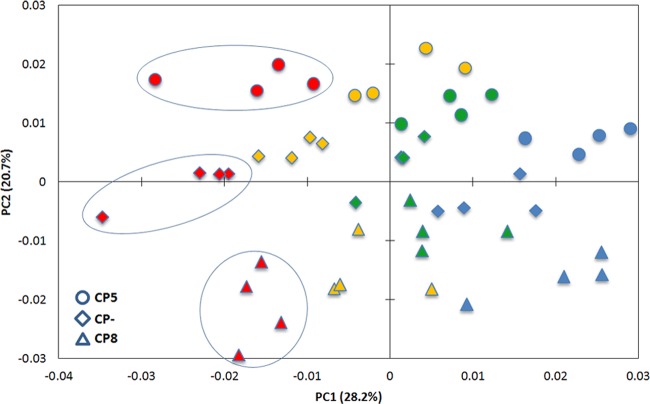
The control strains Reynolds CP5, CP8, and CP− were used to determine the culture condition providing the highest discriminatory power for differentiation of CP types by FTIR spectroscopy. Strains were grown for 24 h under the following conditions: (i) TSA at 30°C, (ii) TSA at 37°C, (iii) TSA supplemented with 2% NaCl at 37°C, and (iv) Columbia base agar supplemented with 2% NaCl at 37°C. Bacteria were subsequently subjected to FTIR spectroscopic analysis (for details, see Materials and Methods). TSA at 30°C (condition i) was included since this culture condition is routinely used for bacterial species identification by means of FTIR spectroscopy (38) and has the advantage of allowing species identification and CP biotyping of S. aureus strains using a single FTIR spectrum, recorded only once. Columbia base agar supplemented with 2% NaCl at 37°C (condition iv) was included in the set of tested cultivation conditions because it is well established for immune-based capsule serotyping. Four independent experiments were performed for each growth condition. PCA, an unsupervised multivariate statistical method, was employed to investigate the discriminatory features of the different growth conditions for S. aureus capsule typing. The score plot for PC1 and PC2 revealed a clear clustering of spectral data according to growth conditions and S. aureus CP types, respectively. As shown in Fig. 1, all growth conditions tested are suitable for discrimination of the Reynolds CP5, CP8, and CP− strains. However, the distance between the different strains depended upon the chosen growth conditions. The determination of spectral distance values, which are a measure of dissimilarity corresponding to the nonoverlapping areas of the spectra, confirmed these findings: (i) TSA at 30°C, 0.55; (ii) TSA at 37°C, 0.33; (iii) TSA supplemented with 2% NaCl at 37°C, 0.41; and (iv) Columbia base agar supplemented with 2% NaCl at 37°C, 0.36. Hence, for FTIR spectroscopy-based CP determination, TSA growth medium is superior to Columbia agar base and a growth temperature of 30°C revealed a better resolution than 37°C. Overall, the best discrimination between the different CP types was achieved for growth on TSA at 30°C (Fig. 1; depicted in red), and these conditions were selected for developing the FTIR capsule serotyping system.
Fig 1.
PCA was performed on 2nd-derivative and vector-normalized spectra in the spectral range of 1,200 to 800 cm−1 recorded from the Reynolds CP5 strain and its isogenic capsule mutants CP8 and CP− grown at the following conditions: (i) TSA at 30°C and 24 h (red), (ii) TSA at 37°C and 24 h (green), (iii) TSA and 2% NaCl at 37°C and 24 h (yellow), and (iv) Columbia base and 2% NaCl at 37°C and 24 h (blue). PC1 was plotted against PC2. PC1 shows a distinct separation of the different growth conditions, whereas PC2 discriminates the Reynolds CP5, CP8, and CP− strains.
Reliable discrimination between encapsulated S. aureus CP5 and CP8 by FTIR spectroscopy using HCA.
Hierarchical cluster analysis (HCA) was employed to assess the overall similarity between isolates of the different CP serotypes. In order to extract spectroscopically relevant information and to increase the discriminatory power, the whole recorded spectral range of 4,000 to 500 cm−1 was limited to 1,200 to 800 cm−1. This spectral region contains two blocks of relevant biochemical information: (i) 1,200 to 900 cm−1, the polysaccharide region, and (ii) 900 to 800 cm−1, which forms part of the so-called “fingerprint” region (22). The polysaccharide region is dominated by C-O-C and C-O-P stretching vibrations of polysaccharides and has been used previously to discriminate the E. coli serotypes O:123 and O:4 (27) as well as for the determination of L. monocytogenes serogroups (29). Several spectral differences in this spectral region were also observed between CP5 and CP8 strains. The most prominent differences were located at 1,110 cm−1, 1,097 cm−1, 1,070 cm−1, 1,058 cm−1, 1,030 cm−1, and 975 cm−1 (data not shown). However, it is still unclear which cellular components are responsible for the differences observed.
A detailed manual analysis of the 2nd derivative of the recorded spectra from the different S. aureus strains revealed a highly discriminatory spectral window in the “fingerprint” region, not yet known to be suitable for serotyping. The spectral region of 845 to 810 cm−1 especially showed remarkable differences between CP5 and CP8 strains (Fig. 2a). Specific spectral differences between the two groups are found at 834 cm−1 and 823 cm−1. The same spectral wavenumbers are also highly discriminatory for the encapsulated Reynolds strain (CP5) and its isogenic mutant CP8 (Fig. 2b). In order to gain a better insight into this highly discriminatory spectral region and to test if these differences can be assigned to CP, spectra of the crude polysaccharide capsule extract from the Reynolds strain and its isogenic mutant CP8 were analyzed by FTIR spectroscopy (Fig. 2c) and compared with spectra obtained from intact cells. Since the highest discriminatory power for differentiation of the two serotypes was achieved for the capsule extract, it can be assumed that the major discriminatory components in the spectral range of 845 to 810 cm−1 for discrimination of CP5 and CP8 are related to capsule compounds. Interestingly, it has been described that the spectral region at 860 to 825 cm−1 is specific for an α-anomeric configuration of carbohydrates (39). An anomeric region absorption band located at 834 cm−1 has been reported to be characteristic for α-glycosidic linkage of aldopyranoses in carbohydrates (40). Therefore, it is tempting to speculate that the highly discriminatory spectral band at 834 cm−1 relates to different specific and structural α-and β-glycosidic linkages in S. aureus capsules. And indeed, a specific α-glycosidic linkage type of the N-acetyl-d-fucosamine (D-FucNAc) residues has been previously described for CP8 capsular polysaccharides (41, 42) as follows: type 5, →4)-β-d-ManNAcA-(1→4)-α-l-FucNAc(3OAc)-(1→3)-β-d-FucNAc-(1→, and type 8, →3)-β-d-ManNAcA(4OAc)-(1→3)-α-l-FucNAc-(1→3)-α-d-FucNAc-(1→.
Fig 2.
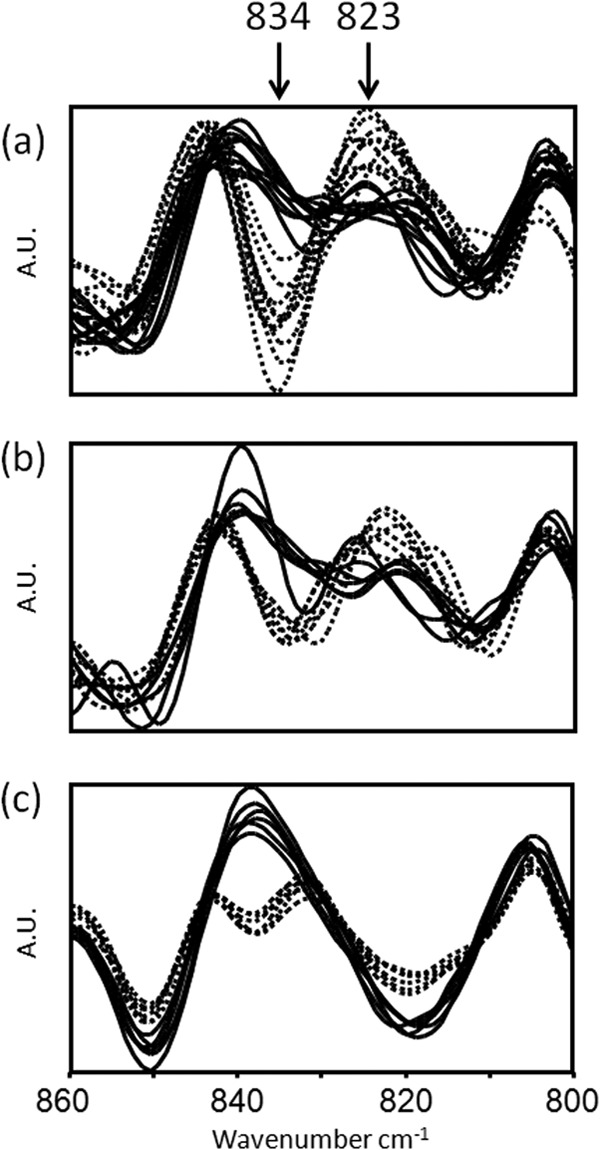
FTIR spectra of S. aureus intact cells and capsule extracts in the spectral range of 860 to 800 cm−1, showing the discrimination between CP5 (solid line) and CP8 (dashed line) at 834 cm−1 and 823 cm−1. All isolates were grown under standardized conditions on TSA at 30°C for 24 h. (a) Clinical CP5 (n = 10) and CP8 (n = 10) isolates included in the reference data set. Whole bacteria. (b) Reynolds prototype strain CP5 and its isogenic mutant CP8. Whole bacteria. (c) Reynolds prototype strain CP5 and its isogenic mutant CP8. Crude capsule extracts. (b and c) Spectral data derived from three independent experiments. Each experiment was performed in technical duplicates. A.U., arbitrary units.
As shown in the dendrogram depicted in Fig. 3, HCA of the 2nd derivatives of FTIR spectral data obtained from the compiled S. aureus strain set revealed two major clusters. Cluster A covers all CP5 strains and cluster B covers all CP8 strains, while the NT isolates are interspersed between the clusters. Subcluster A2 is predominated by NT isolates with different cap gene allelic backgrounds (NTcap5, -cap8, and -nt), but some NT isolates are clustered to the CP5 subcluster A1 (one isolate of NTcap5) and to the CP8 subcluster B2 (two isolates of NTcap8).
Fig 3.
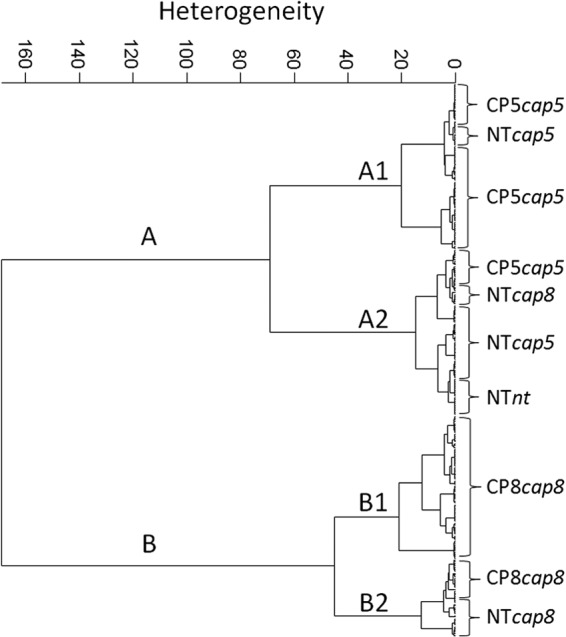
FTIR spectroscopy-based dendrogram of the reference strain set, comprising 10 isolates from serotypes CP5, CP8, and NT. Second derivatives were calculated from 10 replicate spectra of each isolate with a 9-point Savitzky-Golay filter, and subsequent vector normalization was performed for the whole spectral range. Dendrograms were obtained using Ward's algorithm in the spectral range of 1,200 to 800 cm−1 at repro level 30.
Based on their metabolic fingerprints, generated by FTIR spectroscopy, encapsulated CP5 and CP8 strains can be clearly discriminated from each other. But these findings also show that HCA alone is insufficient to discriminate between encapsulated CP5/CP8 and nonencapsulated NT isolates carrying the respective cap5 or cap8 allele or none or both alleles. With respect to implementation in routine diagnostics, an artificial neural network (ANN)-assisted method would also simplify the readout of results because data are clearly classified by the latter method (37).
Identification of S. aureus CP5, CP8, and NT using ANN-assisted FTIR spectroscopy.
It has been shown that the discriminatory power of FTIR spectroscopy can significantly be improved by combining it with appropriate chemometrics, especially by employing supervised learning methods, such as ANNs (see, for instance, references 24, 30, and 43). We therefore used an ANN-based approach to optimize our FTIR spectroscopic serotyping system. To establish the ANNs, a panel of human and veterinary clinical strains with known serotypes was compiled (for details, see Materials and Methods). ANNs are simplified models of neural learning processes, which simulate the behavior of biological neural nets. For the used feed-forward three-layer ANN, the information propagates from the input layer, through the hidden layers, to the output layers. The ANN in our study was trained with selected spectral signatures as input data (input neurons) paired with the predefined output classes CP5, CP8, and NT (output neurons). During the iterative training process, the connection weights (numbers of input and hidden neurons) are automatically adjusted until the global error is minimized (44). In this work, several single ANNs and consecutive combinations of ANNs (modular ANNs) were tested to achieve an optimal network performance. The ANN training resulted in a single-level ANN using the 25 most discriminative wavenumbers (input neurons), three hidden neurons, and three output neurons, one for each CP type. For internal validation, one randomly selected spectrum of each isolate from the training set (n = 30) was tested for the correctness of the classification. For 29 out of 30 strains used for the internal validation, a correct classification was achieved. One strain yielded an uncertain result, but it was not incorrectly classified. To assess the ANN performance, an external validation was performed (Table 2). The external validation comprises only isolates (n = 57) which were not included in the training set to develop the ANN method. To simulate routine diagnostic procedures, strains were measured one time for the external validation. In total, 98.2% (56/57) of the isolates were correctly identified. One isolate was not able to be clearly assigned to one of the three CP types (1.8%), but none of the isolates were incorrectly identified. In particular, the strain showing an unknown classification was a nonencapsulated NT isolate.
Table 2.
Results of external validation of the ANN-assisted FTIR spectroscopy CP typing system
| Capsule serotype | No. of isolates | % with correct identification | % with incorrect identification | % unknown |
|---|---|---|---|---|
| CP5 | 13 | 100 | 0 | 0 |
| CP8 | 17 | 100 | 0 | 0 |
| NT | 27 | 96.3 | 0 | 3.7 |
| Total | 57 | 98.2 | 0 | 1.8 |
In general, similar results for internal (96.7%) and external (98.2%) validation of the ANN are a good indicator for the stability of the established ANN model. It also indicates that a significant part of the microbial diversity was covered by the selected strain set (43). This underpins the reliability and robustness of the established typing system.
CP serotyping is commonly performed in combination with cap allele determination, requiring about 3 days for the immune-based detection of CP expression. Usually, isolates are first serotyped by colony immunoblot (1 day) and NT isolates are subsequently confirmed by the double immunodiffusion assay (2 days). FTIR spectroscopy-based capsule biotyping requires only 1 day for growth of bacteria and less than 5 min of sample preparation per strain. In addition, time and resources for animal experiments for CP-specific antibody production are not needed and only a minimum of bacterial biomass (about 108 bacteria) is required. Since the sample preparation procedure is simple and data analysis is automated by the software, no specific training is necessary. Operating costs for FTIR spectroscopy are very low because it does not require specific reagents and ZnSe optical plates used for the measurements are reusable. Overall, FTIR spectroscopy is, compared to the current gold standard for CP serotyping by immune-based detection, much faster, cheaper, and easier to perform. In addition, due to its high-throughput capacities, it may also provide valuable epidemiological information about the chronicity of specific S. aureus infections.
In conclusion, this study demonstrated that ANN-assisted FTIR spectroscopy is a suitable tool for a rapid, cost-effective, and reliable discrimination of S. aureus capsular serotypes CP5, CP8, and NT. Since it is based on the metabolic fingerprint of the bacterium itself, it enables a routine high-throughput screening and can be applied to monitor pathogen adaptation in host environments. Biotyping by FTIR spectroscopy therefore might not only represent an interesting tool for clinical diagnostics but also may contribute to the better understanding of the adaptation mechanisms of S. aureus to the host, which would allow the development of improved treatment regimes in the future, especially for chronic infections.
ACKNOWLEDGMENTS
We are grateful to Jean C. Lee for providing us the Reynolds strains and their isogenic mutant strains and the protocol for capsule extraction. Furthermore, we thank Luis Calvinho for the kindly supply of Argentinian mastitis isolates.
Financial support by the Vetmeduni Vienna (start-up project for T.G.) is kindly acknowledged. D.O.S. and F.R.B. are supported by ANPCyT (PICT 2010-01039 and PICT 2010-0733) and UBACyT.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee CY, Lee JC. 2006. Staphylococcal capsule, p 456–463 In Fischetti RPN, Ferretti JJ, Portnoy DA, Rood JI. (ed), Gram-positive pathogens. ASM Press, Washington, DC [Google Scholar]
- 3. Sompolinsky D, Samra Z, Karakawa WW, Vann WF, Schneerson R, Malik Z. 1985. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J. Clin. Microbiol. 22:828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. 2006. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol. Microbiol. 59:948–960 [DOI] [PubMed] [Google Scholar]
- 5. Arbeit RD, Karakawa WW, Vann WF, Robbins JB. 1984. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2:85–91 [DOI] [PubMed] [Google Scholar]
- 6. Fournier JM, Bouvet A, Boutonnier A, Audurier A, Goldstein F, Pierre J, Bure A, Lebrun L, Hochkeppel HK. 1987. Predominance of capsular polysaccharide type 5 among oxacillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 25:1932–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochkeppel HK, Braun DG, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan EL, Boutonnier A, Fournier JM. 1987. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J. Clin. Microbiol. 25:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Na'was T, Hawwari A, Hendrix E, Hebden J, Edelman R, Martin M, Campbell W, Naso R, Schwalbe R, Fattom AI. 1998. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J. Clin. Microbiol. 36:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roghmann M, Taylor KL, Gupte A, Zhan M, Johnson JA, Cross A, Edelman R, Fattom AI. 2005. Epidemiology of capsular and surface polysaccharide in Staphylococcus aureus infections complicated by bacteraemia. J. Hosp. Infect. 59:27–32 [DOI] [PubMed] [Google Scholar]
- 10. Sordelli DO, Buzzola FR, Gomez MI, Steele-Moore L, Berg D, Gentilini E, Catalano M, Reitz AJ, Tollersrud T, Denamiel G, Jeric P, Lee JC. 2000. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: genetic and epidemiologic analyses. J. Clin. Microbiol. 38:846–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuchscherr L, Loffler B, Buzzola FR, Sordelli DO. 2010. Staphylococcus aureus adaptation to the host and persistence: role of loss of capsular polysaccharide expression. Future Microbiol. 5:1823–1832 [DOI] [PubMed] [Google Scholar]
- 12. von Eiff C, Taylor KL, Mellmann A, Fattom AI, Friedrich AW, Peters G, Becker K. 2007. Distribution of capsular and surface polysaccharide serotypes of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 58:297–302 [DOI] [PubMed] [Google Scholar]
- 13. Sutter DE, Summers AM, Keys CE, Taylor KL, Frasch CE, Braun LE, Fattom AI, Bash MC. 2011. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol. Med. Microbiol. 63:16–24 [DOI] [PubMed] [Google Scholar]
- 14. Kenny K, Tollersrud T. 1999. Questions uniqueness of surface polysaccharide. Am. J. Vet. Res. 60:530. [PubMed] [Google Scholar]
- 15. Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, Fattom AI, Etienne J. 2007. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J. Clin. Microbiol. 45:725–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guidry A, Fattom A, Patel A, O'Brien C. 1997. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet. Microbiol. 59:53–58 [DOI] [PubMed] [Google Scholar]
- 17. Lattar SM, Tuchscherr LP, Caccuri RL, Centron D, Becker K, Alonso CA, Barberis C, Miranda G, Buzzola FR, von Eiff C, Sordelli DO. 2009. Capsule expression and genotypic differences among Staphylococcus aureus isolates from patients with chronic or acute osteomyelitis. Infect. Immun. 77:1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proctor RA. 2012. Is there a future for a Staphylococcus aureus vaccine? Vaccine 30:2921–2927 [DOI] [PubMed] [Google Scholar]
- 19. Karakawa WW, Fournier JM, Vann WF, Arbeit R, Schneerson RS, Robbins JB. 1985. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J. Clin. Microbiol. 22:445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JC, Liu MJ, Parsonnet J, Arbeit RD. 1990. Expression of type 8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 28:2612–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albus A, Arbeit RD, Lee JC. 1991. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect. Immun. 59:1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naumann D, Helm D, Labischinski H. 1991. Microbiological characterizations by FT-IR spectroscopy. Nature 351:81–82 [DOI] [PubMed] [Google Scholar]
- 23. Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindback T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Martlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197 [DOI] [PubMed] [Google Scholar]
- 24. Bosch A, Minan A, Vescina C, Degrossi J, Gatti B, Montanaro P, Messina M, Franco M, Vay C, Schmitt J, Naumann D, Yantorno O. 2008. Fourier transform infrared spectroscopy for rapid identification of nonfermenting gram-negative bacteria isolated from sputum samples from cystic fibrosis patients. J. Clin. Microbiol. 46:2535–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wenning MSS, Naumann D. 2008. Infrared spectroscopy in the identification of microorganisms, p 71–96 In Diem M, Griffiths P, Chalmers JM. (ed), Vibrational spectroscopy for medical diagnosis. John Wiley & Sons, Ltd, Chichester, United Kingdom [Google Scholar]
- 26. Amiali NM, Golding GR, Sedman J, Simor AE, Ismail AA. 2011. Rapid identification of community-associated methicillin-resistant Staphylococcus aureus by Fourier transform infrared spectroscopy. Diagn. Microbiol. Infect. Dis. 70:157–166 [DOI] [PubMed] [Google Scholar]
- 27. Helm D, Labischinski H, Schallehn G, Naumann D. 1991. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J. Gen. Microbiol. 137:69–79 [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Reuhs BL, Mauer LJ. 2005. Use of Fourier transform infrared spectra of crude bacterial lipopolysaccharides and chemometrics for differentiation of Salmonella enterica serotypes. J. Appl. Microbiol. 99:411–417 [DOI] [PubMed] [Google Scholar]
- 29. Rebuffo-Scheer CA, Schmitt J, Scherer S. 2007. Differentiation of Listeria monocytogenes serovars by using artificial neural network analysis of Fourier-transformed infrared spectra. Appl. Environ. Microbiol. 73:1036–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuhm AE, Suter D, Felleisen R, Rau J. 2009. Identification of Yersinia enterocolitica at the species and subspecies levels by Fourier transform infrared spectroscopy. Appl. Environ. Microbiol. 75:5809–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 73:3502–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pohlmann-Dietze P, Ulrich M, Kiser KB, Doring G, Lee JC, Fournier JM, Botzenhart K, Wolz C. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865–4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cunnion KM, Lee JC, Frank MM. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 69:6796–6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tollersrud T, Kenny K, Reitz AJ, Jr, Lee JC. 2000. Genetic and serologic evaluation of capsule production by bovine mammary isolates of Staphylococcus aureus and other Staphylococcus spp. from Europe and the United States. J. Clin. Microbiol. 38:2998–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alvarez LP, Barbagelata MS, Gordiola M, Cheung AL, Sordelli DO, Buzzola FR. 2010. Salicylic acid diminishes Staphylococcus aureus capsular polysaccharide type 5 expression. Infect. Immun. 78:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kummerle M, Scherer S, Seiler H. 1998. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl. Environ. Microbiol. 64:2207–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Udelhoven T, Novozhilov M, Schmitt J. 2003. The NeuroDeveloper®: a tool for modular neural classification of spectroscopic data. Chemom. Intell. Lab. Syst. 66:219–226 [Google Scholar]
- 38. Oberreuter H, Seiler H, Scherer S. 2002. Identification of coryneform bacteria and related taxa by Fourier-transform infrared (FT-IR) spectroscopy. Int. J. Syst. Evol. Microbiol. 52:91–100 [DOI] [PubMed] [Google Scholar]
- 39. Synytsya A, Copikova J, Matejka P, Machovic V. 2003. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 54:97–106 [Google Scholar]
- 40. Kacurakova M, Capek P, Sasinkova V, Wellner N, Ebringerova A. 2000. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 43:195–203 [Google Scholar]
- 41. Moreau M, Richards JC, Fournier JM, Byrd RA, Karakawa WW, Vann WF. 1990. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 201:285–297 [DOI] [PubMed] [Google Scholar]
- 42. Jones C. 2005. Revised structures for the capsular polysaccharides from Staphylococcus aureus types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res. 340:1097–1106 [DOI] [PubMed] [Google Scholar]
- 43. Rebuffo CA, Schmitt J, Wenning M, von Stetten F, Scherer S. 2006. Reliable and rapid identification of Listeria monocytogenes and Listeria species by artificial neural network-based Fourier transform infrared spectroscopy. Appl. Environ. Microbiol. 72:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naumann D. 2000. Infrared spectroscopy in microbiology, p 102–131 In Meyers RA. (ed), Encyclopedia of analytical chemistry. John Wiley & Sons Ltd, Chichester, United Kingdom [Google Scholar]