Abstract
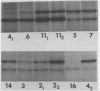
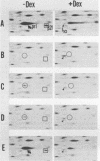
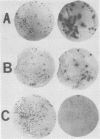
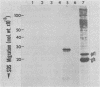
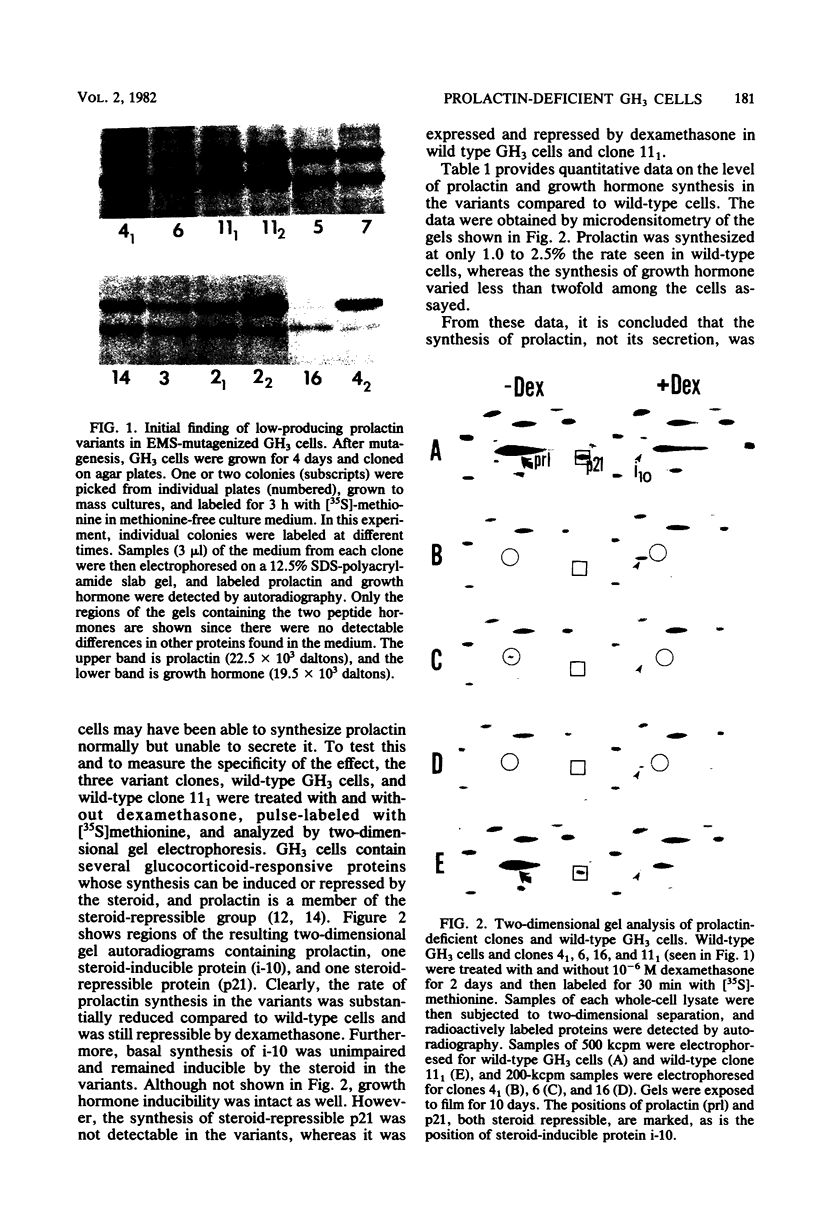
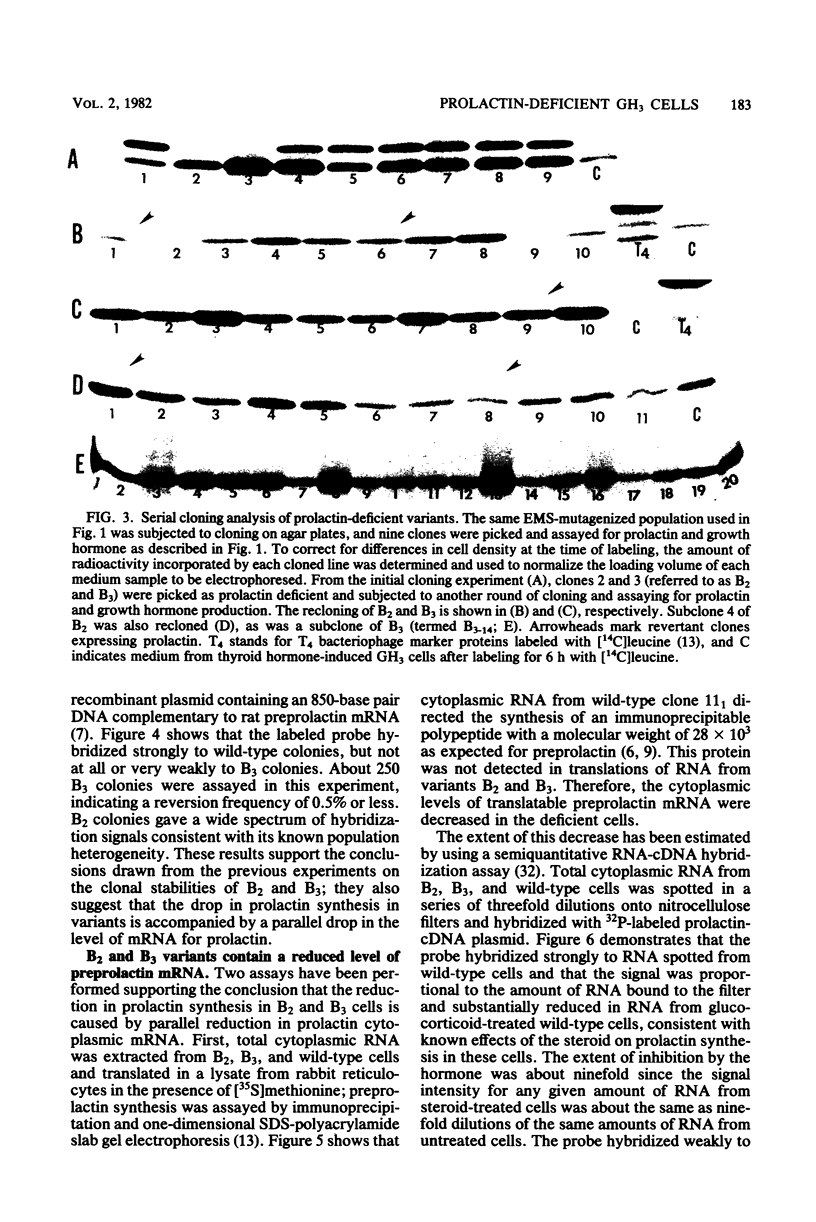
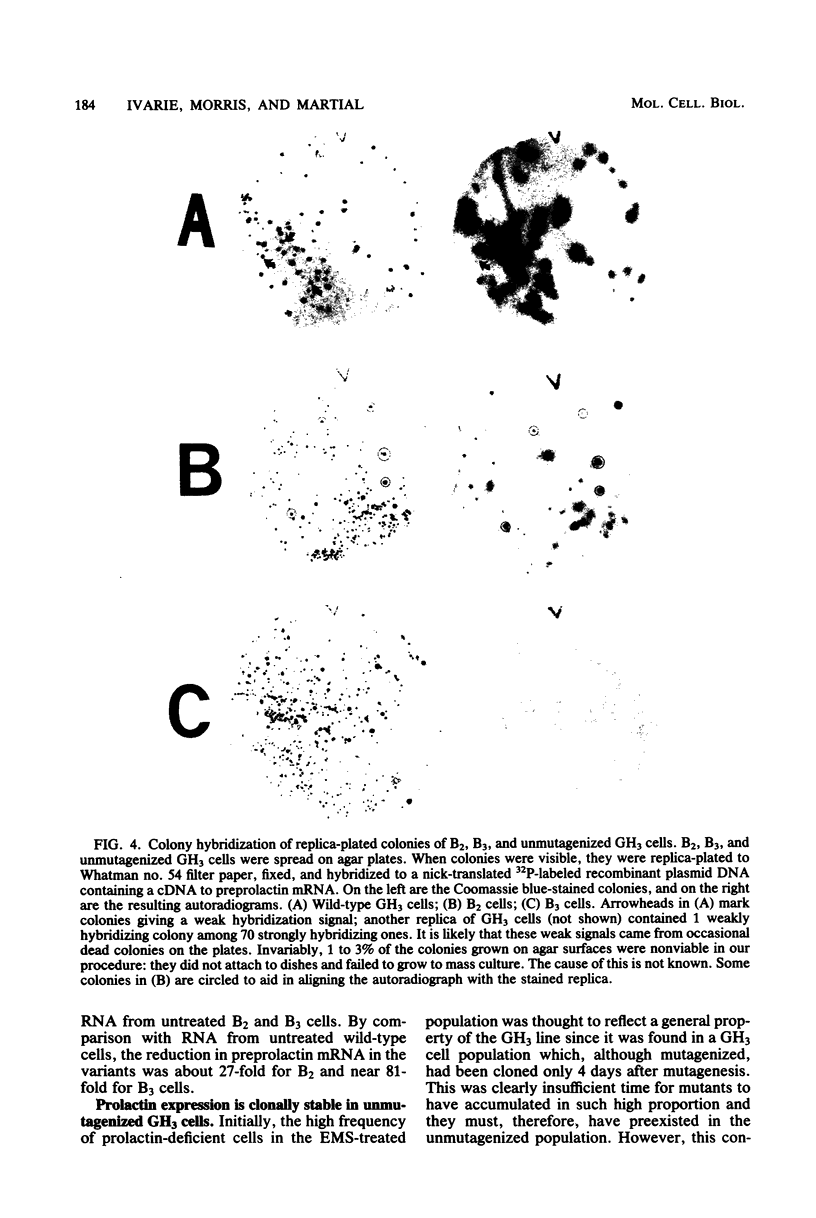
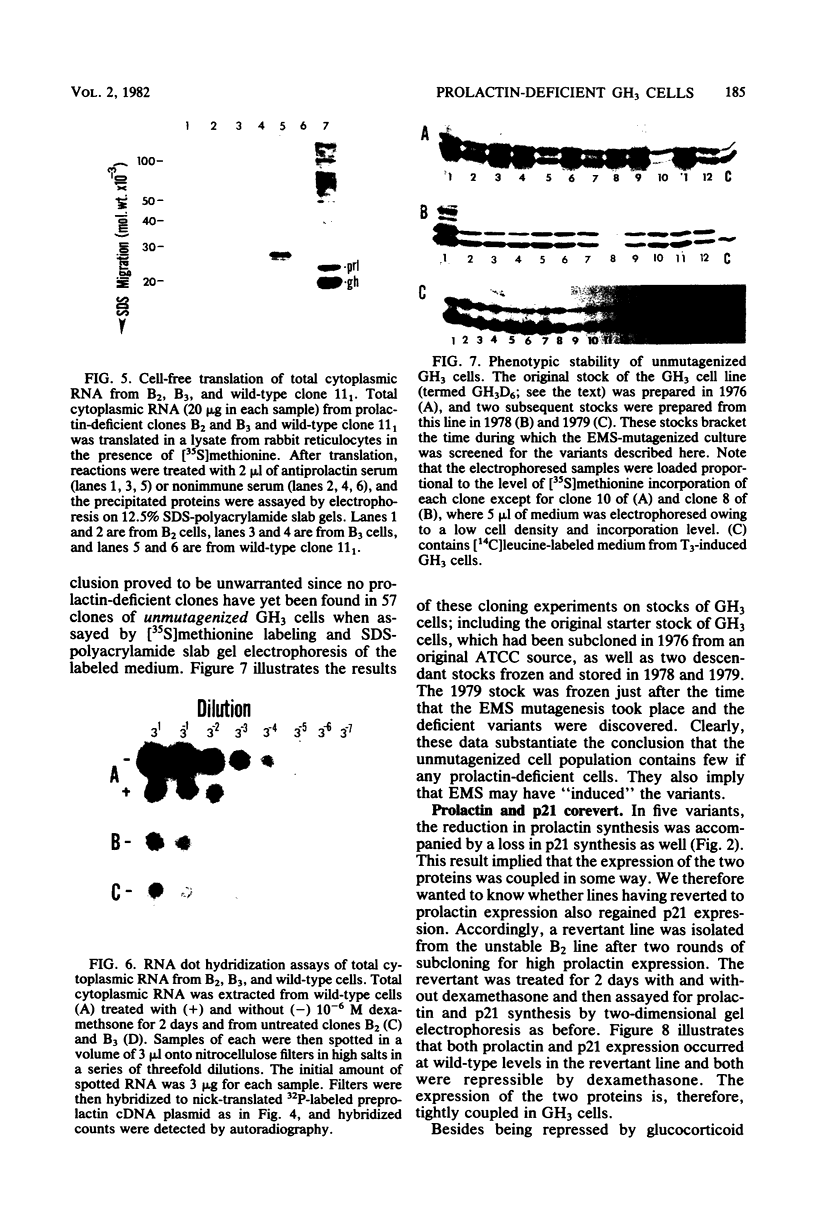
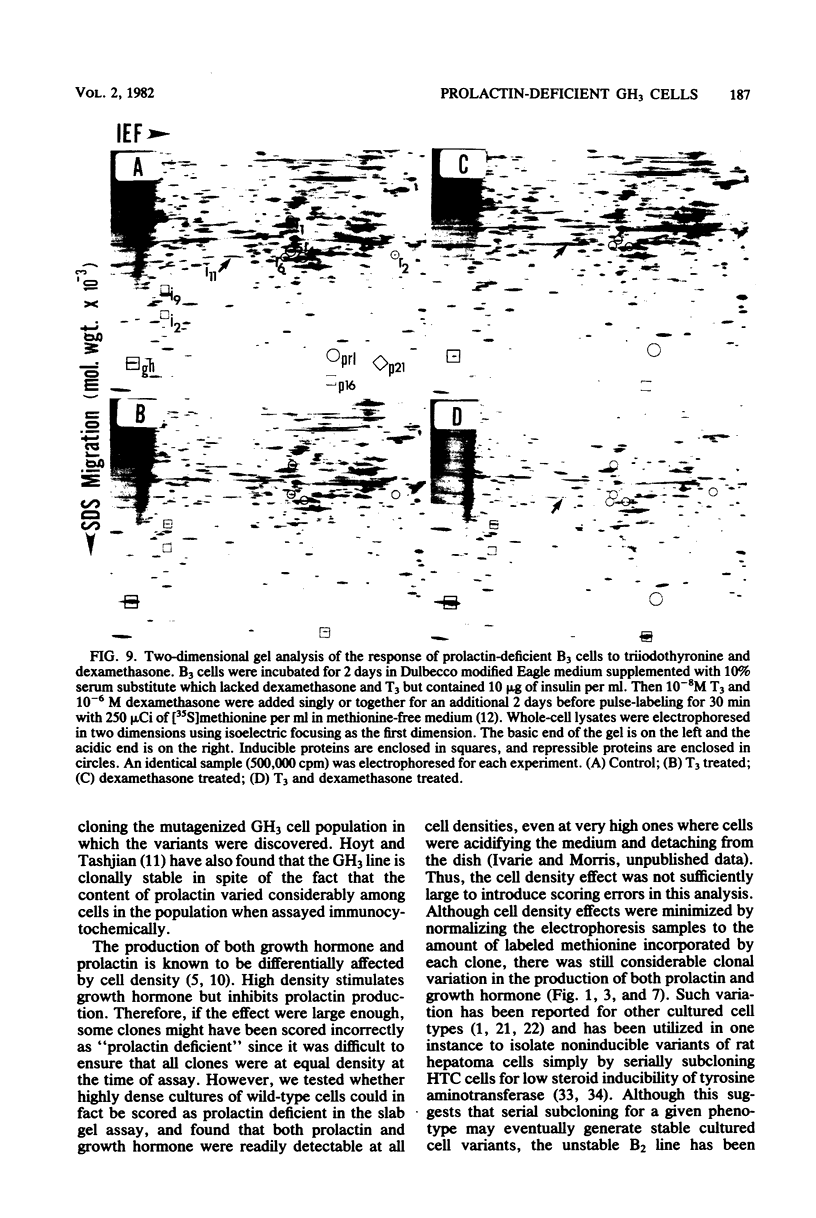
GH3 cells normally synthesize and secrete two pituitary polypeptide hormones, prolactin and growth hormone. From an ethyl methane sulfonate-mutagenized population, prolactin low-producing variants have been isolated at a frequency near 20%. Intracellular prolactin synthesis in the variants was reduced 40- to 100-fold compared to wild-type cells while growth hormone synthesis varied less than 2-fold. This decrease was paralleled by a decrease in intracellular preprolactin mRNA. Although reduced, prolactin synthesis was still repressible by glucocorticoids. There was a coordinate loss of expression of p21, a thyroid and glucocorticoid hormone-regulated protein, in GH3 cells, whereas the synthesis and regulation of other hormonally responsive proteins were unimpaired in the variants. Since p21 expression was coordinately regained in a high-producing prolactin revertant cell, expression of the two proteins is tightly coupled in GH3 cells. The stability of the low-producing phenotype differed among variants. One (B2) gave rise to revertants at about 20% frequency even after two rounds of subcloning, whereas another (B3) was more stable in that only 1 weak revertant was found in 47 subclones. The reversion frequency of B3 cells was also measured at less than 0.5%. Unmutagenized GH3 cells were phenotypically stable in that no prolactin-deficient variant was found among 57 subclones. Since variants were ony found after ethyl methane sulfonate mutagenesis, the DNA alkylating agent appears to have promoted an epigenetic change in pituitary gene expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv D., Thompson E. B. Variation in tyrosine aminotrasferase induction in HTC cell clones. Science. 1972 Sep 29;177(4055):1201–1203. doi: 10.1126/science.177.4055.1201. [DOI] [PubMed] [Google Scholar]
- Bancroft F. C. Measurement of growth hormone synthesis by rat pituitary cells in culture. Endocrinology. 1973 Apr;92(4):1014–1021. doi: 10.1210/endo-92-4-1014. [DOI] [PubMed] [Google Scholar]
- Bauer R. F., Arthur L. O., Fine D. L. Propagation of mouse mammary tumor cell lines and production of mouse mammary tumor virus in a serum-free medium. In Vitro. 1976 Aug;12(8):558–563. doi: 10.1007/BF02797439. [DOI] [PubMed] [Google Scholar]
- Biswas D. K., Lyons J., Tashjian A. H., Jr Induction of prolactin synthesis in rat pituitary tumor cells by 5-bromodeoxyuridine. Cell. 1977 Jun;11(2):431–439. doi: 10.1016/0092-8674(77)90061-7. [DOI] [PubMed] [Google Scholar]
- Bolton W. E., Boyd A. E., 3rd Evaluation of growth hormone production from GH1 cells in vitro: effect of culture media and time in culture. In Vitro. 1980 Apr;16(4):330–336. doi: 10.1007/BF02618339. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Thompson E. B. Genomic organization of rat prolactin and growth hormone genes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4583–4587. doi: 10.1073/pnas.77.8.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Dannies P. S., Tashjian A. R., Jr Effects of thyrotropin-releasing hormone and hydrocortisone on synthesis and degradation of prolactin in a rat pituitary cell strain. J Biol Chem. 1973 Sep 10;248(17):6174–6179. [PubMed] [Google Scholar]
- Evans G. A., Rosenfeld M. G. Regulation of prolactin mRNA analyzed using a specific cDNA probe. J Biol Chem. 1979 Aug 25;254(16):8023–8030. [PubMed] [Google Scholar]
- Haug E., Tjernshaugen H., Gautvik K. M. Variations in prolactin and growth hormone production during cellular growth in clonal strains of rat pituitary cells. J Cell Physiol. 1977 Apr;91(1):15–29. doi: 10.1002/jcp.1040910103. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Tashjian A. H., Jr Immunocytochemical analysis of prolactin production by monolayer cultures of GH3 rat anterior pituitary tumor cells: II. Variation in prolactin content of individual cell colonies, and dynamics of stimulation with thyrotropin-releasing hormone (TRH). Anat Rec. 1980 Jun;197(2):163–181. doi: 10.1002/ar.1091970206. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A., Eberhardt N. L. Hormonal domains of response: actions of glucocorticoid and thyroid hormones in regulating pleiotropic responses in cultured cells. Recent Prog Horm Res. 1980;36:195–239. doi: 10.1016/b978-0-12-571136-4.50012-7. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial J. A., Seeburg P. H., Guenzi D., Goodman H. M., Baxter J. D. Regulation of growth hormone gene expression: synergistic effects of thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4293–4295. doi: 10.1073/pnas.74.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Gubbins E. J., Erwin C. R., Donelson J. E. Comparison of potential nuclear precursors for prolactin and growth hormone messenger RNA. J Biol Chem. 1980 Mar 25;255(6):2243–2246. [PubMed] [Google Scholar]
- Nakane P. K. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem. 1970 Jan;18(1):9–20. doi: 10.1177/18.1.9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A. Analysis of discontinuous variation in albumin production by hepatoma cells at the cellular level. Somatic Cell Genet. 1979 Sep;5(5):641–651. doi: 10.1007/BF01542700. [DOI] [PubMed] [Google Scholar]
- Peterson J. A. Clonal variation in albumin messenger RNA activity in hepatoma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2056–2060. doi: 10.1073/pnas.73.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Roos B. A., Ong E. S., Evans R. M. Altered expression of the calcitonin gene associated with RNA polymorphism. Nature. 1981 Mar 5;290(5801):63–65. doi: 10.1038/290063a0. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Horwitz Z. D., Stanley F., Casanova J., Shapiro L. E. Thyroid hormone controls glucocorticoid action in cultured GH1 cells. Nature. 1977 Jul 21;268(5617):254–257. doi: 10.1038/268254a0. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Shapiro L. E. Thyroid hormone stimulates de novo growth hormone synthesis in cultured GH1 cells: evidence for the accumulation of a rate limiting RNA species in the induction process. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3369–3373. doi: 10.1073/pnas.73.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Levine L. Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol. 1970 Oct;47(1):61–70. doi: 10.1083/jcb.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Aviv D., Lippman M. E. Variants of HTC cells with low tyrosine aminotransferinase inducibility and apparently normal glucorticoid receptors. Endocrinology. 1977 Feb;100(2):406–419. doi: 10.1210/endo-100-2-406. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Granner D. K., Gelehrter T., Erickson J., Hager G. L. Unlinked control of multiple glucocorticoid-induced processes in HTC cells. Mol Cell Endocrinol. 1979 Sep;15(3):135–150. doi: 10.1016/0303-7207(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Chandler V. L., Ross S. R., Ucker D. S., Ring J. C., Feinstein S. C. Integration and activity of mammary tumor virus genes: regulation by hormone receptors and chromosomal position. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):687–697. doi: 10.1101/sqb.1981.045.01.086. [DOI] [PubMed] [Google Scholar]
- Yasamura Y., Tashjian A. H., Jr, Sato G. H. Establishment of four functional, clonal strains of animal cells in culture. Science. 1966 Dec 2;154(3753):1186–1189. doi: 10.1126/science.154.3753.1186. [DOI] [PubMed] [Google Scholar]