Abstract
Anaplasmosis and ehrlichiosis are emerging tick-borne diseases with clinically similar presentations caused by closely related pathogens. Currently, laboratories rely predominantly on blood smear analysis (for the detection of intracellular morulae) and on serologic tests, both of which have recognized limitations, for diagnostic purposes. We compared the performance of a published real-time PCR assay that incorporates melt curve analysis to differentiate Anaplasma and Ehrlichia species with blood smear and serologic methods in an upper Midwest population. Overall, 38.5% of the specimens selected for evaluation had one or more tests that were positive for anaplasmosis. The PCR positivity for all specimens was maximal (21.2%; 29/137) during the early acute phase of illness (0 to 4 days since illness onset) and significantly less frequent (11.5%; 20/174) during later phases (>4 days since illness onset). All positive specimens were Anaplasma phagocytophilum; no Ehrlichia species were identified. The real-time PCR detected 100% of infections that were detected by blood smear analysis (14/14) and broadened the detection window from a maximum of 14 days for smear positivity to 30 days for PCR. Additional infections were detected by real-time PCR in 12.9% (11/85) of smear-negative patients. There was poor agreement between the real-time PCR assay and serologic test results: 19.8% (19/96) and 13.7% (29/212) of seropositive and -negative patients, respectively, were PCR positive. Seropositivity increased with increasing days of illness, demonstrating that serologic detection methods are best utilized during presumed convalescence. Our results indicate that the optimal performance and utilization of laboratory tests for the diagnosis of anaplasmosis require knowledge regarding time of symptom onset or days of illness.
INTRODUCTION
Members of the genera Anaplasma and Ehrlichia (order Rickettsiales, family Anaplasmataceae) are obligate intracellular alphaproteobacteria that are transmitted to vertebrate hosts by ticks of the family Ixodidae and cause clinically similar febrile diseases in humans and domestic animals. In the United States, most human infections are caused by Anaplasma phagocytophilum and Ehrlichia chaffeensis and produce the nationally reportable diseases referred to as human anaplasmosis (human granulocytic anaplasmosis) and ehrlichiosis (human monocytic ehrlichiosis), respectively (1, 2). Although some differences in clinical presentation have been reported for these diseases, they are largely indistinguishable. Symptoms may be mild to severe and frequently include fever, chills, headache, myalgia, nausea, and cough, as well as laboratory-detected features of leukopenia, thrombocytopenia, and elevated hepatic transaminase levels (2, 3). Differences in the disease presentations include rashes frequently reported in ehrlichiosis, but rarely for anaplasmosis, patients (1, 4). Ehrlichiosis may also involve higher rates of severe manifestations, including disseminated intravascular coagulopathy and meningoencephalitis (1). Although most anaplasmosis and ehrlichiosis infections resolve, mortality occurs in 1 to 2% of cases (1). In addition, although anaplasmosis and ehrlichiosis are both effectively treated with doxycycline and tetracycline antibiotics, not all antibiotics are successful at treating both infections (3, 5).
Given the nonspecific and similar presentations of the illnesses associated with A. phagocytophilum and E. chaffeensis, diagnostic tests that reliably detect and differentiate these infections are needed to adequately diagnose patients and deliver prompt, appropriate treatment. At present, serologic diagnostic methods are most frequently used, but these tests have been shown to have poor sensitivity during the acute phases of infection (<1 week since symptom onset), when tests are most likely to be performed (6). A single positive titer result also is unable to distinguish between current infection and evidence of previous exposure to these pathogens, as IgG antibodies may persist in patients for several years postinfection (3, 7, 8). Thus, evidence of seroconversion or a 4-fold increase in titers between acute and convalescent serologic testing is recommended for confirming the diagnosis (according to the CDC case definitions for anaplasmosis [see http://www.cdc.gov/anaplasmosis/] and ehrlichiosis [see http://www.cdc.gov/ehrlichiosis/]); however, physicians rarely test a patient twice (1). Moreover, serologic tests developed for one specific agent (e.g., A. phagocytophilum) may be unable to detect infections from the other closely related species, thereby limiting the ability to adequately identify infections in areas where the pathogens overlap in distribution or where they just may be emerging. Conversely, cross-reactivity between serologic tests for A. phagocytophilum and E. chaffeensis has been demonstrated in about 15% of patients, suggesting that testing for both pathogens should be performed in geographic areas where both pathogens may be present in order to differentiate between the diseases (9, 10).
Microscopic detection of the intracellular clusters of bacteria, called morulae, via blood smear analysis is also possible and may provide presumptive differentiation of anaplasmosis versus ehrlichiosis infections, as A. phagocytophilum preferentially infects neutrophils, whereas E. chaffeensis infects monocytes (2, 3). Another pathogen closely related to E. chaffeensis, Ehrlichia ewingii, may also cause infections in susceptible humans, but rather than infect monocytes, this Ehrlichia species infects granulocytes (11). In geographic regions where A. phagocytophilum, E. chaffeensis, and E. ewingii coexist (e.g., the central Midwest and southeastern United States [1, 12]), the visualization of morulae in granulocytes would be insufficient for the differentiation of A. phagocytophilum and E. ewingii infections. Blood smear analysis also has poor sensitivity due to the transient nature of bacteremia, and it provides only supportive evidence of infection because of the potential for misinterpreting toxic granulation, various cytoplasmic inclusions, and staining artifacts on slides as bacterial morulae (3, 7; see also the CDC case definitions for anaplasmosis and ehrlichiosis).
The use of PCR-based diagnostic tests for the detection of these infections offers several advantages over the traditional serologic and blood smear tests. PCR tests have sensitivity and specificity rates that approach 100% (13), tend to have a higher degree of sensitivity during the acute phase of illness (6, 14), and have the potential to detect coinfections when configured in multiplexed reactions (13). In particular, a real-time assay developed by Bell and Patel (15) represents an attractive alternative to serologic and blood smear analyses for clinical diagnosis because of its ability to detect and differentiate at least 4 species, including A. phagocytophilum and E. chaffeensis, in a single reaction assay. Therefore, in areas where the distributions of these organisms may overlap, the use of this test can be expected to improve the time to diagnosis and accuracy. The analytical sensitivity of the test was demonstrated to be high, with the ability to detect between 5 and 10 copies of E. chaffeensis or A. phagocytophilum target DNA, respectively; however, the evaluation of this test in detecting infections in clinical samples was limited to a comparison of the real-time PCR test results with a conventional PCR method (15). Here, we report the performance of the real-time PCR assay compared to serologic and blood smear methods in detecting infections in clinical samples obtained from patients within an area of high A. phagocytophilum incidence rates in the upper Midwest.
MATERIALS AND METHODS
Patient specimens.
Remainder EDTA-preserved and serum blood samples were collected from patients for whom diagnostic tests (blood smear analysis and serology) for human anaplasmosis were ordered between April and December 2011 by health care providers at a largely rural health care system in central and northern Wisconsin, an area where A. phagocytophilum is known to be endemic (1, 16). Upon completion of routine testing, specimens were aliquoted into 1.5-ml cryovial tubes and frozen at −80°C. Chart reviews, approved by the institutional review board of the Marshfield Clinic, were performed and clinical data were abstracted, including patient age, gender, date of symptom onset, and the signs and symptoms associated with patient illness. The CDC case definition for human anaplasmosis was used as a guide to categorize patients into those with signs and symptoms that were clinically compatible and incompatible with anaplasmosis. Cases were considered consistent with anaplasmosis (n = 311) when the patients presented with at least two of the following symptoms: fever, chills and/or sweats, headache, myalgia, anemia, leukopenia, thrombocytopenia, or elevated hepatic transaminase levels. An additional 50 control patients with symptoms that were inconsistent with anaplasmosis were also included to evaluate the specificity of the assay; these patients reported either no symptoms or fatigue, myalgia, or joint pain without evidence of fever. In total, specimens from 361 patients were tested using the real-time PCR assay.
When available, the results from physician-ordered serologic and blood smear tests were also recorded for the patients. The serologic test used was an indirect fluorescent-antibody assay (IFA) that detected IgG antibodies specific for A. phagocytophilum using a polyvalent anti-human conjugate, with a titer of ≥1:64 being considered positive (Focus Diagnostics, Cypress, CA). Blood smear slides were made from whole-blood samples, stained by Wright's method, and examined microscopically under oil immersion for leukocytic intracellular morulae by trained clinical staff.
Although CDC guidelines require a 4-fold rise in titers between acute and convalescent serologic tests to confirm a case of anaplasmosis, only 1.6% of our patients were tested more than once by physicians; therefore, we considered patients with a positive serologic test as being positive for anaplasmosis, while recognizing that a single positive titer cannot be considered sufficient evidence of a current infection. Therefore, in this study, we did not have a true reference method against which to compare the performance of the PCR assay and blood smear analysis. By limiting our analysis to patients with clinical symptoms that were compatible with anaplasmosis, however, the positive predictive value of the serologic test may be elevated, and thus, errors comparing the serologic test with the PCR assay or blood smear analysis may be reduced.
DNA extraction.
Frozen blood samples were thawed at 37°C, and DNA was extracted from 200 μl using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). DNA was then eluted into 200 μl in AE buffer, as recommended by the manufacturer.
Real-time PCR.
The extracted DNA was amplified by using a previously described real-time PCR (15). This assay was designed to amplify and detect a segment of the heat shock protein operon groEL in three closely related target organisms: A. phagocytophilum, E. chaffeensis, and E. ewingii. Recently, the assay also was demonstrated to detect a novel Ehrlichia species identified in Minnesota and Wisconsin patients (18). The assay incorporates the primers ESp-F (5′-TACTCAGAGTGCTTCTCAATGT-3′) and ESp-R (5′-GCATACCATCAGTTTTTTCAAC-3′) and fluorescence resonance energy transfer (FRET)-labeled probes. A single acceptor probe (ESp-RD) hybridizes to the amplified DNA of all 4 species. Two separate donor probes were designed to allow for differentiation of the organisms. Specifically, the donor probe Aph-FL hybridizes to amplified product from A. phagocytophilum and the donor probe Ec/e-FL hybridizes to amplified product from E. chaffeensis, E. ewingii, and the new Ehrlichia sp. Wisconsin. Mismatches in the sequences of the corresponding donor probe regions of the 4 organisms permit their differentiation using melting curve analysis (15, 18).
For the assay, 5 μl of extracted DNA was added to 15 μl of master mix that contained 10 μl of 2× Platinum quantitative PCR supermix-uracil DNA glycosylase (UDG) (Invitrogen, Carlsbad, CA), 0.4 μl of 50 mM MgCl2, 0.5 μl of each of the primers, 0.8 μl of the acceptor probe, 0.4 μl of each of the donor probes, and 2 μl of sterile water. DNA was then amplified using a 480 LightCycler instrument (Roche Applied Sciences) under the following conditions: 1 cycle of uracil N-glycosylase (UNG) treatment at 50°C for 2 min (4.4°C/s) and 95°C for 3 min (4.4°C/s), followed by 45 amplification cycles at 95°C for 10 s (4.4°C/s), 55°C for 25 s (2.2°C/s), and 72°C for 22 s (4.4°C/s), a single melting curve cycle at 95°C for 1 min (4.4°C/s), 40°C for 2 min (1.0°C/s), and 80°C for 0 s (0.11°C/s), and a single cooling cycle at 40°C for 30 s (1.5°C/s). A negative control of sterile water and 2 positive controls, one containing pooled positive plasmid DNA for A. phagocytophilum, E. chaffeensis, and E. ewingii and a second containing positive plasmid DNA for only Ehrlichia sp. Wisconsin, were included in each run of the assay. The positive controls were generated by inserting amplified products from each species into plasmid vector pCR2.1 (Invitrogen, Carlsbad, CA). We confirmed the analytical sensitivity of the assay by testing dilutions of 5 to 1 × 106 copies of each positive control and obtained detection limits consistent with those described previously (15).
Statistical methods.
Only patients who had an illness that was clinically compatible with the CDC case definition for anaplasmosis were included in our statistical analyses. Categorical descriptive variables were compared between PCR-positive and PCR-negative patients using 2 by 2 contingency tables, whereas continuous variables were compared between the groups using Wilcoxon rank sum tests. Test outcomes were also compared across time intervals termed early acute and later phases (≤4 days and >4 days since illness onset, respectively), and agreement among the PCR, serologic, and blood smear tests was evaluated using sets of 2 by 2 contingency tables and the kappa statistic (19). A P value of ≤0.05 was considered significant.
RESULTS
Detection of A. phagocytophilum and Ehrlichia spp. in clinical samples.
Of the total 361 patient specimens that were included in our evaluation of the real-time PCR assay, a positive PCR result for A. phagocytophilum was obtained in 49 (13.6%) patients. An additional 90 patients were negative by PCR but were seropositive for A. phagocytophilum; this gave an overall positivity rate of 38.5% in our sample population. Positive blood smear results were found only in patients who also had either a positive PCR or serologic test result. Among the patients who had serologic test results (n = 358), 109 (30.4%) were seropositive; blood smear analyses were positive in 14 of 106 (13.2%) patients with blood smear results. No infections with Ehrlichia spp. were detected in our study.
Demographic and clinical characteristics of PCR-positive patients.
Only patients with clinical signs and symptoms compatible with anaplasmosis were PCR positive. Moreover, the majority of PCR-positive patients (48 of 49) had symptoms consistent with the CDC case definition for anaplasmosis. One PCR-positive patient reported headache and myalgia, consistent with anaplasmosis, but failed to report the occurrence of fever, chills, or sweats and had no evidence of fever upon presentation at the clinic, although the patient was leukopenic and thrombocytopenic and had elevated liver enzymes, which is consistent with anaplasmosis.
A comparison of the clinical characteristics of PCR-positive and PCR-negative patients indicated that positive patients tended to be male, be in the acute phase of infection (≤1 week since the onset of symptoms), and require hospitalization. They were also more likely to present with fever, sweats, or chills, urinary tract-related complaints, leukopenia, thrombocytopenia, and elevated liver enzymes, and to have reported tick exposure (Table 1). Although being aged ≥50 years was not associated with being PCR positive (Table 1), PCR-positive patients did tend to be slightly older (Wilcoxon rank sum test, P = 0.029), with a median (range) age of 57.8 (13.7 to 85.8) years, versus 53.7 (2.2 to 96.1) years for PCR-negative patients.
Table 1.
Demographic and clinical characteristics of patients who tested positive or negative with the PCR assay
| Patient characteristic | PCR-positive result |
PCR-negative result |
P valuea | ||
|---|---|---|---|---|---|
| Sample size (n)b | n (%) | Sample size (n)b | n (%) | ||
| Total | 311 | 49 (15.7) | 311 | 262 (86.4) | |
| Serology-positive result | 48 | 19 (39.6) | 260 | 77 (29.6) | 0.171 |
| Smear-positive result | 25 | 14 (56.0) | 74 | 0 (0) | <0.0001 |
| Gender, male | 49 | 36 (73.5) | 262 | 142 (54.2) | 0.012 |
| Age ≥50 yr | 49 | 33 (67.4) | 262 | 137 (52.3) | 0.052 |
| Acute infections | 49 | 38 (77.6) | 262 | 163 (62.2) | 0.039 |
| Hospitalized | 49 | 16 (32.7) | 262 | 52 (19.9) | 0.047 |
| Positive for symptoms | |||||
| Fever/sweats/chills | 49 | 47 (95.9) | 262 | 224 (85.5) | 0.046 |
| Headache | 49 | 28 (57.1) | 262 | 147 (56.1) | 0.893 |
| Myalgias | 49 | 31 (63.3) | 262 | 154 (58.8) | 0.557 |
| Cold symptomsc | 49 | 18 (36.7) | 262 | 88 (33.6) | 0.670 |
| GI symptomsd | 49 | 22 (44.9) | 262 | 118 (45.0) | 0.986 |
| Urinary symptomse | 49 | 22 (44.9) | 262 | 80 (30.5) | 0.049 |
| Leukopenia | 44 | 26 (59.1) | 238 | 43 (18.1) | <0.0001 |
| Thrombocytopenia | 44 | 37 (84.1) | 237 | 83 (35.0) | <0.0001 |
| Elevated liver enzyme levels | 33 | 22 (66.7) | 168 | 60 (35.7) | 0.0009 |
| Reported tick exposure | 49 | 27 (55.1) | 262 | 104 (39.7) | 0.045 |
Based on chi-square analysis; significant variables (P < 0.05) are indicated by bold type.
Sample sizes refer to the numbers of patients who were PCR positive or PCR negative and had data available on the various factors listed (and therefore were included in the comparisons).
Included cough, sore throat, and sinus congestion.
GI, gastrointestinal; symptoms included nausea, vomiting, diarrhea, and abdominal pain.
Included positive culture for urinary tract infection; urinary incontinence, urgency, frequency, or dysuria; hematuria; and hemoglobinuria/myoglobinuria.
Twelve of our 361 patients who were tested for anaplasmosis/ehrlichiosis using the PCR assay had evidence of Lyme disease exposure; eight of these patients could be considered seropositive based on the CDC two-tier testing algorithm for Lyme disease (17), and 6 patients had a physician-diagnosed erythema migrans rash. Four of the seropositive Lyme disease patients were included in the 49 PCR-positive A. phagocytophilum patients identified in our study, suggesting that these patients either had evidence of coinfection or were recently sequentially infected with A. phagocytophilum and Borrelia burgdorferi. Two other patients who were PCR positive for A. phagocytophilum also had antibody titers consistent with Babesia microti exposure. Because these patients had symptoms consistent with our inclusion criteria, and seropositivity does not necessarily mean that patients were infected with more than one tick-borne disease, they were retained in our analysis.
Comparison of positivity among detection methods in patients with anaplasmosis-compatible symptoms.
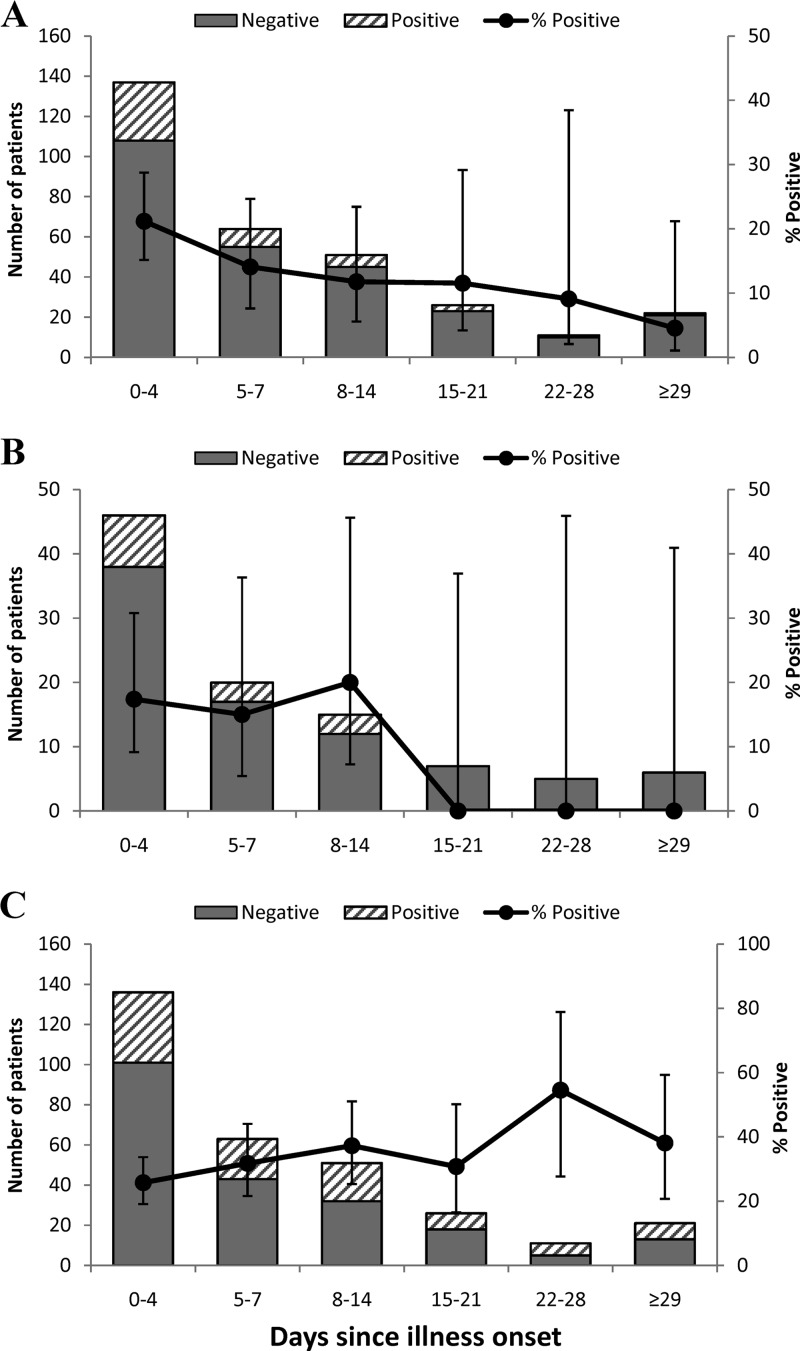
Of the patients with clinical symptoms that were compatible with anaplasmosis, the real-time PCR assay detected A. phagocytophilum infections in patients who had reported being ill beginning on the date of the clinic visit (i.e., 0 days since illness onset) through 1 month prior to the clinic visit (30 days since illness onset) (Fig. 1A). The median number of days that PCR-positive patients reported being ill prior to testing was 4 days. This was in contrast to blood smear-positive patients who had a range of detection between 2 and 14 days post-illness onset (Fig. 1B), although the median number of days that they reported being ill was also 4 days. Serology-positive patients reported being ill between 0 and 81 days prior to testing (Fig. 1C), with a median number of days ill of 6. Based on these results, we constructed two groups to compare the abilities of the 3 detection methods for identifying anaplasmosis-positive patients: patients tested in the early acute phase of infection (0 to 4 days since illness onset) and patients tested in the later phase of infection (>4 days since illness onset).
Fig 1.
Frequency histograms showing the number of patients with positive and negative test results and percentages of positive tests (error bars show 95% confidence intervals) by intervals of time since illness onset for the real-time PCR assay (A), blood smear analysis (B), and the serologic test (C).
The timing of testing was significantly associated with a PCR-positive test result. Patients tested in the early acute phase of infection were more likely to be PCR positive than patients tested later (29 of 137 [21.2%] versus 20 of 174 [11.5%]; χ2 = 7.26, P = 0.020). Positivity for blood smear analyses was also higher in the early acute phase of illness, although patients tested during this phase were not significantly more likely to be smear positive than those tested later (8 of 46 patients [17.4%] versus 6 of 53 [11.3%]; χ2 = 0.74, P = 0.387) (Fig. 1B). Patients with a positive serologic test tended to be in the later stage of infection (>4 days since illness onset; χ2 = 3.35, P = 0.067), with maximum positivity observed for patients tested between 22 and 28 days post-illness onset (6 of 11 [54.5%]) (Fig. 1C). In addition, the antibody titers for seropositive patients were significantly higher in patients who were in the later phase of illness (Wilcoxon rank sum statistic = 1,419.5, P = 0.029).
Patients who were seropositive and PCR positive tended to have higher antibody titers than seropositive patients who were PCR negative (Wilcoxon rank sum statistic = 1,194, P = 0.0096). Titers for both groups of patients ranged between 1:64 and >1:512, though those who were seropositive and PCR positive had a median titer of 1:512 and geometric mean titer of 1:355; those who were seropositive and PCR negative had a median titer of 1:128 and a geometric mean titer of 1:170. The highest median and geometric mean titers were observed in patients (n = 15) who were PCR positive and in the later phase of illness (median titer of >1:512, geometric mean titer of 1:512), and these titers were significantly higher than the titers for the 4 patients who were both seropositive and PCR positive in the early acute phase of illness (2 had titers of 1:64 and 2 had titers of 1:128; Wilcoxon rank sum statistic = 15, P = 0.0078).
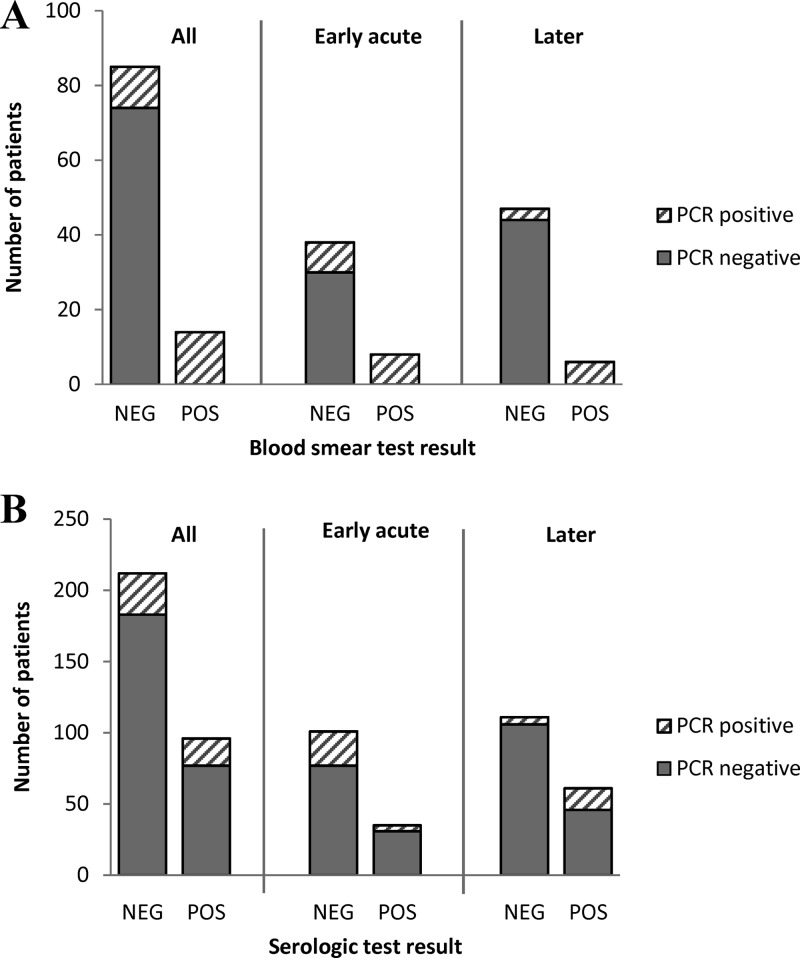
There was moderate agreement between the PCR and blood smear analysis test results (kappa = 0.66; 95% confidence interval [CI], 0.48 to 0.84). All of the blood smear-positive patients were PCR positive (Table 1, Fig. 2A). Among the smear-negative patients, an additional 11 were PCR positive, representing 11.1% of the 99 patients who had blood smear analysis results. Compared to the PCR assay, the sensitivity of the blood smear analysis was 56%. There was little agreement between the PCR and serologic test results (kappa = 0.071; 95% CI, −0.036 to 0.18) (Fig. 2B). Of the 96 patients who were seropositive, only 19 (19.8%) had a PCR-positive result. Likewise, the majority of PCR-positive patients (60.4%; 29/48) were seronegative. Disparity between the PCR and serologic test results was particularly high during the early acute phase of infection (Fig. 2B), as only 4 of the 28 PCR-positive patients (14.3%) in this phase were seropositive, and 31 of 35 (88.6%) seropositive patients were PCR negative. Agreement between the two tests improved during the later phases of infection (Fig. 2B), as 75% (15/20) of the PCR-positive patients were also seropositive in this phase; however, the majority of seropositive patients (46/61, 75.4%) continued to be PCR negative. Overall, about 31% (96/308) of the patients who were considered to have an illness compatible with anaplasmosis and had serologic test results available were seropositive. Of the 103 patients who had results from all 3 tests, the PCR assay detected 7 additional A. phagocytophilum infections that were not detected by either blood smear or serologic tests, representing 29.2% of the 24 patients in this sample who had a positive PCR test.
Fig 2.
Comparisons of positive and negative blood smear (A) and serologic (B) tests with that of the real-time PCR assay for detecting A. phagocytophilum infection in all patients, and for those in the early acute (0 to 4 days since illness onset) and later (>4 days since illness onset) phases of infection.
Patients with anaplasmosis-incompatible illness.
All of the 50 patients with symptoms that were incompatible with anaplasmosis had a negative PCR test result. Likewise, all blood smear tests for these patients were negative. Twenty-six percent (13/50) of these patients, however, were seropositive.
DISCUSSION
Although serologic assays and microscopic examination of stained blood smears are practical and reliable diagnostic methods to detect infections of A. phagocytophilum and Ehrlichia spp., the tests do have recognized limitations (20, 21). Blood smear analysis is insensitive due to the small number of circulating cells that can be practically examined during routine microscopy, the rarity of infected cells, lack of expertise among personnel performing smear examination, and the occurrence of intracellular artifacts that may mimic morulae (22, 23). Serologic tests have limited utility in the early acute phase of infection, when the majority of patients seek care, because this is prior to the production of detectable antibodies. The available assays also suffer from lack of specificity and may have considerable turnaround times (3, 6). Importantly, IgG-only assays lack the ability to distinguish between active and resolved infections, as IgG antibodies may persist in patients for long periods of time after infections have resolved (6, 24).
Based upon recognized improvements in the sensitivity, specificity, and turnaround times for PCR-based assays (25, 26), we evaluated the performance of a real-time PCR assay (15) to detect infections with A. phagocytophilum compared to the performance of serologic and blood smear examination tests. The timing of specimen collection relative to the patient-reported onset of symptoms was subsequently examined for each diagnostic method to better assess optimal test utilization in each stage of infection. The real-time PCR assay performed similarly to other PCR assays that have been developed for A. phagocytophilum detection (2, 13). As with other studies, we found that maximum positivity was obtained in the early phase of illness, when morulae were likely to be visible in blood smear analysis (50% of our PCR-positive patients in the early phase were also smear positive) and before an effective antibody response had been mounted by patients (74% of these PCR-positive results were seronegative). The positivity rate for the real-time PCR assay also declined with the number of days patients reported being ill (Fig. 1A) (7). This decline likely reflects the natural clearance of morulae from circulating neutrophils that has been described with the simultaneous appearance of infection-specific neutralizing antibodies (22). Despite this decline in positivity with increasing length of patient illness, the sensitivity of the PCR assay was 100% compared to blood smear analysis and it detected infections over a wider window of time (0 to 30 days versus 2 to 14 days post-illness onset, respectively), suggesting that it would provide more reliable results for anaplasmosis diagnosis in the early stage of infection. Improving diagnosis during acute infection has clinical relevance, as the majority of patients seek care within the first few days of an acute febrile illness. Of the 361 patients included in our study, 219 (60.7%) visited a clinic within 7 days of illness onset. Thus, a more-sensitive diagnostic method during this phase would ensure proper diagnosis and the initiation of antibiotic treatment in more patients at a time when failure to treat may lead to more severe manifestations of anaplasmosis (4, 7).
In the absence of paired acute and convalescent serology tests to confirm A. phagocytophilum infection, it is difficult to compare the sensitivity of the PCR assay with the IFA serologic test as used in our study. Overall, a higher percentage of patients were seropositive than PCR positive, although the seropositivity rate is indicative of antibody responses to active infections and provides evidence of previous exposure. Seroprevalence studies previously performed in Wisconsin have demonstrated background seropositivity rates of around 15% (27). In our study, we included 50 control patients with symptoms that were incompatible with anaplasmosis; none of these patients were PCR positive, although 13 (26%) of them were seropositive, suggestive of prior exposure. This background seropositivity may be higher than what was previously reported because we selected from a pool of patients who had been tested for anaplasmosis rather than from a pool of completely healthy individuals. Thus, although their clinical symptoms were not compatible with the case definition for anaplasmosis, they presented with symptoms that caused enough suspicion to be tested by their health care providers. It may be that some of these control patients were actually still recovering from a recent anaplasmosis infection.
Characteristics of patients with PCR-positive test results were largely consistent with what has been reported previously for anaplasmosis. The PCR assay detects pathogen DNA in the blood of infected patients. Anaplasmosis patients are most likely to be bacteremic during acute infection, and our data supported this observation, with 78.6% of our positive blood smears detected during the first week of patient illness. This period of illness has also been shown to coincide with hematologic abnormalities, including leukopenia and thrombocytopenia, which are associated with anaplasmosis infections (20, 28). In our study, the association between urinary tract-related complaints and positive PCR status has not previously been reported. An abnormal urinalysis result indicating hemoglobinuria/myoglobinuria (the laboratory urinary test used did not distinguish between them) was reported for 15 of 49 (30.6%) patients who were PCR positive for A. phagocytophilum infection, and 6 additional patients reported dark urine, which may be a sign of hemoglobinuria/myoglobinuria, as well as of dehydration. Hemoglobinuria/myoglobinuria, in particular, may result from cell lysis or the proinflammatory immune response that occurs in A. phagocytophilum infection (29, 30). In more severe cases, acute renal failure may also occur, such that hemoglobinuria/myoglobinuria may be a precursor to renal dysfunction. Additionally, rhabdomyolysis has been described as a complication in association with some cases of anaplasmosis (31); hemoglobinuria/myoglobinuria may be supporting evidence of this condition. Whether hemoglobinuria/myoglobinuria is another clinical sign that will be consistently supportive of an anaplasmosis diagnosis or be indicative of the severity of infection should be examined in future studies.
Although we did not detect any Ehrlichia infections in our study population, the ability of the real-time PCR assay to detect these pathogens represents a significant advantage over traditional serologic and blood smear tests and will permit species-specific surveillance in the future. Currently, the geographic distributions and life cycles of the two most common pathogens, A. phagocytophilum and E. chaffeensis, are largely distinct, allowing for presumptive diagnoses of infections based upon the geographic location of patient exposure. A. phagocytophilum is transmitted primarily by black-legged ticks (Ixodes scapularis in the eastern United States and Ixodes pacificus in the western United States), with the highest incidence rates reported in the northern Midwest and northern Atlantic seaboard states, whereas E. chaffeensis is transmitted by the Lone Star tick (Amblyomma americanum) and most frequently causes infections in the central Midwest and southeastern states (1). Range expansions of the black-legged and Lone Star ticks present an increasing risk of exposure to these infections in new areas and a greater potential for misdiagnosis as the distributions of the pathogens overlap more (32, 33). Having the ability to detect and differentiate between them will be important for their accurate diagnosis and treatment, as all antibiotic therapies are not effective against both pathogen groups (3, 4). In particular, E. chaffeensis appears to be resistant to fluoroquinolone antibiotics (5). Moreover, we now know that Wisconsin is an area of endemicity for not only A. phagocytophilum but also the newly described Ehrlichia sp. Wisconsin (18). To date, PCR is the only known method available for its detection. There have not been any diagnoses of Ehrlichia sp. Wisconsin infections by blood smear analysis (B. Pritt, personal communication). A diagnostic method capable of detecting this new pathogen will aid in understanding its epidemiology. In addition, evidence suggests the species is transmitted by the black-legged tick (18, 34), and therefore, the potential for coinfections with A. phagocytophilum exists. The use of this real-time PCR assay enables the detection of coinfections in a single run of the assay.
In summary, utilization of the described real-time PCR assay has enabled us to rapidly and accurately identify infections with A. phagocytophilum and potentially other species of Anaplasmataceae that are known to occur in the upper Midwest. The assay is more sensitive than blood smear analysis and extends the window of direct organism detection from 14 days to 30 days from the onset of symptoms. Serologic detection, not surprisingly, correlates poorly with PCR or blood smear analysis and more accurately reflects the collective exposure history occurring from late in the acute infection period into convalescence. Depending upon patient history, symptomatology, and hematologic and chemical metabolic profiles, the PCR assay may be an appropriate diagnostic adjunct. Proper selection of currently available diagnostic assays for maximal diagnostic potential does depend, however, upon taking a detailed clinical history that identifies the time interval from the onset of symptoms to the submission of clinical specimens.
ACKNOWLEDGMENTS
We thank the Marshfield Clinic Research Foundation and Marshfield Labs for providing financial and technical support for this study.
We also thank Sandor Karpathy and Marina Eremeeva of the Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention, for providing testing control materials. Technical advice and support were also kindly supplied by Bobbi Pritt and Lynne Sloan of Mayo Medical Laboratories and Diep (Zip) Hoang Johnson of the Wisconsin Division of Public Health.
Footnotes
Published ahead of print 1 May 2013
REFERENCES
- 1. Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. 2011. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 85:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dumler JS, Madigan JE, Pusterla N, Bakken JS. 2007. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 45(Suppl 1):S45–S51. 10.1086/518146 [DOI] [PubMed] [Google Scholar]
- 3. Thomas RJ, Dumler JS, Carlyon JA. 2009. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev. Anti Infect. Ther. 7:709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA, Tickborne Rickettsial Diseases Working Group, CDC 2006. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis–United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm. Rep. 55:1–27 [PubMed] [Google Scholar]
- 5. Maurin M, Abergel C, Raoult D. 2001. DNA gyrase-mediated natural resistance to fluoroquinolones in Ehrlichia spp. Antimicrob. Agents Chemother. 45:2098–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakken JS, Haller I, Riddell D, Walls JJ, Dumler JS. 2002. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin. Infect. Dis. 34:22–27 [DOI] [PubMed] [Google Scholar]
- 7. Bakken JS, Dumler JS. 2006. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann. N. Y. Acad. Sci. 1078:236–247 [DOI] [PubMed] [Google Scholar]
- 8. Walls JJ, Aguero-Rosenfeld M, Bakken JS, Goodman JL, Hossain D, Johnson RC, Dumler JS. 1999. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J. Clin. Microbiol. 37:2968–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comer JA, Nicholson WL, Olson JG, Childs JE. 1999. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J. Clin. Microbiol. 37:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comer JA, Nicholson WL, Sumner JW, Olson JG, Childs JE. 1999. Diagnosis of human ehrlichiosis by PCR assay of acute-phase serum. J. Clin. Microbiol. 37:31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148–155 [DOI] [PubMed] [Google Scholar]
- 12. Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Iazbik C, Kania SA, Lathan P, Little SE, Roy A, Sayler KA, Stillman BA, Welles EG, Wolfson W, Yabsley MJ. 2012. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit. Vectors 5:29. 10.1186/1756-3305-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumler JS, Brouqui P. 2004. Molecular diagnosis of human granulocytic anaplasmosis. Expert Rev. Mol. Diagn. 4:559–569 [DOI] [PubMed] [Google Scholar]
- 14. Edelman DC, Dumler JS. 1996. Evaluation of an improved PCR diagnostic assay for human granulocytic ehrlichiosis. Mol. Diagn. 1:41–49 [DOI] [PubMed] [Google Scholar]
- 15. Bell CA, Patel R. 2005. A real-time combined polymerase chain reaction assay for the rapid detection and differentiation of Anaplasma phagocytophilum, Ehrlichia chaffeensis, and Ehrlichia ewingii. Diagn. Microbiol. Infect. Dis. 53:301–306 [DOI] [PubMed] [Google Scholar]
- 16. Chen SM, Dumler JS, Bakken JS, Walker DH. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 44:590–591 [PubMed] [Google Scholar]
- 18. Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 365:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stokes ME, Davis CS, Koch GG. 2000. Categorical data analysis using the SAS system, 2nd ed SAS Institute Inc., Cary, NC [Google Scholar]
- 20. Aguero-Rosenfeld ME. 2002. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2:233–239 [DOI] [PubMed] [Google Scholar]
- 21. Walker D, Task Force on Consensus Approach for Ehrlichiosis 2000. Diagnosing human ehrlichioses: current status and recommendations. ASM News 66:287–290 [Google Scholar]
- 22. Bakken JS, Aguero-Rosenfeld ME, Tilden RL, Wormser GP, Horowitz HW, Raffalli JT, Baluch M, Riddell D, Walls JJ, Dumler JS. 2001. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin. Infect. Dis. 32:862–870 [DOI] [PubMed] [Google Scholar]
- 23. Bakken JS, Dumler JS. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554–560 [DOI] [PubMed] [Google Scholar]
- 24. Aguero-Rosenfeld ME, Kalantarpour F, Baluch M, Horowitz HW, McKenna DF, Raffalli JT, Hsieh TC, Wu J, Dumler JS, Wormser GP. 2000. Serology of culture-confirmed cases of human granulocytic ehrlichiosis. J. Clin. Microbiol. 38:635–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muldrew KL. 2009. Molecular diagnostics of infectious diseases. Curr. Opin. Pediatr. 21:102–111 [DOI] [PubMed] [Google Scholar]
- 26. Weile J, Knabbe C. 2009. Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal. Bioanal. Chem. 394:731–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bakken JS, Goellner P, Van Etten M, Boyle DZ, Swonger OL, Mattson S, Krueth J, Tilden RL, Asanovich K, Walls J, Dumler JS. 1998. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin. Infect. Dis. 27:1491–1496 [DOI] [PubMed] [Google Scholar]
- 28. Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199–205 [PubMed] [Google Scholar]
- 29. Dumler JS, Barat NC, Barat CE, Bakken JS. 2007. Human granulocytic anaplasmosis and macrophage activation. Clin. Infect. Dis. 45:199–204 [DOI] [PubMed] [Google Scholar]
- 30. Goodman JL. 2005. Human granulocytic anaplasmosis, p 218–238 In Goodman JL, Dennis DT, Sonenshine DE. (ed), Tick-borne diseases of humans. ASM Press, Washington, DC [Google Scholar]
- 31. Talsness SR, Shukla SK, Mazza JJ, Yale SH. 2011. Rhabdomyolysis-induced acute kidney injury secondary to Anaplasma phagocytophilum and concomitant statin use. WMJ 110:82–84 [PubMed] [Google Scholar]
- 32. Mixson TR, Ginsberg HS, Campbell SR, Sumner JW, Paddock CD. 2004. Detection of Ehrlichia chaffeensis in adult and nymphal Amblyomma americanum (Acari: Ixodidae) ticks from Long Island, New York. J. Med. Entomol. 41:1104–1110 [DOI] [PubMed] [Google Scholar]
- 33. Paddock CD, Childs JE. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Telford SR, III, Goethert HK, Cunningham JA. 2011. Prevalence of Ehrlichia muris in Wisconsin deer ticks collected during the mid-1990s. Open Microbiol. J. 5:18–20 [DOI] [PMC free article] [PubMed] [Google Scholar]