Abstract
Background
Because declining glucose levels should be detected quickly in persons with Type 1 diabetes, a lag between blood glucose and subcutaneous sensor glucose can be problematic. It is unclear whether the magnitude of sensor lag is lower during falling glucose than during rising glucose.
Methods
Initially, we analysed 95 data segments during which glucose changed and during which very frequent reference blood glucose monitoring was performed. However, to minimize confounding effects of noise and calibration error, we excluded data segments in which there was substantial sensor error. After these exclusions, and combination of data from duplicate sensors, there were 72 analysable data segments (36 for rising glucose, 36 for falling). We measured lag in two ways: (1) the time delay at the vertical mid-point of the glucose change (regression delay); and (2) determination of the optimal time shift required to minimize the difference between glucose sensor signals and blood glucose values drawn concurrently.
Results
Using the regression delay method, the mean sensor lag for rising vs. falling glucose segments was 8.9 min (95% CI 6.1–11.6) vs. 1.5 min (95% CI −2.6 to 5.5, P < 0.005). Using the time shift optimization method, results were similar, with a lag that was higher for rising than for falling segments [8.3 (95% CI 5.8–10.7) vs. 1.5 min (95% CI −2.2 to 5.2), P < 0.001]. Commensurate with the lag results, sensor accuracy was greater during falling than during rising glucose segments.
Conclusions
In Type 1 diabetes, when noise and calibration error are minimized to reduce effects that confound delay measurement, subcutaneous glucose sensors demonstrate a shorter lag duration and greater accuracy when glucose is falling than when rising.
Keywords: biotechnology, continuous blood glucose monitoring, Type 1 diabetes
Introduction
Amperometric continuous glucose monitors that are designed for use in the subcutaneous space are intended to measure glucose in interstitial fluid. During changing glucose levels, there is typically a lag of the sensor signal behind blood glucose, as recently reviewed [1]. The physiologic component of this lag results from the time required to transport glucose from the plasma to the interstitial fluid. The instrumental (analytic) component [2] results from the time required for analytes to diffuse through sensor elements before signal transduction. The data processing component results from noise filtering [1].
Ideally, the use of continuous glucose monitors in persons with diabetes provides early warning for extremes of glycaemia. However, lag impairs accuracy during changing glucose at times when accuracy is most needed. Failure to detect falling glucose can lead to confusion, stupor and coma.
Some workers found that the magnitude of sensor lag was less during falling glucose than during rising glucose. This was first observed in humans [3] and shortly thereafter in animals [4]. It was suggested that, during high insulin effect, the decline in plasma glucose results from glucose transport from the interstitial fluid into cells. Thus, it made sense that the decline in interstitial fluid glucose in this state precedes the decline in plasma. In contrast, during low insulin effect and low peripheral glucose uptake, glucose is transported from the liver or gut into plasma, then later diffuses into the interstitial fluid [4,5]. Findings similar to those of Sternberg et al. and Thome-Duret et al. were found with a transdermal sensor [6].
In contrast with these findings, other groups reported that sensor glucose lagged behind plasma glucose, regardless of whether glucose was rising or falling and regardless of insulin effect [2,7,8]. Table 1 is a summary of the literature on the topic of sensor delay. The methods used in these studies varied widely and it is likely that there are many sources of error that can confound lag measurement. For example, in a recent study, we found that calibration error can perturb measurements of sensor lag [9].
Table 1.
A summary of published reports that have compared the magnitude of sensor lag measured during rising glucose (or low insulin effect) to lag measured during falling glucose (or high insulin effect)
| Authors, year | Reference | Type of sensor | Subjects | Lower lag found during high insulin effect? |
|---|---|---|---|---|
| Sternberg et al., 1996 | [3] | Microdialysis | Type 1 diabetes— humans | Yes, but only in ~50% of subjects |
| Thome-Duret et al., 1996 | [4] | Wilson-Reach sensor(GOX, non-mediated) | Type 1 diabetes—rats | Yes |
| Wilhelm et al., 2006 | [21] | MiniMed (GOX, non-mediated) | Type 1 diabetes— humans | * |
| Schmidke et al., 1998 | [7] | Heller sensor (GOX, osmium-mediated) | Non-diabetic rats | No |
| Aussedat et al., 2000 | [5] | Wilson-Reach | Non-diabetic rats | Yes |
| Rebrin et al., 1999 | [8] | MiniMed | Non-diabetic dogs | No |
| Kulcu et al., 2003 | [6] | Cygnus, GOX, transdermal | Type 1 and Type 2 diabetes— humans | Yes |
| Boyne et al., 2003 | [2] | MiniMed | Type 1 diabetes— humans | No |
| Kamath et al., 2009 | [12] | Dexcom Seven+ (GOX, non-mediated) | Type 1diabetes— humans | Apparently yes, although not directly stated |
Compared with the rising glucose phase, during rapid decline after oral glucose and insulin administration, there was less of a lag of the sensor (vs. capillary blood as a reference), but there was also consistent calibration error (the sensor substantially underestimated blood glucose at high glucose levels), so this finding is difficult to interpret.
GOX, glucose oxidase.
The first purpose of the present study was to compare sensor lag during falling vs. rising glucose. Because we were interested in studying lag during substantial rates of change, we also asked whether meal carbohydrate of high glycaemic index would increase the glucose rate of change and thus create more opportunities to measure sensor lag. We were also interested in the magnitude of the difference in glucose levels resulting from meals of differing glycaemic index values.
Subjects and methods
Meal study
Seven subjects with Type 1 diabetes mellitus on subcutaneous insulin pump therapy were recruited from clinics in Portland, Oregon, USA. Patients who were pregnant or had cardiovascular, cerebrovascular, kidney or liver disease were excluded. Other exclusion criteria included oral or parenteral corticosteroid use, visual or physical impairments that impede the use of a continuous glucose monitoring device, insulin allergy, hypoglycaemia unawareness, insulin resistance defined as requiring more than 200 units of insulin per day or gastroparesis.
A total of 14 experiments, each with two meals, were completed in three men and four women aged 45.0 ± 4.5 years, whose duration of diabetes was 28.3 ± 3.8 years. HbA1c was 58 ± 2 mmol/mol (7.3 ± 0.2%) and BMI 26.1 ± 1.2 kg/m2. For each experiment, two meals were served 195 min apart and both were accompanied by continuous glucose monitoring. On one study day, two high glycaemic index meals were given and, on the other study day, two low glycaemic index meals were given. The glycaemic index is the area under the 2-h blood glucose response curve for a given food, compared with glucose [10]. The glycaemic index was 75 for high glycaemic index meals and 30 for low glycaemic index meals. The order (high glycaemic index vs. low glycaemic index) was randomized.
The Dexcom Seven® Plus subcutaneous sensor (Dexcom Inc, San Diego, Ca) was inserted the day prior to the study to allow time for signal stabilization. Subjects took no bolus insulin after 05.00 h on the day of the study. Aspart insulin (Novo-Nordisk Inc., Princeton, NJ, USA) was delivered subcutaneously by a portable insulin pump. Each subject’s standard basal insulin infusion rates were used throughout the day and were identical for both study days. Arterialized venous blood glucose samples were obtained every 15 min using a HemoCue Glucose 201 Analyzer (Hemocue AB, Engelholm, Sweden). After blood glucose remained stable at 3.8–9.4 mmol/l (70–170 mg/dl) for 30 min, the first meal was given.
Pre-meal insulin dosing was based on each subject’s usual insulin:carbohydrate ratio and was the same for all four meals. Sensors were calibrated using the Hemocue blood glucose analyser just prior to the first meal.
Closed-loop study
In order to further investigate sensor lag and to obtain additional comparisons between Dexcom Seven Plus continuous glucose monitor data and corresponding blood glucose, we also evaluated data from a previously published closed-loop study [11]. A total of 24 sensor data sets were analysed (12 closed-loop experiments in which each subject wore two sensors), with a mean study duration of 25 h. A data set is defined as all data for one sensor obtained over the course of one experiment. The two sensors were widely separated, on either side of the abdomen. Sensors were placed before the experiment started, as above, and sensors were calibrated every 6 h. Blood glucose was obtained every 10 min.
Data analysis
In order to measure the lag of sensor glucose behind blood glucose, there must be a change in glucose. Therefore, a data segment is defined as the data collected over a time period during which the magnitude of the change in sensor signal is sufficient to calculate sensor delay. All data sets in both studies were analysed in order to capture all data segments in which blood glucose was changing at a rate of ≥ 0.028 mmol/l (+0.5 mg/dl) or ≤ −0.028 mmol/l (−0.5 mg/dl) per min over a time period of at least 25 min. For a data segment to be included in the analysis, it was necessary to have a consistent monotonic rise or fall of glucose with a coefficient of determination (r2) of ≥ 0.82 for the changing blood glucose and sensor glucose values. In addition, to avoid confounding effects on sensor lag, we did not include sensor data segments with substantial calibration error, which can occur by drift or incorrect designation of background current. In particular, the data from the two plateaus (i.e. before and after the change segment) were analysed for sensor accuracy. Specifically, we included only those data segments whose mean absolute relative difference values obtained at the lower and upper plateaus were ≤ 15%. The data from the change segment itself was not subjected to this accuracy test, because such a test would inappropriately exclude data sets with a large lag. The rationale for excluding inaccurate sensor records is explained further by the three panels in the Supporting Information (Fig. S1), which show how calibration error impairs sensor accuracy. As shown in panels (b) and (c), data segments with substantial sensor calibration error (shown here as error in the upper plateau segments) make computation of lag duration difficult.
For the meal study, there were a total of 18 rising glucose sensor data segments and 19 falling glucose segments that met the criteria for rate of change, duration and r2. Of these segments, there were 13 rising and 12 falling segments that also met the plateau accuracy criteria. For the closed-loop study, there were 51 rising sensor data segments and 56 falling glucose segments that met the criteria for rate of change, duration and r2. Of these segments, there were 34 rising and 36 falling segments that also met the plateau accuracy criteria.
The calculated lag results of both sensors in the same subject over the same period of time are not independent observations. For this reason, when both sensors at any given time point met all the acceptance criteria, the two lag calculations were combined (averaged) and reported as a single observation. After combining the lag results from the duplicate sensors and taking closed-loop data and the meal study data together, there were a total of 36 rising and 36 falling segments. Table 2 shows the interrelationships among numbers of subjects, experiments, data sets and data segments.
Table 2.
Numerical interrelationships among number of subjects, experiments, data sets and glucose data segments
| Meal study | Comments | |
|---|---|---|
| Subjects | 7 | One sensor per subject |
| Experiments | 14 | Two experiments per subject (one high glycaemic index, one low glycaemic index) |
| Data sets | 14 | One sensor data set per experiment |
| Rising glucose data segments | 13 | Of the 14 data sets, there were 11 that had one evaluable rising segment, one that had two evaluable rising segments and two that had no evaluable rising segment |
| Rising segments after combination of duplicate sensors | 13 | (No change, single sensor per experiments) |
| Falling glucose data segments | 12 | Of the 14 data sets, there were six that had one evaluable falling segment, three that had two evaluable falling segments and five that had no evaluable falling segment |
| Falling segments after combination of duplicate sensors | 12 | (No change, single sensor per experiments) |
| Closed-loop study | Comments |
|---|---|
| 14 | Two sensors per subject |
| 23 | Five subjects had one experiment, nine subjects had two experiments |
| 46 | Two sensor data sets per experiment |
| 34 | Of the 46 data sets, there were nine that had one evaluable rising segment, five that had two evaluable rising segments, five that had three evaluable rising segments and 27 that had no evaluable rising segment |
| 23 | In 11 sensor pairs, both sensors met all inclusion criteria and thus were combined (averaged), leaving 23 |
| 36 | Of the 46 data sets, there were seven that had one evaluable falling segment, four that had two evaluable falling segments, three that had three evaluable falling segments, three that had four evaluable falling segments and 29 that had no evaluable falling segment |
| 24 | In 12 sensor pairs, both sensors met all inclusion criteria and thus were combined (averaged), leaving 24 |
| Combined data from both studies | ||
|---|---|---|
| Total rising data segments | 36 | 13 (meal) + 23 (closed loop) = 36 |
| Total falling data segments | 36 | 12 (meal) + 24 (closed loop) = 36 |
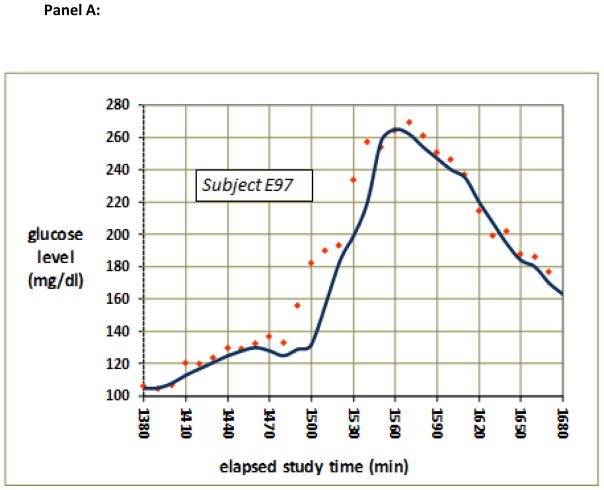
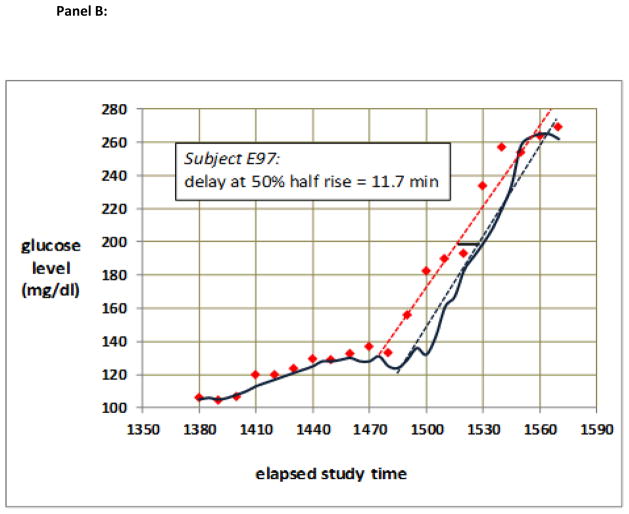
Sensor time lag was determined for each sensor using two methods. The first method was the linear regression delay method. For each rising glucose and for each falling glucose sensor data set, excluding the plateau segments, least-squares linear regression lines were calculated for the blood glucose values (vs. time) and for the sensor values (vs. time). The lag time was defined as the time difference between the two regression lines at the vertical (glucose level) midpoint between the low plateau and the high plateau. An example of measurement of delay in this fashion is shown in Fig. 1.
Figure 1.
(a) Typical raw data from a period during which glucose rose then fell. Note that the sensor glucose values (blue) lagged behind blood glucose (red) as glucose was rising, but not as glucose was falling. (b) Quantitative measurement of the lag obtained during rising glucose from the data shown in (a). The time difference between the regression lines for changing blood glucose (dotted red line) and changing sensor glucose (dotted blue line) obtained halfway between the lower plateau and the upper plateau (vertical midpoint) is indicated as the dark horizontal bar, the length of which is calculated to be 11.7 min.
The second method was the time-shift optimization method, as reported by Kamath et al. [12] and similar to the method of Breton and Kovatchev [13] and Kovatchev et al. [14]. In this method, least-squares regression lines were initially calculated as above, with no time shift. Then, the sensor data were time shifted in 5-min increments and decrements in order to obtain the optimal time shift which maximized the coefficient of determination calculated for the combined blood glucose and sensor glucose readings. For each data set, a total of 15 coefficients of determination were calculated for time shifts from −35 to 35 min.
For determination of the effect of glycaemic index on glycaemia, we compared blood glucose area under the curve between the two groups. Area under the curve was calculated over 3 h after meals as described [15].
Sensor accuracy was determined by calculating the absolute relative (per cent) difference and the relative difference between sensor glucose and blood glucose. The absolute relative difference is the percentage difference between the sensor glucose value and the arterialized venous reference blood glucose value, calculated as follows: [(reference glucose − sensor glucose)/reference glucose] ×100. The relative difference (per cent bias) is a signed value and calculated as follows: (reference glucose − sensor glucose) × 100. Positive and negative relative difference values indicate overestimates and underestimates, respectively.
Statistics
Data for the outcome variables (duration of lag as measured both by regression delay and by time shift optimization; and measures of sensor accuracy and error) were analysed for distribution and symmetry in the rising and falling data sets. In these data sets, the shape of the distribution was not perfectly consistent, in part because the size of the data sets was not large. The magnitude of the Pearson skewness coefficients was generally between 0 and 0.5 (indicating a substantial degree of symmetry) although one lag set coefficient was 0.8. As, in some cases, there was more than one evaluable segment per subject in any given day, we used generalized estimating equations for comparison of sensor lag and sensor accuracy (Stata, version 10; StataCorp, College Station, TX, USA). The generalized estimating equation method of analysis is designed primarily for Gaussian data sets, although it can handle some non-Gaussian behaviour. To be consistent with generalized estimating equations, we report central tendency as mean and 95% confidence intervals. For other comparisons, including glucose slope during glycaemic index studies, t-tests were used, as indicated.
In addition, to further address the issue of independence of data (potential correlation within data sets obtained in individual subjects), when there were two sensors for any given change segment, the data from the two were combined (averaged).
Results
High glycaemic index vs. low glycaemic index conditions
In high glycaemic index meals (vs. low glycaemic index meals), we found only a small, non-significant difference in the number of evaluable glucose change segments and no difference in absolute relative difference or relative difference. For these reasons, for all subsequent data analysis in this paper, the high and low glycaemic index conditions were analysed together.
Sensor lag: rising vs. falling glucose
Using the linear regression delay method, the lag time of the sensor glucose behind blood glucose was substantially greater for rising segments than for falling segments [8.9 min (6.1–11.6) vs. 1.5 min (−2.6 to 5.5), P < 0.005]. Using the time shift optimization method, results were similar, with a lag that was higher for rising than for falling segments [8.3 min (5.8–10.7) vs. 1.5 min (−2.2 to 5.2), P < 0.001].
An example of sensor lag during rising and falling glucose levels is shown in Fig. 1. Panel (a) shows the raw sensor and raw blood glucose data and panel (b) shows the method of lag measurement in the rising segment using the linear regression delay method. The least-squares regression lines are fit to the ascending blood glucose and sensor data, not including the plateau periods. The lag value (difference between the two regression lines) is taken at the vertical midpoint.
We also observed that, for the glucose change segments, the slope of rising glucose tended to be steeper than that of falling glucose. The magnitude of the glucose rate of change was 0.075 ± 0.026 mmol/l (1.36 ± 0.46 mg/dl) (rising, mean ± SD) vs. 0.05 ± 0.017 mmol/l (0.92 ± 0.30 mg/dl) per min (falling, P < 0.001, unpaired t-test). Because of this finding, we wondered whether high rates of change contributed to the greater lag in rising vs. falling segments. However, there was no correlation between the absolute value of the rate of change and the lag for all segments (r2 = 0.027 for rising segments and r2 = 0.020 for falling segments, neither significant).
Comparison of sensor error during rising vs. falling glucose
Sensor error data are shown in Table 3. As the direction of error was important, the primary metric of sensor error was the (signed) relative difference, i.e. per cent bias. We also calculated the absolute relative difference (per cent difference), a more global, unsigned metric of accuracy. For all data pairs of each rising and falling segment that met all inclusion criteria, a mean and median relative difference and absolute relative difference were calculated. The relative difference values were greater during rising glucose because sensor delays during rising glucose predictably led to underestimates (positive values). Although delays that occur during falling glucose will lead to sensor overestimates (negative values), the small delays that occurred during glucose decline minimized this effect. As shown in Table 3, the general metric of accuracy, the absolute relative difference, was lower (indicating greater accuracy) during falling glucose than during rising glucose.
Table 3.
Sensor accuracy and error
| Glucose sensor error/accuracy metrics | |||
|---|---|---|---|
| Relative difference* | Absolute relative difference† | ||
| Rising | Mean | −8.1 | 11.0 |
| 95% CI | −5.8 to −10.3 | 9.3–12.6 | |
| Median | −6.6 | 9.7 | |
| n (data pairs) | 36 | 36 | |
| Falling | Mean | 2.3 | 8.5 |
| 95% CI | 0.02–4.6 | 7.0–9.9 | |
| Median | 1.2 | 7.4 | |
| n (data pairs) | 36 | 36 | |
| P rising vs. falling | < 0.001 | < 0.001 | |
Relative (per cent) difference is defined as [(sensor glucose − reference glucose)/reference glucose] ×100. The magnitude of relative difference was greater (and more negative) during rising vs. falling glucose, indicating that the sensor error was substantially greater when glucose was rising, at a time when lag was greater.
Absolute relative difference refers to absolute value of the relative difference and was lower when glucose was falling, indicating greater accuracy in that condition.
Effect of glycaemic index on postprandial glucose rise
The high glycaemic index breakfast, compared with the low glycaemic index breakfast, produced a much greater rise in blood glucose as measured as the area under the curve [10 497 ± 2028 vs. 1096 ± 2428 min ×(mg/dl), P = 0.01]. The slope of the blood glucose from 20 to 80 m after the high glycaemic index breakfast was similarly steeper than after the low glycaemic index breakfast [1.3 ± 0.2 vs. 0.2 ± 0.3 (mg/dl)/min, P = 0.002, paired t-test]. There was not a significant difference in postprandial glucose area under the curve or post-meal glucose slopes between the high and low glycaemic index treatments for the lunch meal [4314 ± 3837 vs. 2579 ± 817 min ×(mg/dl), P = NS]. These results showed that the glycaemic index effect was greater with the first meal than with the second.
Discussion
We compared the temporal relationship of sensor glucose values to simultaneously obtained, frequently sampled blood glucose values in persons with Type 1 diabetes when glucose was rising vs. falling. We allowed glucose level to vary rather than use glucose clamps for this study, as we wished to study situations encountered during typical living conditions. The clinical relevance of this study pertains to the accuracy of such devices in situations during which glucose is changing. If there were substantial and fixed sensor lag under all conditions, there would be a systematic error that could be clinically problematic as glucose declines. Because hypoglycaemia can have dangerous consequences, early warning alarms from the device are very important.
In general, the degree of lag in this study was relatively short, as was shown with the same continuous sensor studied by Kamath et al. [12]. When we compared different directions of glucose change, we found that the duration of lag during falling glucose was significantly shorter than during rising glucose. In many cases, the lag during falling glucose was less than zero, suggesting that sensor glucose changed before blood glucose changed. The difference in lag (rising vs. falling) was highly significant when measured either with a linear regression delay method or a time-shift optimization method, the latter being similar to a method used by others [12,14]. The lag results and statistical results using these two methods yielded very similar results.
In order to ensure sufficient rates of change and to minimize calibration error (to minimize confounding of lag measurement), we excluded those glucose change segments of shallow slope, those of short duration, those in which the rise or fall was inconsistent, and those with substantial sensor inaccuracy. The fact that we selected segments with low noise and high sensor accuracy may contribute to the differences of our findings compared with those of other groups [2,7,8]. In addition, these groups used sensors made by different manufacturers and, in some cases, studied animals instead of humans [7,8]. Our finding of a shorter lag during falling glucose, a time of high insulin effect, is in general agreement with those of several other workers [3–6]. A high insulin effect causes glucose to be pulled into cells from the interstitial fluid, causing a reduction of interstitial fluid glucose first, followed by diffusion of glucose down the concentration gradient from plasma to interstitial fluid, leading to decline of plasma glucose [5].
Typically, in our study, we observed a lag of 6–12 min during rising glucose and −6 to +6 min during falling glucose. Using sensors made by the same manufacturer, Kamath and colleagues found a lag of approximately +6 min when all segments were analysed together. They reported that, when glucose was falling rapidly and was normal or low, the sensor read on average 0.2–0.3 mmol/l (4–6 mg/dl) less than the blood glucose [12]. This finding suggests a shorter lag during falling glucose and agrees with our findings.
These findings suggest that, when glucose is falling, amperometric sensors may be capable of providing early warning of impending hypoglycaemia, although such accuracy cannot be guaranteed. Early warning is beneficial for persons with Type 1 diabetes whose glucose can fall rapidly and unexpectedly. Calibration error, which we were careful to minimize by selection criteria, will tend to introduce noise in to the system and reduce accuracy during glucose change, although we should add that that calibration error can be minimized by frequent calibration [9]. We also acknowledge that, in our study, the sensors were calibrated by a laboratory device (HemoCue 201; Hemocue AB) and that subjects remained largely inactive. In free-living subjects who use standard capillary glucose meters, sensor accuracy will often be lower, which may impair early detection of falling glucose.
As would be expected with a shorter lag duration, sensor accuracy was greater (lower absolute relative difference values) during falling glucose. During rising glucose, the signed values of relative difference (bias) were larger in magnitude and were more negative (indicating sensor underestimates). When glucose was falling, the relative difference was smaller in magnitude, a finding that was predictable in view of the shorter delays in that setting.
After the first meal of the day (but not the second), high glycaemic index meals resulted in much higher postprandial glucose levels vs. low glycaemic index. Several investigators have commented on such differences between meals and have used terms such as the ‘breakfast effect’ or ‘second meal phenomenon’ to describe this effect [16–18], which might be attributable to continued presence of the pre-breakfast insulin bolus or a diurnal change in insulin sensitivity or other metabolic factor [19,20]. In our study, the change in glucose after meals was easily detectable using the sensor.
Limitations of this study included inclusion of data from two different experimental protocols and the presence of some cases of repeated measures in a single individual (although that statistical method, generalized estimating equations, is designed to allow comparisons in the presence of such repetitions). In addition, some criteria for choosing glucose change segments (e.g. the coefficient of variation) are admittedly arbitrary.
We conclude that subcutaneous amperometric glucose sensors, when well-calibrated and functioning accurately, demonstrate a lower lag duration when glucose is falling vs. rising, a finding consistent with a greater insulin effect in that condition. When glucose is falling and lag is minimal, sensor accuracy is greater.
Supplementary Material
Acknowledgments
We thank the Juvenile Diabetes Research Foundation and the Legacy Good Samaritan Foundation for their generous financial support of this project and Legacy Clinical Research Support Services for their technical assistance. We also thank the clinical research centre at Oregon Health and Science University (the Oregon Clinical and Translational Research Institute, OCTRI) for their assistance in carrying out the closed-loop studies. We thank Michael Lasarev for his statistical expertise, and the journal reviewers. The NIH provided support for OCTRI (grant UL1 RR024140) and for JRC’s salary (grant T32 DK 007674).
Footnotes
Competing interests
Nothing to declare.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Simulations of estimated lag times during conditions in which sensor accuracy is impaired by calibration errors.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol. 2009;3:1207–1214. doi: 10.1177/193229680900300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52:2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg F, Meyerhoff C, Mennel FJ, Mayer H, Bischof F, Pfeiffer EF. Does fall in tissue glucose precede fall in blood glucose? Diabetologia. 1996;39:609–612. doi: 10.1007/BF00403309. [DOI] [PubMed] [Google Scholar]
- 4.Thome-Duret V, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, et al. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal Chem. 1996;68:3822–3826. doi: 10.1021/ac960069i. [DOI] [PubMed] [Google Scholar]
- 5.Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab. 2000;278:E716–728. doi: 10.1152/ajpendo.2000.278.4.E716. [DOI] [PubMed] [Google Scholar]
- 6.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26:2405–2409. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 7.Schmidtke DW, Freeland AC, Heller A, Bonnecaze RT. Measurement and modeling of the transient difference between blood and subcutaneous glucose concentrations in the rat after injection of insulin. Proc Natl Acad Sci U S A. 1998;95:294–299. doi: 10.1073/pnas.95.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277:E561–571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 9.El Youssef JE, Castle JR, Engle JM, Massoud RG, Ward WK. Continuous glucose monitoring in subjects with type 1 diabetes: improvement in accuracy by correcting for background current. Diabetes Technol Ther. 2010;12:921–928. doi: 10.1089/dia.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 11.Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–1287. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath A, Mahalingam A, Brauker J. Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11:689–695. doi: 10.1089/dia.2009.0060. [DOI] [PubMed] [Google Scholar]
- 13.Breton M, Kovatchev B. Analysis, modeling, and simulation of the accuracy of continuous glucose sensors. J Diabetes Sci Technol. 2008;2:853–862. doi: 10.1901/jaba.2008.2-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovatchev BP, Shields D, Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11:139–143. doi: 10.1089/dia.2008.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolever TM, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr. 2006;83:1306–1312. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care. 2009;32:1199–1201. doi: 10.2337/dc08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanovic A, Leverton E, Solanky B, Ravikumar B, Snaar JE, Morris PG, et al. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin Sci (Lond) 2009;117:119–127. doi: 10.1042/CS20080542. [DOI] [PubMed] [Google Scholar]
- 18.Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care. 1991;14:95–101. doi: 10.2337/diacare.14.2.95. [DOI] [PubMed] [Google Scholar]
- 19.Schulz B, Ratzmann KP, Albrecht G, Bibergeil H. Diurnal rhythm of insulin sensitivity in subjects with normal and impaired glucose tolerance. Exp Clin Endocrinol. 1983;81:263–272. doi: 10.1055/s-0029-1210235. [DOI] [PubMed] [Google Scholar]
- 20.Bolli GB, Gerich JE. The ‘dawn phenomenon’—a common occurrence in both NIDDM and IDDM. N Engl J Med. 1984;310:746–750. doi: 10.1056/NEJM198403223101203. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm B, Forst S, Weber MM, Larbig M, Pfutzner A, Forst T. Evaluation of continuous glucose monitors during rapid blood glucose changes in patients with type 1 diabetes. Diabetes Technol Ther. 2006;8:146–155. doi: 10.1089/dia.2006.8.146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.