Abstract
Cerebral amyloid angiopathy is caused by deposition of the amyloid β protein in the cerebral vasculature. In analogy to previous observations in Alzheimer disease, we hypothesized that analysis of amyloid β40 and β42 proteins in the cerebrospinal fluid might serve as a molecular biomarker. We observed strongly decreased cerebrospinal fluid amyloid β40 (p < 0.01 vs controls or Alzheimer disease) and amyloid β42 concentrations (p < 0.001 vs controls and p < 0.05 vs Alzheimer disease) in cerebral amyloid angiopathy patients. The combination of amyloid β42 and total tau discriminated cerebral amyloid angiopathy from controls, with an area under the receiver operator curve of 0.98. Our data are consistent with neuropathological evidence that amyloid β40 as well as amyloid β42 protein are selectively trapped in the cerebral vasculature from interstitial fluid drainage pathways that otherwise transport amyloid β proteins toward the cerebrospinal fluid.
The amyloid β-peptide (Aβ) not only accumulates in the brain parenchyma as senile plaques in Alzheimer disease (AD), but also in brain vessels as cerebral amyloid angiopathy (CAA). In contrast to the parenchymal depositions, CAA contains both Aβ40 and Aβ42,1,2 with Aβ40 as the major isoform.3 Progression of CAA is associated with increased Aβ40 accumulation.4
The cerebrospinal fluid (CSF) concentration of Aβ42 is significantly decreased in AD patients5 and in patients with mild cognitive impairment that progress into clinical AD after several years6 compared with controls, whereas the Aβ40 concentration remains unaltered. Furthermore, the increased CSF total tau (t-tau) and phosphorylated tau (p-tau) concentrations in AD5 are likely related to the pathological accumulation of these proteins in neurofibrillary tangles.
CAA can occur in the setting of AD or as an isolated finding. Patients with primary CAA clinically present with multiple large or small hemorrhages restricted to lobar brain regions7; these hemorrhages may be directly related to cognitive decline.8 In addition, autosomal dominantly inherited mutations in the amyloid precursor protein (APP) may cause familial CAA, such as the E693Q mutation causing hereditary cerebral hemorrhage with amyloidosis of the Dutch type (HCHWAD)9 or the D694N mutation causing CAA in an Iowa family.10
In this study, we examined if we could identify a specific pattern of Aβ and tau protein concentrations in CSF of CAA patients. Our a priori hypothesis was that CAA without dementia might be characterized by a CSF pattern, distinct from either controls or AD, of decreased Aβ40 with normal or only marginally elevated tau protein. We discuss our findings in the context of the pathophysiology of CAA as described in literature.
Patients and CSF Analysis
We included 72 AD patients (probable diagnosis according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria), 58 control subjects, and 17 patients with cerebral amyloid angiopathy (CAA; diagnosed according to the Boston criteria7) in this study. Lumbar punctures were performed after informed consent was obtained from the patients themselves or from the patients' legal representatives. CSF was analyzed for Aβ40, Aβ42, t-tau, and p-tau181 by enzyme-linked immunosorbent assay.11 Unpaired t tests for comparison between two groups was used. Chi-square test was used to study gender distribution in the groups. Receiver operator characteristic (ROC) analysis was used to evaluate the prognostic ability of the individual biochemical variables to discriminate between CAA and either controls or AD. Forward linear regression was performed using SPSS (SPSS Inc., Chicago, IL). ROC analysis was also performed for the equation resulting from multivariate logistic regression. More detailed information on the patients included in this study and the CSF and statistical analyses can be found in the supplementary text available online.
Results
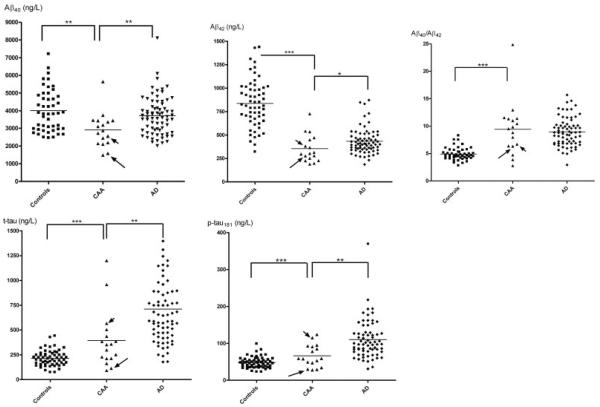
Age, gender, and an overview of the CSF results are provided in the Table and Figures 1 and 2. The age distribution was similar in the CAA and control groups, whereas AD patients were significantly older than CAA patients (p = 0.008; t = 2.71). CSF Aβ42 was decreased in both AD (p < 0.0001; t = 11.7) and CAA (p < 0.0001; t = 7.48) patients compared with control subjects and was even lower in the CAA group compared with the AD group (p = 0.039; t = 2.10). The mean Aβ42 concentration in the CAA group was 42.4% of the controls; in the AD group this was 51.6% of control values. In contrast, however, CSF Aβ40 concentrations were decreased only in the CAA group compared with the AD (p = 0.0063; t = 2.80) and control (p = 0.0012; t = 3.40) groups (CAA: 72.7% of control; AD: 92.8% of control). Both CSF t-tau (p = 0.002; t = 3.19) and p-tau181 (p = 0.0013; t = 3.33) concentrations were significantly higher in the AD patients compared with the CAA group, although the concentrations in the latter group (p = 0.0009; t = 3.46) were also higher than in controls (CAA: t-tau, 184% of control; p-tau181, 138% of control; AD: t-tau, 332% of control; p-tau181, 230% of control). The Aβ40/Aβ42 ratio was clearly elevated in both the AD (p < 0.0001; t = 10.2) and CAA (p < .0001; t = 5.98) groups as compared with the control group (CAA: 192% of control; AD: 182% of control) and did not differentiate between CAA and AD (p = 0.57; t = 0.57). The results were essentially unchanged when the two familial CAA cases were omitted from the analyses and were also unaffected by controlling for subject age.
Table.
Overview of Gender and Age Distribution in the Patient Groups and Results of CSF t-tau, p-tau181, Aβ42, and Aβ40 Analyses
| Gender (M/F; n) | Age (yr)a | Aβ42 (pg/ml)a | Aβ40 (pg/ml)a | Aβ40/ Aβ42a | t-tau (pg/ml)a | p-tau181 (pg/ml)a | |
|---|---|---|---|---|---|---|---|
| CAA | 11/6 | 62.8 ± 11.9 (n = 17) | 355 ± 146 (n = 17) | 2912 ± 977 (n = 17) | 9.43 ± 4.95 (n = 17) | 395 ± 292 (n = 17) | 66.3 ± 30.2 (n = 17) |
|
| |||||||
| AD | 34/38b | 69.4 ± 8.3 (n = 72)c | 433 ± 135 (n = 72)d | 3713 ± 1081 (n = 72)c | 8.95 ± 2.57 (n = 72)b | 712 ± 384 (n = 72)c | 110 ± 51.8 (n = 69)c |
|
| |||||||
| Controls | 28/30b | 61.0 ± 8.7 (n = 58)b | 838 ± 253 (n = 58)e | 4003 ± 1185 (n = 48)c | 4.91 ± 1.13 (n = 48)e | 215 ± 78.3 (n = 58)e | 47.9 ± 14.8 (n = 58)e |
Tests for statistical significance are shown for CAA vs AD and for CAA vs controls. Gender distribution was tested by chi-square analysis; other comparisons by t-tests.
Mean ± standard deviation is shown.
Not significantly different.
p < 0.01.
p < 0.05.
p < 0.001.
CSF = cerebrospinal fluid; t-tau = total tau; p-tau = phosphorylated tau; Aβ = amyloid β-peptide; M = male; F = female; CAA = cerebral amyloid angiopathy; AD = Alzheimer disease.
Fig 1.
Scatter plots of amyloid β-peptide (Aβ)42, Aβ40, total tau, phosphorylated tau181, and Aβ40/Aβ42 in the control, cerebral amyloid angiopathy (CAA) and Alzheimer disease (AD) groups. The CAA patient with the Iowa mutation is indicated by a short arrow, the CAA patient with the Dutch mutation by a long arrow. Mean concentration is indicated by a horizontal line. *p < 0.05; **p < 0.01; ***p < 0.001 (t tests).
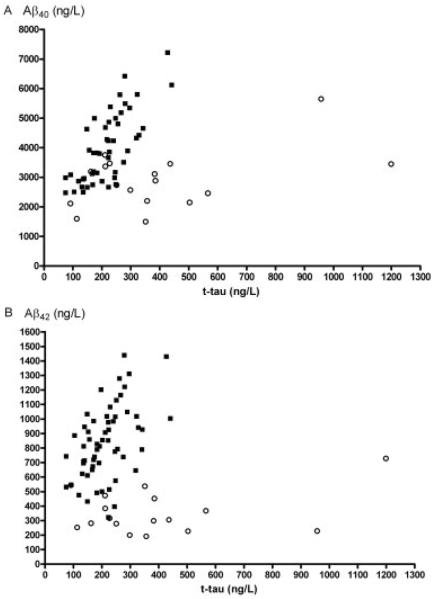
Fig 2.
Combination of amyloid β-peptide (Aβ)40 and total tau (t-tau) analysis (A) or Aβ42 and t-tau analysis (B) in the cerebral amyloid angiopathy (open circles) and control groups (closed squares).
ROC analysis for optimal differentiation of CAA from controls yielded the following results for sensitivity (Se), specificity (Sp), and area under the curves (AUC) for Aβ40 (Se: 87.5%; Sp: 47.1%; AUC: 0.76), Aβ42 (Se: 86.2%; Sp: 94.1%; AUC: 0.96), t-tau (Se: 96.5%; Sp: 52.9%; AUC: 0.73), and p-tau181 (Se: 77.6%; Sp: 58.8%; AUC: 0.67). When Aβ42 and t-tau (Fig 2B) were selected in a forward linear regression procedure (y = −4.264 + 0.014 × Aβ42 − 0.009 × t-tau), ROC analysis resulted in an AUC of 0.98. ROC analysis for optimal differentiation of CAA from AD yielded the following results for Aβ40 (Se: 88.2%; Sp: 59.7%; AUC: 0.74), Aβ42 (Se: 58.8%; Sp: 86.1%; AUC: 0.68), t-tau (Se: 76.5%; Sp: 76.4%; AUC: 0.80), and p-tau181 (Se: 88.2%; Sp: 56.5%; AUC: 0.79). When Aβ42 and p-tau181 were selected in a forward linear regression procedure (y = −3.586 + 0.037 × p-tau181 + 0.005 × Aβ42), ROC analysis resulted in an AUC of 0.82.
Discussion
The major findings of our study are the decreased CSF Aβ40 and Aβ42 concentrations in CSF of CAA patients relative to controls and AD. Our results are particularly striking given the known overlap between CAA and AD pathologies,12 such that the majority of subjects diagnosed with AD have some degree of cerebrovascular amyloid, and the majority of those diagnosed with CAA have some senile plaque pathology, but not to such an extent that a clinical diagnosis of dementia was applicable. The fact that significant differences could nonetheless be identified suggests that the predominance of one pathology relative to the other is strong enough to generate distinct CSF profiles.
The observation of low CSF Aβ40 and Aβ42 possibly relates to the specific mechanisms of Aβ deposition that are involved in the development of CAA. Neurons are the main source of cerebral Aβ, and it has been proposed that Aβ secreted by neurons is dragged along interstitial fluid drainage pathways along the cerebral vasculature toward the subarachnoid space, where it is collected in the CSF.13 Indeed, in transgenic mice models in which neuronally expressed APP leads to excessive production of Aβ, abundant CAA may develop, suggesting a nonvascular source of Aβ.14 The deposition of Aβ in the vasculature is counterbalanced by its transport out of the brain by passage across the blood-brain barrier, for example, by interaction with specific Aβ receptors such as the low-density lipoprotein receptor related protein-1, which mediate the transendothelial transport of Aβ and degradation by proteases such as neprilysin, angiotensin-converting enzyme, or insulin-degrading enzyme.15 The balance between Aβ production, clearance from the brain by transport across the blood-brain barrier or proteolytic degradation, and retention within the brain in the form of senile plaques or CAA determines what amounts of soluble Aβ will finally reach the CSF. Alterations in this dynamic equilibrium of production, clearance, and accumulation may lead to altered Aβ concentrations in the CSF. Thus, the decreased Aβ40 and Aβ42 concentration in CSF of CAA patients is the result of a net reduction in both Aβ peptides eluted with interstitial fluid toward the CSF. Very likely, the most important cause is the selective trapping of Aβ40 and Aβ42 isoforms in the cerebral vasculature. These data are in line with neuropathological data of the deposition of both Aβ40 and Aβ42 proteins in CAA,1,2 whereas the predominant deposition of Aβ42 in diffuse senile plaques in AD leads to selective reduction in CSF levels of this protein in AD.5
In AD CSF t-tau and p-tau181 concentrations are clearly elevated; this has been related to the formation of neurofibrillary tangles and might be a nonspecific consequence of neural damage. In contrast, however, neurofibrillary tangles are not among the pathological hallmarks of CAA patients.16,17 CSF t-tau and p-tau181 concentrations were increased in CAA patients, but not to the same degree as in AD. A plausible explanation for this observation is the presence of some accompanying AD pathology even in these nondemented CAA subjects.
Our studies suggest that CSF analysis may be helpful in the identification of CAA patients, but the diagnostic accuracy to differentiate CAA from AD is limited so far. CSF analysis appears to be more useful for differentiating CAA from non-AD controls, especially the analysis of Aβ42 in combination with t-tau. This observation raises the possibility that CSF testing could be useful in clinical situations where the diagnosis of CAA based on magnetic resonance imaging (MRI) findings alone is not clear-cut. This possibility assumes that potential mimics of CAA (such as hypertensive vasculopathy or multiple cavernous hemangiomas) will show similar CSF Aβ and tau profiles to that seen in our elderly control subjects, an assumption that will need to be assessed in future analyses. Along with this limitation to the current study, we also note that our AD subjects did not undergo T2*-weighted gradient-echo MRI, and therefore could not be excluded from having the CAA-related microbleeds seen in a substantial proportion of AD patients.18 This source of error makes the observed differences in CSF profile between CAA and AD more striking, as any contamination of the AD group with advanced CAA would be expected to bias against significant differences.
Together with recent studies using the Aβ-binding agent Pittsburgh Compound B,19 these results suggest that biomarkers might be used to identify cerebrovascular Aβ. MRI has been the primary noninvasive diagnostic method for CAA,7 but it detects only the secondary consequences of CAA such as microbleeding and white matter hyperintensities, not the vascular amyloid itself. Future studies will determine whether CSF can be used as a quantitative measure of CAA severity, and thus potentially a predictor of the effects of CAA on vascular function and small vessel brain injury.20
Supplementary Material
Acknowledgments
We thank the technicians of the Laboratory of Pediatrics and Neurology for CSF analysis.
Supported by a grant from Zon-MW (Vidi-Vernieuwingsimpuls, 917.46.331) to M.M.V. and the US National Institutes of Health (R01 AG26484) to S.M.G.
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Ab42(43) and Ab40 in senile plaques with end-specific monoclonals: evidence that an initially deposited species is Ab42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 2.Savage MJ, Kawooya JK, Pinsker LR, et al. Elevated Ab levels in Alzheimer's disease brain are associated with selective accumulation of Ab42 in parenchymal amyloid plaques and both Ab40 and Ab42 in cerebrovascular deposits. Amyloid. 1995;2:234–240. [Google Scholar]
- 3.Miller DL, Papayannopoulos IA, Styles J, et al. Peptide composition of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- 4.Alonzo NC, Hyman BT, Rebeck GW, et al. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol. 1998;57:353–359. doi: 10.1097/00005072-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 5.de Jong D, Kremer BP, Olde Rikkert MG, et al. Current state and future directions of neurochemical biomarkers for Alzheimer's disease. Clin Chem Lab Med. 2007;45:1421–1434. doi: 10.1515/CCLM.2007.320. [DOI] [PubMed] [Google Scholar]
- 6.Fagan AM, Roe CM, Xiong C, et al. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 7.Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg SM, Gurol ME, Rosand J, et al. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 9.Levy E, Carman MD, Fernandez-Madrid IJ, et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski TJ, Cho HS, Vonsattel JP, et al. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 11.Reijn TS, Olde Rikkert M, van Geel WJ, et al. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta(42) and tau proteins. Clin Chem. 2007;53:859–865. doi: 10.1373/clinchem.2006.081679. [DOI] [PubMed] [Google Scholar]
- 12.Vinters HV, Wang ZZ, Secor DL. Brain parenchymal and microvascular amyloid in Alzheimer's disease. Brain Pathol. 1996;6:179–195. doi: 10.1111/j.1750-3639.1996.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 13.Weller RO, Subash M, Preston SD, et al. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis J, Xu F, Deane R, et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 15.Miners JS, Baig S, Palmer J, et al. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45:79–90. [PubMed] [Google Scholar]
- 17.Maat-Schieman MLC, Radder CM, van Duinen SG, et al. Hereditary cerebral hemorrhage with amyloidosis (Dutch): a model for congophilic plaque formation without neurofibrillary pathology. Acta Neuropathol. 1994;88:371–378. doi: 10.1007/BF00310382. [DOI] [PubMed] [Google Scholar]
- 18.Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 19.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 20.Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow response in cerebral amyloid angiopathy. Neurology. 2008;71:1424–1430. doi: 10.1212/01.wnl.0000327887.64299.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.